Metabolic Profiling and Potential Taste Biomarkers of Two Rambutans during Maturation
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Qualities of BY2 and BY7 Rambutans at Different Growth Stages
2.2. Sugars and Organic Acids of BY2 and BY7 Rambutans at Different Growth Stages
2.3. Amino Acids of BY2 and BY7 Rambutans at Different Growth Stages
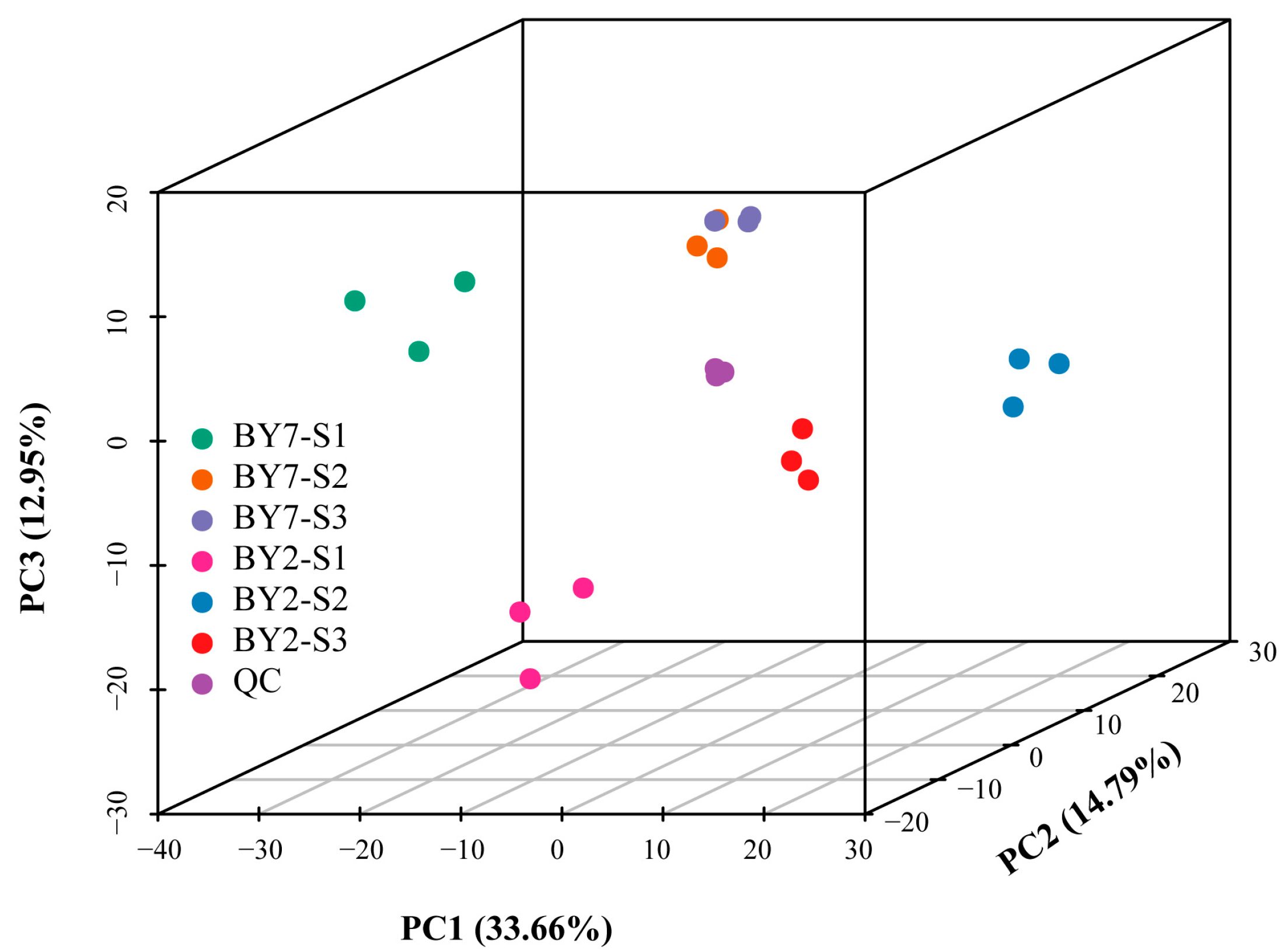
2.4. Widely Targeted Metabolomics Analysis
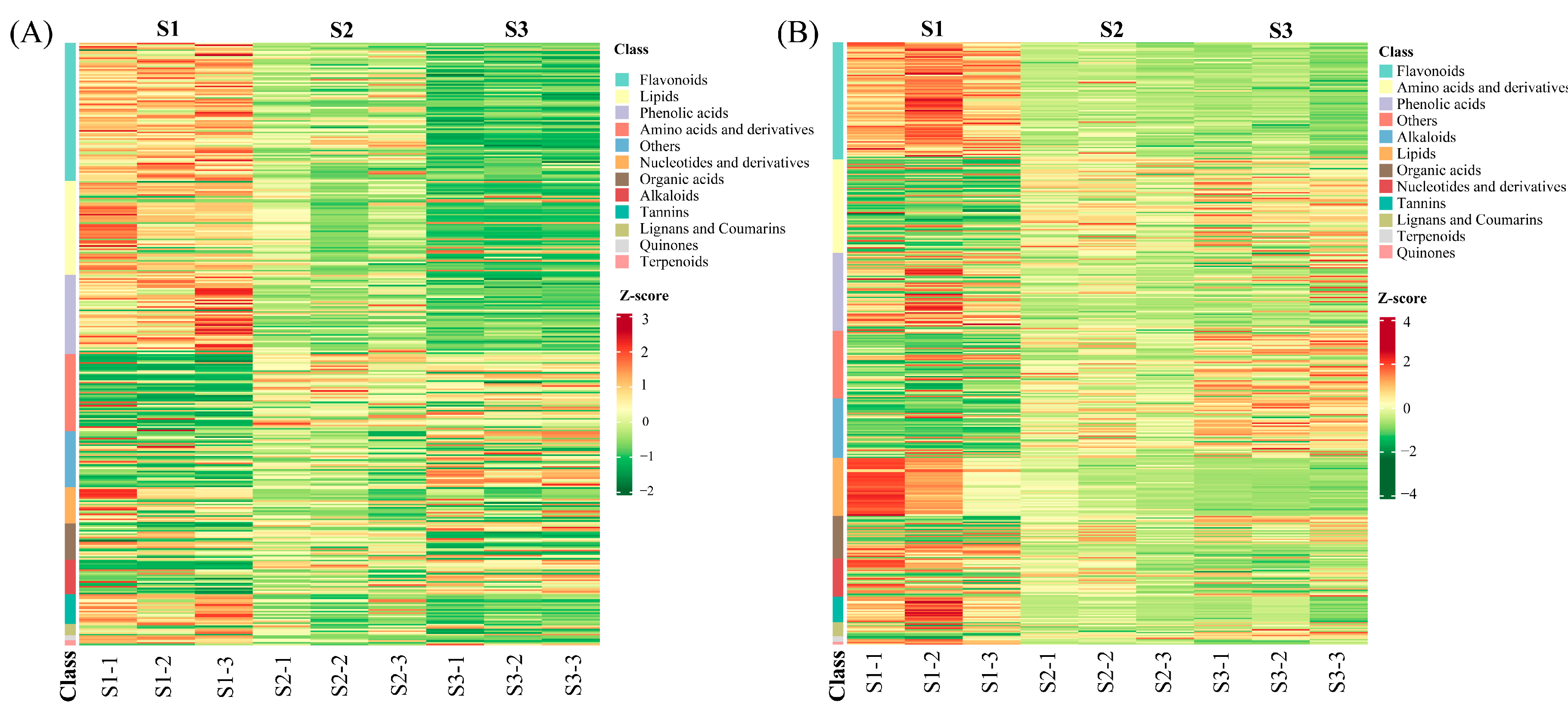
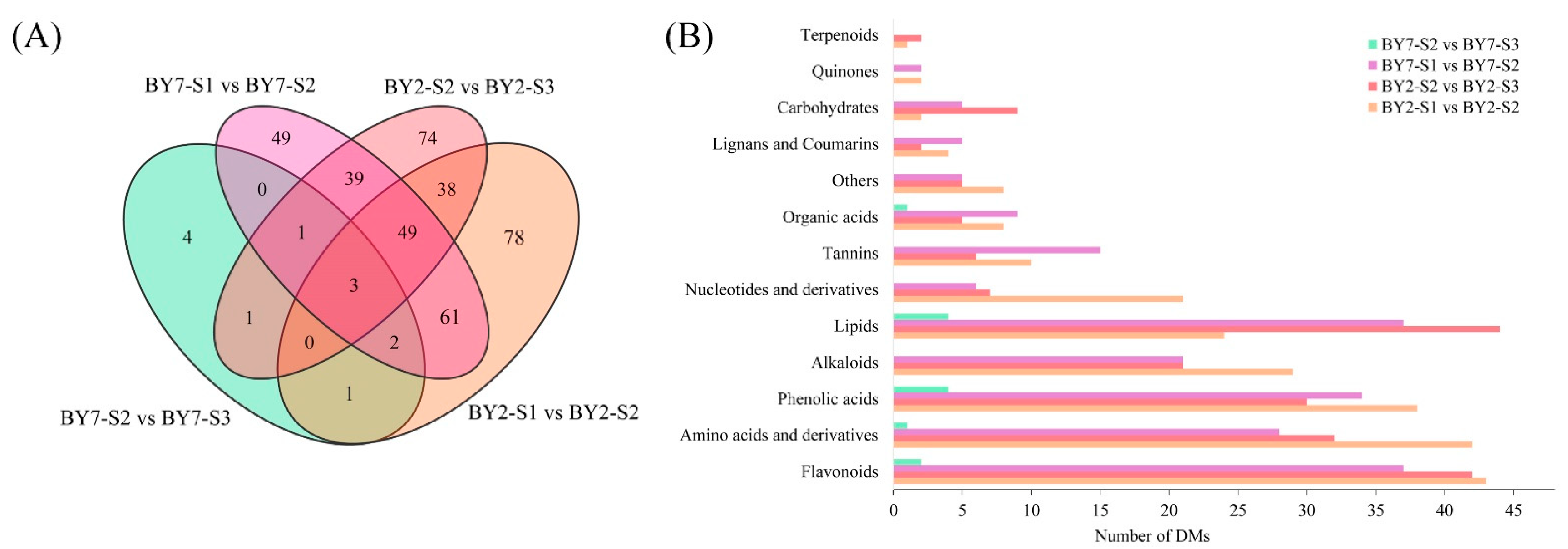
2.5. Identification and Classification of DMs
2.6. Correlation Analysis of Taste Indicators and Metabolites
2.7. Verification of Potential Sugar-Acid Ratio Biomarker
2.8. Verification of Potential Amino Acid Biomarkers
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Materials and Sampling
3.3. Basic Quality Analysis of Rambutans
3.4. Sugar and Organic Acid Analysis
3.5. Amino Acid Analysis
3.6. Widely Targeted Metabolomics Analysis
3.7. Identification and Quantification of Differential Metabolites
3.8. Verification of Potential Taste Biomarkers
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wall, M.M. Ascorbic acid and mineral composition of longan (Dimocarpus longan), lychee (Litchi chinensis) and rambutan (Nephelium lappaceum) cultivars grown in hawaii. J. Food Compos. Anal. 2006, 19, 655–663. [Google Scholar] [CrossRef]
- O’hare, T.J. Postharvest physiology and storage of rambutan. Postharvest Biol. Technol. 1995, 6, 189–199. [Google Scholar] [CrossRef]
- Kong, F.C.; Mohd Adzahan, N.; Karim, R.; Rukayadi, Y.; Mohd Ghazali, H. Selected physicochemical properties of registered clones and wild types rambutan (Nephelium lappaceum L.) fruits and their potentials in food products. Sains Malays 2018, 47, 1483–1490. [Google Scholar]
- Lee, P.R.; Tan, R.M.; Yu, B.; Curran, P.; Liu, S.Q. Sugars, organic acids, and phenolic acids of exotic seasonable tropical fruits. Nutr. Food Sci. 2013, 43, 267–276. [Google Scholar] [CrossRef]
- Deewatthanawong, R.; Meeploy, M. Effect of maturity stages and postharvest treatments on levels of gaba and amino acids in rambutan fruit cv Rongrean. In Proceedings of the 2nd International Conference on Agriculture and Agro-Industry 2014—“Fresh Produce, Novel Process and Health Product”, Chiang Rai, Thailand, 20–21 November 2014. [Google Scholar]
- Yang, R.; Li, Y.; Zhang, Y.; Huang, J.; Liu, J.; Lin, Z.; Yu, Q.; Wu, A.; Wang, B. Widely targeted metabolomics analysis reveals key quality-related metabolites in kernels of sweet corn. Int. J. Genomics 2021, 2021, 2654546. [Google Scholar] [CrossRef]
- Maurer, H.H.; Muddiman, D.C. High-resolution mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hu, O.; Fu, H.; Ouyang, L.; Gong, X.; Meng, P.; Wang, Z.; Dai, M.; Guo, X.; Wang, Y. Uplc-q-tof/ms-based untargeted metabolomics coupled with chemometrics approach for tieguanyin tea with seasonal and year variations. Food Chem. 2019, 283, 73–82. [Google Scholar] [CrossRef]
- Zhou, W.; Niu, Y.; Ding, X.; Zhao, S.; Li, Y.; Fan, G.; Zhang, S.; Liao, K. Analysis of carotenoid content and diversity in apricots (prunus armeniaca L.) grown in China. Food Chem. 2020, 330, 127223. [Google Scholar] [CrossRef]
- Aubert, C.; Chalot, G.; Lurol, S.; Ronjon, A.; Cottet, V. Relationship between fruit density and quality parameters, levels of sugars, organic acids, bioactive compounds and volatiles of two nectarine cultivars, at harvest and after ripening. Food Chem. 2019, 297, 124954. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef]
- Aksic, M.F.; Tosti, T.; Sredojevic, M.; Milivojevic, J.; Meland, M.; Natic, M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Jia, H.; Okamoto, G.; Hirano, K. Effect of amino acid composition on the taste of’hakuho’peaches (prunus persica batsch) grown under different fertilizer levels. J. Jap. Soc. Hortic. Sci. 2000, 69, 135–140. [Google Scholar] [CrossRef]
- Perez, A.G.; Rios, J.J.; Sanz, C.; Olias, J.M. Aroma components and free amino acids in strawberry variety chandler during ripening. J Agri. Food Chem. 2002, 40, 2232–2235. [Google Scholar] [CrossRef]
- Chilakala, S.; Mehtab, V.; Tallapally, M.; Vemula, M.; Shaikh, A.S.; Chenna, S.; Upadhyayula, V. Sec-ms/ms determination of amino acids from mango fruits and application of the method for studying amino acid perturbations due to post harvest ripening. LWT 2021, 138, 110680. [Google Scholar] [CrossRef]
- Hussain, J.; Bukhari, N.; Hussain, H.; Bano, N.; Naeem, A.; Green, I.R. Flavonoids and terpenoids from phlomis cashmeriana and their chemotaxonomic significance. Rec. Nat. Prod. 2010, 4, 242–249. [Google Scholar]
- Adam, K.P. Phenolic constituents of the fern phegopteris connectilis. Phytochemistry 1999, 52, 929–934. [Google Scholar] [CrossRef]
- Schmid, J.; Amrhein, N. Molecular organization of the shikimate pathway in higher plants. Phytochemistry 1995, 39, 737–749. [Google Scholar] [CrossRef]
- Hahlbrock, K. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Phys. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Marsh, K.B.; Boldingh, H.L.; Shilton, R.S.; Laing, W.A. Changes in quinic acid metabolism during fruit development in three kiwifruit species. Funct. Plant Biol. 2009, 36, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Faeh, D.; Chiolero, A.; Paccaud, F. Homocysteine as a risk factor for cardiovascular disease: Should we (still) worry about? Swiss Med. Wkly. 2007, 136, 745–756. [Google Scholar]
- Phuangsombut, K.; Phuangsombut, A.; Terdwongworakul, A. Combination of visible reflectance and acoustic response to improve non-destructive assessment of maturity and indirect prediction of internal quality of red-fleshed pomelo. Int. J. Food Sci. Technol. 2020, 56, 936–944. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Xue, C.; Xu, L.; Sun, H.; Qin, M.F.; Zhang, S.; Wu, J. Distinct transcriptome profiles reveal gene expression patterns during fruit development and maturation in five main cultivated species of pear (Pyrus L.). Sci. Rep. 2016, 6, 28130. [Google Scholar] [CrossRef] [PubMed]
- Rolin, D.; Baldet, P.; Just, D.; Chevalier, C.; Biran, M.; Raymond, P. Nmr study of low subcellular ph during the development of cherry tomato fruit. Aust. J. Plant Physiol. 2000, 27, 61–69. [Google Scholar]
- Lin, C.C.; Yang, Y.C.; Chen, C.Y.; Yin, M.C. Combination of s-methyl cysteine and protocatechuic acid provided greater lipid-lowering and anti-inflammatory effects in mice liver against chronic alcohol consumption. Iran. J. Basic Med. Sci. 2021, 24, 1146–1152. [Google Scholar]
- Ashiq, K.; Tanveer, S.; Qayyum, M.; Bajwa, M.; Ashiq, S.; Shahzad, A.; Tariq, Z.; Faisal, M.; Ahmad, N.; Arsahd, A.; et al. Common herbal plants and their role in control of obesity. Int. J. Biosci. 2019, 15, 23–32. [Google Scholar]
- Diptendu, S.; Amritendu, G. Molecular docking studies with garlic phytochemical constituents to inhibit the human EGFR protein for lung cancer therapy. Int. J. Pharm. Sci. 2022, 13, B1–B14. [Google Scholar]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- GB5009.8-2016; Determination of Fructose, Glucose, Sucrose, Maltose and Lactose in Foods. National Health and Family Planning Commission/State Food and Drug Administration: Beijing, China, 2016.
- GB5009.157-2016; National Food Safety Standards: Determination of Organic Acids in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- GB/T30987-2020; National Food Safety Standards: Determination of Free Amino Acids in Plan. State Adminstration for Market Regulation/National Standardization Administration: Beijing, China, 2020.
- Deng, H.; Yin, Q.C.; Lin, Y.Q.; Feng, J.C.; Chen, Z.; Zhang, R.H. Analysis on quality differences associated with metabolomics of rambutan during different temperature storage. Food Chem. Mol. Sci. 2021, 3, 100036. [Google Scholar] [CrossRef]
- Yin, Q.C.; Zhang, R.H.; Wu, G.; Chen, Z.; Deng, H. Comparative metabolomics analysis reveals the taste variations among three selected wampee cultivars. Plant Foods Hum. Nutr. 2022, 77, 250–257. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Wu, G.; Guo, L.; Hu, F.; Zhou, L.; Xu, B.; Yin, Q.; Chen, Z. Metabolic Profiling and Potential Taste Biomarkers of Two Rambutans during Maturation. Molecules 2023, 28, 1390. https://doi.org/10.3390/molecules28031390
Deng H, Wu G, Guo L, Hu F, Zhou L, Xu B, Yin Q, Chen Z. Metabolic Profiling and Potential Taste Biomarkers of Two Rambutans during Maturation. Molecules. 2023; 28(3):1390. https://doi.org/10.3390/molecules28031390
Chicago/Turabian StyleDeng, Hao, Guang Wu, Li Guo, Fuchu Hu, Liying Zhou, Bin Xu, Qingchun Yin, and Zhe Chen. 2023. "Metabolic Profiling and Potential Taste Biomarkers of Two Rambutans during Maturation" Molecules 28, no. 3: 1390. https://doi.org/10.3390/molecules28031390
APA StyleDeng, H., Wu, G., Guo, L., Hu, F., Zhou, L., Xu, B., Yin, Q., & Chen, Z. (2023). Metabolic Profiling and Potential Taste Biomarkers of Two Rambutans during Maturation. Molecules, 28(3), 1390. https://doi.org/10.3390/molecules28031390






