Analyzing Citramalic Acid Enantiomers in Apples and Commercial Fruit Juice by Liquid Chromatography–Tandem Mass Spectrometry with Pre-Column Derivatization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Optical Rotations
2.3. Sample Preparation
2.4. LC–MS/MS
2.5. Validation
2.5.1. Calibration Curves
2.5.2. Intra and Inter-Day Precisions
2.5.3. Recovery
3. Results
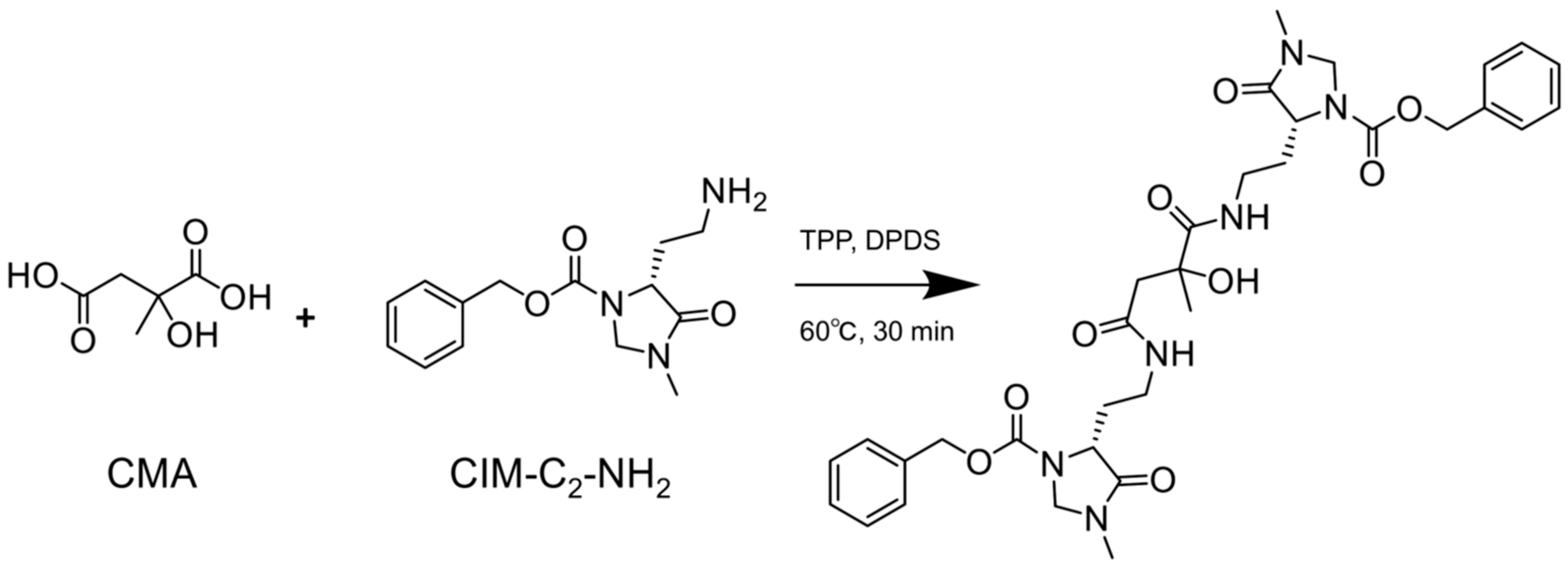
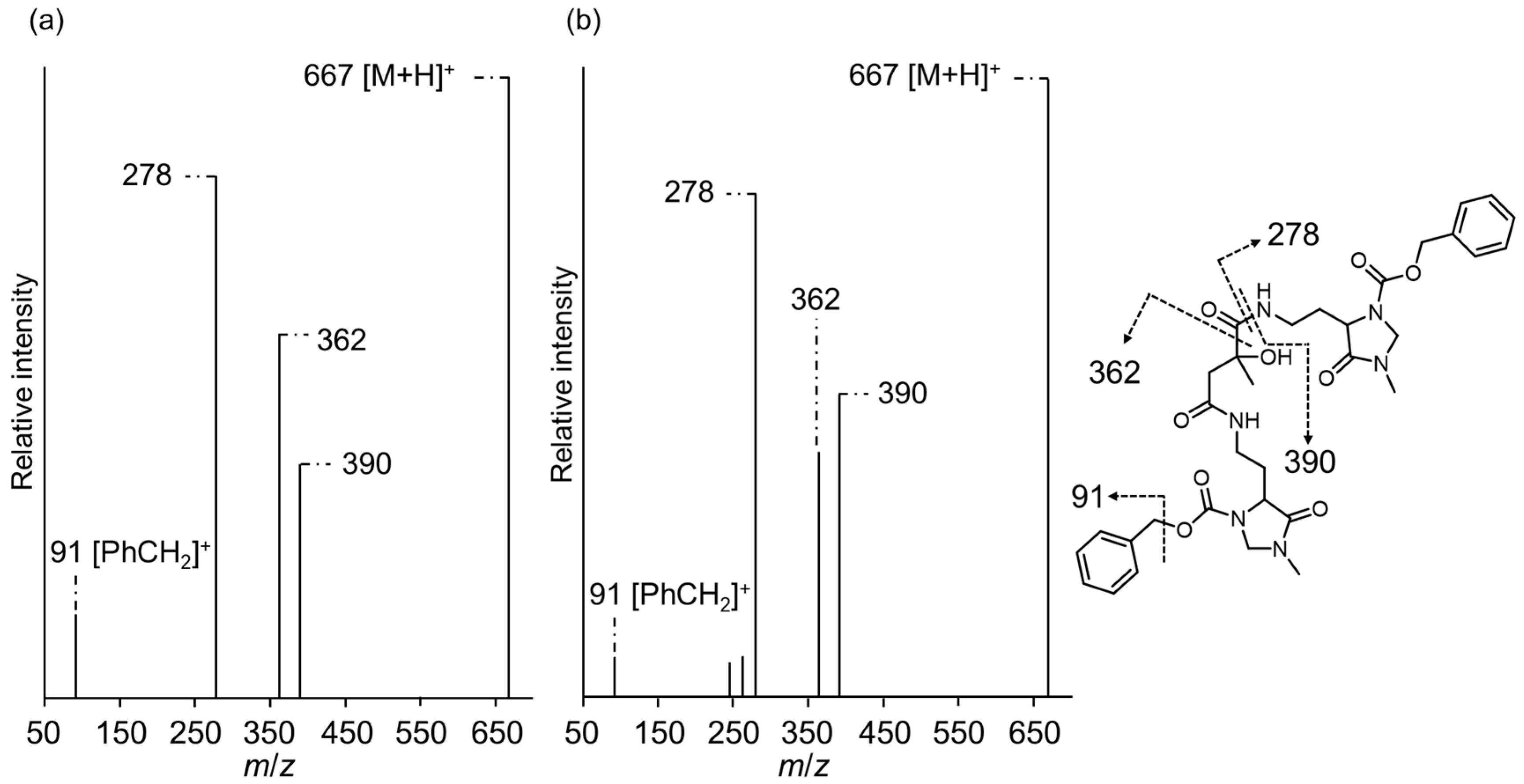
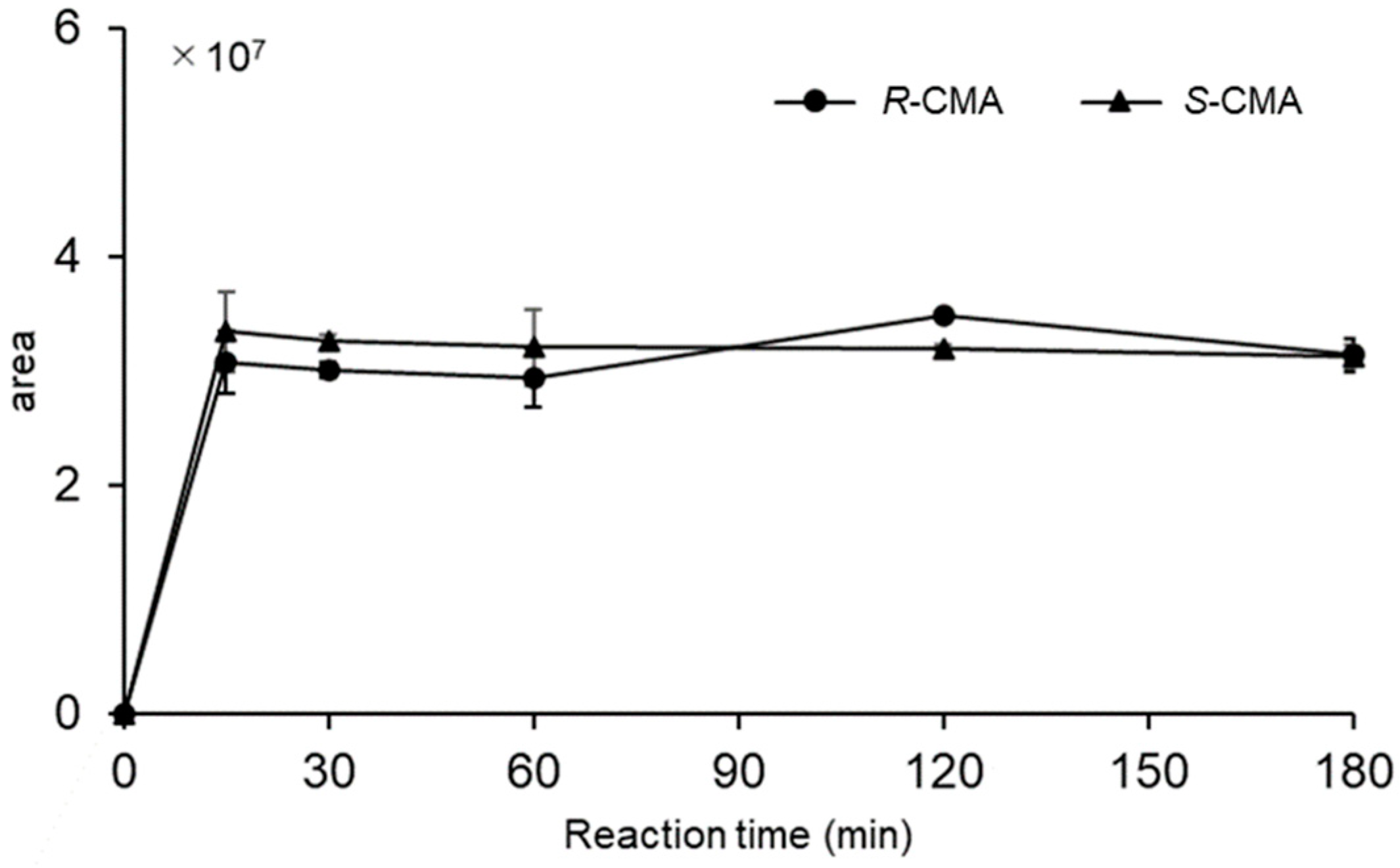
3.1. R- and S-CMA Standards
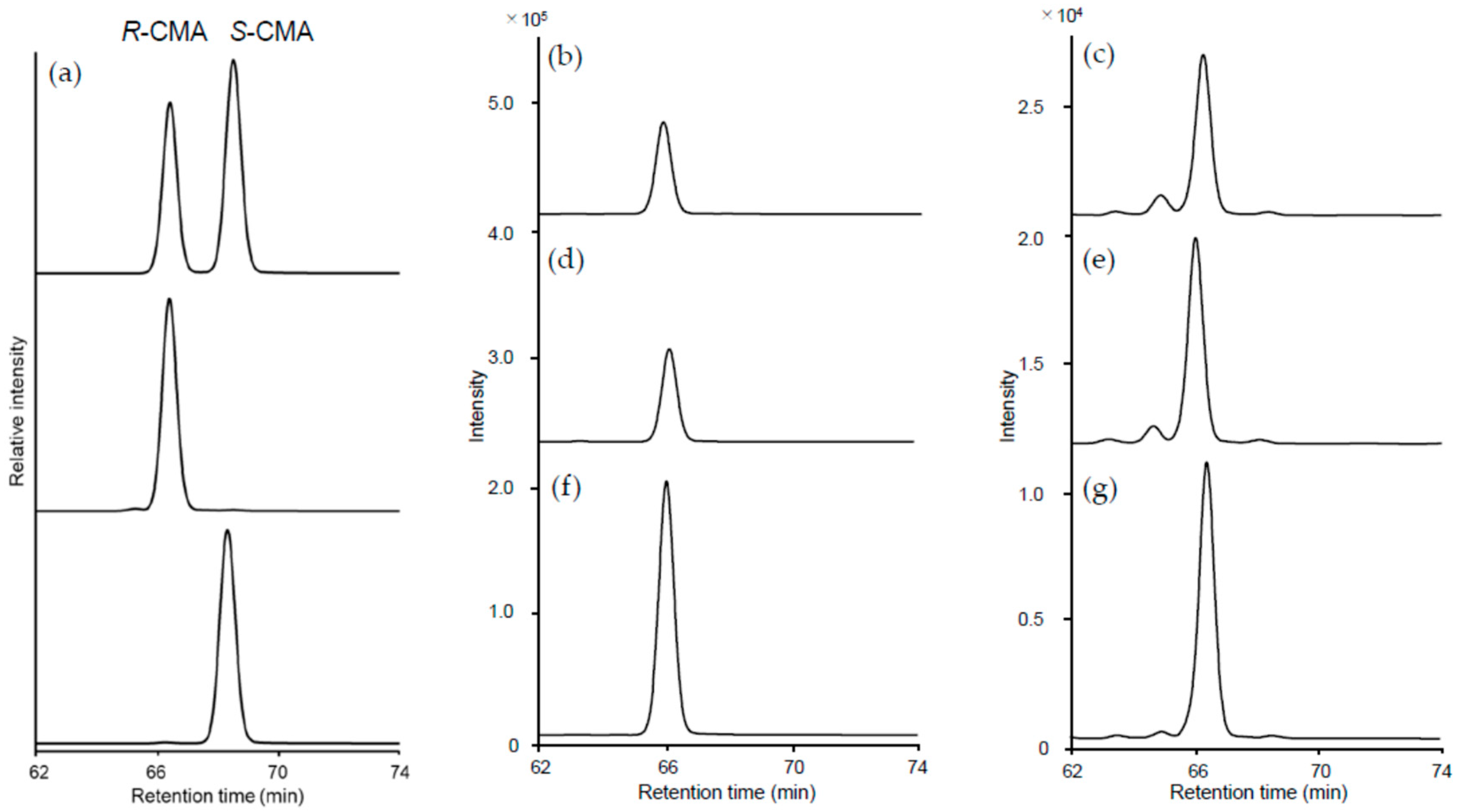
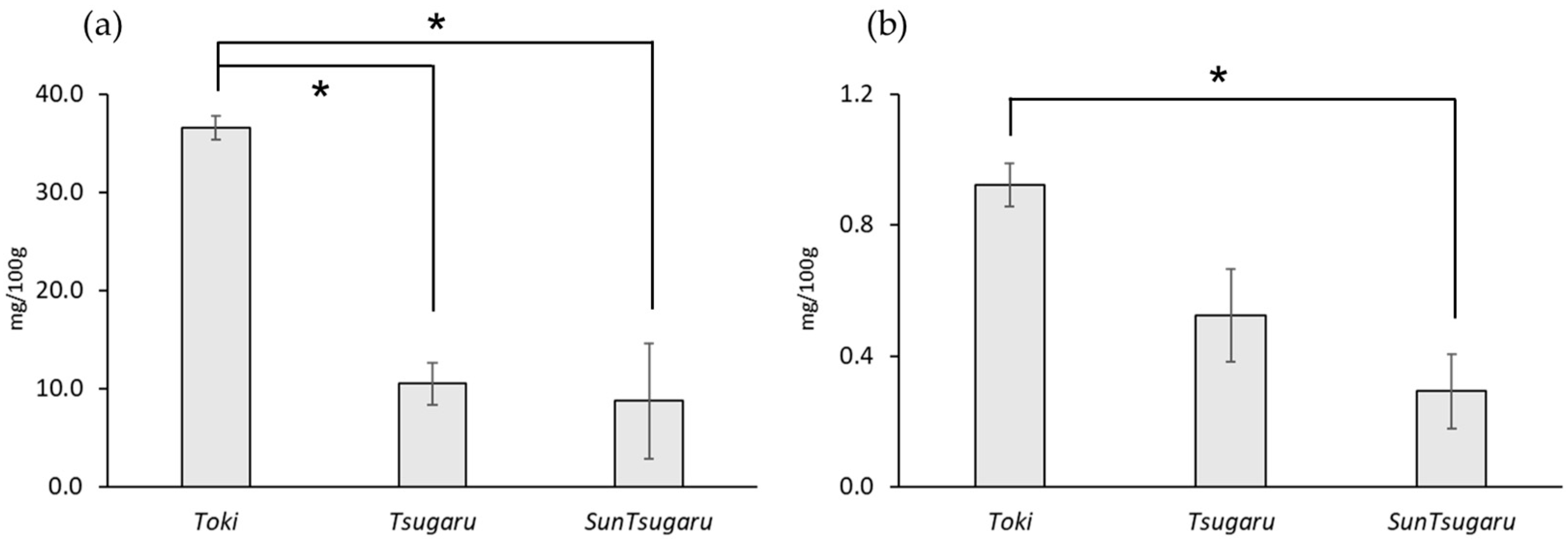
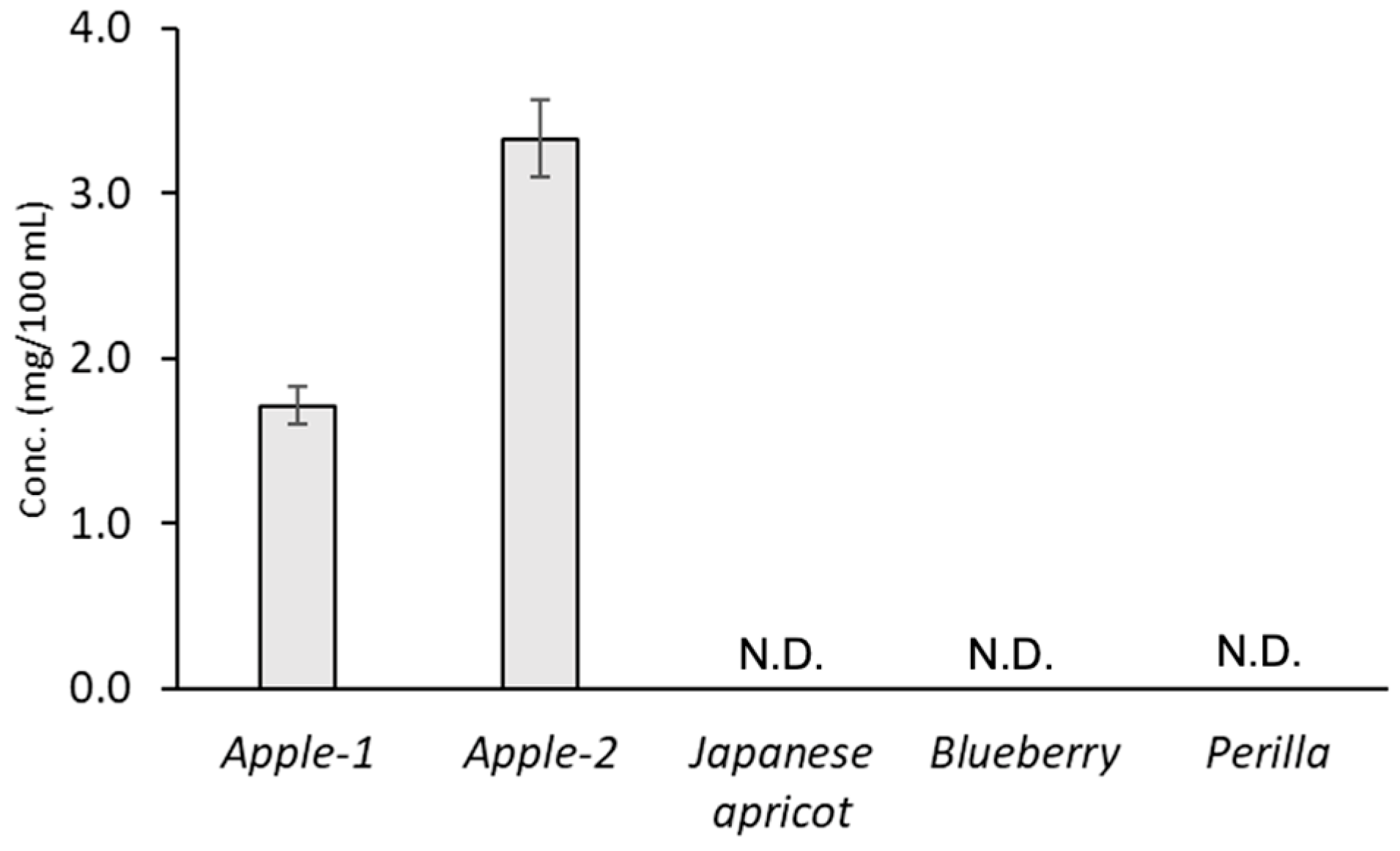
3.2. CMA in Apples and Commercial Fruit Juice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulme, A.C. The isolation of l-citramalic acid from the peel of the apple fruit. Biochim. Biophys. Acta 1954, 14, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.T.; Kornberg, H.L. Enzymic formation of citramalate from acetyl-coenzyme A and pyruvate in Pseudomonas ovalis Chester, catalysed by “pyruvate transacetase”. Biochim. Biophys. Acta 1960, 42, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Engelgau, P.; Jones, A.D.; Song, J.; Beaudry, R. Citramalate synthase yields a biosynthetic pathway for isoleucine and straight- and branched-chain ester formation in ripening apple fruit. Proc. Natl. Acad. Sci. USA 2021, 118, e2009988118. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.H.; Hendrikx, A.; Punt, P.J. Identification of novel citramalate biosynthesis pathways in Aspergillus niger. Fungal Biol. Biotechnol. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- He, B.F.; Ozawa, T.; Nakajima-Kambe, T.; Nakahara, T. Efficient conversion of itaconic acid to (S)-(+)-citramalic acid by Alcaligenes xylosoxydans IL142. J. Biosci. Bioeng. 2000, 89, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.H.; Barker, H.A. Assay and purification of (+)-citramalate hydro-lyase components from Clostridium tetanomorphum. J. Biol. Chem. 1966, 241, 400–408. [Google Scholar] [CrossRef]
- Umino, M.; Sakamoto, T.; Onozato, M.; Fukushima, T. Preparation of imidazolidinone compounds as derivatization reagent for diastereomerization and chromatographic separation of chiral organic acids. J. Chromatogr. A 2022, 1675, 463159. [Google Scholar] [CrossRef]
- Sakamoto, T.; Onozato, M.; Uekusa, S.; Ichiba, H.; Umino, M.; Shirao, M.; Fukushima, T. Development of derivatization reagents bearing chiral 4-imidazolidinone for distinguishing primary amines from other amino acids and application to the liquid chromatography-tandem mass spectrometric analysis of miso. J. Chromatogr. A 2021, 1652, 462341. [Google Scholar] [CrossRef]
- Wu, X.; Eiteman, M.A. Production of citramalate by metabolically engineered Escherichia coli. Biotechnol. Bioeng. 2016, 113, 2670–2675. [Google Scholar] [CrossRef]
- Lebeau, J.; Efromson, J.P.; Lynch, M.D. A review of the biotechnological production of methacrylic acid. Front. Bioeng. Biotechnol. 2020, 8, 207. [Google Scholar] [CrossRef]
- Wu, X.; Eiteman, M.A. Synthesis of citramalic acid from glycerol by metabolically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2017, 44, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.P.; Arnold, S.A.; Baxter, S.; Hall, S.J.; Eastham, G.; Stephens, G. Efficient bio-production of citramalate using an engineered Escherichia coli strain. Microbiol. 2018, 164, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Jones, A.D.; Beaudry, R. Changes in free amino acid content in ‘Jonagold’ apple fruit as related to branched-chain ester production, ripening, and senescence. J. Am. Soc. Hortic. Sci. 2011, 136, 429–440. [Google Scholar] [CrossRef]
- Yin, Q.C.; Ji, J.B.; Zhang, R.H.; Duan, Z.W.; Xie, H.; Chen, Z.; Hu, F.C.; Deng, H. Identification and verification of key taste components in wampee using widely targeted metabolomics. Food Chem. 2022, 13, 100261. [Google Scholar] [CrossRef]
- Amaha, M.; Sai, T. Some aspects of (−)-citramalic acid accumulation by respiration-deficient mutants of yeasts. Antonie Leeuwenhoek 1969, 35, G15-6. [Google Scholar]
- Howell, D.M.; Xu, H.M.; White, R.H. (R)-citramalate synthase in methanogenic archaea. J. Bacteriol. 1999, 181, 331–333. [Google Scholar] [CrossRef]
- Calderon, C.; Santi, C.; Lammerhofer, M. Chiral separation of disease biomarkers with 2-hydroxycarboxylic acid structure. J. Sep. Sci. 2018, 41, 1224–1231. [Google Scholar] [CrossRef]
- Hyun, M.H.; Kim, J.I.; Cho, Y.J.; Han, S.C. Liquid chromatographic resolution of racemic alpha-hydroxycarboxylic acids on ligand exchange chiral stationary phases. Chromatographia 2004, 60, 275–280. [Google Scholar] [CrossRef]
- Onozato, M.; Kanda, R.; Sato, Y.; Sakamoto, T.; Umino, M.; Fukushima, T. Column-switching high-performance liquid chromatography-fluorescence detection method for malic acid enantiomers in commercial wines. J. Food Compos. Anal. 2022, 106, 104282. [Google Scholar] [CrossRef]
- Noro, S.; Kudo, N.; Kitsuwa, T. Differences in sugars and organic acids between red and yellow apple cultivars at time of coloring, and effect of citramalic acid on development of anthocyanin. J. Jpn. Soc. Hort. Sci. 1988, 57, 381–389. [Google Scholar] [CrossRef]
- Di Matteo, G.; Spano, M.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L.; Ingallina, C.; Sobolev, A.P. NMR Characterization of ten apple cultivars from the Piedmont region. Foods 2021, 10, 289. [Google Scholar] [CrossRef] [PubMed]
| ☐ | Linearity | ☐ | Sensitivity | ☐ | Precision | ☐ | Accuracy | ||||||
| ☐ | Equation | R2 | ☐ | LOD a | ☐ | Intra-day | Inter-day | ☐ | Recovery | ||||
| ☐ | (fmol, S/N b = 3) | RSD c % | RSD c % | (%, ± SD) | |||||||||
| ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | low | high | |||||
| R-CMA in peel | 7.52 × 10−4 | x | + | 0.0204 | 0.9996 | ☐ | 15.43 | ☐ | 1.33 | 1.72 | ☐ | 93 ± 4.6 | 93 ± 1.1 |
| R-CMA in fruit | 8.83 × 10−4 | x | + | 0.000599 | 0.9968 | ☐ | ☐ | 1.84 | 7.89 | ☐ | 87 ± 2.4 | 86 ± 2.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umino, M.; Onozato, M.; Sakamoto, T.; Koishi, M.; Fukushima, T. Analyzing Citramalic Acid Enantiomers in Apples and Commercial Fruit Juice by Liquid Chromatography–Tandem Mass Spectrometry with Pre-Column Derivatization. Molecules 2023, 28, 1556. https://doi.org/10.3390/molecules28041556
Umino M, Onozato M, Sakamoto T, Koishi M, Fukushima T. Analyzing Citramalic Acid Enantiomers in Apples and Commercial Fruit Juice by Liquid Chromatography–Tandem Mass Spectrometry with Pre-Column Derivatization. Molecules. 2023; 28(4):1556. https://doi.org/10.3390/molecules28041556
Chicago/Turabian StyleUmino, Maho, Mayu Onozato, Tatsuya Sakamoto, Mikoto Koishi, and Takeshi Fukushima. 2023. "Analyzing Citramalic Acid Enantiomers in Apples and Commercial Fruit Juice by Liquid Chromatography–Tandem Mass Spectrometry with Pre-Column Derivatization" Molecules 28, no. 4: 1556. https://doi.org/10.3390/molecules28041556
APA StyleUmino, M., Onozato, M., Sakamoto, T., Koishi, M., & Fukushima, T. (2023). Analyzing Citramalic Acid Enantiomers in Apples and Commercial Fruit Juice by Liquid Chromatography–Tandem Mass Spectrometry with Pre-Column Derivatization. Molecules, 28(4), 1556. https://doi.org/10.3390/molecules28041556







