p-Synephrine Indicates Internal Maturity of Citrus grandis (L.) Osbeck cv. Mato Peiyu—Reclaiming Functional Constituents from Nonedible Parts
Abstract
1. Introduction
2. Results
2.1. Weight and Size Variation
2.2. Extractability of Essential Oil
2.3. Volatile Components in Essential Oil
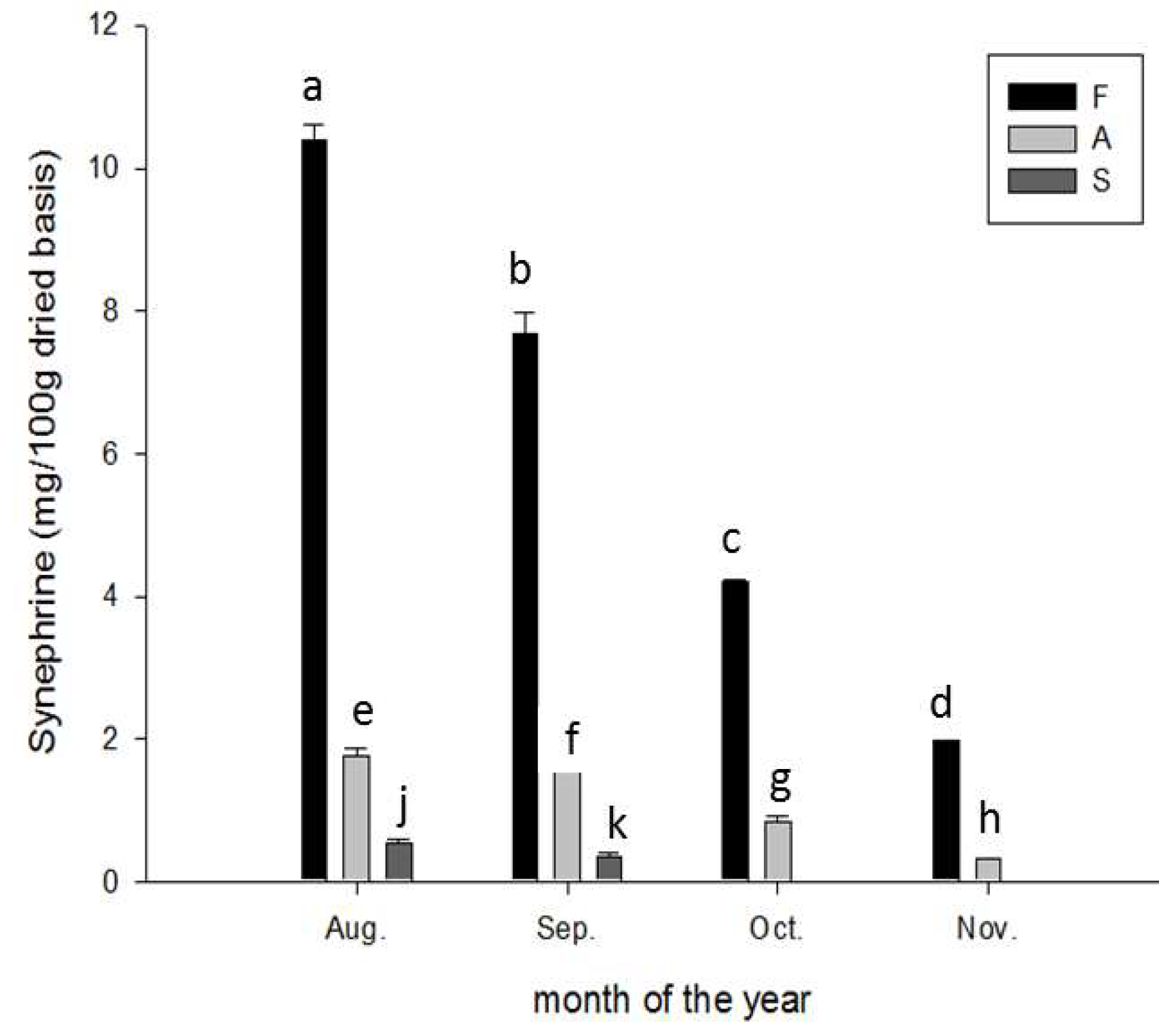
2.4. Content of p-Synephrine
2.5. Limonin and Nomilin Contents
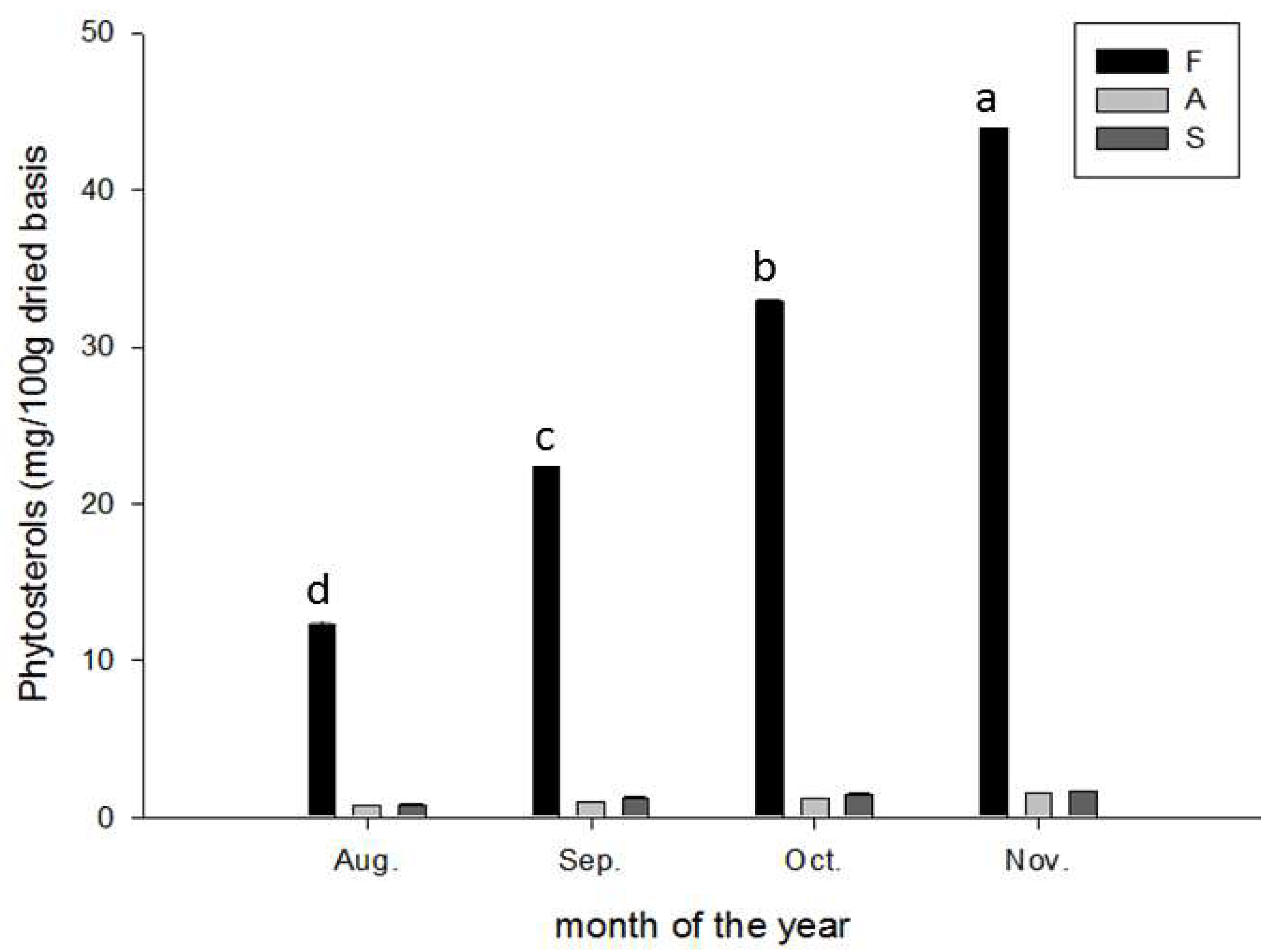
2.6. Phytosterol Content
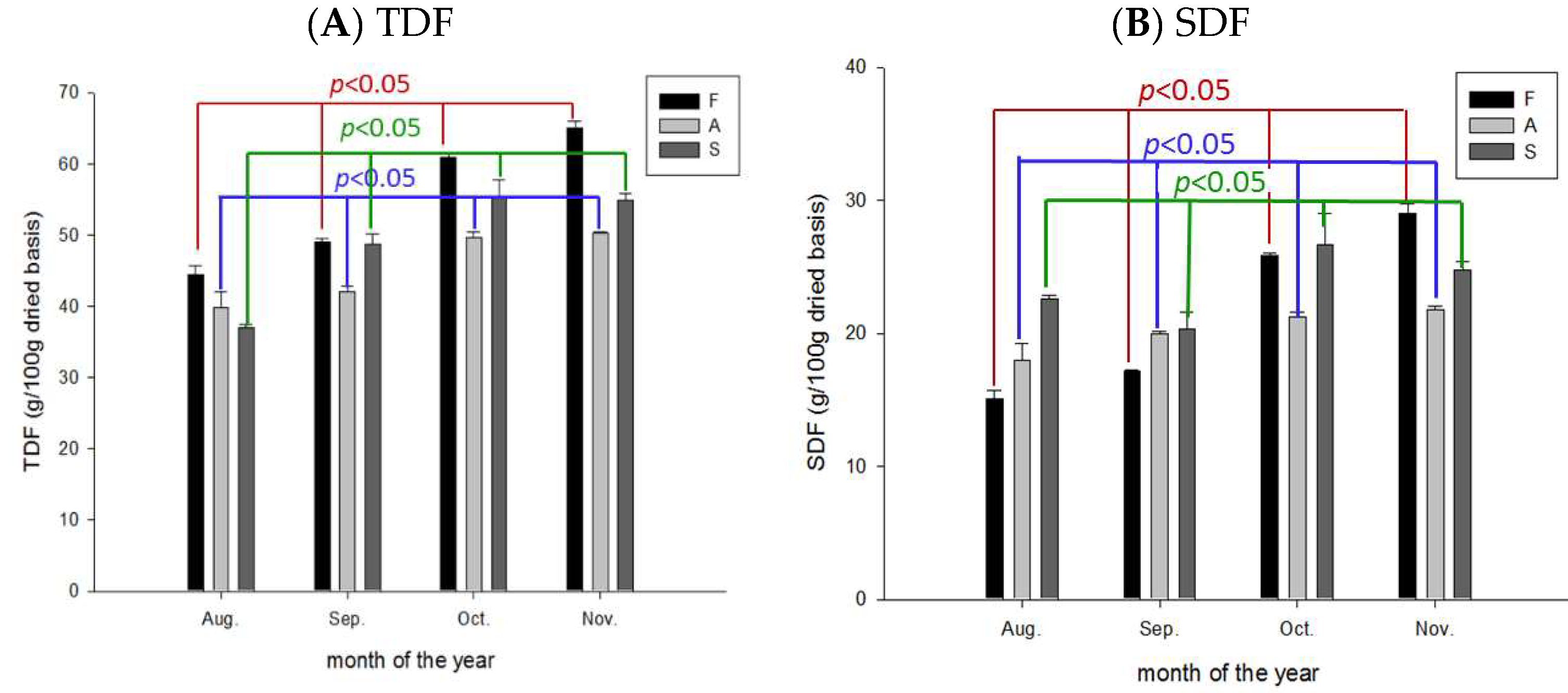
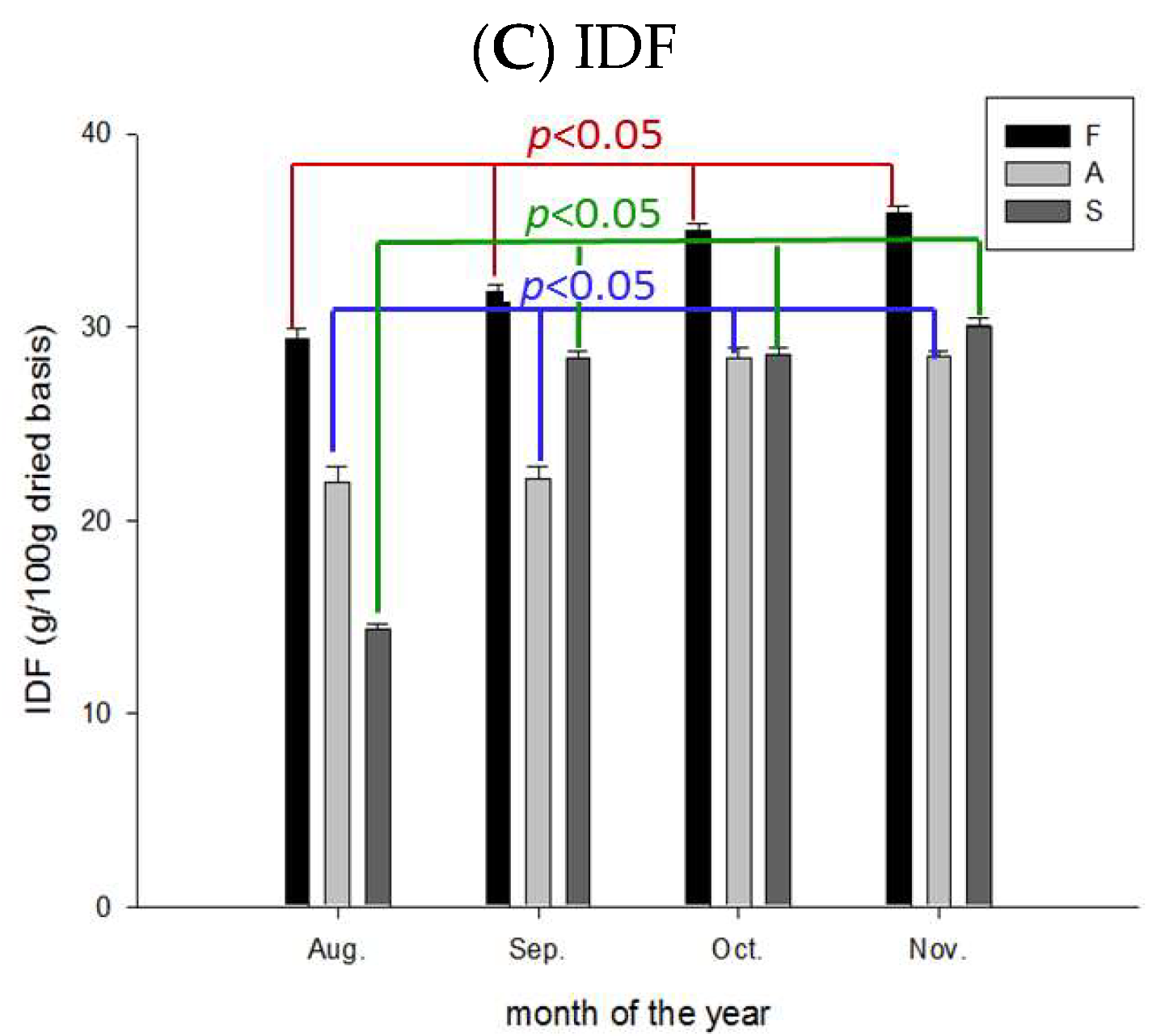
2.7. Variation of Total-, Soluble-, and Insoluble DFs
2.8. Polyphenolic and Flavonoid Contents
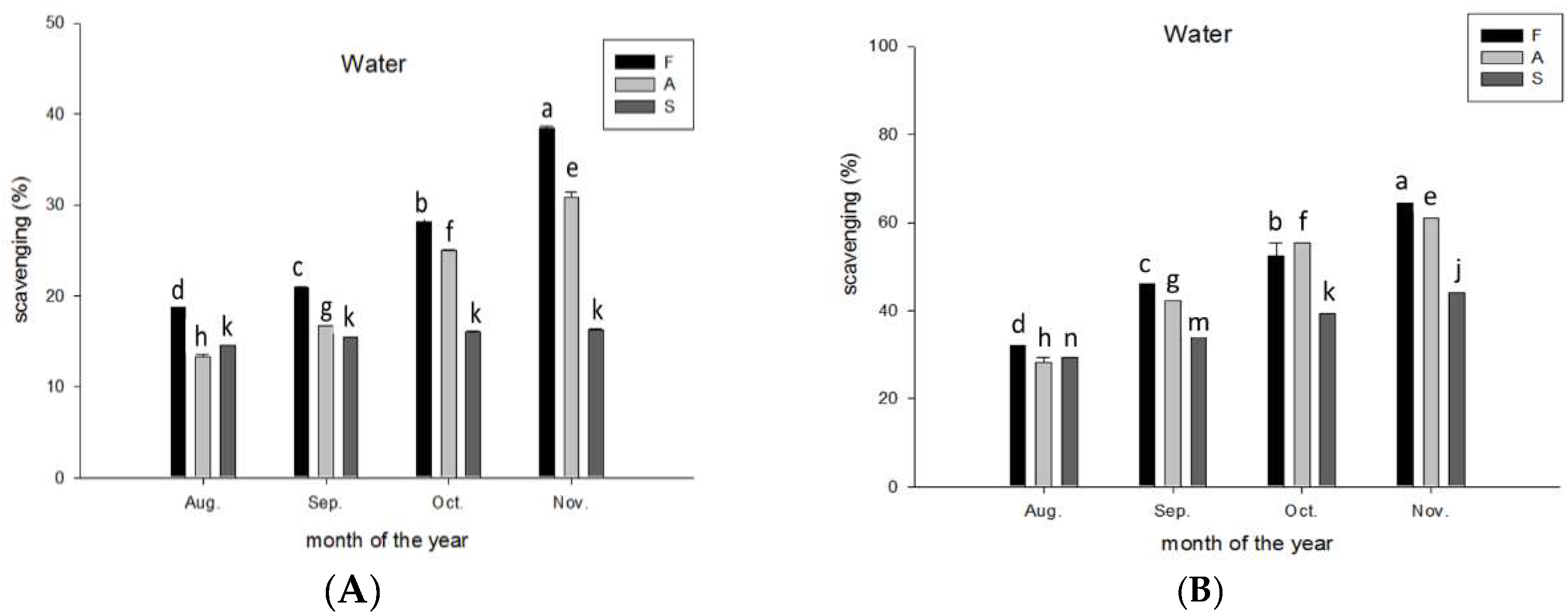
2.9. Antioxidant Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Source of Peiyu Fruits
3.3. Extraction of Peiyu Essential Oil
3.4. Desiccation of the Samples
3.5. Analysis of Flavor Compounds
3.6. Determination of Phenolics
3.7. Determination of Flavonoids
3.8. Assay for p-Synephrine
3.9. Determination of Limonin and Nomilin Contents
3.10. Determination of Phytosterols
3.11. Analysis of DFs
3.12. Determination of Antioxidant Activity
3.12.1. DPPH Free Radical Scavenging Activity
3.12.2. ABTS+• Free Radical Scavenging Activity
3.13. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chang, W.C. The Common Citrus Species in Taiwan; Tainan District Agricultural Research and Extension Station: Taiwan, China, 2011; Volume 381, pp. 80–86.
- Chang, W.C.; Lin, M.Y.; Zuo, J.R. The Cultivation and Management Technology of High Quality Mato Peiyu; Taiwan Agricultural Research Institute, Council of Agriculture (COA): Taiwan, China, 2011.
- Caccioni, D.R.; Guizzardi, M.; Biondi, D.M.; Renda, A.; Ruberto, G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and penicillium italicum. Int. J. Food Microbiol. 1998, 43, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-L.; Lin, C.-D.; Khoo, K.A.; Wang, M.-Y.; Kuan, T.-K.; Lin, W.-C.; Zhang, Y.-N.; Wang, Y.-Y. Composition and Bioactivity of Essential Oil from Citrus grandis (L.) Osbeck ‘Mato Peiyu’ Leaf. Molecules 2017, 22, 2154. [Google Scholar] [CrossRef] [PubMed]
- Anmol, R.J.; Marium, S.; Hiew, F.T.; Han, W.C.; Kwan, L.K.; Wong, A.K.Y.; Khan, F.; Sarker, M.M.R.; Chan, S.Y.; Kifli, N.; et al. Phytochemical and Therapeutic Potential of Citrus grandis (L.) Osbeck: A Review. J. Evid. Based Integr. Med. 2021, 26, 2515690X211043741. [Google Scholar] [CrossRef]
- Gursoy, N.; Tepe, B.; Sokmen, M. Evaluation of the Chemical Composition and Antioxidant Activity of the Peel Oil of Citrus nobilis. Int. J. Food Prop. 2010, 13, 983–991. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Arbo, M.D.; Larentis, E.R.; Linck, V.M.; Aboy, A.L.; Pimentel, A.L.; Henriques, A.T.; Dallegrave, E.; Garcia, S.C.; Leal, M.B.; Limberger, R.P. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem. Toxicol. 2008, 46, 2770–2775. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Xiong, B.; Qiu, X.; Sun, G.; Wang, X.; Zhang, X.; Dong, Z.; Wang, Z. Variation in limonin and nomilin content in citrus fruits of eight varieties determined by modified HPLC. Food Sci. Biotechnol. 2019, 28, 641–647. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Lamonica, G.; Dugo, G. Characterization of Cold-Pressed Key and Persian Lime Oils by Gas Chromatography, Gas Chromatography/Mass Spectroscopy, High-Performance Liquid Chromatography, and Physicochemical Indices. J. Agric. Food Chem. 1997, 45, 3608–3616. [Google Scholar] [CrossRef]
- Minh Tu, N.T.; Onishi, Y.; Choi, H.S.; Kondo, Y.; Bassore, S.M.; Ukeda, H.; Sawamura, M. Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. J. Agric. Food Chem. 2002, 50, 2908–2913. [Google Scholar] [CrossRef]
- Buettner, A.; Mestres, M.; Fischer, A.; Guasch, J.; Schieberle, P. Evaluation of the most odour-active compounds in the peel oil of Clementines (Citrus reticulata Blanco cv. Clementine). Eur. Food Res. Technol. 2003, 216, 11–14. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. J. Agric. Food Chem. 2005, 53, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.-G.; Liu, Y.-J. Chemical Composition and Antimicrobial Activity of the Essential Oil from the Peel of Shatian Pummelo (Citrus grandis Osbeck). Int. J. Food Prop. 2012, 15, 709–716. [Google Scholar] [CrossRef]
- Choi, H.-S.; Sawamura, M. Composition of the Essential Oil of Citrus tamurana Hort. ex Tanaka (Hyuganatsu). J. Agric. Food Chem. 2000, 48, 4868–4873. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.; Suharoschi, R.; Pop, O.L. Dietary Fiber and Prebiotic Compounds in Fruits and Vegetables Food Waste. Sustainability 2021, 13, 7219. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Leporini, M.; Tundis, R.; Vincenzo, S.; Loizzo, M. Citrus species: Modern functional food and nutraceutical-based product ingredient—Review. Ital. J. Food Sci. 2021, 33, 63–107. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Chapter 2—Classification, Technological Properties, and Sustainable Sources, in Dietary Fiber: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 27–58. [Google Scholar]
- Ilce Gabriela, M.-M.; Girish, G. Fruit Processing By-Products: A Rich Source for Bioactive Compounds and Value Added Products. In Food Processing By-Products and Their Utilization; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 11–26. [Google Scholar]
- Fernández-Fernández, A.M.; Dellacassa, E.; Medrano-Fernandez, A.; Del Castillo, M.D. Citrus Waste Recovery for Sustainable Nutrition and Health. In Food Wastes and By-Products; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 193–222. [Google Scholar]
- Feng, M.; Han, J.; Liu, C. The establishment of phytosterols determination in plant oils and the analysis of phytosterols content in edible oil. Chin. J. Hyg. 2006, 18, 197–201. [Google Scholar]
- Heller, S.R.; Milne, G.W.A. EPA/NIH Mass Spectral Data Base; U.S. Department of Commerce, National Bureau of Standards: Gaithersburg, MD, USA, 1978.
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Review of Food Composition Tables and Nutrient Data Banks in Europe. Ann. Nutr. Metab. 1985, 29 (Suppl. 1), 11–45. [CrossRef]
- Deseva, I.; Koleva, E.; Mihaylova, D. HPLC determination of twelve polyphenols: Application in wine analysis. J. Hyg. Eng. Des. 2020, 32, 120–126. [Google Scholar]
- Roman, M.C.; Betz, J.M.; Hildreth, J. Determination of synephrine in bitter orange raw materials, extracts, and dietary supplements by liquid chromatography with ultraviolet detection: Single-laboratory validation. J. AOAC Int. 2007, 90, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis; Association of Official Analytical Collaboration (A.O.A.C.): Washington, DC, USA, 1983. [Google Scholar]
- Lin, L.Y.; Peng, C.C.; Yang, Y.L.; Peng, R.Y. Optimization of bioactive compounds in buckwheat sprouts and their effect on blood cholesterol in hamsters. J. Agric. Food Chem. 2008, 56, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS.+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Julhia, L.; Belmin, R.; Meynard, J.-M.; Pailly, O.; Casabianca, F. Acidity Drop and Coloration in Clementine: Implications for Fruit Quality and Harvesting Practices. Front. Plant Sci. 2019, 10, 754. [Google Scholar] [CrossRef]
- Union, E. Council Regulation (EEC) No 2081/92 ‘Clémentine de Corse’ EC No: FR/00300/02.07.2003; European Union: Brussels, Belgium, 2005. [Google Scholar]
- Blanco Tirado, C.; Stashenko, E.E.; Combariza, M.Y.; Martinez, J.R. Comparative study of Colombian citrus oils by high-resolution gas chromatography and gas chromatography-mass spectrometry. J. Chromatogr. A 1995, 697, 501–513. [Google Scholar] [CrossRef]
- Ou, M.C.; Liu, Y.H.; Sun, Y.W.; Chan, C.F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid. Based Complement Altern. Med. 2015, 2015, 804091. [Google Scholar] [CrossRef]
- Song, H.S.; Sawamura, M.; Ito, T.; Ukeda, H. Chemical compositions of the volatile part of yuzu (Citrus junos Tanaka) peel cold-pressed oils from Japan and Korea. Flavour Fragr. J. 1999, 14, 383–389. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Shara, M. A Review of the Receptor-Binding Properties of p-Synephrine as Related to Its Pharmacological Effects. Oxidative Med. Cell. Longev. 2011, 2011, 482973. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Ruiz-Moreno, C.; Del Coso, J. Acute p-synephrine ingestion increases whole-body fat oxidation during 1-h of cycling at Fatmax. Eur. J. Nutr. 2020, 59, 3341–3345. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hellín, J.; Baltazar-Martins, G.; Rodríguez, I.; Lara, B.; Ruiz-Moreno, C.; Aguilar-Navarro, M.; Del Coso, J. p-Synephrine, the main protoalkaloid of Citrus aurantium, raises fat oxidation during exercise in elite cyclists. Eur. J. Sport Sci. 2021, 21, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Li, J.-M.; Chen, B.-Z.; Zhang, X.-M.; Wu, R.-H.; Wu, P.-P.; Li, C.; Chen, W.-H. Recent Advance in the biological activity of synephrine in Citri Reticulatae Pericarpium. Eur. J. Med. Chem. 2022, 5, 100061. [Google Scholar] [CrossRef]
- Schmitt, G.C.; Arbo, M.D.; Lorensi, A.L.; Maciel, E.S.; Krahn, C.L.; Mariotti, K.C.; Dallegrave, E.; Leal, M.B.; Limberger, R.P. Toxicological effects of a mixture used in weight loss products: p-Synephrine associated with ephedrine, salicin, and caffeine. Int. J. Toxicol. 2012, 31, 184–191. [Google Scholar] [CrossRef]
- Lutsch, D.J.; Camic, C.L.; Jagim, A.R.; Stefan, R.R.; Cox, B.J.; Tauber, R.N.; Henert, S.E. Effects of a Multi-Ingredient Preworkout Supplement Versus Caffeine on Energy Expenditure and Feelings of Fatigue during Low-Intensity Treadmill Exercise in College-Aged Males. Sports 2020, 8, 132. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; McCollum, G.; Plotto, A.; Manthey, J.A.; Widmer, W.W.; Luzio, G.; Cameron, R. Changes in Volatile and Non-Volatile Flavor Chemicals of “Valencia” Orange Juice over the Harvest Seasons. Foods 2016, 5, 4. [Google Scholar] [CrossRef]
- Zhang, X.; Breksa, A.P., III; Mishchuk, D.O.; Slupsky, C.M. Elevation, Rootstock, and Soil Depth Affect the Nutritional Quality of Mandarin Oranges. J. Agric. Food Chem. 2011, 59, 2672–2679. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Jayaprakasha, G.K.; Jifon, J.; Patil, B.S. Production system and storage temperature influence grapefruit vitamin C, limonoids, and carotenoids. J. Agric. Food Chem. 2012, 60, 7096–7103. [Google Scholar] [CrossRef]
- Awad, A.B.; Downie, A.; Fink, C.S.; Kim, U. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer. Res. 2000, 20, 821–824. [Google Scholar]
- Berger, A.; Jones, P.J.; Abumweis, S.S. Plant sterols: Factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004, 3, 5. [Google Scholar] [CrossRef]
- Jones, P.J.H. Cholesterol-lowering action of plant sterols. Curr. Atheroscler. Rep. 1999, 1, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Garoufi, A.; Vorre, S.; Soldatou, A.; Tsentidis, C.; Kossiva, L.; Drakatos, A.; Marmarinos, A.; Gourgiotis, D. Plant sterols-enriched diet decreases small, dense LDL-cholesterol levels in children with hypercholesterolemia: A prospective study. Ital. J. Pediatr. 2014, 40, 42. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
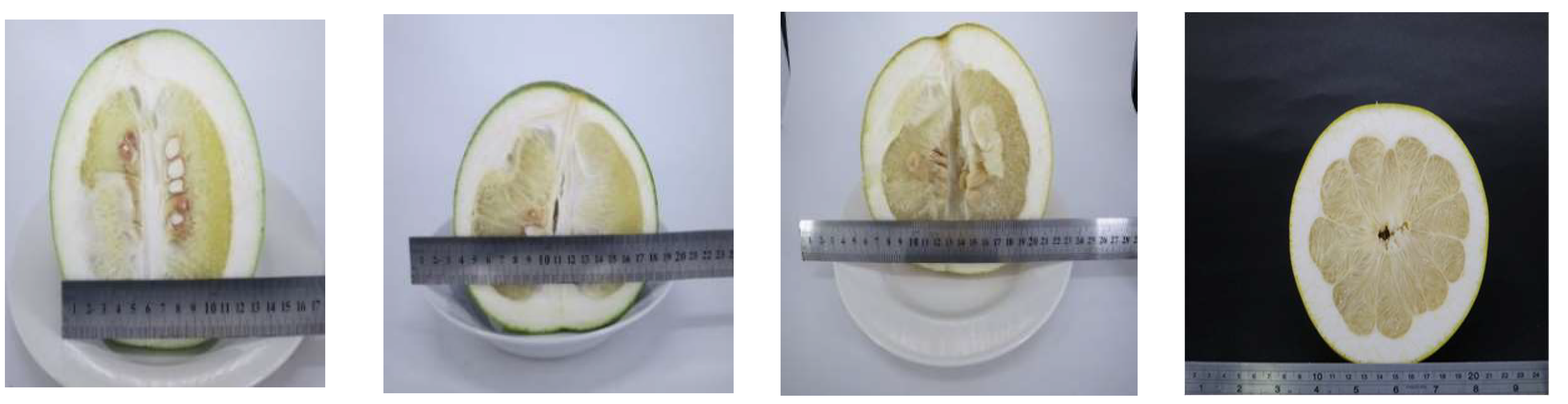
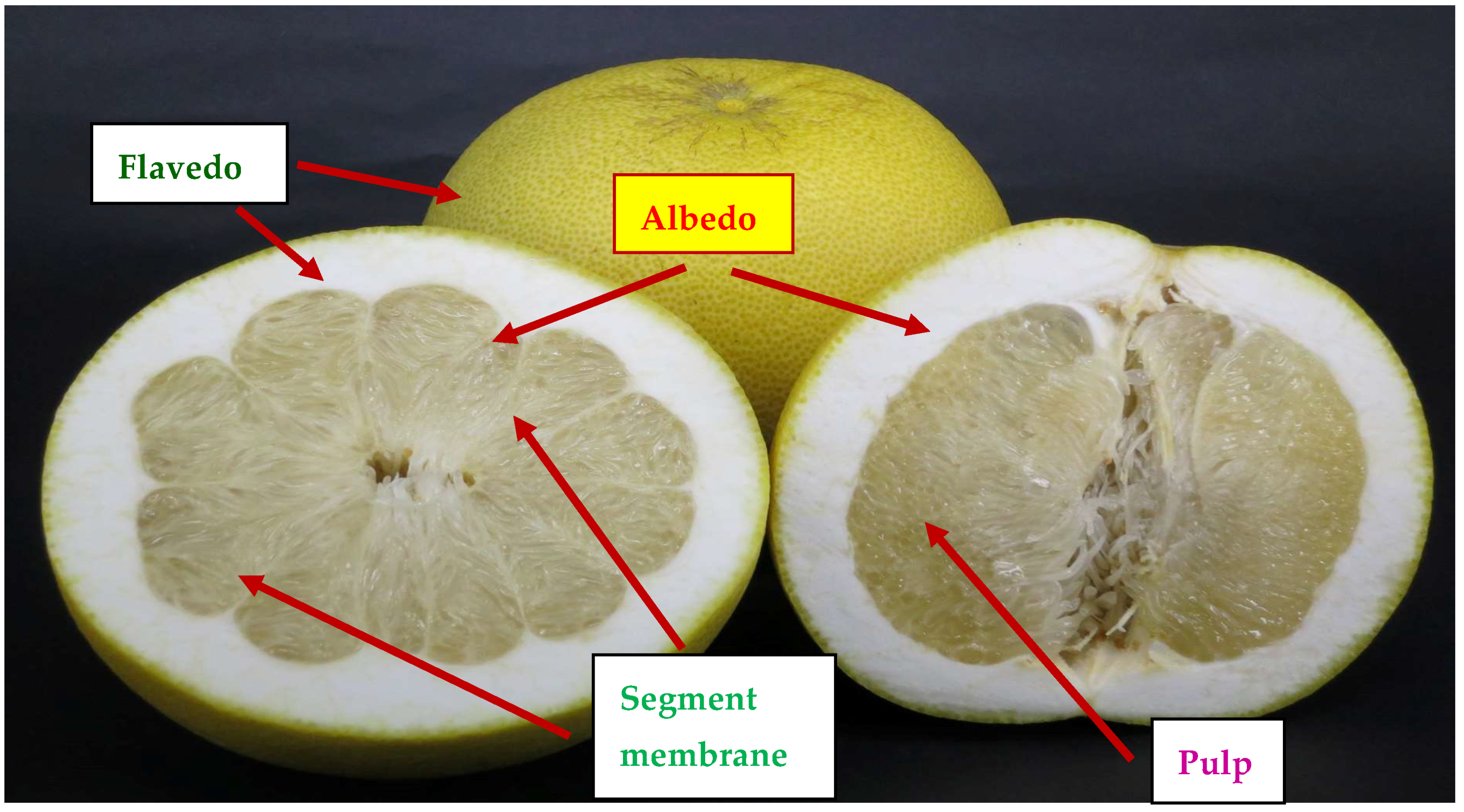


| Months | Pejyu | ||
|---|---|---|---|
| Wt (g) | Width [23] | Length [23] | |
| August | 990 ± 8.32 c | 13.12 ± 1.87 a | 13.89 ± 1.83 a |
| September | 1246 ± 9.35 b | 14.26 ± 3.98 a | 14.02 ± 1.36 a |
| October | 1356 ± 15.98 a | 15.00 ± 2.56 a | 14.98 ± 3.56 a |
| November | 1356 ± 15.99 a | 15.96 ± 5.21 a | 15.10 ± 1.32 a |
| Compounds | RI | CAS-NO | Formula | MW | Composition (%) | |||
|---|---|---|---|---|---|---|---|---|
| Monoterpene | August | September | October | November | ||||
| α-thujene | 927 | 002867-5-2 | C10H16 | 136 | 0.01 | 0.02 | nd | nd |
| α-pinene | 933 | 00080-56-8 | C10H16 | 136 | 1.09 | 1.08 | 1.05 | 0.95 |
| camphene | 948 | 000079-92-5 | C10H16 | 136 | 0.02 | 0.01 | 0.01 | nd |
| sabinene | 968 | 003387-41-5 | C10H16 | 136 | 0.50 | 0.44 | 0.42 | 0.23 |
| β-pinene | 973 | 000127-91-3 | C10H16 | 136 | 1.37 | 1.08 | 0.84 | 0.14 |
| β-myrcene | 982 | 000123-35-3 | C10H16 | 136 | 2.99 | 3.17 | 3.12 | 2.92 |
| α-phellandrene | 997 | 000099-83-2 | C10H16 | 136 | 0.07 | 0.02 | 0.07 | 0.02 |
| limonene | 1042 | 005989-54-8 | C10H16 | 136 | 87.08 | 85.57 | 85.42 | 81.12 |
| β-ocimene | 1045 | 003779-61-1 | C10H16 | 136 | 0.46 | 0.37 | 0.57 | 0.06 |
| γ-terpinene | 1055 | 000099-85-4 | C10H16 | 136 | 0.03 | 0.03 | 0.04 | 0.01 |
| α-terpinolen | 1082 | 000586-62-9 | C10H16 | 136 | 0.03 | 0.02 | 0.02 | 0.02 |
| α-terpinene | 1335 | 000099-86-5 | C10H16 | 136 | 0.10 | 0.08 | 0.10 | 0.09 |
| total | 93.75 | 91.88 | 91.66 | 85.56 | ||||
| Sesquiterpene | ||||||||
| α-copaene | 1376 | 003856-25-5 | C15H24 | 204 | 0.03 | 0.01 | 0.01 | nd |
| β-elemene | 1387 | 000515-13-9 | C15H24 | 204 | 0.08 | 0.07 | 0.07 | 0.08 |
| γ-cadinene | 1417 | 039029-41-9 | C15H24 | 204 | 0.07 | 0.06 | 0.05 | 0.03 |
| β-caryophyllene | 1419 | 000087-44-5 | C15H24 | 204 | 0.18 | 0.11 | 0.08 | 0.06 |
| β-cubebene | 1443 | 013744-15-5 | C15H24 | 204 | 0.02 | 0.02 | 0.02 | 0.10 |
| α-caryophyllene | 1453 | 006753-98-6 | C15H24 | 204 | 0.02 | 0.01 | 0.01 | 0.06 |
| α-amorphene | 1472 | 000483-75-0 | C15H24 | 204 | 0.02 | 0.01 | 0.02 | nd |
| germacrene-D | 1479 | 023986-74-5 | C15H24 | 204 | 1.76 | 1.50 | 1.43 | 1.15 |
| δ-cadinene | 1514 | 000483-76-1 | C15H24 | 204 | 0.03 | 0.02 | 0.03 | 0.02 |
| total | 2.21 | 1.81 | 1.72 | 1.50 | ||||
| Terpene aldehydes | ||||||||
| β-citronellal | 1133 | 000106-23-0 | C10H18O | 154 | 0.03 | 0.03 | 0.04 | 0.04 |
| β-citral | 1218 | 000106-26-3 | C10H16O | 152 | 0.19 | 0.22 | 0.19 | 0.84 |
| α-citral | 1247 | 000141-27-5 | C10H16O | 152 | 0.25 | 0.3 | 0.72 | 0.93 |
| total | 0.47 | 0.55 | 1.55 | 1.81 | ||||
| Terpene alcohols | ||||||||
| linalol | 1087 | 000078-70-6 | C10H18O | 154 | 0.30 | 0.29 | 0.28 | 0.21 |
| 4-terpeneol | 1166 | 000562-74-3 | C10H18O | 154 | 0.05 | 0.05 | 0.04 | 0.03 |
| α-terpineol | 1177 | 010482-56-1 | C10H18O | 154 | 0.18 | 0.16 | 0.15 | 0.14 |
| trans-carveol | 1202 | 001197-07-5 | C10H16O | 152 | 0.05 | 0.07 | 0.05 | 0.02 |
| cis-geraniol | 1213 | 000106-25-2 | C10H18O | 154 | 0.20 | 0.54 | 0.27 | 0.26 |
| α-cadinol | 1632 | 000481-34-5 | C25H26O | 222 | 0.01 | 0.02 | 0.04 | 0.01 |
| total | 0.79 | 1.13 | 0.83 | 0.67 | ||||
| Other | ||||||||
| cis-linalool oxide | 1077 | 005989-33-3 | C10H18O2 | 170 | 0.48 | 0.22 | 0.21 | 0.17 |
| limonene oxide | 1119 | 004680-24-4 | C10H16O | 152 | 0.03 | 0.05 | 0.04 | 0.01 |
| trans-limonene oxide | 1124 | 004959-35-7 | C10H16O | 152 | 0.03 | 0.04 | 0.03 | 0.03 |
| total | 0.54 | 0.31 | 0.28 | 0.21 | ||||
| 1 Months | Hydroxybenzoic Acid | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid (μg/g) | Protocatechuic Acid (μg/g) | ||||||||
| F | A | S | F | A | S | ||||
| August | 110.25 ± 3.56 a | 252.89 ± 4.23 a | 187.36 ± 2.56 a | 65.32 ± 2.36 d | 26.35 ± 2.36 c | 214.98 ± 4.23 c | |||
| September | 97.34 ± 0.58 b | 175.32 ± 1.25 b | 126.36 ± 1.65 b | 138.19 ± 165 c | 46.84 ± 4.32 b | 282.17 ± 3.54 b | |||
| October | 90.99 ± 1.36 c | 107.65 ± 2.02 c | 119.35 ± 0.98 c | 173.36 ± 3.25 b | 68.64 ± 1.87 a | 299.88 ± 1.00 a | |||
| November | 70.32 ± 2.35 d | 99.27 ± 3.21 d | 105.78 ± 1.69 d | 204.94 ± 1.52 a | 72.35 ± 1.65 a | 302.65 ± 4.87 a | |||
| 1 Months | Hydroxycinnamic Acid | ||||||||
| p-Coumaric Acid (μg/g) | Ferulic Acid (μg/g) | Chlorogenic Acid (μg/g) | |||||||
| F | A | S | F | A | S | F | A | S | |
| August | 169.13 ± 2.89 a | 17.00 ± 2.01 a | 34.32 ± 0.36 a | 12.36 ± 1.32 d | 1.21 ± 0.32 c | 17.07 ± 2.56 d | 59.19 ± 2.54 d | 33.081.10 d | 65.32 ± 2.03 d |
| September | 72.24 ± 3.02 b | 13.48 ± 0.52 b | 18.23 ± 3.32 b | 20.35 ± 3.32 c | 3.32 ± 1.00 b | 24.23 ± 1.33 c | 98.66 ± 1.23 c | 65.51 ± 3.21 c | 88.36 ± 0.32 c |
| October | 42.98 ± 4.12 c | 7.35 ± 1.25 c | 10.36 ± 3.02 c | 34.32 ± 1.45 b | 9.58 ± 0.48 a | 29.01 ± 1.21 b | 123.87 ± 3.20 b | 84.33 ± 4.23 b | 133.11 ± 4.20 b |
| November | 30.63 ± 3.58 d | 4.32 ± 0.75 d | 6.68 ± 1.02 d | 39.36 ± 2.54 a | 10.25 ± 1.00 a | 39.63 ± 3.26 a | 199.36 ± 1.20 a | 108.57 ± 1.54 a | 150.14 ± 3.02 a |
| 1 Months | Glucosides | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Naringin (μg/g) | Hesperidin (mg/g) | Diosmin (mg/g) | |||||||
| F | A | S | F | A | S | F | A | S | |
| August | 283.19 ± 4.65 a | nd | nd | 6.87 ± 0.56 a | 13.34 ± 1.22 a | 7.64 ± 0.58 a | 5.19 ± 0.54 a | nd | nd |
| September | 213.53 ± 5.32 b | nd | nd | 4.10 ± 0.65 b | 7.17 ± 0.78 b | 5.96 ± 0.98 b | 4.66 ± 0.32 a | nd | nd |
| October | 101.76 ± 1.32 c | nd | nd | 3.20 ± 0.32 bc | 4.51 ± 0.65 c | 5.05 ± 0.47 b | 1.36 ± 0.85 b | nd | nd |
| November | 89.32 ± 2.32 d | nd | nd | 2.73 ± 0.85 c | 3.61 ± 0.21 c | 4.79 ± 0.00 b | 0.85 ± 0.69 b | nd | nd |
| 1 Months | Aglycone | Polymethoxylated | |||||||
| Quercetin (μg/g) | Nobiletin (μg/g) | Tangeritin (μg/g) | |||||||
| F | A | S | F | A | S | F | A | S | |
| August | nd | nd | nd | 289.36 ± 1.32 a | nd | nd | 563.7 ± 7.63 a | nd | nd |
| September | nd | nd | nd | 156.34 ± 3.20 b | nd | nd | 351.18 ± 7.25 b | nd | nd |
| October | 181.05 ± 5.32 | nd | nd | 116.31 ± 2.01 c | nd | nd | 323.39 ± 10.25 c | nd | nd |
| November | 248.51 ± 2.02 | nd | nd | 80.36 ± 1.65 d | nd | nd | 259.75 ± 2.03 d | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.-Y.; Peng, C.C.; Huang, Y.-P.; Chen, K.-C.; Peng, R.Y. p-Synephrine Indicates Internal Maturity of Citrus grandis (L.) Osbeck cv. Mato Peiyu—Reclaiming Functional Constituents from Nonedible Parts. Molecules 2023, 28, 4244. https://doi.org/10.3390/molecules28104244
Lin L-Y, Peng CC, Huang Y-P, Chen K-C, Peng RY. p-Synephrine Indicates Internal Maturity of Citrus grandis (L.) Osbeck cv. Mato Peiyu—Reclaiming Functional Constituents from Nonedible Parts. Molecules. 2023; 28(10):4244. https://doi.org/10.3390/molecules28104244
Chicago/Turabian StyleLin, Li-Yun, Chiung Chi Peng, Yi-Ping Huang, Kuan-Chou Chen, and Robert Y. Peng. 2023. "p-Synephrine Indicates Internal Maturity of Citrus grandis (L.) Osbeck cv. Mato Peiyu—Reclaiming Functional Constituents from Nonedible Parts" Molecules 28, no. 10: 4244. https://doi.org/10.3390/molecules28104244
APA StyleLin, L.-Y., Peng, C. C., Huang, Y.-P., Chen, K.-C., & Peng, R. Y. (2023). p-Synephrine Indicates Internal Maturity of Citrus grandis (L.) Osbeck cv. Mato Peiyu—Reclaiming Functional Constituents from Nonedible Parts. Molecules, 28(10), 4244. https://doi.org/10.3390/molecules28104244






