Acid-Triggered Switchable Near-Infrared/Shortwave Infrared Absorption and Emission of Indolizine-BODIPY Dyes
Abstract
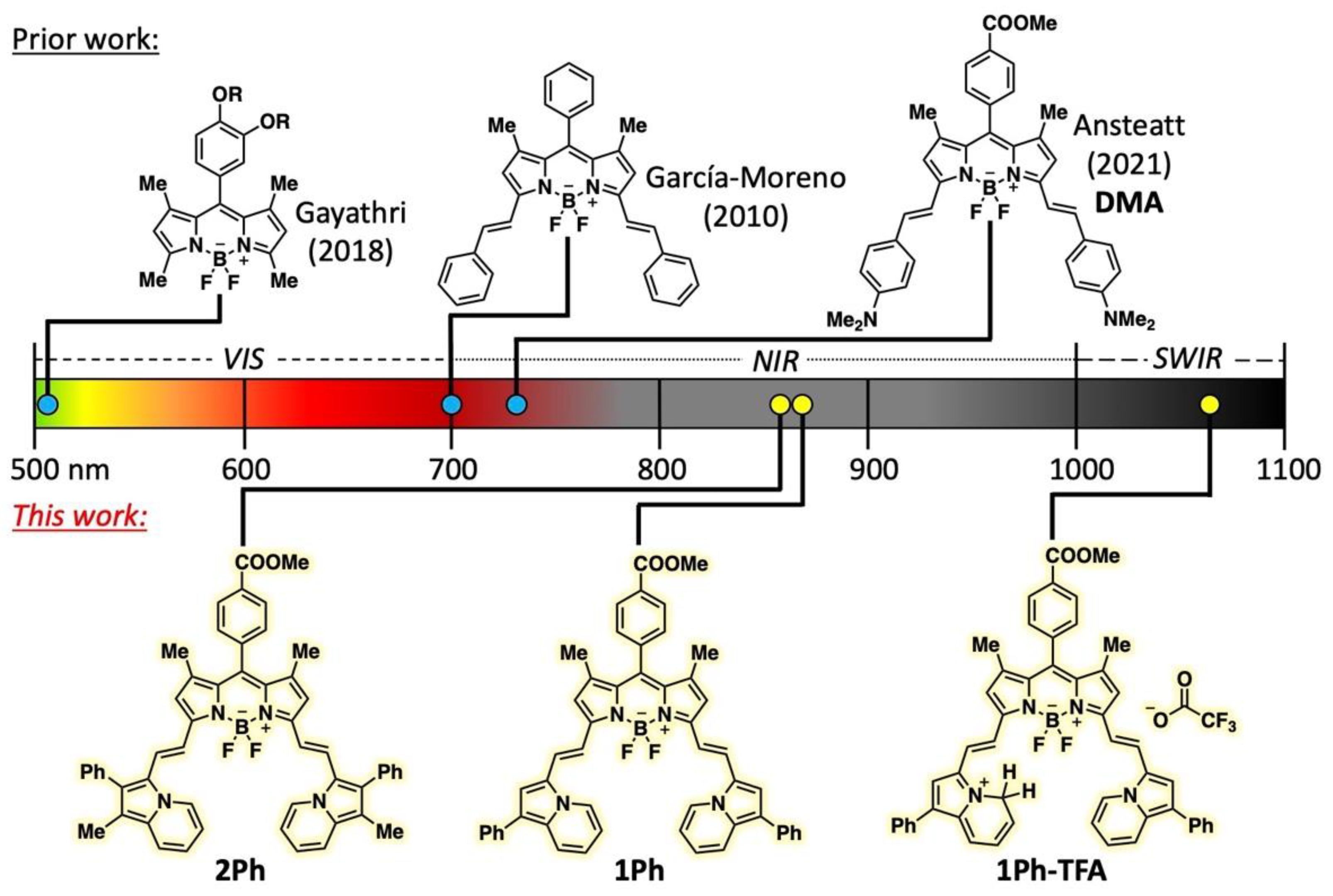
1. Introduction
2. Results and Discussion
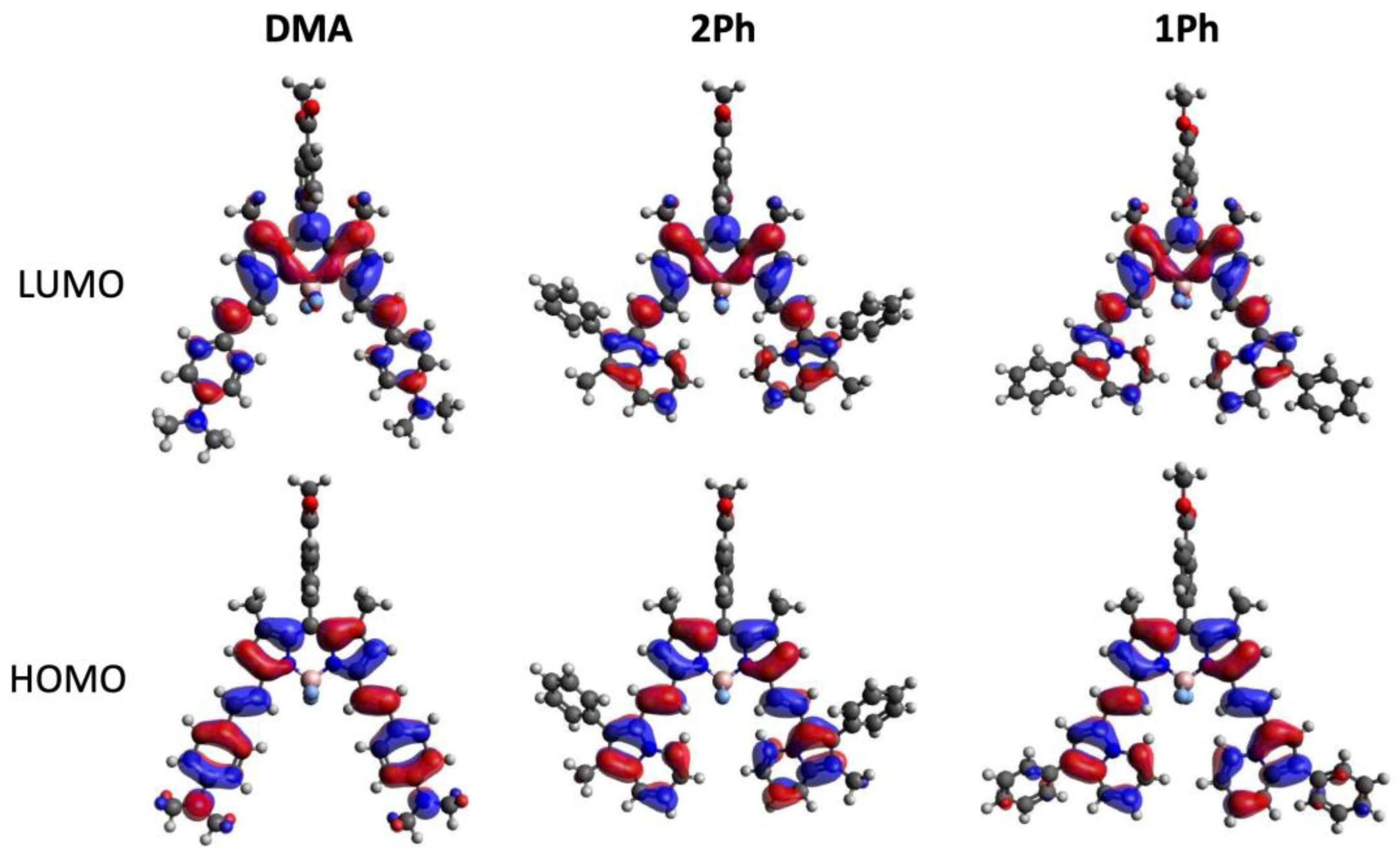
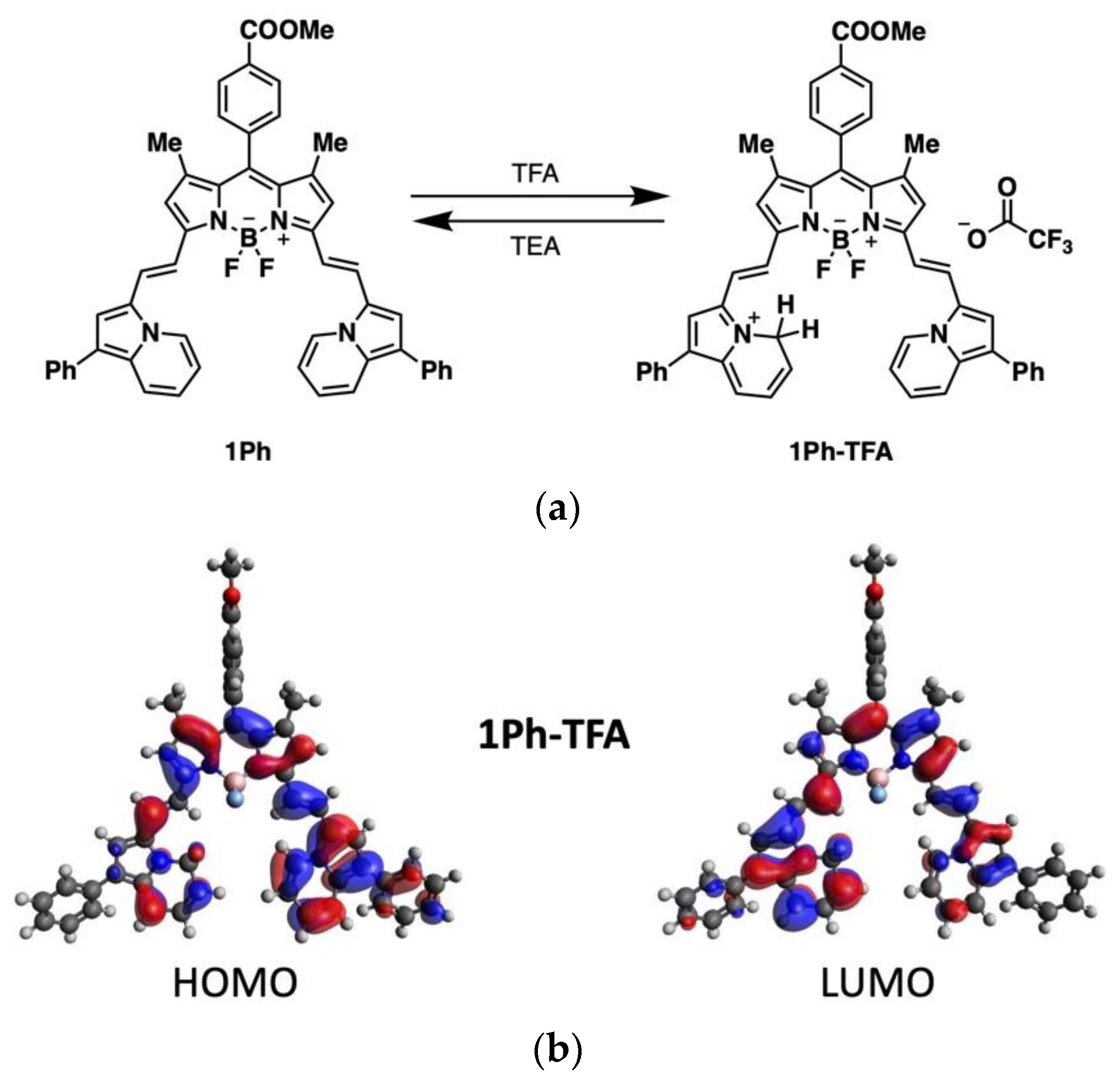
2.1. Computational Studies
2.2. Synthesis
2.3. Photophysical Studies
3. Experimental Details
3.1. General Experimental and Computational Information
3.2. Procedure for the Preparation of 1-Phenylindolizine-3-Carbaldehyde (1Ph-CHO)
3.3. General Procedure for the Preparation of Indolizine-BODIPY Dyes 2Ph and 1Ph
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 1–22. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, F. Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew. Chem. Int. Ed. 2021, 60, 16294–16308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Wan, J.-B.; Xu, F.; Zhao, N.; Chen, M. Optical Imaging in the Second Near Infrared Window for Vascular Bioimaging. Small 2021, 17, 2103780. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Bellotti, E. Numerical Study of the Intrinsic Recombination Carriers Lifetime in Extended Short-Wavelength Infrared Detector Materials: A Comparison between InGaAs and HgCdTe. J. Appl. Phys. 2016, 119, 205702. [Google Scholar] [CrossRef]
- Vittadello, L.; Klenen, J.; Koempe, K.; Kocsor, L.; Szaller, Z.; Imlau, M. NIR-to-NIR Imaging: Extended Excitation Up to 2.2 Μm Using Harmonic Nanoparticles with a Tunable HIGh EneRgy (TIGER) Widefield Microscope. Nanomaterials 2021, 11, 3193. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, D.C.; Sordillo, L.A.; Sordillo, P.P.; Shi, L.; Alfano, R.R. Short Wavelength Infrared Optical Windows for Evaluation of Benign and Malignant Tissues. J. Biomed. Opt. 2017, 22, 045002. [Google Scholar] [CrossRef]
- Barton, J.B.; Demro, J.C.; Amber, R.; Gasparian, G.; Lange, M. Performance of an Uncooled Camera Utilizing an SWIR InGaAs 256x256 FPA for Imaging in the 1.0 Um-1.7 Um Spectral Band. In Defense Public Release Technical Report; ADA399438; Defense Technical Information Center: Fort Belvoir, VA, USA, 1998. [Google Scholar]
- Li, H.; Wang, X.; Li, X.; Zeng, S.; Chen, G. Clearable Shortwave-Infrared-Emitting NaErF4 Nanoparticles for Noninvasive Dynamic Vascular Imaging. Chem. Mater. 2020, 32, 3365–3375. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent Advances on Fluorescent Biomarkers of Near-Infrared Quantum Dots for in Vitro and in Vivo Imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef]
- Carr, J.A.; Aellen, M.; Franke, D.; So, P.T.C.; Bruns, O.T.; Bawendi, M.G. Absorption by Water Increases Fluorescence Image Contrast of Biological Tissue in the Shortwave Infrared. Proc. Natl. Acad. Sci. USA 2018, 115, 9080–9085. [Google Scholar] [CrossRef]
- Sun, C.; Li, B.; Zhao, M.; Wang, S.; Lei, Z.; Lu, L.; Zhang, H.; Feng, L.; Dou, C.; Yin, D.; et al. J-Aggregates of Cyanine Dye for NIR-II in Vivo Dynamic Vascular Imaging beyond 1500 Nm. J. Am. Chem. Soc. 2019, 141, 19221–19225. [Google Scholar] [CrossRef]
- Ansteatt, S.; Meares, A.; Ptaszek, M. Amphiphilic Near-IR-Emitting 3,5-Bis(2-Pyrrolylethenyl)BODIPY Derivatives: Synthesis, Characterization, and Comparison with Other (Hetero)Arylethenyl-Substituted BODIPYs. J. Org. Chem. 2021, 86, 8755–8765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Guo, M.; Pan, Q.; Zhou, M.; Xu, L.; Rao, Y.; Wang, K.; Yin, B.; Zhou, J.; Song, J. Rhodium-Catalyzed Annulation of Pyrrole Substituted BODIPYs with Alkynes to Access π-Extended Polycyclic Heteroaromatic Molecules and NIR Absorption. Org. Chem. Front. 2021, 8, 868–875. [Google Scholar] [CrossRef]
- Kubota, Y.; Kimura, K.; Jin, J.; Manseki, K.; Funabiki, K.; Matsui, M. Synthesis of Near-Infrared Absorbing and Fluorescing Thiophene-Fused BODIPY Dyes with Strong Electron-Donating Groups and Their Application in Dye-Sensitised Solar Cells. New J. Chem. 2019, 43, 1156–1165. [Google Scholar] [CrossRef]
- Umezawa, K.; Nakamura, Y.; Makino, H.; Citterio, D.; Suzuki, K. Bright, Color-Tunable Fluorescent Dyes in the Visible–Near-Infrared Region. J. Am. Chem. Soc. 2008, 130, 1550–1551. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gérard, L.; Vasseur, W.; Scherninski, F.; Andrioletti, B. Recent Advances in the Synthesis of [a]-Benzo-Fused BODIPY Fluorophores. Chem. Commun. 2018, 54, 12914–12929. [Google Scholar] [CrossRef]
- Liu, D.; He, Z.; Zhao, Y.; Yang, Y.; Shi, W.; Li, X.; Ma, H. Xanthene-Based NIR-II Dyes for In Vivo Dynamic Imaging of Blood Circulation. J. Am. Chem. Soc. 2021, 143, 17136–17143. [Google Scholar] [CrossRef]
- Chatterjee, S.; Meador, W.E.; Smith, C.; Chandrasiri, I.; Zia, M.F.; Nguyen, J.; Dorris, A.; Flynt, A.; Watkins, D.L.; Hammer, N.I.; et al. SWIR Emissive RosIndolizine Dyes with Nanoencapsulation in Water Soluble Dendrimers. RSC Adv. 2021, 11, 27832–27836. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-H.; Zhang, Z.; Yang, Y.-C.; Chan, Y.-H. Polymethine-Based Semiconducting Polymer Dots with Narrow-Band Emission and Absorption/Emission Maxima at NIR-II for Bioimaging. Angew. Chem. Int. Ed. 2021, 60, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Cosco, E.D.; Spearman, A.L.; Ramakrishnan, S.; Lingg, J.G.P.; Saccomano, M.; Pengshung, M.; Arús, B.A.; Wong, K.C.Y.; Glasl, S.; Ntziachristos, V.; et al. Shortwave Infrared Polymethine Fluorophores Matched to Excitation Lasers Enable Non-Invasive, Multicolour in Vivo Imaging in Real Time. Nat. Chem. 2020, 12, 1123–1130. [Google Scholar] [CrossRef]
- Li, B.; Zhao, M.; Feng, L.; Dou, C.; Ding, S.; Zhou, G.; Lu, L.; Zhang, H.; Chen, F.; Li, X.; et al. Organic NIR-II Molecule with Long Blood Half-Life for in Vivo Dynamic Vascular Imaging. Nat. Commun. 2020, 11, 3102. [Google Scholar] [CrossRef]
- Huckaba, A.J.; Giordano, F.; McNamara, L.E.; Dreux, K.M.; Hammer, N.I.; Tschumper, G.S.; Zakeeruddin, S.M.; Grätzel, M.; Nazeeruddin, M.K.; Delcamp, J.H. Indolizine-Based Donors as Organic Sensitizer Components for Dye-Sensitized Solar Cells. Adv. Energy Mater. 2015, 5, 1401629. [Google Scholar] [CrossRef]
- McNamara, L.E.; Rill, T.A.; Huckaba, A.J.; Ganeshraj, V.; Gayton, J.; Nelson, R.A.; Sharpe, E.A.; Dass, A.; Hammer, N.I.; Delcamp, J.H. Indolizine–Squaraines: NIR Fluorescent Materials with Molecularly Engineered Stokes Shifts. Chem.-Eur. J. 2017, 23, 12494–12501. [Google Scholar] [CrossRef]
- Meador, W.E.; Autry, S.A.; Bessetti, R.N.; Gayton, J.N.; Flynt, A.S.; Hammer, N.I.; Delcamp, J.H. Water-Soluble NIR Absorbing and Emitting Indolizine Cyanine and Indolizine Squaraine Dyes for Biological Imaging. J. Org. Chem. 2020, 85, 4089–4095. [Google Scholar] [CrossRef]
- Gayton, J.; Autry, S.A.; Meador, W.; Parkin, S.R.; Hill, G.A., Jr.; Hammer, N.I.; Delcamp, J.H. Indolizine-Cyanine Dyes: Near Infrared Emissive Cyanine Dyes with Increased Stokes Shifts. J. Org. Chem. 2019, 84, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ndaleh, D.; Smith, C.; Loku Yaddehige, M.; Shaik, A.K.; Watkins, D.L.; Hammer, N.I.; Delcamp, J.H. Shortwave Infrared Absorptive and Emissive Pentamethine-Bridged Indolizine Cyanine Dyes. J. Org. Chem. 2021, 86, 15376–15386. [Google Scholar] [CrossRef]
- Rathnamalala, C.S.L.; Gayton, J.N.; Dorris, A.L.; Autry, S.A.; Meador, W.; Hammer, N.I.; Delcamp, J.H.; Scott, C.N. Donor–Acceptor–Donor NIR II Emissive Rhodindolizine Dye Synthesized by C–H Bond Functionalization. J. Org. Chem. 2019, 84, 13186–13193. [Google Scholar] [CrossRef]
- Gayathri, T.; Karnewar, S.; Kotamraju, S.; Singh, S.P. High Affinity Neutral Bodipy Fluorophores for Mitochondrial Tracking. ACS Med. Chem. Lett. 2018, 9, 618–622. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, I.; Zhang, D.; Costela, Á.; Martín, V.; Sastre, R.; Xiao, Y. Red-Edge Laser Action from Borondipyrromethene Dyes. J. Appl. Phys. 2010, 107, 073105. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent Molecular Orbital Methods 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Pascual-ahuir, J.L.; Silla, E.; Tuñon, I. GEPOL: An improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J. Comput. Chem. 1994, 15, 1127–1138. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilizaion of AB Initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Miertuš, S.; Tomasi, J. Approximate Evaluations of the Electrostatic Free Energy and Internal Energy Changes in Solution Processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Pohjala, E. A Facile Synthesis of Stable Dihydroindolizines via Intramolecular 1,5-Cyclization of Ylides. Tetrahedron Lett. 1972, 13, 2585–2588. [Google Scholar] [CrossRef]
- Cheng, J.M.H.; Chee, S.H.; Dölen, Y.; Verdoes, M.; Timmer, M.S.M.; Stocker, B.L. An Efficient Synthesis of a 6″-BODIPY-α-Galactosylceramide Probe for Monitoring α-Galactosylceramide Uptake by Cells. Carbohydr. Res. 2019, 486, 107840. [Google Scholar] [CrossRef]
- Kalinin, A.A.; Smirnov, M.A.; Islamova, L.N.; Fazleeva, G.M.; Vakhonina, T.A.; Levitskaya, A.I.; Fominykh, O.D.; Ivanova, N.V.; Khamatgalimov, A.R.; Nizameev, I.R.; et al. Synthesis and Characterization of New Second-Order NLO Chromophores Containing the Isomeric Indolizine Moiety for Electro-Optical Materials. Dyes Pigments 2017, 147, 444–454. [Google Scholar] [CrossRef]
- Peterson, J.A.; Wijesooriya, C.; Gehrmann, E.J.; Mahoney, K.M.; Goswami, P.P.; Albright, T.R.; Syed, A.; Dutton, A.S.; Smith, E.A.; Winter, A.H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. [Google Scholar] [CrossRef]
- Mula, S.; Elliott, K.; Harriman, A.; Ziessel, R. Energy Transfer by Way of an Exciplex Intermediate in Flexible Boron Dipyrromethene-Based Allosteric Architectures. J. Phys. Chem. A 2010, 114, 10515–10522. [Google Scholar] [CrossRef]
- Brzeczek, A.; Piwowar, K.; Domagala, W.; Mikołajczyk, M.M.; Walczak, K.; Wagner, P. Systematic Elongation of Thienyl Linkers and Their Effect on Optical and Electrochemical Properties in Carbazole–BODIPY Donor–Acceptor Systems. RSC Adv. 2016, 6, 36500–36509. [Google Scholar] [CrossRef]
- Hoogendoorn, S.; Blom, A.E.M.; Willems, L.I.; van der Marel, G.A.; Overkleeft, H.S. Synthesis of PH-Activatable Red Fluorescent BODIPY Dyes with Distinct Functionalities. Org. Lett. 2011, 13, 5656–5659. [Google Scholar] [CrossRef]
- Fedeli, S.; Paoli, P.; Brandi, A.; Venturini, L.; Giambastiani, G.; Tuci, G.; Cicchi, S. Azido-Substituted BODIPY Dyes for the Production of Fluorescent Carbon Nanotubes. Chem.-Eur. J. 2015, 21, 15349–15353. [Google Scholar] [CrossRef]
- James, N.S.; Chen, Y.; Joshi, P.; Ohulchanskyy, T.Y.; Ethirajan, M.; Henary, M.; Strekowsk, L.; Pandey, R.K. Evaluation of Polymethine Dyes as Potential Probes for Near Infrared Fluorescence Imaging of Tumors: Part-1. Theranostics 2013, 3, 692–702. [Google Scholar] [CrossRef]
- Rurack, K.; Spieles, M. Fluorescence Quantum Yields of a Series of Red and Near-Infrared Dyes Emitting at 600–1000 Nm. Anal. Chem. 2011, 83, 1232–1242. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, B.; Yang, J.; Baryshnikov, G.V.; Zhou, Y.; Ågren, H.; Zou, Q.; Zhu, L. Large Red-Shifted NIR Absorption in Azulenyl- and Iodinated-Modified BODIPYs Sensitive to Aggregation and Protonation Stimuli. Dyes Pigments 2022, 197, 109867. [Google Scholar] [CrossRef]
- Cosco, E.D.; Caram, J.R.; Bruns, O.T.; Franke, D.; Day, R.A.; Farr, E.P.; Bawendi, M.G.; Sletten, E.M. Flavylium Polymethine Fluorophores for Near- and Shortwave Infrared Imaging. Angew. Chem. Int. Ed. 2017, 56, 13126–13129. [Google Scholar] [CrossRef]
- Dou, K.; Feng, W.; Fan, C.; Cao, Y.; Xiang, Y.; Liu, Z. Flexible Designing Strategy to Construct Activatable NIR-II Fluorescent Probes with Emission Maxima beyond 1200 Nm. Anal. Chem. 2021, 93, 4006–4014. [Google Scholar] [CrossRef]
- Parker, C.A.; Rees, W.T. Correction of Fluorescence Spectra and Measurement of Fluorescence Quantum Efficiency. Analyst 1960, 85, 587–600. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent Molecular Orbital Methods. XXIII. A Polarization-type Basis Set for Second-row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]

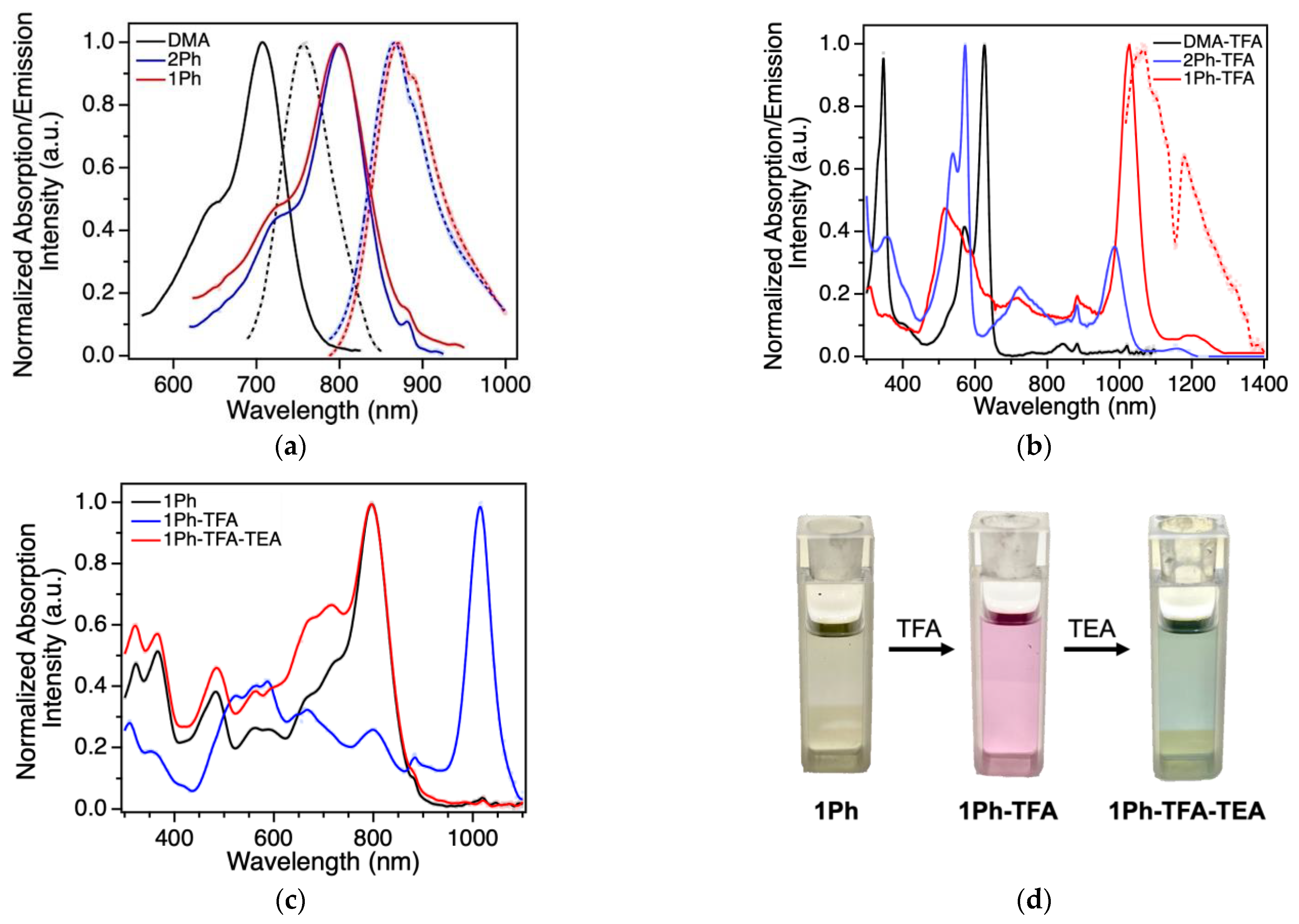
| Dye | λmaxabs (nm|eV) | λmaxemis (nm|eV) | ε (M−1 cm−1) | Φ (%) | MB (M−1 cm−1) | Stokes Shift (eV) |
|---|---|---|---|---|---|---|
| DMA | 707|1.75 | 760|1.63 | 87,500 | 34.6 ± 1.8 | 30,300 | 0.12 |
| 2Ph | 798|1.55 | 867|1.43 | 97,000 | 3.5 ± 0.3 | 3400 | 0.12 |
| 1Ph | 797|1.56 | 872|1.42 | 121,000 | 5.6 ± 0.6 | 6800 | 0.14 |
| 1Ph-TFA 1 | 1027|1.21 | 1061|1.17 | 133,500 | 0.0020 ± 0.0001 | 2.7 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saucier, M.A.; Smith, C.; Kruse, N.A.; Hammer, N.I.; Delcamp, J.H. Acid-Triggered Switchable Near-Infrared/Shortwave Infrared Absorption and Emission of Indolizine-BODIPY Dyes. Molecules 2023, 28, 1287. https://doi.org/10.3390/molecules28031287
Saucier MA, Smith C, Kruse NA, Hammer NI, Delcamp JH. Acid-Triggered Switchable Near-Infrared/Shortwave Infrared Absorption and Emission of Indolizine-BODIPY Dyes. Molecules. 2023; 28(3):1287. https://doi.org/10.3390/molecules28031287
Chicago/Turabian StyleSaucier, Matthew A., Cameron Smith, Nicholas A. Kruse, Nathan I. Hammer, and Jared H. Delcamp. 2023. "Acid-Triggered Switchable Near-Infrared/Shortwave Infrared Absorption and Emission of Indolizine-BODIPY Dyes" Molecules 28, no. 3: 1287. https://doi.org/10.3390/molecules28031287
APA StyleSaucier, M. A., Smith, C., Kruse, N. A., Hammer, N. I., & Delcamp, J. H. (2023). Acid-Triggered Switchable Near-Infrared/Shortwave Infrared Absorption and Emission of Indolizine-BODIPY Dyes. Molecules, 28(3), 1287. https://doi.org/10.3390/molecules28031287







