Self-Emulsifying Micellization of Crude Extracts from Apple (Malus domestica cv. Anna), Plum (Prunus domestica cv. Satsuma), and Guava (Psidium guajava L.) Fruits
Abstract
1. Introduction
2. Results
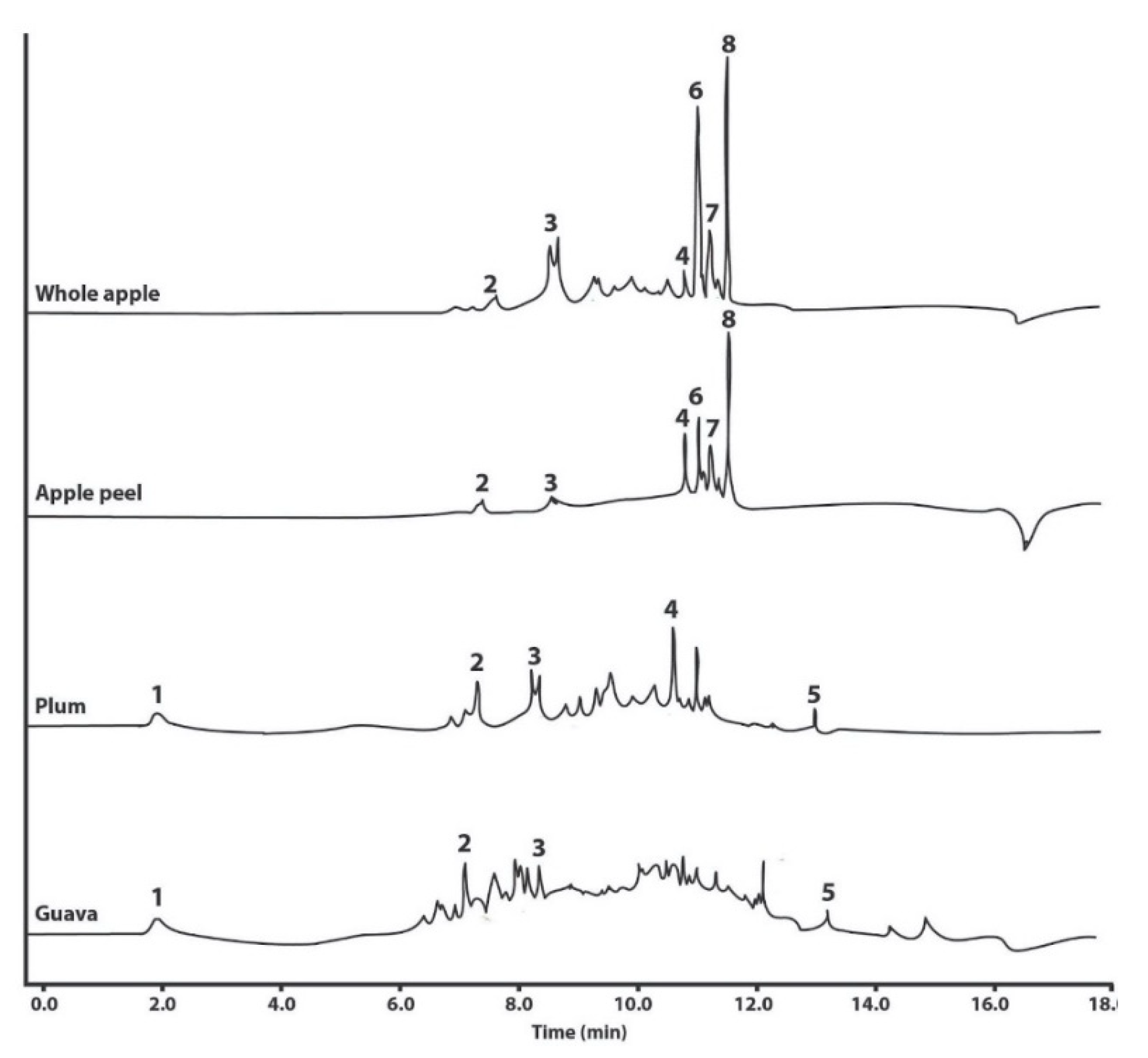
2.1. Phytochemical Analysis
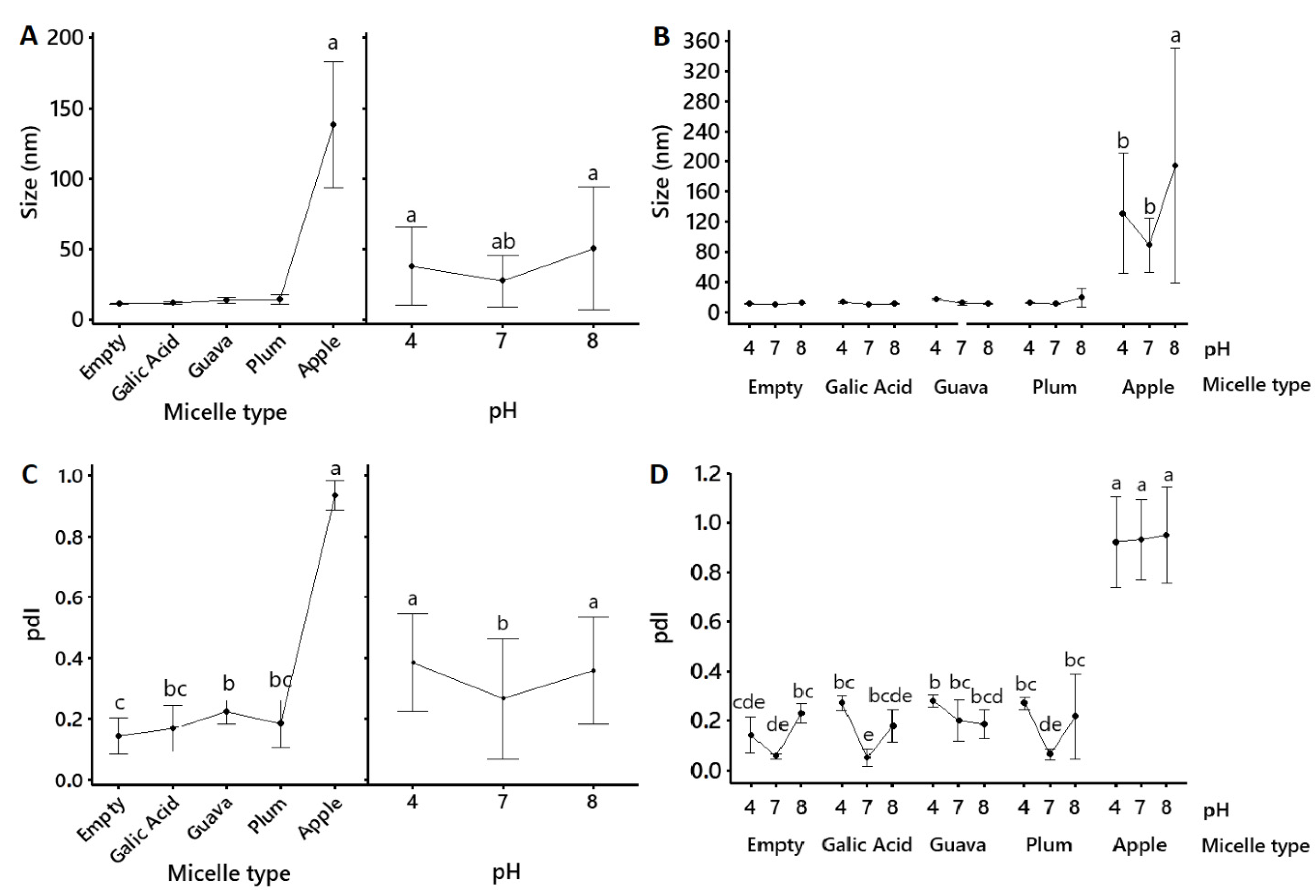
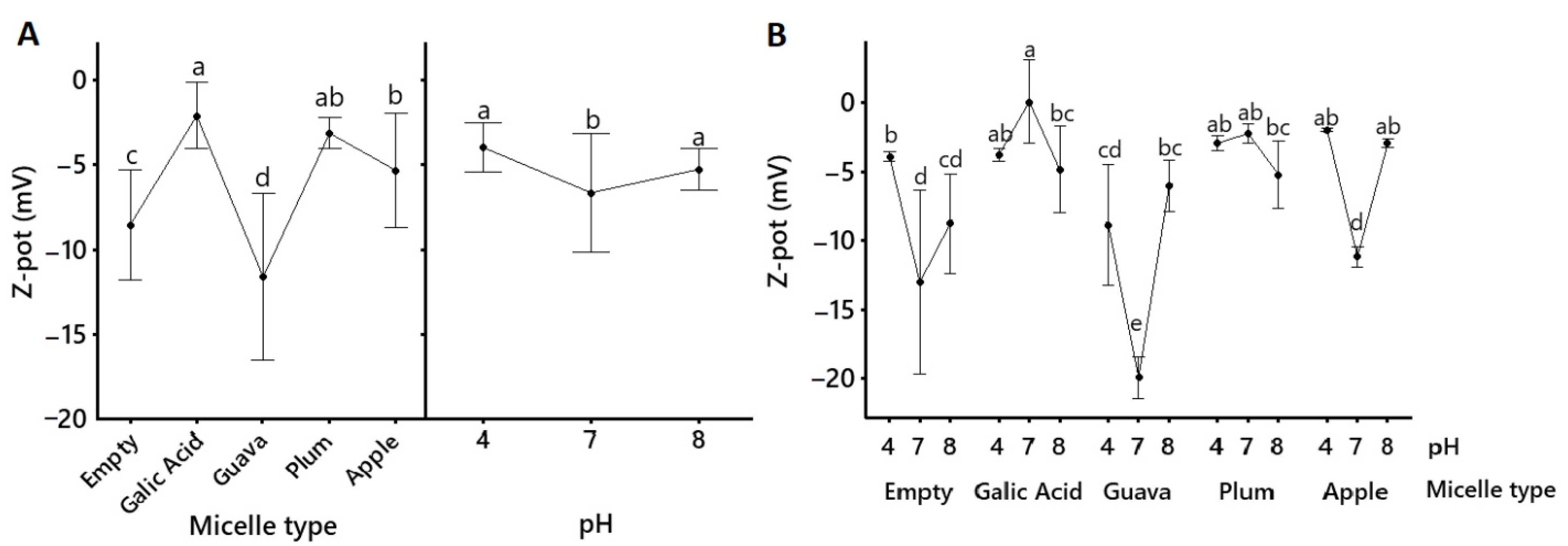
2.2. Micellar Characterization
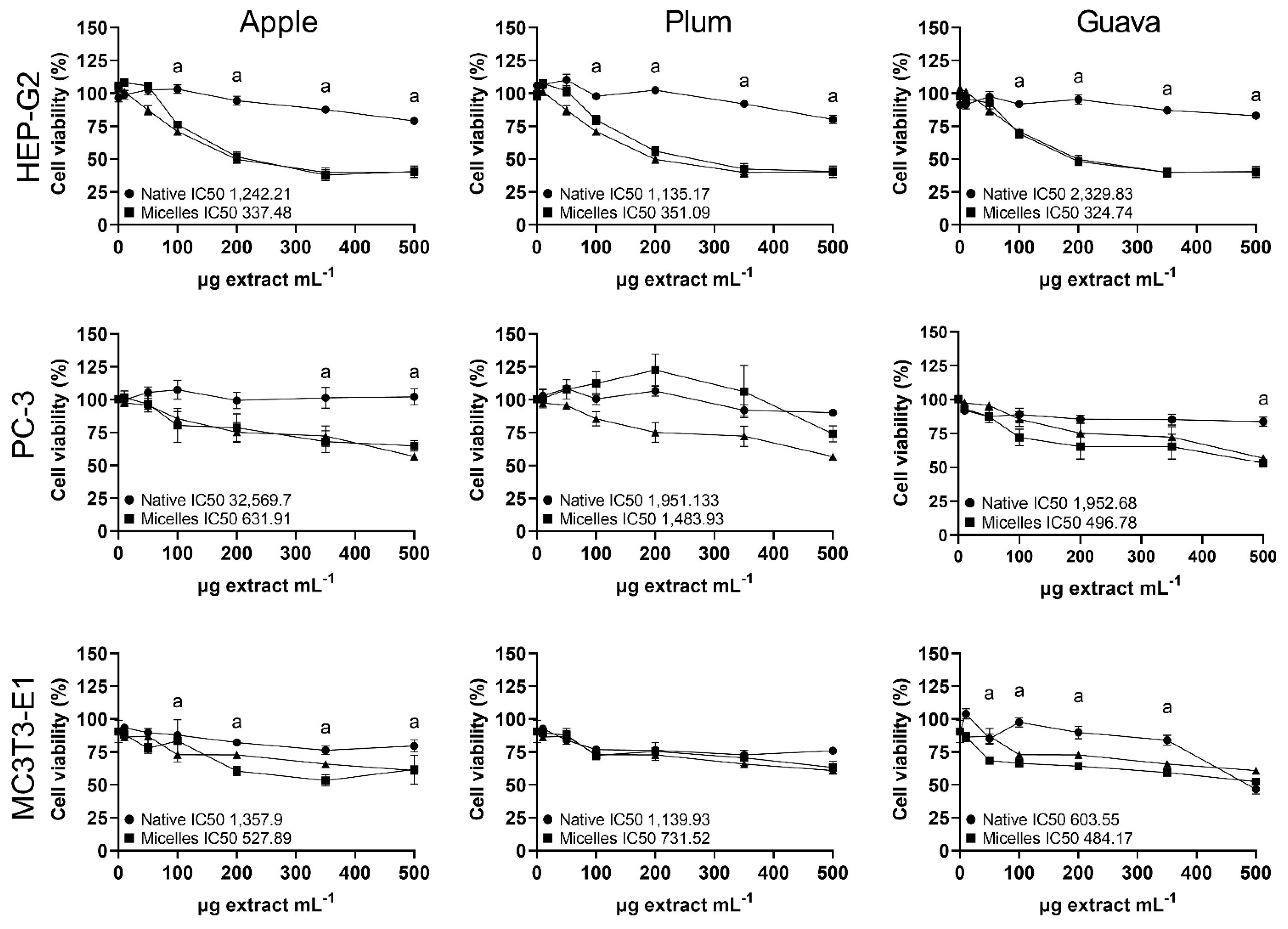
2.3. Cytotoxicity
3. Materials and Methods
3.1. Extract Preparation
3.2. Micellar Formulations
3.3. Phytochemical Analysis
3.4. Antioxidant Activity (DPPH Assay)
3.5. Cell Culture
3.6. Cell Viability
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Harish, K.; Neha, C.; Varsha, N.; Naveen, K.; Raman, S. Phenolic compounds and their health benefits: A review. J. Food Res. Technol. 2014, 2, 46–59. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. A Review of Strawberry Photobiology and Fruit Flavonoids in Controlled Environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef] [PubMed]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple (Malus × domestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic Characterization and Antioxidant Activity of Malus domestica and Prunus domestica Cultivars from Costa Rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Queirós-Reis, L.; Gomes da Silva, P.; Gonçalves, J.; Brancale, A.; Bassetto, M.; Mesquita, J.R. SARS-CoV-2 Virus−Host Interaction: Currently Available Structures and Implications of Variant Emergence on Infectivity and Immune Response. Int. J. Mol. Sci. 2021, 22, 10836. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. Polyphenolic QTOF-ESI MS Characterization and the Antioxidant and Cytotoxic Activities of Prunus domestica Commercial Cultivars from Costa Rica. Molecules 2021, 26, 6493. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Lobo-Vázquez, M.; Gómez-González, J.C.; Arnáez-Serrano, E.; Zamora-Fallas, G.; Sánchez-Zúñiga, K.; Centeno-Cerdas, C. Bioactive Potential of Tropical Highland Apple (Malus domestica cv. Anna) Crude Extract: Opportunities for Food Waste Revalorization. Futur. J. Pharm. Sci. 2022, 8, 57. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegria, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Behnam, D.; Hayward, M.A. Resveratrol Solubilisation Product For Pharmaceutical Purposes. United States Patent No. US010780056B2, 22 September 2020. Available online: https://patents.google.com/patent/US10780056B2/en.
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic Profiles in Eight Apple Cultivars Using High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Pérez, A.M.; Vaillant, F.; Pineda-Castro, M.L. Physicochemical and antioxidant composition of fresh peach palm (Bactris gasipaes Kunth) fruits in Costa Rica. Braz. J. Food Technol. 2016, 19, e2015097. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; de Batista, R.S.A.; de Medeiros, F.D.; Santos, F.S.; Medeiros, A.C.D. Development of a rapid and simple HPLC-UV method for determination of gallic acid in Schinopsis brasiliensis. Rev. Bras. Farmacogn. 2015, 25, 208–211. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2018, 96, 128–136. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak-Wilke, E.; Polkowska, Ż.; Koprowski, M.; Owsianik, K.; Mitchell, A.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Garbanzo, C.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Characterization of phenolic and other polar compounds in peel and flesh of pink guava (Psidium guajava L. cv. ‘Criolla’) by ultra-high performance liquid chromatography with diode array and mass spectrometric detection. Food Res. Int. 2017, 100, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Miletić, N.; Mitrović, O.; Popović, B.; Nedović, V.; Zlatković, B.; Kandić, M. Polyphenolic Content and Antioxidant Capacity in Fruits of Plum (Prunus domestica L.) Cultivars “Valjevka” and “Mildora” as Influenced by Air Drying. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Francia, V.; Montizaan, D.; Salvati, A. Interactions at the cell membrane and pathways of internalization of nano-sized materials for nanomedicine. Beilstein J. Nanotechnol. 2020, 11, 338–353. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Evans, D.; Wennerström, H. The Colloidal Domain—Where Physics, Chemistry, Biology and Technology Meet, 2nd ed.; Wiley-VCH: Hoboken, NJ, USA, 1999. [Google Scholar]
- Pool, R.; Bolhuis, P.G. The influence of micelle formation on the stability of colloid surfactant mixtures. Phys. Chem. Chem. Phys. 2010, 12, 14789–14797. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Kabat, M.; Węgrzyn, E.; Zielina, M.; Banach, M. Encapsulation of antioxidant compounds in biopolymer micelles. Chem. Eng. Commun. 2019, 207, 393–412. [Google Scholar] [CrossRef]
- Frank, J.; Schiborr, C.; Kocher, A.; Meins, J.; Behnam, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Transepithelial Transport of Curcumin in Caco-2 Cells Is significantly Enhanced by Micellar Solubilisation. Plant Foods Hum. Nutr. 2016, 72, 48–53. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral Bioavailability of Trans -Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A. Improving the Oral Bioavailability of Dietary Polyphenols by Micellar Solubilization: Investigations on 6- and 8-Prenylnaringenin, Trans-Resveratrol and ferulic Acid. Doctoral Dissertation, University of Hohenheim, Stuttgart, Germany, 2018. [Google Scholar]
- Nordin, M.; Kadir, A.; Zakaria, Z.; Abdullah, R.; Nazrul, M.; Abdullah, H. In vitro investigation of cytotoxic and anti-oxidative activities of Ardisia crispa against breast cancer cell lines, MCF-7 and MDA-MB-231. BMC Complement. Altern. Med. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-Soluble Drugs. J. Drug Deliv. 2013, 2013, 340315. [Google Scholar] [CrossRef]
| Extract | TPC (µg GAE mg−1 Extract) | TFC (µg QE mg−1 Extract) | DPPH (IC50) (µg Extract mL−1) |
|---|---|---|---|
| Whole Apple (M. domestica cv. Anna) | |||
| High ethanol | 15.70 ± 3.99 a | 1.09 ± 0.25 a | 55.66 ± 19.33 a |
| Low ethanol | 22.23 ± 12.66 a | 0.32 ± 0.27 a | 30.26 ± 0.23 b |
| Apple Peel (M. domestica cv. Anna) | |||
| High ethanol | 25.72 ± 5.99 a | 2.93 ± 1.84 a | 19.06 ± 5.52 b |
| Low ethanol | 23.53 ± 11.45 a | 1.11 ± 0.16 a | 25.43 ± 26.38 b |
| Whole Plum (P. domestica cv. Satsuma) | |||
| High ethanol | 20.29 ± 4.68 a | 1.84 ± 0.77 a | 15.84 ± 1.62 b |
| Low ethanol | 23.45 ± 3.87 a | 0.18 ± 0.44 a | 15.39 ± 2.35 b |
| Guava (P. guajava L.) | |||
| High ethanol | 15.17 ± 3.20 a | 5.85 ± 1.12 b | 14.28 ± 3.99 b |
| Low ethanol | 24.27 ± 5.19 a | 1.46 ± 0.44 a | 16.04 ± 5.87 b |
| Compound | Retention Time (min) | UV/Vis Wavelength (nm) | Polyphenol Concentration [ug mg−1 Extract] in Native Extracts | Proportion of Polyphenols in Micelles/Native Extract | |||||
|---|---|---|---|---|---|---|---|---|---|
| Whole Apple | Apple Peel | Plum | Guava | Apple Peel | Plum | Guava | |||
| Gallic acid | 2.0 | 201/220/270 | n.d. | n.d. | 0.81 ± 0.49 a | 2.65 ± 0.10 b | n.d. | 19.29 | 4.97 |
| Catechin | 7.7 | 201/278 | 0.83 ± 0.49 a | 1.27 ± 0.42 a | 9.44 ± 6.87 a | 24.07 ± 0.88 b | n.d. | 2.91 | n.d. |
| Epicatechin | 9.0 | 201/281/324 | 2.68 ± 1.34 a | 2.91 ± 0.36 a | 7.05 ± 1.25 b | 2.26 ± 0.08 a | 2.56 | 1.09 | n.d. |
| Rutin | 11.2 | 201/256/355 | 1.08 ± 0.07 a | 5.54 ± 0.79 b | 5.97 ± 1.21 b | n.d. | 1.14 | 0.45 | n.d. |
| Quercetin | 13.1 | 201/255/370 | n.d. | n.d. | 0.55 ± 0.30 a | 1.09 ± 0.04 b | n.d. | 17.26 | n.d. |
| Phloretin xyloglucoside ti | 11.4 | 191/220/286 | n.c. | n.c. | n.d. | n.d. | 0.94 | n.d. | n.d. |
| Quercetin glucoside ti | 11.6 | 199/257/353 | n.c. | n.c. | n.d. | n.d. | 1.01 | n.d. | n.d. |
| Phloridzin ti | 11.9 | 191/221/286 | n.c. | n.c. | n.d. | n.d. | 1.28 | n.d. | n.d. |
| Sample | Formulation | Size (nm) | pdI | ζ–Potentials (mV) |
|---|---|---|---|---|
| Gallic Acid | ||||
| Commercial standard | Native | 156.93 ± 4.51 a | 0.239 ± 0.017 a | −15.27 ± 8.59 b |
| Micelles | 10.56 ± 0.04 b | 0.054 ± 0.014 b | 0.04 ± 2.87 a | |
| Apple (M. domestica cv. Anna) | ||||
| Whole fruit | Native | 228.30 ± 28.50 a | 0.361 ± 0.048 b | −13.87 ± 3.91 a |
| Apple peel | Native | 244.60 ± 12.81 a | 0.313 ± 0.008 b | −24.28 ± 2.95 a |
| Micelles | 138.18 ± 6.19 b | 0.935 ± 0.014 a | −5.36 ± 0.48 b | |
| Plum (P. domestica cv. Satsuma) | ||||
| Whole fruit | Native | 139.53 ± 1.33 a | 0.189 ± 0.019 a | −11.43 ± 1.47 b |
| Micelles | 10.68 ± 0.03 b | 0.066 ± 0.009 b | −2.25 ± 0.66 a | |
| Guava (P. guajava L.) | ||||
| Whole fruit | Native | 245.63 ± 15.07 a | 0.265 ± 0.025 a | −53.17 ± 0.68 b |
| Micelles | 11.89 ± 1.02 b | 0.202 ± 0.033 a | −19.90 ± 0.60 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Castro, L.A.; Irías-Mata, A.; Cano-Contreras, D.; Arnáez-Serrano, E.; Chacón-Cerdas, R.; Starbird-Pérez, R.; Morales-Sánchez, J.; Centeno-Cerdas, C. Self-Emulsifying Micellization of Crude Extracts from Apple (Malus domestica cv. Anna), Plum (Prunus domestica cv. Satsuma), and Guava (Psidium guajava L.) Fruits. Molecules 2023, 28, 1297. https://doi.org/10.3390/molecules28031297
Calvo-Castro LA, Irías-Mata A, Cano-Contreras D, Arnáez-Serrano E, Chacón-Cerdas R, Starbird-Pérez R, Morales-Sánchez J, Centeno-Cerdas C. Self-Emulsifying Micellization of Crude Extracts from Apple (Malus domestica cv. Anna), Plum (Prunus domestica cv. Satsuma), and Guava (Psidium guajava L.) Fruits. Molecules. 2023; 28(3):1297. https://doi.org/10.3390/molecules28031297
Chicago/Turabian StyleCalvo-Castro, Laura A., Andrea Irías-Mata, Daronne Cano-Contreras, Elizabeth Arnáez-Serrano, Randall Chacón-Cerdas, Ricardo Starbird-Pérez, Johan Morales-Sánchez, and Carolina Centeno-Cerdas. 2023. "Self-Emulsifying Micellization of Crude Extracts from Apple (Malus domestica cv. Anna), Plum (Prunus domestica cv. Satsuma), and Guava (Psidium guajava L.) Fruits" Molecules 28, no. 3: 1297. https://doi.org/10.3390/molecules28031297
APA StyleCalvo-Castro, L. A., Irías-Mata, A., Cano-Contreras, D., Arnáez-Serrano, E., Chacón-Cerdas, R., Starbird-Pérez, R., Morales-Sánchez, J., & Centeno-Cerdas, C. (2023). Self-Emulsifying Micellization of Crude Extracts from Apple (Malus domestica cv. Anna), Plum (Prunus domestica cv. Satsuma), and Guava (Psidium guajava L.) Fruits. Molecules, 28(3), 1297. https://doi.org/10.3390/molecules28031297









