In Vitro Evaluation of Antiprotozoal Properties, Cytotoxicity Effect and Anticancer Activity of New Essential-Oil Based Phytoncide Mixtures
Abstract
1. Introduction
2. Results
2.1. Activity against Selected Protozoa
2.2. Compositions Analysis (GC-MS)
2.2.1. Clove Essential Oil
| No. | Peak Name | KI Exp. 1 | KI Adams 2 | KI NIST 3 | CAS 4 | Content [%] 5 | Identification |
|---|---|---|---|---|---|---|---|
| 1 | Chavicol | 1258 | 1255 | 501-92-8 | 0.03 | S, MS, KI | |
| 2 | Eugenol | 1364 | 1359 | 1357 | 97-53-0 | 71.45 | S, MS, KI |
| 3 | α-Copaene | 1379 | 1375 | 1376 | 3856-25-5 | 0.10 | MS, KI |
| 4 | trans-Caryophyllene | 1423 | 1419 | 1419 | 13877-93-5 | 14.08 | S, MS, KI |
| 5 | α-Humulene | 1457 | 1454 | 1454 | 6753-98-6 | 3.45 | S, MS, KI |
| 6 | Zonarene | 1527 | 1529 | 1527 | 41929-05-9 | 0.53 | MS, KI |
| 7 | Eugenyl acetate | 1532 | 1524 | 93-28-7 | 9.11 | S, MS, KI | |
| 8 | Unknown | 1557 | 0.29 | - | |||
| 9 | Caryophyllene oxide | 1587 | 1583 | 1581 | 1139-30-6 | 0.96 | S, MS, KI |
2.2.2. Garlic Essential Oil
| No. | Peak Name | KI Exp. 1 | KI NIST 2 | CAS 3 | Content [%] 4 |
|---|---|---|---|---|---|
| 1 | Diallyl sulfide | 860 | 861 | 592-88-1 | 2.62 |
| 2 | Disulfide, methyl 2-propenyl | 923 | 920 | 2179-58-0 | 0.6 |
| 3 | 3H-1,2-Dithiole | 949 | 952 | 288-26-6 | 0.9 |
| 4 | Diallyl disulphide | 1081 | 1081 | 2444-49-7 | 35.2 |
| 5 | Trisulfide, methyl 2-propenyl | 1140 | 1142 | 34135-85-8 | 1.18 |
| 6 | 4-Methyl-1,2,3-trithiolane | 1154 | 1154 | 116664-29-0 | 6.21 |
| 7 | 4H-1,2,3-Trithiine | 1202 | 1202 | 290-30-2 | 0.25 |
| 8 | Trisulfide, di-2-propenyl | 1300 | 1297 | 2050-87-5 | 28.75 |
| 9 | 5-Methyl-1,2,3,4-tetrathiane | 1364 | 1364 | 116664-30-3 | 3.72 |
| 10 | 1-(1-(Methylthio)propyl)-2-propyldisulfane | 1440 | 1431 | 126876-22-0 | 0.53 |
| 11 | Diallyl tetrasulfide | 1542 | 1540 | 2444-49-7 | 16.59 |
| 12 | Disulfide, 1-(1-propenylthio)propyl propyl | 1585 | 1592 | 143193-11-7 | 0.66 |
| 13 | 1-Allyl-2-(1-(allylthio)propan-2-yl)disulfane | 1594 | 1597 | 116664-22-3 | 1.54 |
| 14 | 1,2,3,5-Tetrathiane, 4,6-diethyl-, trans- | 1641 | 1640 | 137363-93-0 | 1.25 |
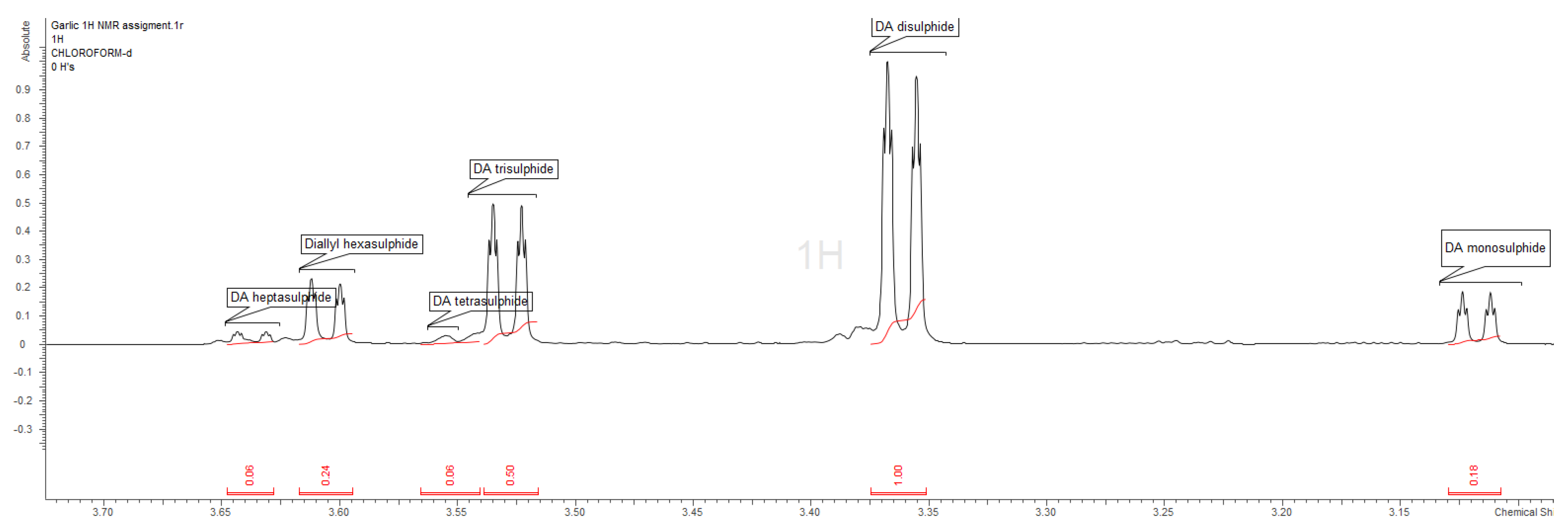
| Precentage a | 1 H Multiplet (ppm) | 13 C (ppm) | Reference | |
|---|---|---|---|---|
| DA monosulphide | 8.8 | 3.12 (dt, J = 7.1, 1.1 Hz, 2H) | 33.35 | [34] |
| DA disulphide | 49.0 | 3.36 (dt, J = 7.4, 1.1 Hz, 2H) | 42.33 | [35] |
| DA trisulphide | 24.5 | 3.53 (dt, J = 7.3, 1.1 Hz, 2H) | 42.12 | [35] |
| DA tetrasulphide | - b | 3.58 (d, J= 7.2 Hz, 2H) | - | [35] |
| DA pentasulphide | 2.9 | 3.36 (dt, J = 7.4, 1.1 Hz, 2H) | 42.50 | [35] |
| DA hexasulphide | 11.8 | 3.61 (dt, J = 7.3, 1.0 Hz, 2H) | 42.47 | [35] |
| DA heptasulphide c | 2.9 | 3.64 (dt, J = 7.3, 1.1 Hz, 2H) | 42.62 | - |

2.2.3. Ceylon Cinnamon Essential Oil
| No. | Peak Name | KI Exp. 1 | KI Adams 2 | KI NIST 3 | CAS 4 | Content [%] 5 | Identification |
|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 939 | 939 | 937 | 80-56-8 | 1.44 | S, MS, KI |
| 2 | Benzaldehyde | 966 | 960 | 962 | 100-52-7 | 0.88 | S, MS, KI |
| 3 | β-Pinene | 980 | 979 | 979 | 127-91-3 | 0.68 | S, MS, KI |
| 4 | p-Cymene | 1028 | 1024 | 1025 | 99-87-6 | 2.23 | S, MS, KI |
| 5 | Limonene | 1032 | 1029 | 1030 | 138-86-3 | 0.68 | S, MS, KI |
| 6 | Eucalyptol | 1035 | 1031 | 1034 | 470-82-6 | 3.49 | S, MS, KI |
| 7 | γ-Terpinene | 1063 | 1059 | 1060 | 99-85-4 | 1.83 | S, MS, KI |
| 8 | Terpinolene | 1090 | 1088 | 1088 | 586-62-9 | 0.87 | S, MS, KI |
| 9 | Linalool | 1100 | 1090 | 1099 | 78-70-6 | 5.50 | S, MS, KI |
| 10 | Phenethyl alcohol | 1116 | 1116 | 60-12-8 | 1.26 | S, MS, KI | |
| 11 | Terpinen-4-ol | 1180 | 1177 | 1177 | 562-74-3 | 0.71 | S, MS, KI |
| 12 | α-Terpineol | 1192 | 1188 | 1189 | 98-55-5 | 2.94 | S, MS, KI |
| 13 | γ-Terpineol | 1198 | 1199 | 1197 | 586-81-2 | 0.21 | S, MS, KI |
| 14 | cis-Cinnamaldehyde | 1224 | 1219 | 1219 | 57194-69-1 | 0.22 | S, MS, KI |
| 15 | Geraniol | 1259 | 1252 | 1255 | 106-24-1 | 0.58 | S, MS, KI |
| 16 | 2-Phenethyl acetate | 1261 | 1258 | 103-45-7 | 1.42 | S, MS, KI | |
| 17 | trans-Cinnamaldehyde | 1277 | 1270 | 1270 | 14371-10-9 | 54.70 | S, MS, KI |
| 18 | trans-Anethole | 1289 | 1284 | 1284 | 104-46-1 | 2.44 | S, MS, KI |
| 19 | trans-Cinnamyl alcohol | 1308 | 1304 | 1312 | 4407-36-7 | 0.44 | S, MS, KI |
| 20 | Limonenal | 1329 | 1326 | 6784-13-0 | 0.16 | MS, KI | |
| 21 | α-Terpinyl acetate | 1353 | 1349 | 1350 | 80-26-2 | 1.66 | S, MS, KI |
| 22 | α-Longipinene | 1355 | 1352 | 1353 | 5989-08-2 | 0.15 | MS, KI |
| 23 | Eugenol | 1362 | 1359 | 1357 | 97-53-0 | 3.20 | S, MS, KI |
| 24 | α-Copaene | 1379 | 1376 | 1376 | 3856-25-5 | 1.37 | S, MS, KI |
| 25 | trans-Caryophyllene | 1422 | 1419 | 1419 | 87-44-5 | 4.14 | S, MS, KI |
| 26 | α-trans-Bergamotene | 1439 | 1432 | 1435 | 13474-59-4 | 0.57 | S, MS, KI |
| 27 | trans-Cinnamyl acetate | 1449 | 1446 | 1446 | 21040-45-9 | 4.27 | MS, KI |
| 28 | α-Humulene | 1457 | 1454 | 1454 | 6753-98-6 | 0.23 | S, MS, KI |
| 29 | Chavibetol acetate | 1532 | 1525 | 61499-22-7 | 0.17 | MS, KI | |
| 30 | γ-trans-Bisabolene | 1546 | 1531 | 1533 | 70286-32-7 | 0.24 | MS, KI |
| 31 | Caryophyllene oxide | 1587 | 1583 | 1581 | 1139-30-6 | 0.18 | S, MS, KI |
| 32 | Benzyl benzoate | 1769 | 1760 | 1762 | 120-51-4 | 1.14 | S, MS, KI |
2.2.4. Rosemary Essential Oil
| No. | Peak Name | KI Exp. 1 | KI Adams 2 | KI NIST 3 | CAS 4 | Content [%] 5 | Identification |
|---|---|---|---|---|---|---|---|
| 1 | Tricyclene | 926 | 925 | 508-32-7 | 0.13 | S, MS, KI | |
| 2 | α-Thujene | 932 | 930 | 929 | 2867-05-2 | 0.15 | S, MS, KI |
| 3 | α-Pinene | 939 | 939 | 937 | 80-56-8 | 13.09 | S, MS, KI |
| 4 | Camphene | 954 | 954 | 952 | 79-92-5 | 5.09 | S, MS, KI |
| 5 | Benzaldehyde | 960 | 960 | 962 | 100-52-7 | 0.00 | S, MS, KI |
| 6 | Sabinene | 977 | 975 | 974 | 3387-41-5 | 0.01 | S, MS, KI |
| 7 | β-Pinene | 980 | 979 | 979 | 127-91-3 | 6.51 | S, MS, KI |
| 8 | β-Myrcene | 993 | 990 | 991 | 123-35-3 | 1.07 | S, MS, KI |
| 9 | α-Fellandrene | 1006 | 1002 | 1005 | 99-83-2 | 0.17 | S, MS, KI |
| 10 | 3-Carene | 1013 | 1011 | 1011 | 13466-78-9 | 0.03 | S, MS, KI |
| 11 | α-Terpinene | 1020 | 1017 | 1017 | 99-86-5 | 0.47 | S, MS, KI |
| 12 | p-Cymene | 1028 | 1024 | 1025 | 99-87-6 | 2.31 | S, MS, KI |
| 13 | Limonene + Eucalyptol | 1036 | 1029/1031 | 1030/1032 | 138-86-3 | 47.93 | S, MS, KI |
| 14 | γ-Terpinene | 1063 | 1059 | 1060 | 99-85-4 | 0.52 | S, MS, KI |
| 15 | trans-Sabinene hydrate | 1071 | 1070 | 1070 | 17699-16-0 | 0.04 | MS, KI |
| 16 | Terpinolene | 1091 | 1088 | 1088 | 586-62-9 | 0.30 | S, MS, KI |
| 17 | Linalool | 1100 | 1096 | 1099 | 78-70-6 | 0.82 | S, MS, KI |
| 18 | Fenchol | 1116 | 1116 | 1113 | 1632-73-1 | 0.04 | S, MS, KI |
| 19 | trans-Sabinol | 1143 | 1142 | 1142 | 471-16-9 | 0.05 | MS, KI |
| 20 | Camphor | 1148 | 1146 | 1144 | 464-49-3 | 11.75 | S, MS, KI |
| 21 | Isoborneol | 1160 | 1160 | 1157 | 124-76-5 | 0.17 | MS, KI |
| 22 | endo-Borneol | 1169 | 1169 | 1167 | 507-70-0 | 2.55 | MS, KI |
| 23 | Terpinen-4-ol | 1180 | 1177 | 1177 | 562-74-3 | 0.40 | S, MS, KI |
| 24 | α-Terpineol | 1192 | 1188 | 1189 | 98-55-5 | 1.22 | S, MS, KI |
| 25 | Verbenone | 1211 | 1205 | 1205 | 80-57-9 | 0.11 | S, MS, KI |
| 26 | Bornyl acetate | 1287 | 1285 | 1285 | 76-49-3 | 0.63 | S, MS, KI |
| 27 | α-Copaene | 1380 | 1375 | 1376 | 3856-25-5 | 0.27 | MS, KI |
| 28 | β-trans-Caryophyllene | 1424 | 1417 | 1419 | 87-44-5 | 3.30 | S, MS, KI |
| 29 | Aromandendrene | 1444 | 1441 | 1440 | 489-39-4 | 0.05 | MS, KI |
| 30 | Humulene | 1458 | 1454 | 1454 | 6753-98-6 | 0.26 | S, MS, KI |
| 31 | γ-Muurolene | 1480 | 1479 | 1477 | 30021-74-0 | 0.20 | MS, KI |
| 32 | γ-Cadinene | 1517 | 1513 | 1513 | 39029-41-9 | 0.12 | MS, KI |
| 33 | δ-Cadinene | 1526 | 1523 | 1524 | 483-76-1 | 0.25 | MS, KI |
2.3. Evaluation of Biological Activity In Vitro
3. Discussion
4. Materials and Methods
4.1. Maintenance of Parasite Cultures and Evaluation of Antiprotozoal Activity
4.2. Essential Oils
4.3. Chemicals and Reagents
4.4. Phytoncides Mixture Preparation
4.5. GC-MS Analysis
4.6. NMR Measurement
4.7. Cell Culture
4.8. Evaluation of Biological Activity on Cell Cultures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Malaria Report 2022; WHO: Geneva, Switzerland, 2022; Volume 2022, p. 372. [Google Scholar]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 23 January 2023).
- Adams, D.S.; Kulkarni, R.R.; Mohammed, J.P.; Crespo, R. A flow cytometric method for enumeration and speciation of coccidia affecting broiler chickens. Vet. Parasitol. 2022, 301, 109634. [Google Scholar] [CrossRef]
- de Andrade, R.M.; Pagnussatt, H.; Talian, L.E.; Santo, A.D.; Ribeiro, A.B.; Leite, F.; Mis, G.; Hoinoski, G.; Aniecevski, E.; Fabiani, L.M.; et al. Interaction between live vaccines for coccidiosis and phytogenic compounds in the diet of broilers. Parasitol. Int. 2022, 89, 102584. [Google Scholar] [CrossRef]
- Su, R.; Jiang, N.; Lu, Y.; Jian, F.; Wang, H.; Zhang, G.; Zhang, L.; Yang, Y. Low prevalence of viable Toxoplasma gondii in swine from slaughter houses in the central of China. Parasitol. Int. 2020, 76, 102090. [Google Scholar] [CrossRef]
- Chomel, B.B. Control and prevention of emerging parasitic zoonoses. Int. J. Parasitol. 2008, 38, 1211–1217. [Google Scholar] [CrossRef]
- Pramanik, P.K.; Alam, M.N.; Roy Chowdhury, D.; Chakraborti, T. Drug Resistance in Protozoan Parasites: An Incessant Wrestle for Survival. J. Glob. Antimicrob. Resist. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Sato, D.; Kobayashi, S.; Yasui, H.; Shibata, N.; Toru, T.; Yamamoto, M.; Tokoro, G.; Ali, V.; Soga, T.; Takeuchi, T.; et al. Cytotoxic effect of amide derivatives of trifluoromethionine against the enteric protozoan parasite Entamoeba histolytica. Int. J. Antimicrob. Agents 2010, 35, 56–61. [Google Scholar] [CrossRef]
- World Health Organization. Strategy to Respond to Antimalarial Drug Resistance in Africa; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Steverding, D.; Rushworth, S.A. Front-line glioblastoma chemotherapeutic temozolomide is toxic to Trypanosoma brucei and potently enhances melarsoprol and eflornithine. Exp. Parasitol. 2017, 178, 45–50. [Google Scholar] [CrossRef]
- Vandekerckhove, S.; D’hooghe, M. Quinoline-based antimalarial hybrid compounds. Bioorganic Med. Chem. 2015, 23, 5098–5119. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Malaria; WHO: Geneva, Switzerland, 2022; p. 240. [Google Scholar]
- Pérez, S.; Ramos-Lopez, M.; Sánchez-Miranda, E.; Fresán-Orozco, M.; Pérez-Ramos, J. Antiprotozoa activity of some essential oils. J. Med. Plant Res. 2012, 6, 2901–2908. [Google Scholar]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Valerino-Díaz, A.B.; Zanatta, A.C.; Gamiotea-Turro, D.; Candido, A.C.B.B.; Magalhães, L.G.; Vilegas, W.; Santos, L.C.d. An enquiry into antileishmanial activity and quantitative analysis of polyhydroxylated steroidal saponins from Solanum paniculatum L. leaves. J. Pharm. Biomed. Anal. 2020, 191, 113635. [Google Scholar] [CrossRef]
- Sahu, A.; Agrawal, R.K.; Pandey, R. Synthesis and systemic toxicity assessment of quinine-triazole scaffold with antiprotozoal potency. Bioorganic Chem. 2019, 88, 102939. [Google Scholar] [CrossRef] [PubMed]
- Ávila Brustolin, A.; Fernandes Herculano Ramos-Milaré, Á.C.; Perles de Mello, T.F.; Alessi Aristides, S.M.; Campana Lonardoni, M.V.; Verzignassi Silveira, T.G. In vitro activity of cinnamaldehyde on Leishmania (Leishmania) amazonensis. Exp. Parasitol. 2022, 236–237, 108244. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, E.M.; Semeih, M.Y. Phyto—Monoterpene linalool as precursor to synthesis epoxides and hydroperoxides as anti carcinogenic agents via thermal and photo chemical oxidation reactions. Arab. J. Chem. 2019, 12, 966–973. [Google Scholar] [CrossRef]
- Manikandan, P.; Vinothini, G.; Vidya Priyadarsini, R.; Prathiba, D.; Nagini, S. Eugenol inhibits cell proliferation via NF-κB suppression in a rat model of gastric carcinogenesis induced by MNNG. Investig. New Drugs 2011, 29, 110–117. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S.K. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci. Rep. 2018, 8, 15787. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci. Rep. 2018, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Mohd Izham, M.N.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials 2019, 9, 1028. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol. 2019, 123, 837–845. [Google Scholar] [CrossRef]
- Das, B.K.; Swamy, A.H.M.V.; Koti, B.C.; Gadad, P.C. Experimental evidence for use of Acorus calamus (asarone) for cancer chemoprevention. Heliyon 2019, 5, e01585. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.N.; S Pillai, G.; Babu, K. Under-utilized herbs and spices. In Handbook Herbs Spices; Woodhead Publishing: Sawston, UK, 2004; Volume 2, pp. 53–103. [Google Scholar]
- Rahamooz Haghighi, S.; Asadi, M.H.; Akrami, H.; Baghizadeh, A. Anti-carcinogenic and anti-angiogenic properties of the extracts of Acorus calamus on gastric cancer cells. Avicenna J. Phytomedicine 2017, 7, 145–156. [Google Scholar]
- Bains, J.S.; Dhuna, V.; Singh, J.; Kamboj, S.S.; Nijjar, K.K.; Agrewala, J.N. Novel lectins from rhizomes of two Acorus species with mitogenic activity and inhibitory potential towards murine cancer cell lines. Int. Immunopharmacol. 2005, 5, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Shi, L.; Zhang, W.; Du, X.; Wang, Z.; Zhang, Y. β-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine 2013, 20, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Koca, H.; Koken, T.; Ozkurt, M.; Kuş, G.; Kus, G.; Erkasap, N.; Çolak, Ö. Effects of Acorus calamus plant extract on prostate cancer cell culture. Anatol. J. Bot. 2018, 2, 46–51. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Malaikozhundan, B.; Saravanakumar, K.; Durán-Lara, E.F.; Wang, M.-H.; Vaseeharan, B. Garlic clove extract assisted silver nanoparticle—Antibacterial, antibiofilm, antihelminthic, anti-inflammatory, anticancer and ecotoxicity assessment. J. Photochem. Photobiol. B Biol. 2019, 198, 111558. [Google Scholar] [CrossRef]
- Salim, A.A.; Bakhtiar, H.; Dawood, D.A.S.; Ghoshal, S.K. Anticancer and cytotoxicity evaluation of silver-cinnamon nanoshells. Mater. Lett. 2023, 334, 133671. [Google Scholar] [CrossRef]
- Block, E. Flavor artifacts. J. Agric. Food Chem. 1993, 41, 692. [Google Scholar] [CrossRef]
- Baker, A.; Graz, M.; Saunders, R.; Evans, G.J.S.; Kaul, S.; Wirth, T. Flow Synthesis of Symmetrical Di- and Trisulfides Using Phase-Transfer Catalysis. J. Flow Chem. 2013, 3, 118–121. [Google Scholar] [CrossRef]
- Bhattacherjee, D.; Sufian, A.; Mahato, S.K.; Begum, S.; Banerjee, K.; De, S.; Srivastava, H.K.; Bhabak, K.P. Trisulfides over disulfides: Highly selective synthetic strategies, anti-proliferative activities and sustained H2S release profiles. Chem. Commun. 2019, 55, 13534–13537. [Google Scholar] [CrossRef]
- Al-Khodir, F.A.I.; Refat, M.S. Investigation of coordination ability of Mn(II), Fe(III), Co(II), Ni(II), and Cu(II) with metronidazole, the antiprotozoal drug, in alkaline media: Synthesis and spectroscopic studies. Russ. J. Gen. Chem. 2017, 87, 873–879. [Google Scholar] [CrossRef]
- Becco, L.; Rodríguez, A.; Bravo, M.E.; Prieto, M.J.; Ruiz-Azuara, L.; Garat, B.; Moreno, V.; Gambino, D. New achievements on biological aspects of copper complexes Casiopeínas®: Interaction with DNA and proteins and anti-Trypanosoma cruzi activity. J. Inorg. Biochem. 2012, 109, 49–56. [Google Scholar] [CrossRef]
- Monzote, L.; Alarcón, O.; Setzer, W. Antiprotozoal Activity of Essential Oils. Agric. Conspec. Sci. 2012, 77, 167–175. [Google Scholar]
- Santoro, G.F.; Cardoso, M.G.; Guimarães, L.G.L.; Mendonça, L.Z.; Soares, M.J. Trypanosoma cruzi: Activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 2007, 116, 283–290. [Google Scholar] [CrossRef]
- Islamuddin, M.; Sahal, D.; Afrin, F. Apoptosis-like death in Leishmania donovani promastigotes induced by eugenol-rich oil of Syzygium aromaticum. J. Med. Microbiol. 2014, 63, 74–85. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical Composition, Antioxidant and Antifungal Potential of Clove (Syzygium aromaticum) Essential Oil, its Major Compound and its Derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Agüero, J.; Fuentes, V. Essential Oil from Buds and Leaves of Clove (Syzygium aromaticum (L.) Merr. et Perry) Grown in Cuba. J. Essent. Oil Res. 2001, 13, 278–279. [Google Scholar] [CrossRef]
- Amelia, B.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.; Sulistyoningrum, A.S.; Haib, J. GC-MS analysis of clove (Syzygium aromaticum) bud essential oil from Java and Manado. AIP Conf. Proc. 2017, 1862, 030082. [Google Scholar] [CrossRef]
- Pepeljnjak, S. Antimicrobial and Antitumor Properties of Medicinal and Spice Plants from Croatia. Biomed. J. Sci. Tech. Res. 2020, 31, 24029–24032. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Horváth, G.; Böszörményi, A.; Ott, P.G. Detection and Identification of Antibacterial and Antioxidant Components of Essential Oils by TLC-Biodetection and GC-MS. Nat. Prod. Commun. 2016, 11, 1934578X1601101120. [Google Scholar] [CrossRef]
- Li, Y.-q.; Kong, D.-x.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Martiniaková, S.; Ácsová, A.; Hojerová, J.; Krepsová, Z.; Kreps, F. Ceylon cinnamon and clove essential oils as promising free radical scavengers for skin care products. Acta Chim. Slovaca 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Behnia, M.; Haghighi, A.; Komeilizadeh, H.; Tabaei, S.J.S.; Abadi, A. In Vitro Antiamoebic Activity of Iranian Allium sativum in Comparison with Metronidazole against Entamoeba histolytica. Iran. J. Parasitol. 2008, 3, 32–38. [Google Scholar]
- Navarro, M.; Gabbiani, C.; Messori, L.; Gambino, D. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives. Drug Discov. Today 2010, 15, 1070–1078. [Google Scholar] [CrossRef]
- Vigan, M. Essential oils: Renewal of interest and toxicity. Eur. J. Dermatol. EJD 2010, 20, 685–692. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Eid, A.M.; Jaradat, N.; Issa, L.; Abu-Hasan, A.; Salah, N.; Dalal, M.; Mousa, A.; Zarour, A. Evaluation of anticancer, antimicrobial, and antioxidant activities of rosemary (Rosmarinus Officinalis) essential oil and its Nanoemulgel. Eur. J. Integr. Med. 2022, 55, 102175. [Google Scholar] [CrossRef]
- Kaushal, S.; Rashmi. Chapter 27—Composition and functionality of clove (Syzygium aromaticum) essential oil. In Clove (Syzygium aromaticum); Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 461–483. [Google Scholar]
- Haase, H.; Hieke, N.; Plum, L.M.; Gruhlke, M.C.H.; Slusarenko, A.J.; Rink, L. Impact of allicin on macrophage activity. Food Chem. 2012, 134, 141–148. [Google Scholar] [CrossRef]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef]
- Dogiel, W.A. Zoologia Bezkręgowców, 3rd ed.; Państwowe Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 1972. [Google Scholar]
- Hempel-Zawitkowska, J. Zoologia dla Uczelni Rolniczych; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2006. [Google Scholar]
- Pawlaczyk-Szpilowa, M. Ćwiczenia z Mikrobiologii Wody i Ścieków; PWN: Warszawa, Poland, 1980. [Google Scholar]
- Feng, J.; Zhu, H.; Lukeš, J.; Korabečná, M.; Fohlerová, Z.; Mei, T.; Chang, H.; Neužil, P. Nanowatt simple microcalorimetry for dynamically monitoring the defense mechanism of Paramecium caudatum. Sens. Actuators A Phys. 2021, 323, 112643. [Google Scholar] [CrossRef]
- Sonneborn, T.M. Chapter 12 Methods in Paramecium Research. In Methods in Cell Biology; Prescott, D.M., Ed.; Academic Press: New York, NY, USA, 1970; Volume 4, pp. 241–339. [Google Scholar]
- Wu, M.; Qin, H.; Deng, J.; Liu, Y.; Lei, A.; Zhu, H.; Hu, Z.; Wang, J. A new pilot-scale fermentation mode enhances Euglena gracilis biomass and paramylon (β-1,3-glucan) production. J. Clean. Prod. 2021, 321, 128996. [Google Scholar] [CrossRef]
- Chomicz, L.; Padzik, M.; Laudy, A.; Kozłowska, M.; Pietruczuk, A.; Piekarczyk, J.; Godineau, N.; Olędzka, G.; Kazimierczuk, Z. Anti-Pentatrichomonas hominis activity of newly synthesized benzimidazole derivatives—In vitro studies. Acta Parasitol. 2009, 54, 165–171. [Google Scholar] [CrossRef]
- Yudin, A.L. Amoeba and Other Protozoa. In Animal Species for Developmental Studies: Volume 1 Invertebrates; Dettlaff, T.A., Vassetzky, S.G., Eds.; Springer: Boston, MA, USA, 1990; pp. 1–11. [Google Scholar]
- Demin, S.Y.; Berdieva, M.A.; Podlipaeva, Y.I.; Goodkov, A.V. Karyotypic instability of endoprophase and mitotic cells of Amoeba sp. strain Cont from the “proteus-type” group (Amoebozoa, Euamoebida, Amoebidae). Eur. J. Protistol. 2020, 74, 125691. [Google Scholar] [CrossRef] [PubMed]
- Moraczewski, J. Ćwiczenia z Zoologii Bezkręgowców, 1st ed.; PWN: Warsaw, Poland, 1974; Volume 29–31, pp. 285–292. [Google Scholar]
- Łyczko, J.; Pawlak, A.; Augustyński, I.; Okińczyc, P.; Szperlik, J.; Kulma, A.; Różański, H.; Obmińska-Mrukowicz, B.; Szumny, A. Chemical profiling and cytotoxic activity of 150-year old original sample of Jerusalem Balsam. Food Chem. Toxicol. 2020, 138, 111183. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Ion Trap Mass Spectroscopy; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Arendt-Pindel, A.; Marszałek-Harych, A.; Gębarowska, E.; Gębarowski, T.; Jędrzkiewicz, D.; Pląskowska, E.; Zalewski, D.; Gulia, N.; Szafert, S.; Ejfler, J. Design and functionalization of bioactive benzoxazines. An unexpected ortho-substitution effect. New J. Chem. 2019, 43, 12042–12053. [Google Scholar] [CrossRef]
| Essential Oil | Acetic Acid (A) | Propionic Acid (P) | Lactic Acid (L) | Mixture of Acids (M) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Mn | Zn | Cu | Mn | Zn | Cu | Mn | Zn | Cu | Mn | Zn | |
| Clove (Syzygium aromaticum (L.) Merr. and Perry) (S) | SACu | SAMn | SAZn | SPCu | SPMn | SPZn | SLCu | SLMn | SLZn | SMCu | SMMn | SMZn |
| Garlic (Allium sativum L.) (G) | GACu | GAMn | GAZn | GPCu | GPMn | GPZn | GLCu | GLMn | GLZn | GMCu | GMMn | GMZn |
| Ceylon cinnamon (Cinnamomum verum J. Presl) (C) | CACu | CAMn | CAZn | CPCu | CPMn | CPZn | CLCu | CLMn | CLZn | CMCu | CMMn | CMZn |
| Rosemary (Rosmarinus officinalis L.) (R) | RACu | RAMn | RAZn | RPCu | RPMn | RPZn | RLCu | RLMn | RLZn | RMCu | RMMn | RMZn |
| Protozoa | CH a | M b | Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids c | MnCl2 Solution d | CH2Cu2O5 Solution e | ZnCO3 Solution f | Catalyst Solution g | Clove Essential Oil (Syzygium aromaticum (L.) Merr. and Perry) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Euglena gracilis | LD50: 0.05 LD100: 0.09 | LD50: n.t LD100: n.t | LD50: 0.8 LD100: 1.1 | LD50: 0.5 LD100: 1.1 | LD50: 0.6 LD100: 1.3 | LD50: 0.5 LD100: 0.9 | LD50: 0.5 LD100: 0.9 | LD50: 0.5 LD100: 0.7 | LD50: 0.1 LD100: 0.2 | LD50: 0.5 LD100: 0.1 | LD50: 0.2 LD100: 0.3 |
| Gregarina blattarum | LD50: n.t LD100: n.t | LD50: 0.1 LD100: 0.3 | LD50: 0.9 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 1.0 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 0.9 LD100: 1.0 | LD50: 0.4 LD100: 0.7 | LD50: 0.1 LD100: 0.4 | LD50: 0.7 LD100: 0.3 | LD50: 0.1 LD100: 0.2 |
| Amoeba proteus | LD50: 0.07 LD100: 0.15 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.6 LD100: 1.0 | LD50: 0.9 LD100: 1.4 | LD50: 0.5 LD100: 1.0 | LD50: 0.5 LD100: 1.0 | LD50: 0.5 LD100: 1.0 | LD50: 0.1 LD100: 0.2 | LD50: 0.5 LD100: 1.0 | LD50: 0.1 LD100: 0.2 |
| Paramecium caudatum | LD50: 0.001 LD100: 0.006 | LD50: n.t LD100: n.t | LD50: 1.0 LD100: 1.3 | LD50: 0.8 LD100: 1.2 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.8 LD100: 1.2 | LD50: 0.8 LD100: 1.2 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.2 LD100: 0.3 |
| Pentatrichomonas hominis | LD50: n.t LD100: n.t | LD50: 0.05 LD100: 0.14 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.3 | LD50: 0.8 LD100: 1.0 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.1 | LD50: 0.1 LD100: 0.3 | LD50: 0.9 LD100: 1.1 | LD50: 0.1 LD100: 0.2 |
| Protozoa | Clove Essential Oil (Syzygium aromaticum (L.) Merr. and Perry) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids a | |||||||||
| Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | |
| SACu | SAMn | SAZn | SPCu | SPMn | SPZn | SLCu | SLMn | SLZn | SMCu | SMMn | SMZn | |
| Euglena gracilis | LD50: 0.01 LD100:0.04 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.001 LD100:0.003 | LD50: 0.001 LD100:0.005 | LD50: 0.001 LD100:0.002 |
| Gregarina blattarum | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.015 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.02 LD100:0.025 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.002 LD100:0.004 | LD50: 0.002 LD100:0.003 | LD50: 0.002 LD100:0.005 |
| Amoeba proteus | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.015 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.015 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.002 LD100:0.004 | LD50: 0.001 LD100:0.002 | LD50: 0.002 LD100:0.004 |
| Paramecium caudatum | LD50: 0.01 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50: 0.02 LD100:0.025 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.001 LD100:0.004 | LD50: 0.002 LD100:0.005 | LD50: 0.002 LD100:0.005 |
| Pentatrichomonas hominis | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.004 LD100:0.006 | LD50: 0.003 LD100:0.005 | LD50: 0.003 LD100:0.004 |
| Protozoa | CH a | M b | Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids c | MnCl2 Solution d | CH2Cu2O5 Solution e | ZnCO3 Solution f | Catalyst Solution g | Garlic Essential Oil (Allium sativum L.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Euglena gracilis | LD50: 0.05 LD100: 0.09 | LD50: n.t LD100: n.t | LD50: 0.8 LD100: 1.1 | LD50: 0.5 LD100: 1.1 | LD50: 0.6 LD100: 1.3 | LD50: 0.5 LD100: 0.9 | LD50: 0.5 LD100: 0.7 | LD50: 0.1 LD100: 0.2 | LD50: 0.1 LD100: 0.3 | LD50: 0.5 LD100: 0.1 | LD50: 0.4 LD100: 0.7 |
| Gregarina blattarum | LD50: n.t LD100: n.t | LD50: 0.1 LD100: 0.3 | LD50: 0.9 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 1.0 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 0.4 LD100: 0.7 | LD50: 0.1 LD100: 0.4 | LD50: 0.2 LD100: 0.4 | LD50: 0.7 LD100: 0.3 | LD50: 0.3 LD100: 0.7 |
| Amoeba proteus | LD50: 0.07 LD100: 0.15 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.6 LD100: 1.0 | LD50: 0.9 LD100: 1.4 | LD50: 0.5 LD100: 1.0 | LD50: 0.5 LD100: 1.0 | LD50: 0.1 LD100: 0.2 | LD50: 0.1 LD100: 0.2 | LD50: 0.5 LD100: 1.0 | LD50: 0.4 LD100: 0.6 |
| Paramecium caudatum | LD50: 0.001 LD100: 0.006 | LD50: n.t LD100: n.t | LD50: 1.0 LD100: 1.3 | LD50: 0.8 LD100: 1.2 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.8 LD100: 1.2 | LD50: 0.3 LD100: 0.5 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.5 LD100: 0.7 |
| Pentatrichomonas hominis | LD50: n.t LD100: n.t | LD50: 0.05 LD100: 0.14 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.3 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.1 | LD50: 0.1 LD100: 0.3 | LD50: 0.2 LD100: 0.4 | LD50: 0.9 LD100: 1.1 | LD50: 0.6 LD100: 0.8 |
| Protozoa | Garlic Essential Oil (Allium sativum L.) (G) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids a | |||||||||
| Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | |
| GACu | GAMn | GAZn | GPCu | GPMn | GPZn | GLCu | GLMn | GLZn | GMCu | GMMn | GMZn | |
| Euglena gracilis | LD50: 0.03 LD100:0.04 | LD50: 0.02 LD100:0.05 | LD50: 0.03 LD100:0.05 | LD50: 0.02 LD100:0.06 | LD50: 0.04 LD100:0.06 | LD50: 0.05 LD100:0.06 | LD50: 0.03 LD100:0.05 | LD50: 0.02 LD100:0.04 | LD50: 0.02 LD100:0.03 | LD50:0.005 LD100:0.007 | LD50:0.003 LD100:0.005 | LD50:0.004 LD100:0.006 |
| Gregarina blattarum | LD50: 0.01 LD100:0.05 | LD50: 0.04 LD100:0.06 | LD50: 0.03 LD100:0.06 | LD50: 0.01 LD100:0.05 | LD50: 0.02 LD100:0.04 | LD50: 0.04 LD100:0.05 | LD50: 0.01 LD100:0.04 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.03 | LD50:0.003 LD100:0.006 | LD50:0.003 LD100:0.005 | LD50:0.003 LD100:0.006 |
| Amoeba proteus | LD50: 0.01 LD100:0.05 | LD50: 0.03 LD100:0.05 | LD50: 0.03 LD100:0.04 | LD50: 0.01 LD100:0.02 | LD50: 0.03 LD100:0.05 | LD50: 0.02 LD100:0.04 | LD50: 0.01 LD100:0.03 | LD50: 0.01 LD100:0.03 | LD50: 0.01 LD100:0.04 | LD50:0.004 LD100:0.005 | LD50:0.001 LD100:0.003 | LD50:0.004 LD100:0.006 |
| Paramecium caudatum | LD50: 0.03 LD100:0.06 | LD50: 0.02 LD100:0.03 | LD50: 0.03 LD100:0.04 | LD50: 0.04 LD100:0.05 | LD50: 0.02 LD100:0.06 | LD50: 0.02 LD100:0.04 | LD50: 0.01 LD100:0.03 | LD50: 0.01 LD100:0.04 | LD50: 0.01 LD100:0.02 | LD50:0.004 LD100:0.006 | LD50:0.002 LD100:0.004 | LD50:0.004 LD100:0.007 |
| Pentatrichomonas hominis | LD50: 0.02 LD100:0.04 | LD50: 0.04 LD100:0.05 | LD50: 0.04 LD100:0.05 | LD50: 0.03 LD100:0.065 | LD50: 0.04 LD100:0.06 | LD50: 0.04 LD100:0.07 | LD50: 0.02 LD100:0.06 | LD50: 0.01 LD100:0.05 | LD50: 0.03 LD100:0.05 | LD50:0.03 LD100:0.06 | LD50:0.03 LD100:0.05 | LD50:0.003 LD100:0.004 |
| Protozoa | CH a | M b | Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids c | MnCl2 Solution d | CH2Cu2O5 Solution e | ZnCO3 Solution f | Catalyst Solution g | Ceylon Cinnamon Essential Oil (Cinnamomum verum J. Presl) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Euglena gracilis | LD50: 0.05 LD100: 0.09 | LD50: n.t LD100: n.t | LD50: 0.8 LD100: 1.1 | LD50: 0.5 LD100: 1.1 | LD50: 0.6 LD100: 1.3 | LD50: 0.5 LD100: 0.9 | LD50: 0.5 LD100: 0.7 | LD50: 0.1 LD100: 0.2 | LD50: 0.1 LD100: 0.3 | LD50: 0.5 LD100: 0.1 | LD50: 0.2 LD100: 0.3 |
| Gregarina blattarum | LD50: n.t LD100: n.t | LD50: 0.1 LD100: 0.3 | LD50: 0.9 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 1.0 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 0.4 LD100: 0.7 | LD50: 0.1 LD100: 0.4 | LD50: 0.2 LD100: 0.4 | LD50: 0.7 LD100: 0.3 | LD50: 0.1 LD100: 0.35 |
| Amoeba proteus | LD50: 0.07 LD100: 0.15 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.6 LD100: 1.0 | LD50: 0.9 LD100: 1.4 | LD50: 0.5 LD100: 1.0 | LD50: 0.5 LD100: 1.0 | LD50: 0.1 LD100: 0.2 | LD50: 0.1 LD100: 0.2 | LD50: 0.5 LD100: 1.0 | LD50: 0.5 LD100: 0.6 |
| Paramecium caudatum | LD50: 0.001 LD100: 0.006 | LD50: n.t LD100: n.t | LD50: 1.0 LD100: 1.3 | LD50: 0.8 LD100: 1.2 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.8 LD100: 1.2 | LD50: 0.3 LD100: 0.5 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.2 LD100: 0.45 |
| Pentatrichomonas hominis | LD50: n.t LD100: n.t | LD50: 0.05 LD100: 0.14 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.3 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.1 | LD50: 0.1 LD100: 0.3 | LD50: 0.2 LD100: 0.4 | LD50: 0.9 LD100: 1.1 | LD50: 0.2 LD100: 0.7 |
| Protozoa | Ceylon Cinnamon Essential Oil (Cinnamomum verum J. Presl) (C) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids a | |||||||||
| Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | |
| CACu | CAMn | CAZn | CPCu | CPMn | CPZn | CLCu | CLMn | CLZn | CMCu | CMMn | CMZn | |
| Euglena gracilis | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50:0.005 LD100:0.007 | LD50:0.001 LD100:0.003 | LD50:0.003 LD100:0.006 |
| Gregarina blattarum | LD50: 0.02 LD100:0.04 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.035 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50: 0.01 LD100:0.02 | LD50:0.005 LD100:0.006 | LD50:0.003 LD100:0.006 | LD50:0.003 LD100:0.005 |
| Amoeba proteus | LD50: 0.04 LD100:0.055 | LD50: 0.02 LD100:0.05 | LD50: 0.04 LD100:0.05 | LD50: 0.05 LD100:0.06 | LD50: 0.03 LD100:0.05 | LD50: 0.05 LD100:0.06 | LD50: 0.04 LD100:0.05 | LD50: 0.02 LD100:0.04 | LD50: 0.02 LD100:0.04 | LD50:0.003 LD100:0.005 | LD50:0.001 LD100:0.003 | LD50:0.004 LD100:0.006 |
| Paramecium caudatum | LD50: 0.03 LD100:0.05 | LD50: 0.03 LD100:0.04 | LD50: 0.03 LD100:0.04 | LD50: 0.03 LD100:0.04 | LD50: 0.02 LD100:0.045 | LD50: 0.02 LD100:0.04 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50:0.002 LD100:0.004 | LD50:0.002 LD100:0.006 | LD50:0.004 LD100:0.007 |
| Pentatrichomonas hominis | LD50: 0.06 LD100:0.07 | LD50: 0.05 LD100:0.065 | LD50: 0.04 LD100:0.06 | LD50: 0.03 LD100:0.055 | LD50: 0.04 LD100:0.05 | LD50: 0.05 LD100:0.07 | LD50: 0.04 LD100:0.05 | LD50: 0.04 LD100:0.05 | LD50: 0.04 LD100:0.05 | LD50:0.05 LD100:0.06 | LD50:0.03 LD100:0.05 | LD50:0.04 LD100:0.055 |
| Protozoa | CH a | M b | Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids c | MnCl2 Solution d | CH2Cu2O5 Solution e | ZnCO3 Solution f | Catalyst Solution g | Rosemary Essential Oil (Rosmarinus officinalis L.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Euglena gracilis | LD50: 0.05 LD100: 0.09 | LD50: n.t LD100: n.t | LD50: 0.8 LD100: 1.1 | LD50: 0.5 LD100: 1.1 | LD50: 0.6 LD100: 1.3 | LD50: 0.5 LD100: 0.9 | LD50: 0.5 LD100: 0.7 | LD50: 0.1 LD100: 0.2 | LD50: 0.1 LD100: 0.3 | LD50: 0.5 LD100: 0.1 | LD50: 0.2 LD100: 0.6 |
| Gregarina blattarum | LD50: n.t LD100: n.t | LD50: 0.1 LD100: 0.3 | LD50: 0.9 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 1.0 LD100: 1.1 | LD50: 0.9 LD100: 1.0 | LD50: 0.4 LD100: 0.7 | LD50: 0.1 LD100: 0.4 | LD50: 0.2 LD100: 0.4 | LD50: 0.7 LD100: 0.3 | LD50: 0.2 LD100: 0.4 |
| Amoeba proteus | LD50: 0.07 LD100: 0.15 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.6 LD100: 1.0 | LD50: 0.9 LD100: 1.4 | LD50: 0.5 LD100: 1.0 | LD50: 0.5 LD100: 1.0 | LD50: 0.1 LD100: 0.2 | LD50: 0.1 LD100: 0.2 | LD50: 0.5 LD100: 1.0 | LD50: 0.3 LD100: 0.5 |
| Paramecium caudatum | LD50: 0.001 LD100: 0.006 | LD50: n.t LD100: n.t | LD50: 1.0 LD100: 1.3 | LD50: 0.8 LD100: 1.2 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.8 LD100: 1.2 | LD50: 0.3 LD100: 0.5 | LD50: 0.3 LD100: 0.5 | LD50: 0.8 LD100: 1.2 | LD50: 0.1 LD100: 0.5 |
| Pentatrichomonas hominis | LD50: n.t LD100: n.t | LD50: 0.05 LD100: 0.14 | LD50: 1.0 LD100: 1.5 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.3 | LD50: 0.8 LD100: 1.0 | LD50: 0.9 LD100: 1.1 | LD50: 0.1 LD100: 0.3 | LD50: 0.2 LD100: 0.4 | LD50: 0.9 LD100: 1.1 | LD50: 0.3 LD100: 0.55 |
| Protozoa | Rosemary Essential Oil (Rosmarinus officinalis L.) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Lactic Acid | Mixture of Acids a | |||||||||
| Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | Cu b | Mn c | Zn d | |
| RACu | RAMn | RAZn | RPCu | RPMn | RPZn | RLCu | RLMn | RLZn | RMCu | RMMn | RMZn | |
| Euglena gracilis | LD50: 0.03 LD100:0.05 | LD50: 0.02 LD100:0.05 | LD50: 0.03 LD100:0.06 | LD50: 0.03 LD100:0.05 | LD50: 0.02 LD100:0.045 | LD50: 0.04 LD100:0.05 | LD50: 0.03 LD100:0.05 | LD50: 0.02 LD100:0.04 | LD50: 0.02 LD100:0.03 | LD50: 0.002 LD100:0.005 | LD50: 0.001 LD100:0.004 | LD50: 0.002 LD100:0.004 |
| Gregarina blattarum | LD50: 0.02 LD100:0.04 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.04 | LD50: 0.02 LD100:0.04 | LD50: 0.03 LD100:0.05 | LD50: 0.01 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.01 LD100:0.02 | LD50: 0.002 LD100:0.004 | LD50: 0.002 LD100:0.003 | LD50: 0.003 LD100:0.005 |
| Amoeba proteus | LD50: 0.04 LD100:0.05 | LD50: 0.02 LD100:0.03 | LD50: 0.04 LD100:0.05 | LD50: 0.03 LD100:0.04 | LD50: 0.02 LD100:0.05 | LD50: 0.02 LD100:0.03 | LD50: 0.03 LD100:0.04 | LD50: 0.02 LD100:0.04 | LD50: 0.04 LD100:0.05 | LD50: 0.002 LD100:0.004 | LD50: 0.002 LD100:0.005 | LD50: 0.003 LD100:0.004 |
| Paramecium caudatum | LD50: 0.03 LD100:0.04 | LD50: 0.03 LD100:0.04 | LD50: 0.03 LD100:0.04 | LD50: 0.03 LD100:0.05 | LD50: 0.03 LD100:0.04 | LD50: 0.03 LD100:0.05 | LD50: 0.01 LD100:0.04 | LD50: 0.02 LD100:0.03 | LD50: 0.02 LD100:0.03 | LD50: 0.001 LD100:0.004 | LD50: 0.002 LD100:0.005 | LD50: 0.002 LD100:0.005 |
| Pentatrichomonas hominis | LD50: 0.03 LD100:0.05 | LD50: 0.05 LD100:0.055 | LD50: 0.04 LD100:0.06 | LD50: 0.03 LD100:0.04 | LD50: 0.04 LD100:0.05 | LD50: 0.03 LD100:0.04 | LD50: 0.03 LD100:0.05 | LD50: 0.04 LD100:0.05 | LD50: 0.03 LD100:0.04 | LD50: 0.007 LD100:0.009 | LD50: 0.006 LD100:0.008 | LD50: 0.005 LD100:0.008 |
| Cell Lines | Mixture of Acids | +MnCl2 | +CH2Cu2O5 | +ZnCO3 |
|---|---|---|---|---|
| NHDF | 1.43 (±0.16) | 0.54 (±0.2) | 19.28 (±1.3) | 0.73 (±0.16) |
| A549 | 0.12 (±0.03) | 0.10 (±0.01) | 0.15 (±0.02) | 0.07 (±0.01) |
| MCF7 | 0.34 (±0.09) | 0.70 (±0.09) | 0.67 (±0.15) | 0.15 (±0.03) |
| LOVO | 0.14 (±0.03) | 0.06 (±0.01) | 0.09 (±0.01) | 0.07 (±0.01) |
| HT29 | 1.43 (±0.16) | 0.54 (±0.2) | 19.28 (±1.3) | 0.73 (±0.16) |
| Cell Lines | Mixture of Acids | +MnCl2 | +CH2Cu2O5 | +ZnCO3 |
|---|---|---|---|---|
| NHDF | 0.62 (±0.11) | 0.91 (±0.07) | 0.92 (±0.13) | 1.17 (±0.14) |
| A549 | 0.40 (±0.07) | 0.46 (±0.) | NA | NA |
| MCF7 | 0.13 (±0.03) | 0.07 (±0.) | 0.13 (±0.03) | 0.11 (±0.01) |
| LOVO | NA | 0.39 (±0.) | 0.31 (±0.15) | NA |
| HT29 | 0.46 (±0.12) | 0.06 (±0.01) | NA | NA |
| Cell Lines | Mixture of Acids | +MnCl2 | +CH2Cu2O5 | +ZnCO3 |
|---|---|---|---|---|
| NHDF | 0.51 (±0.22) | 0.71 (±0.12) | 0.38 (±0.12) | 0.71 (±0.21) |
| A549 | 0.38 (±0.10) | 0.22 (±0.02) | NA | 0.13 (±0.03) |
| MCF7 | 0.22 (±0.06) | 0.13 (±0.02) | NA | 0.05 (±0.01) |
| LOVO | 0.37 (±0.01) | 0.13 (±0.03) | 0.08 (±0.02) | 0.05 (±0.01) |
| HT29 | NA | NA | NA | 0.15 (±0.03) |
| Cell Lines | Mixture of Acids | +MnCl2 | +CH2Cu2O5 | +ZnCO3 |
|---|---|---|---|---|
| NHDF | 1.28 (±0.21) | 1.51 (±0.31) | 1.24 (±0.21) | NA |
| A549 | NA | 0.15 (±0.03) | NA | 0.07 (±0.02) |
| MCF7 | NA | 0.11 (±0.01) | 0.35 (±0.03) | NA |
| LOVO | NA | NA | NA | 0.07 (±0.01) |
| HT29 | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwiński, H.; Różański, H.; Pachura, N.; Wojciechowska, A.; Gębarowski, T.; Szumny, A. In Vitro Evaluation of Antiprotozoal Properties, Cytotoxicity Effect and Anticancer Activity of New Essential-Oil Based Phytoncide Mixtures. Molecules 2023, 28, 1395. https://doi.org/10.3390/molecules28031395
Iwiński H, Różański H, Pachura N, Wojciechowska A, Gębarowski T, Szumny A. In Vitro Evaluation of Antiprotozoal Properties, Cytotoxicity Effect and Anticancer Activity of New Essential-Oil Based Phytoncide Mixtures. Molecules. 2023; 28(3):1395. https://doi.org/10.3390/molecules28031395
Chicago/Turabian StyleIwiński, Hubert, Henryk Różański, Natalia Pachura, Aleksandra Wojciechowska, Tomasz Gębarowski, and Antoni Szumny. 2023. "In Vitro Evaluation of Antiprotozoal Properties, Cytotoxicity Effect and Anticancer Activity of New Essential-Oil Based Phytoncide Mixtures" Molecules 28, no. 3: 1395. https://doi.org/10.3390/molecules28031395
APA StyleIwiński, H., Różański, H., Pachura, N., Wojciechowska, A., Gębarowski, T., & Szumny, A. (2023). In Vitro Evaluation of Antiprotozoal Properties, Cytotoxicity Effect and Anticancer Activity of New Essential-Oil Based Phytoncide Mixtures. Molecules, 28(3), 1395. https://doi.org/10.3390/molecules28031395








