The Study of Hypoglycemic Activity of 7-Terpenylcoumarins
Abstract
1. Introduction
2. Results
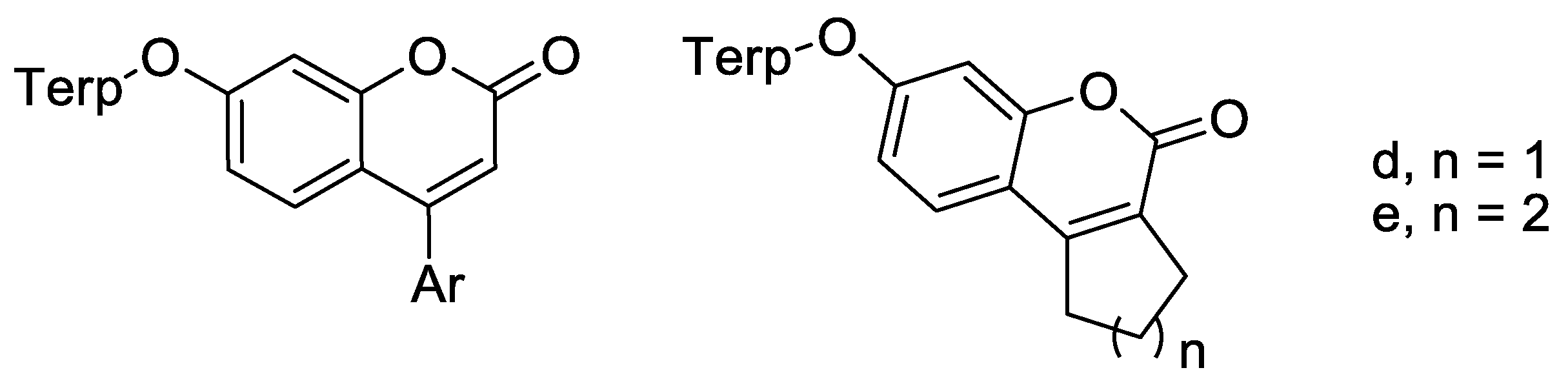
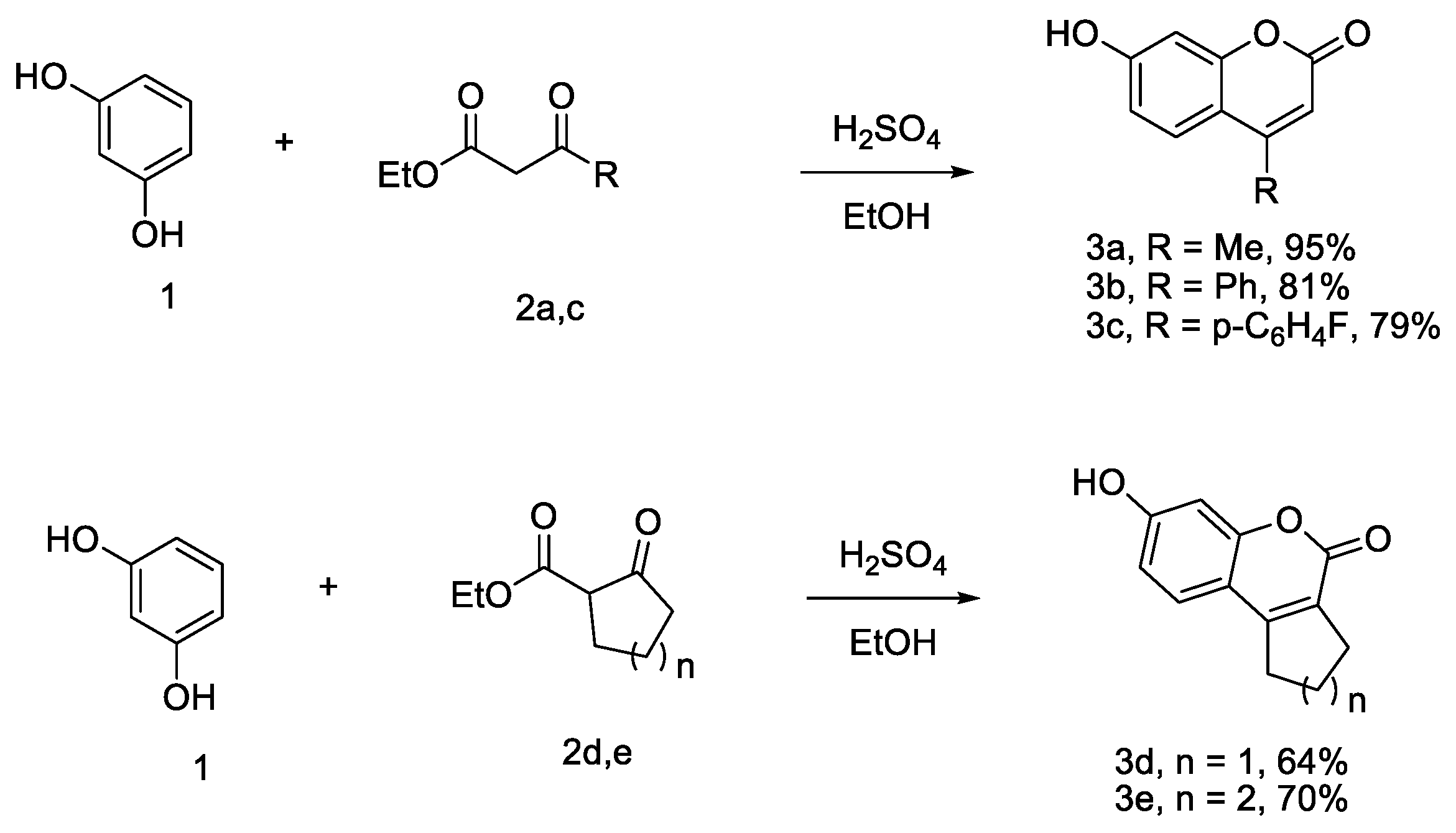
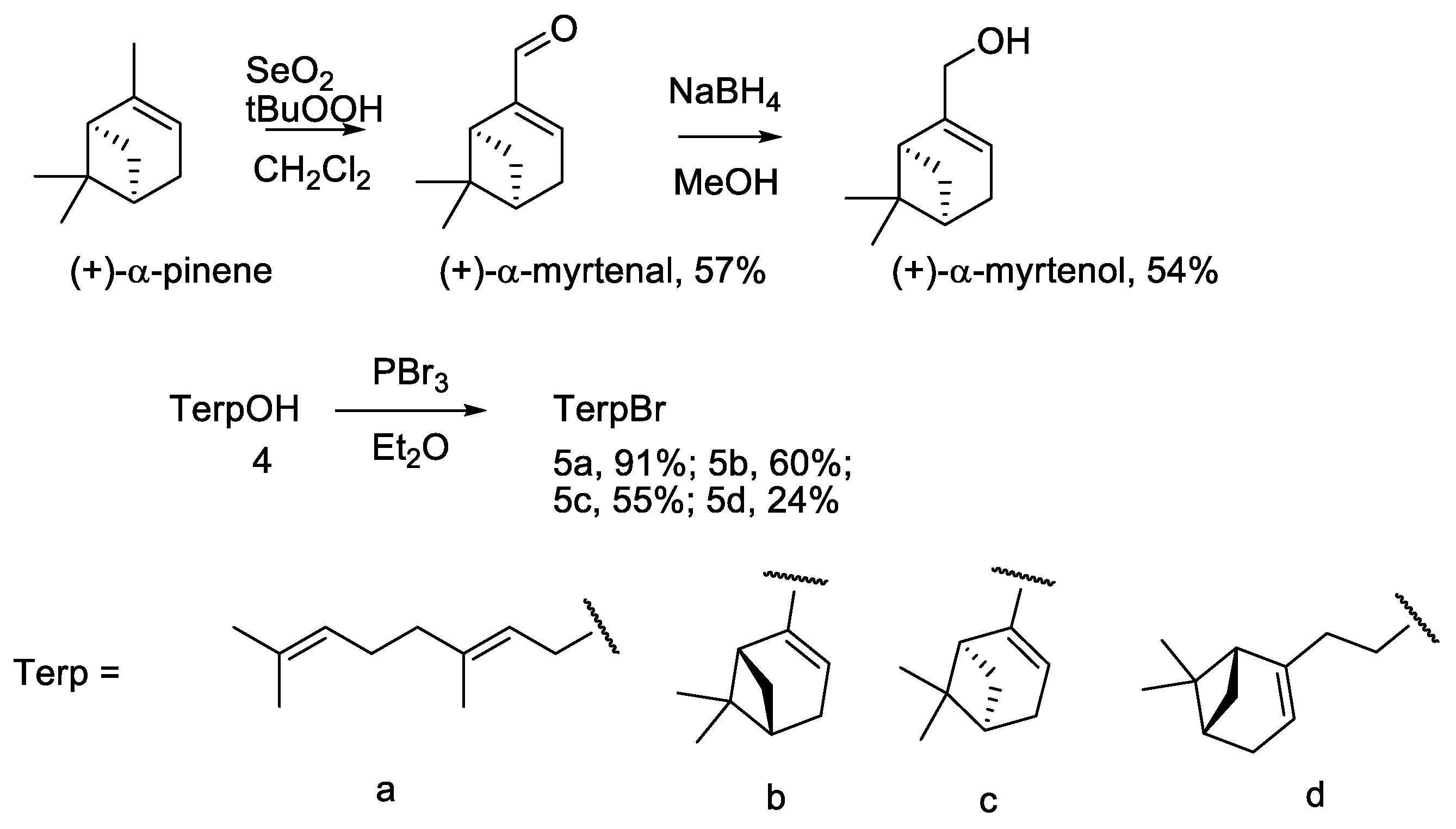
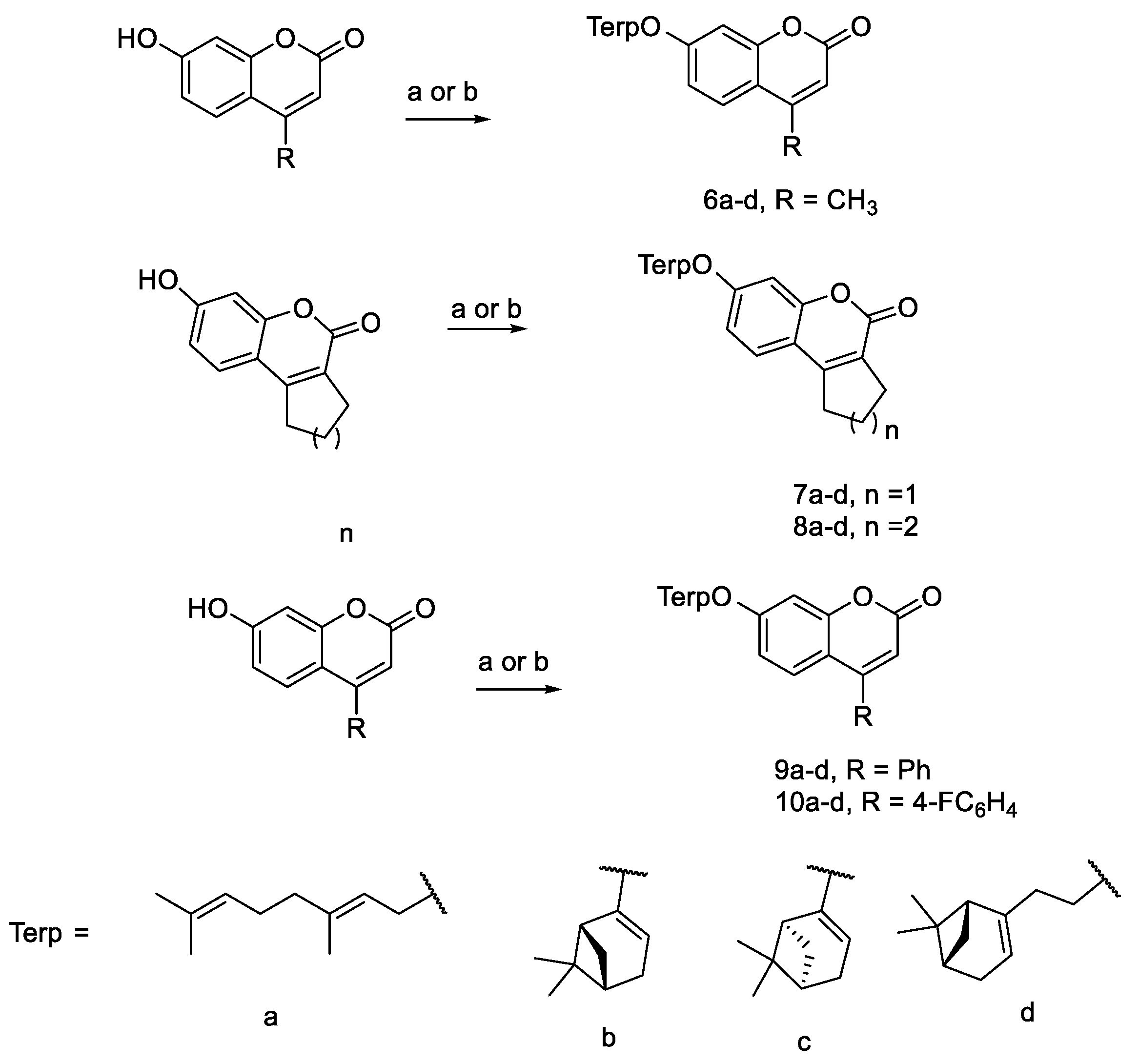
2.1. Chemistry
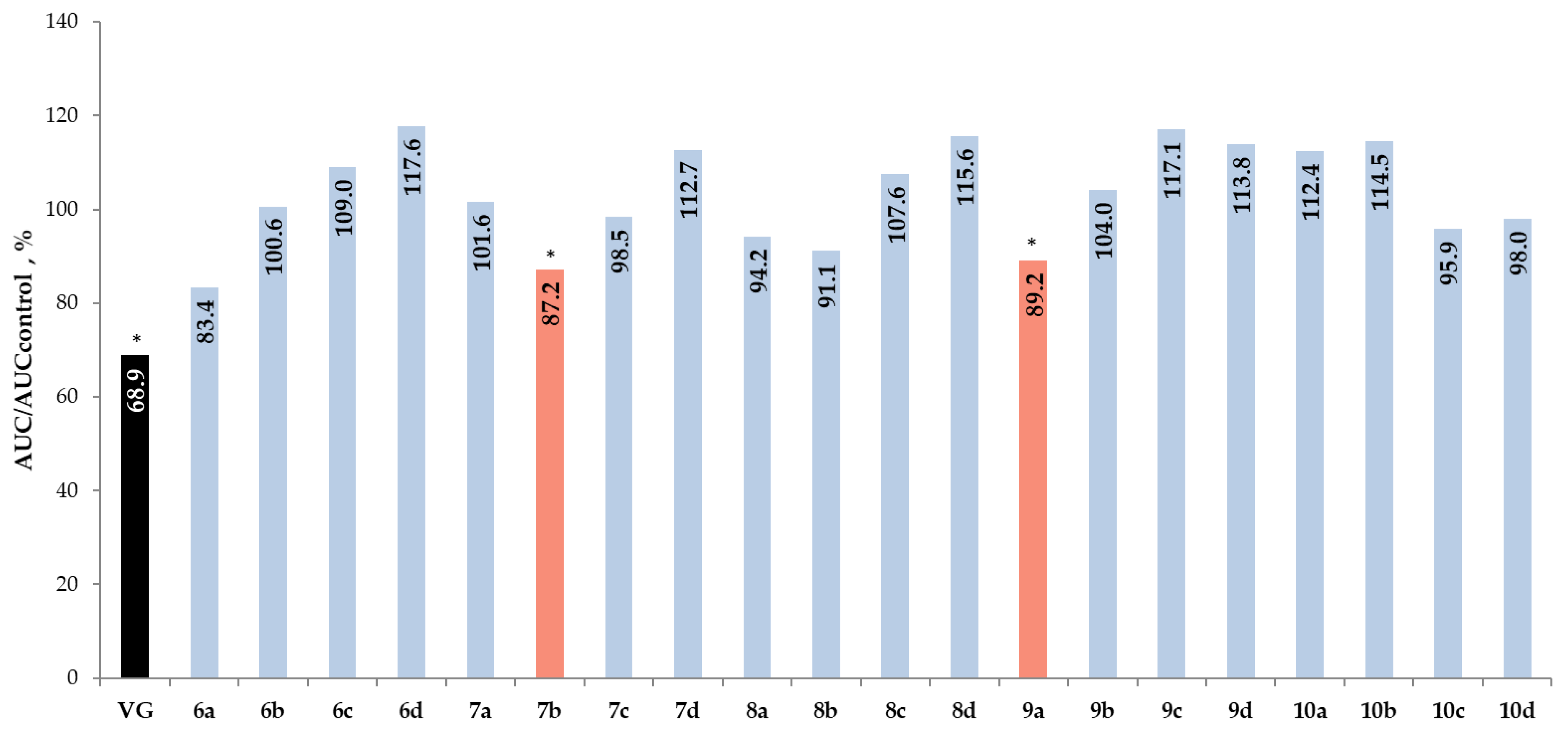
2.2. The OGTT in C57BL/6 Mice
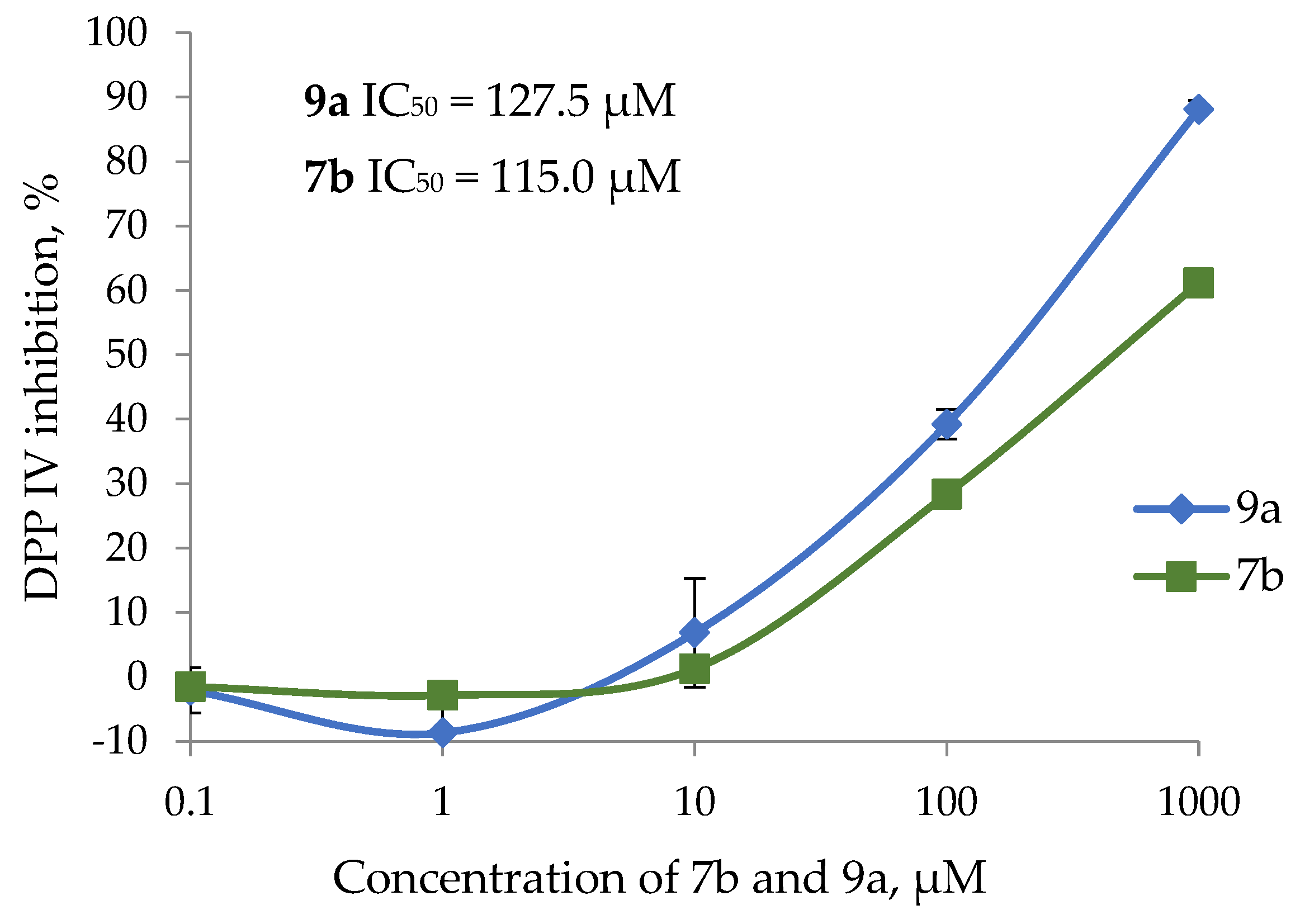
2.3. The In Vitro DPP IV Assay
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Biological Experiments
4.2.1. Animals
4.2.2. The OGTT
4.2.3. In Vitro Investigation
4.2.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Schneider, P.; Schneider, G. Privileged Structures Revisited. Angew. Chem. Int. Ed. 2017, 56, 7971–7974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liang, L.; Guo, Y. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol. Sin. 2012, 33, 1217–1245. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.; Cui, S.; Wu, F.; Zhang, Y.; Su, M.; Gong, Y.; Qiu, S.; Jiao, Q.; Qin, C.; et al. Discovery and Rational Design of Natural-Product-Derived 2-Phenyl-3,4-dihydro-2H-benzo[f]chromen-3-amine Analogs as Novel and Potent Dipeptidyl Peptidase 4 (DPP-4) Inhibitors for the Treatment of Type 2 Diabetes. J. Med. Chem. 2016, 59, 6772–6790. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.M.; Modi, P.; Trivedi, P. Recent Medicinal Chemistry Approach for the Development of Dipeptidyl Peptidase IV Inhibitors. Curr. Med. Chem. 2021, 28, 3595–3621. [Google Scholar] [CrossRef] [PubMed]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Singh, H.; Singh, J.V.; Bhagat, K.; Gulati, H.K.; Sanduja, M.; Kumar, N.; Kinarivala, N.; Sharma, S. Rational approaches, design strategies, structure activity relationship and mechanistic insights for therapeutic coumarin hybrids. Bioorganic Med. Chem. 2019, 27, 3477–3510. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, T.; Wang, X.; Sun, J. Research progress of coumarins and their derivatives in the treatment of diabetes. J. Enzym. Inhib. Med. Chem. 2022, 37, 616–628. [Google Scholar] [CrossRef]
- Habtemariam, S. Antidiabetic Potential of Monoterpenes: A Case of Small Molecules Punching above Their Weight. Int. J. Mol. Sci. 2017, 19, 4. [Google Scholar] [CrossRef]
- Curini, M.; Cravotto, G.; Epifano, F.; Giannone, G. Chemistry and Biological Activity of Natural and Synthetic Prenyloxycoumarins. Curr. Med. Chem. 2006, 13, 199–222. [Google Scholar] [CrossRef]
- Shakeri, A.; Iranshahy, M.; Iranshahi, M. Biological properties and molecular targets of umbelliprenin—A mini-review. J. Asian Nat. Prod. Res. 2014, 16, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Preziuso, F.; Genovese, S.; Marchetti, L.; Sharifi-Rad, M.; Palumbo, L.; Epifano, F.; Fiorito, S. 7-Isopentenyloxycoumarin: What Is New across the Last Decade. Molecules 2020, 25, 5923. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N.; et al. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1) combining 7-hydroxycoumarin and monoterpenoid moieties. Bioorganic Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, T.M.; Zarubaev, V.V.; Orshanskaya, I.R.; Kadyrova, R.A.; Sannikova, V.A.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. Anti-influenza activity of monoterpene-containing substituted coumarins. Bioorganic Med. Chem. Lett. 2017, 27, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, T.M.; Zakharenko, A.L.; Chepanova, A.A.; Ilina, E.S.; Zakharova, O.D.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Korchagina, D.V.; Reynisson, J.; et al. Promising New Inhibitors of Tyrosyl-DNA Phosphodiesterase I (Tdp 1) Combining 4-Arylcoumarin and Monoterpenoid Moieties as Components of Complex Antitumor Therapy. Int. J. Mol. Sci. 2019, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Bo, S.; Kiat Chang, S.; Shan, Y.; Chen, Y.; Liu, H.; Li, B.; Jiang, Y.; Zhu, H.; Yang, B. The bioactivity of prenylated stilbenoids and their structure-activity relationship. Food Res. Int. 2022, 157, 111275. [Google Scholar] [CrossRef]
- Babukumar, S.; Vinothkumar, V.; Sankaranarayanan, C.; Srinivasan, S. Geraniol, a natural monoterpene, ameliorates hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 1442–1449. [Google Scholar] [CrossRef]
- Xuemei, L.; Qiu, S.; Chen, G.; Liu, M. Myrtenol alleviates oxidative stress and inflammation in diabetic pregnant rats via TLR4/MyD88/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22904. [Google Scholar] [CrossRef]
- Couchman, F.M.; Pinder, A.R.; Bromham, N.H. Studies on the essential oil of Cyperus articulatus L. Tetrahedron 1964, 20, 2037–2045. [Google Scholar] [CrossRef]
- Araki, S.; Shimizu, T.; Johar, P.S.; Jin, S.J.; Butsugan, Y. Preparation and some reactions of allylic indium reagents. J. Org. Chem. 1991, 56, 2538–2542. [Google Scholar] [CrossRef]
- Gnerre, C.; Catto, M.; Leonetti, F.; Weber, P.; Carrupt, P.-A.; Altomare, C.; Carotti, A.; Testa, B. Inhibition of Monoamine Oxidases by Functionalized Coumarin Derivatives: Biological Activities, QSARs, and 3D-QSARs. J. Med. Chem. 2000, 43, 4747–4758. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.M. A Mathematical Model for the Determination of Total Area Under Glucose Tolerance and Other Metabolic Curves. Diabetes Care 1994, 17, 152–154. [Google Scholar] [CrossRef] [PubMed]

| Coumarin/Terp | a | b | c | d |
|---|---|---|---|---|
| 6 | 47/64 | 54/70 | 30/60 | 50 |
| 7 | 37/71 | 40 | 55/56 | 29 |
| 8 | 53/69 | 38 | 38/54 | 32 |
| 9 | 56 | 40 | 46/58 | 12/60 |
| 10 | 35 | 53/70 | 35 | 37/47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuranov, S.; Marenina, M.; Ivankin, D.; Blokhin, M.; Borisov, S.; Khomenko, T.; Luzina, O.; Khvostov, M.; Volcho, K.; Tolstikova, T.; et al. The Study of Hypoglycemic Activity of 7-Terpenylcoumarins. Molecules 2022, 27, 8663. https://doi.org/10.3390/molecules27248663
Kuranov S, Marenina M, Ivankin D, Blokhin M, Borisov S, Khomenko T, Luzina O, Khvostov M, Volcho K, Tolstikova T, et al. The Study of Hypoglycemic Activity of 7-Terpenylcoumarins. Molecules. 2022; 27(24):8663. https://doi.org/10.3390/molecules27248663
Chicago/Turabian StyleKuranov, Sergey, Mariya Marenina, Dmitriy Ivankin, Mikhail Blokhin, Sergey Borisov, Tatyana Khomenko, Olga Luzina, Mikhail Khvostov, Konstantin Volcho, Tatyana Tolstikova, and et al. 2022. "The Study of Hypoglycemic Activity of 7-Terpenylcoumarins" Molecules 27, no. 24: 8663. https://doi.org/10.3390/molecules27248663
APA StyleKuranov, S., Marenina, M., Ivankin, D., Blokhin, M., Borisov, S., Khomenko, T., Luzina, O., Khvostov, M., Volcho, K., Tolstikova, T., & Salakhutdinov, N. (2022). The Study of Hypoglycemic Activity of 7-Terpenylcoumarins. Molecules, 27(24), 8663. https://doi.org/10.3390/molecules27248663








