Paired Electrolysis Enabled Cyanation of Diaryl Diselenides with KSCN Leading to Aryl Selenocyanates
Abstract
:1. Introduction
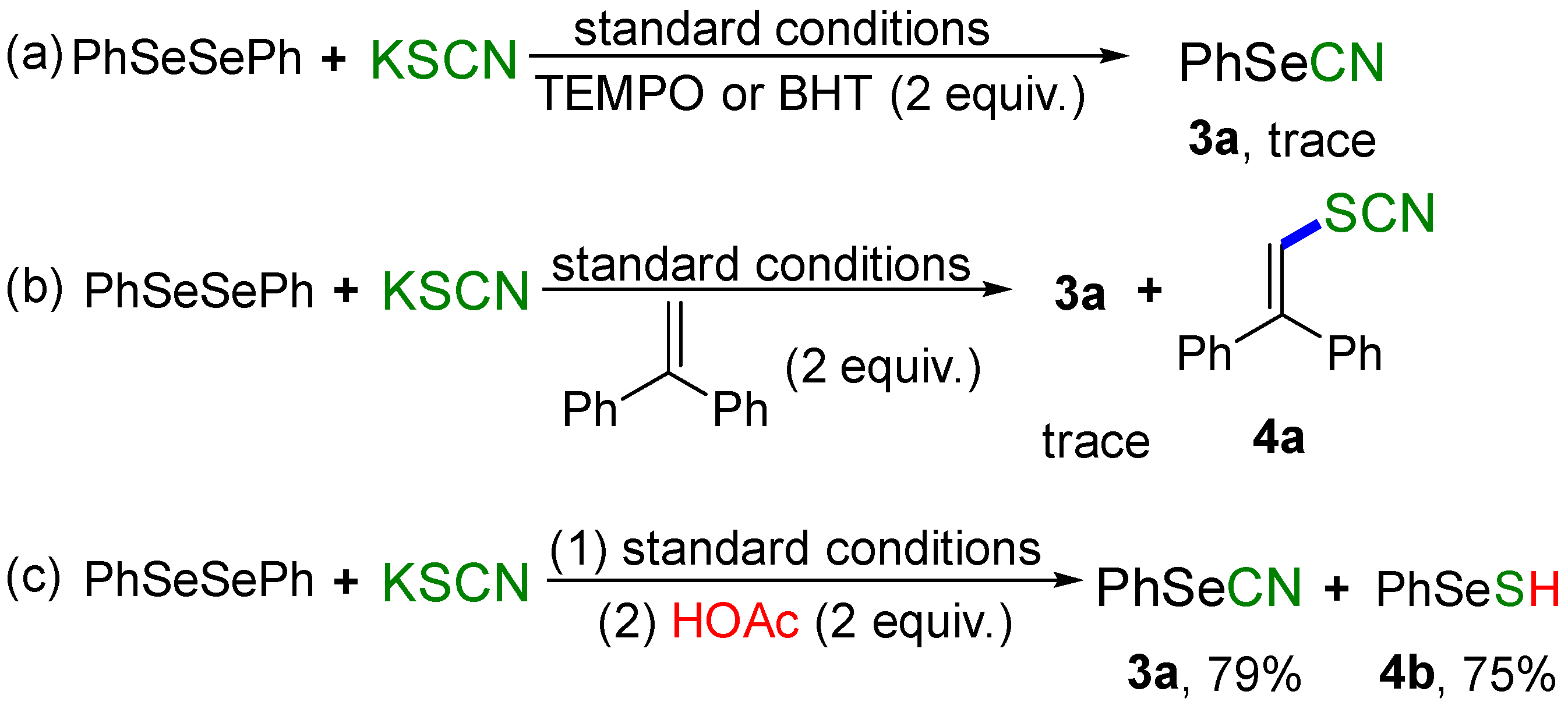
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Typical Procedure for the Synthesis of 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ali, W.; Álvarez-Pérez, M.; Marć, M.A.; Salardón-Jiménez, N.; Handzlik, J.; Domínguez-Álvarez, E. The Anticancer and Chemopreventive Activity of Selenocyanate-Containing Compounds. Curr. Pharmacol. Rep. 2018, 4, 468–481. [Google Scholar] [CrossRef]
- An, B.; Wang, B.; Hu, J.; Xu, S.; Huang, L.; Li, X.; Chan, A.S.C. Synthesis and Biological Evaluation of Selenium-Containing 4-Anilinoquinazoline Derivatives as Novel Antimitotic Agents. J. Med. Chem. 2018, 61, 2571–2588. [Google Scholar] [CrossRef]
- Mukherjee, N.; Kundu, D.; Ranu, B.C. Copper-Silver Dual Catalyzed Decyanative C–Se Cross-Coupling. Adv. Synth. Catal. 2017, 359, 329–338. [Google Scholar] [CrossRef]
- Kalaramna, P.; Bhatt, D.; Sharma, H.; Goswami, A. An Expeditious and Environmentally-Benign Approach to 2-Aryl/Heteroaryl Selenopyridines via Ruthenium Catalyzed [2+2+2] Cycloadditions. Eur. J. Org. Chem. 2019, 2019, 4694–4700. [Google Scholar] [CrossRef]
- Yang, L.; Tian, Z.-Y.; Zhang, C.-P. Transition-Metal-Free Selective Synthesis of (Z)-1,2-Diarylthio-1-arylalkenes, (2-Arylethene-1,1,2-triyl)tris(arylsulfane)s and Alkynyl Sulfides from Thiocyanates and Terminal Arylalkynes. ChemistrySelect 2019, 4, 311–315. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, L.; Yi, W. Synthesis of Monofluoromethyl Selenoethers of Aryl and Alkyl from Organoselenocyanate via One-Pot Reaction. Adv. Synth. Catal. 2019, 361, 4360–4368. [Google Scholar] [CrossRef]
- Liu, X.-S.; Li, M.; Dong, K.; Peng, S.; Liu, L. Highly Stereoselective Synthesis of Tetrasubstituted Vinyl Selenides via Rhodium-Catalyzed [1,4]-Acyl Migration of Selenoesters and Diazo Compounds. Org. Lett. 2022, 24, 2175–2180. [Google Scholar] [CrossRef]
- Jana, S.; Koenigs, R.M. Rhodium-Catalyzed Carbene Transfer Reactions for Sigmatropic Rearrangement Reactions of Selenium Ylides. Org. Lett. 2019, 21, 3653–3657. [Google Scholar] [CrossRef]
- Lin, X.; Tan, Z.; Yang, W.; Yang, W.; Liu, X.; Feng, X. Chiral Cobalt(II) Complex Catalyzed Asymmetric [2,3]-Sigmatropic Rearrangement of Allylic Selenides with α-Diazo Pyrazoleamides. CCS Chem. 2020, 3, 1423–1433. [Google Scholar] [CrossRef]
- Gao, M.; Vuagnat, M.; Chen, M.-Y.; Pannecoucke, X.; Jubault, P.; Besset, T. Design and Use of Electrophilic Thiocyanating and Selenocyanating Reagents: An Interesting Trend for the Construction of SCN- and SeCN-Containing Compounds. Chem.-Eur. J. 2021, 27, 6145–6160. [Google Scholar] [CrossRef]
- Karmaker, P.G.; Huo, F. Organic Selenocyanates: Rapid Advancements and Applicationsin the Field of Organic Chemistry. Asian J. Org. Chem. 2022, 11, e202200226. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Ji, X.-M.; Hu, M.-L.; Tang, R.-Y. Nitromethane as a cyanating reagent for the synthesis of thiocyanates. Tetrahedron Lett. 2015, 56, 5067–5070. [Google Scholar] [CrossRef]
- Frei, R.; Courant, T.; Wodrich, M.D.; Waser, J. General and Practical Formation of Thiocyanates from Thiols. Chem.-Eur. J. 2015, 21, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Peng, Y.; Ding, G.; Li, X.; Cui, C.; Yan, Y. Catalyst and additive-free regioselective oxidative C–H thio/selenocyanation of arenes and heteroarenes with elemental sulfur/selenium and TMSCN. Chem. Commun. 2018, 54, 13367–13370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, X.-B.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Metal-Free Synthesis of Aryl Selenocyanates and Selenaheterocycles with Elemental Selenium. Chem.-Eur. J. 2021, 27, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Redon, S.; Kosso, A.R.O.; Broggi, J.; Vanelle, P. Metal-Free ipso-Selenocyanation of Arylboronic Acids Using Malononitrile and Selenium Dioxide. Synthesis 2019, 51, 3758–3764. [Google Scholar] [CrossRef]
- Nikolaienko, P.; Rueping, M. Trifluoromethylselenolation of Aryldiazonium Salts: A Mild and Convenient Copper-Catalyzed Procedure for the Introduction of the SeCF3 Group. Chem.-Eur. J. 2016, 22, 2620–2623. [Google Scholar] [CrossRef]
- Guan, Y.; Townsend, S.D. Metal-Free Synthesis of Unsymmetrical Organoselenides and Selenoglycosides. Org. Lett. 2017, 19, 5252–5255. [Google Scholar] [CrossRef]
- He, D.; Yao, J.; Ma, B.; Wei, J.; Hao, G.; Tuo, X.; Guo, S.; Fu, Z.; Cai, H. An electrochemical method for deborylative seleno/thiocyanation of arylboronic acids under catalyst- and oxidant-free conditions. Green Chem. 2020, 22, 1559–1564. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Chen, Y.; Xie, H.; Ding, C.; Tan, J.; Xu, K. Electrochemical synthesis of selenocyanated imidazo[1,5-a]quinolines under metal catalyst- and chemical oxidant-free conditions. Chin. Chem. Lett. 2020, 31, 1576–1579. [Google Scholar] [CrossRef]
- Xiao, J.-A.; Liang, J.-S.; Zhang, H.; Meng, R.-F.; Su, W.; Lin, C.; Cui, J.-G.; Huang, Y.-M. Solid-State Direct Electrophilic Selenocyanation of (Hetero)Arenes using Mechanochemistry. Eur. J. Org. Chem. 2022, 2022, e202200902. [Google Scholar] [CrossRef]
- Castanheiro, T.; Suffert, J.; Donnard, M.; Gulea, M. Recent advances in the chemistry of organic thiocyanates. Chem. Soc. Rev. 2016, 45, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Ofial, A.R. Potassium Thiocyanate as Source of Cyanide for the Oxidative α-Cyanation of Tertiary Amines. J. Org. Chem. 2015, 80, 2848–2854. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Zhu, Z.; Zhu, C.; Cen, J.; Li, X.; Wu, W.; Jiang, H. Copper-Catalyzed Cyanation of N-Tosylhydrazones with Thiocyanate Salt as the “CN” Source. J. Org. Chem. 2017, 82, 7621–7627. [Google Scholar] [CrossRef] [PubMed]
- Sardarian, A.R.; Inaloo, I.D.; Modarresi-Alam, A.R.; Kleinpeter, E.; Schilde, U. Metal-Free Regioselective Monocyanation of Hydroxy-, Alkoxy-, and Benzyloxyarenes by Potassium Thiocyanate and Silica Sulfuric Acid as a Cyanating Agent. J. Org. Chem. 2019, 84, 1748–1756. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Zhang, L.; Xu, T.; Xie, Y.; Jin, C. Transition-metal-free direct C-3 cyanation of quinoxalin-2(1H)-ones with ammonium thiocyanate as the “CN” source. Org. Chem. Front. 2020, 7, 113–118. [Google Scholar] [CrossRef]
- Yang, Q.; Yan, X.-T.; Feng, C.-T.; Chen, D.-X.; Yan, Z.-Z.; Xu, K. Tandem Strecker/C(sp3)–H amination reactions for the construction of cyanide-functionalized imidazo[1,5-a]pyridines with NH4SCN as a cyanating agent. Org. Chem. Front. 2021, 8, 6384–6389. [Google Scholar] [CrossRef]
- Guo, W.; Tan, W.; Zhao, M.; Zheng, L.; Tao, K.; Chen, D.; Fan, X. Direct Photocatalytic S–H Bond Cyanation with Green “CN” Source. J. Org. Chem. 2018, 83, 6580–6588. [Google Scholar] [CrossRef]
- Yi, B.; Yan, N.; Yi, N.; Xie, Y.; Wen, X.; Au, C.-T.; Lan, D. Oxidative cyanation of N-aryltetrahydroisoquinoline induced by visible light for the synthesis of α-aminonitrile using potassium thiocyanate as a “CN” agent. RSC Adv. 2019, 9, 29721–29725. [Google Scholar] [CrossRef]
- Dharpure, P.D.; Behera, M.; Khade, V.V.; Thube, A.S.; Bhat, R.G. Direct Access to Thiocyano-Thioesters from Cyclic Thioacetals via Photoredox Catalysis: An Introduction of Two Functional Groups in One Pot. Org. Lett. 2022, 24, 6919–6924. [Google Scholar] [CrossRef]
- Chen, N.; Xu, H.-C. Electrochemically Driven Radical Reactions: From Direct Electrolysis to Molecular Catalysis. Chem. Rec. 2021, 21, 2306–2319. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Fang, P.; Liu, Z.-R.; Xu, S.-S.; Xu, K.; Cheng, X.; Lei, A.; Xu, H.-C.; Zeng, C.; Mei, T.-S. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts. Sci. Bull. 2021, 66, 2412–2429. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Photo-/electrocatalytic functionalization of quinoxalin-2(1H)-ones. Chin. J. Catal. 2021, 42, 1921–1943. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Tan, H.-T.; He, W.-M. Organic Electrochemistry-current transfer future. Chin. J. Catal. 2023, 46, 4–10. [Google Scholar]
- Wu, H.; Yu, X.; Cao, Z. Electrochemical Multicomponent Synthesis of α-Ketoamides from α-Oxocarboxylic Acids, Isocyanides and Water. Chin. J. Org. Chem. 2021, 41, 4712–4717. [Google Scholar] [CrossRef]
- Xu, H.; Meng, X.; Zheng, Y.; Luo, J.; Huang, S. Electrochemical Annulations of o-Alkynylanilines for Synthesis of 3-Iodoindoles. Chin. J. Org. Chem. 2021, 41, 4696–4703. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.-Y.; Liao, H.-R.; Shu, X.-R.; Duan, L.-L.; Yang, X.-F.; He, W.-M. Electrochemical transient iodination and coupling for selenylated 4-anilinocoumarin synthesis. Green Synth. Catal. 2021, 2, 233–236. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wu, H.-Y.; Gui, Q.-W.; Yan, S.-S.; Deng, J.; Lin, Y.-W.; Cao, Z.; He, W.-M. Sustainable Electrochemical Cross-Dehydrogenative Coupling of 4-Quinolones and Diorganyl Diselenides. Chin. J. Catal. 2021, 42, 1445–1450. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Chen, J.-Y.; Tian, X.-Z.; Ouyang, W.-T.; Zhang, Z.-T.; He, W.-M. Electrochemical regioselective synthesis of N-substituted/unsubstituted 4-selanylisoquinolin-1(2H)-ones. Chin. Chem. Lett. 2022, 33, 1501–1504. [Google Scholar] [CrossRef]
- Ding, L.; Niu, K.; Liu, Y.; Wang, Q. Electro-reductive C-H cyanoalkylation of quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2022, 33, 4057–4060. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Zhang, Z.-T.; Wu, H.-Y.; Zhou, M.-H.; Song, H.-Y.; Ji, H.-T.; Jiang, J.; Chen, J.-Y.; He, W.-M. TBAI/H2O-cooperative electrocatalytic decarboxylation coupling-annulation of quinoxalin-2(1H)-ones with N-arylglycines. Chin. Chem. Lett. 2023, 34, 108036. [Google Scholar] [CrossRef]
- Li, L.; Hou, Z.-W.; Li, P.; Wang, L. Site-Selective Electrochemical C–H Cyanation of Indoles. Org. Lett. 2021, 23, 5983–5987. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhu, Y.; Li, H.; Liu, P.; Sun, P. Direct Cyanation of Thiophenols or Thiols to Access Thiocyanates under Electrochemical Conditions. J. Org. Chem. 2022, 87, 10026–10033. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Zhan, Y.; Dai, C.; Lin, J.; Liu, P.; Sun, P. Electrochemical Oxidative Regioselective C–H Cyanation of Imidazo[1,2-a]pyridines. J. Org. Chem. 2021, 86, 15897–15905. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, Y.; Tong, J.; Liu, P.; Sun, P. Electrochemical Oxidative C−H Cyanation of Quinoxalin-2(1H)-ones with TMSCN. Eur. J. Org. Chem. 2021, 2021, 2193–2197. [Google Scholar] [CrossRef]
- Gao, J.; Weng, X.; Ma, C.; Xu, X.; Fang, P.; Mei, T. Electrochemical 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO)-Mediated α-Cyanation and Phosphonylation of Cyclic Amines with Metal-Free Conditions. Chin. J. Org. Chem. 2021, 41, 3223–3234. [Google Scholar] [CrossRef]
- Kong, X.; Chen, X.; Chen, Y.; Cao, Z.-Y. Scalable Electrocatalytic Intermolecular Acylcyanation and Aminocyanation of Alkenes. J. Org. Chem. 2022, 87, 7013–7021. [Google Scholar] [CrossRef]
- Gui, Q.-W.; Xiong, Z.-Y.; Teng, F.; Cai, T.-C.; Li, Q.; Hu, W.; Wang, X.; Yu, J.; Liu, X. Electrochemically promoted oxidative α-cyanation of tertiary and secondary amines using cheap AIBN. Org. Biomol. Chem. 2021, 19, 8254–8258. [Google Scholar] [CrossRef]
- Kong, X.; Wang, Y.; Chen, Y.; Chen, X.; Lin, L.; Cao, Z.-Y. Cyanation and cyanomethylation of trimethylammonium salts via electrochemical cleavage of C–N bonds. Org. Chem. Front. 2022, 9, 1288–1294. [Google Scholar] [CrossRef]
- Kumar, G.S.; Shinde, P.S.; Chen, H.; Muralirajan, K.; Kancherla, R.; Rueping, M. Paired Electrolysis for Decarboxylative Cyanation: 4-CN-Pyridine, a Versatile Nitrile Source. Org. Lett. 2022, 24, 6357–6363. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, N.; Song, L.; Fu, N. Reaching the Full Potential of Electroorganic Synthesis by Paired Electrolysis. Chem. Rec. 2021, 21, 2574–2584. [Google Scholar] [CrossRef]
- Marken, F.; Cresswell, A.J.; Bull, S.D. Recent Advances in Paired Electrosynthesis. Chem. Rec. 2021, 21, 2585–2600. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.-R.; Ma, C.; Jiao, K.-J.; Sun, B.; Wei, L.; Lefranc, J.; Herbert, S.; Mei, T.-S. Nickel-Catalyzed N-Arylation of NH-Sulfoximines with Aryl Halides via Paired Electrolysis. Angew. Chem. Int. Ed. 2021, 60, 9444–9449. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hong, J.; Yao, X.; Liu, C.; Zhang, L.; Fu, Y.; Sun, M.; Cheng, R.; Li, Z.; Ye, J. Aminomethylation of Aryl Bromides by Nickel-Catalyzed Electrochemical Redox Neutral Cross Coupling. Org. Lett. 2021, 23, 9387–9392. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Li, H.; Huang, M.; Zhang, W.; Zhi, S.; Wang, Y.; Pan, Y. Electrochemical-Promoted Nickel-Catalyzed Oxidative Fluoroalkylation of Aryl Iodides. Org. Lett. 2021, 23, 8252–8256. [Google Scholar] [CrossRef] [PubMed]
- Kuciński, K.; Simon, H.; Ackermann, L. Rhoda-Electrocatalyzed C−H Methylation and Paired Electrocatalyzed C−H Ethylation and Propylation. Chem.-Eur. J. 2022, 28, e202103837. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Wei, L.; Jiao, K.-J.; Ma, C.; Mei, T.-S. Nickel-catalyzed decarboxylative cross-coupling of indole-3-acetic acids with aryl bromides by convergent paired electrolysis. Chem. Commun. 2022, 58, 8202–8205. [Google Scholar] [CrossRef]
- Ouyang, W.-T.; Xiao, F.; Ou, L.-J.; He, W.-M. Green Photocatalytic Syntheses Using Water as Solvent/Hydrogen Source/Oxygen Source. Curr. Opin. Green Sust. 2023, 41, 100760. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Li, H.-X.; Mu, S.-Y.; Song, H.-Y.; Wu, Z.-L.; Yang, T.-B.; Jiang, J.; He, W.-M. Electrocatalytic three-component synthesis of 4-halopyrazoles with sodium halide as the halogen source. Org. Biomol. Chem. 2022, 20, 8501–8505. [Google Scholar] [CrossRef]
- Gui, Q.-W.; Teng, F.; Yu, P.; Wu, Y.-F.; Nong, Z.-B.; Yang, L.-X.; Chen, X.; Yang, T.-B.; He, W.-M. Visible light-induced Z-scheme V2O5/g-C3N4 heterojunction catalyzed cascade reaction of unactivated alkenes. Chin. J. Catal. 2023, 44, 111–116. [Google Scholar] [CrossRef]
- Li, H.; Chen, P.; Wu, Z.; Lu, Y.; Peng, J.; Chen, J.; He, W. Electrochemical Oxidative Cross-Dehydrogenative Coupling of Five-Membered Aromatic Heterocycles with NH4SCN. Chin. J. Org. Chem. 2022, 42, 3398–3404. [Google Scholar] [CrossRef]
- Yi, R.; He, W. Visible-Light-Induced Radical Arylation Reactions via Electron Donor-Acceptor Complex. Chin. J. Org. Chem. 2022, 42, 2590–2592. [Google Scholar] [CrossRef]
- Yang, D.; Yan, Q.; Zhu, E.; Lv, J.; He, W.-M. Carbon-sulfur bond formation via photochemical strategies: An efficient method for the synthesis of sulfur-containing compounds. Chin. Chem. Lett. 2022, 33, 1798–1816. [Google Scholar] [CrossRef]
- He, W.-B.; Zhao, S.-J.; Chen, J.-Y.; Jiang, J.; Chen, X.; Xu, X.; He, W.-M. External electrolyte-free electrochemical one-pot cascade synthesis of 4-thiocyanato-1H-pyrazoles. Chin. Chem. Lett. 2023, 34, 107640. [Google Scholar] [CrossRef]
- Song, H.-Y.; Zhang, Z.-T.; Tan, H.-Y.; Lu, Y.-H.; Yang, T.-B.; Chen, J.-Y.; Ji, H.-T.; He, W.-M. Visible-Light-Promoted Electron Donor-Acceptor Complex Enabled Decarboxylative Alkylation of Quinoxalin-2(1H)-ones and N-Hydroxyphthalimide Esters with Na2S as a Catalytic Donor. Asian J. Org. Chem. 2023, 12, e202200658. [Google Scholar]
- Li, N.-B.; Gu, S.; Hu, C.-Q.; Wang, Y.-X.; Zhou, X.; Qiao, J.; Jiang, J.; Ji, H.-T.; He, W.-M. Visible-light-induced mesoporous graphitic carbon nitride-catalyzed trifluoromethylation and perfluoroalkylation of 4-aminocoumarins. New J. Chem. 2023, 47, 660–665. [Google Scholar] [CrossRef]




 | ||
|---|---|---|
| Entry | Variation from the Standard Conditions | Yield (%) b |
| 1 | none | 83% |
| 2 | Pt (+)|Pt (−) instead of C (+)|Pt (−) | 51% |
| 3 | C(+)|C(−) instead of C(+)|Pt (−) | 60% |
| 4 | C(+)|Cu(−) instead of C(+)|Pt (−) | 26% |
| 5 | C(+)|Al(−) instead of C(+)|Pt (−) | 17% |
| 6 | C(+)|Ni (−) instead of C(+)|Pt (−) | trace |
| 7 | C(+)|Mg (−) instead of C(+)|Pt (−) | trace |
| 8 | LiPF6, LiClO4, LiOAc instead of LiBF4 | trace |
| 9 | NaBF4, H4NBF4, nBu4NBF4 instead of LiBF4 | trace |
| 10 | DMF instead of MeCN | 27% |
| 11 | EtOH, DCE, DMSO, THF instead of MeCN | N.R. |
| 12 | 10 mA instead of 15 mA | N.R. |
| 13 | 20 mA instead of 15 mA | 65% |
| 14 | No LiBF4 | N.R. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.-B.; Tang, L.-L.; Jiang, J.; Li, X.; Xu, X.; Yang, T.-B.; He, W.-M. Paired Electrolysis Enabled Cyanation of Diaryl Diselenides with KSCN Leading to Aryl Selenocyanates. Molecules 2023, 28, 1397. https://doi.org/10.3390/molecules28031397
He W-B, Tang L-L, Jiang J, Li X, Xu X, Yang T-B, He W-M. Paired Electrolysis Enabled Cyanation of Diaryl Diselenides with KSCN Leading to Aryl Selenocyanates. Molecules. 2023; 28(3):1397. https://doi.org/10.3390/molecules28031397
Chicago/Turabian StyleHe, Wei-Bao, Luo-Lin Tang, Jun Jiang, Xiao Li, Xinhua Xu, Tian-Bao Yang, and Wei-Min He. 2023. "Paired Electrolysis Enabled Cyanation of Diaryl Diselenides with KSCN Leading to Aryl Selenocyanates" Molecules 28, no. 3: 1397. https://doi.org/10.3390/molecules28031397
APA StyleHe, W.-B., Tang, L.-L., Jiang, J., Li, X., Xu, X., Yang, T.-B., & He, W.-M. (2023). Paired Electrolysis Enabled Cyanation of Diaryl Diselenides with KSCN Leading to Aryl Selenocyanates. Molecules, 28(3), 1397. https://doi.org/10.3390/molecules28031397









