IR Spectroscopic Degradation Study of Thin Organometal Halide Perovskite Films
(This article belongs to the Section Electrochemistry)
Abstract
1. Introduction
2. Results and Discussion
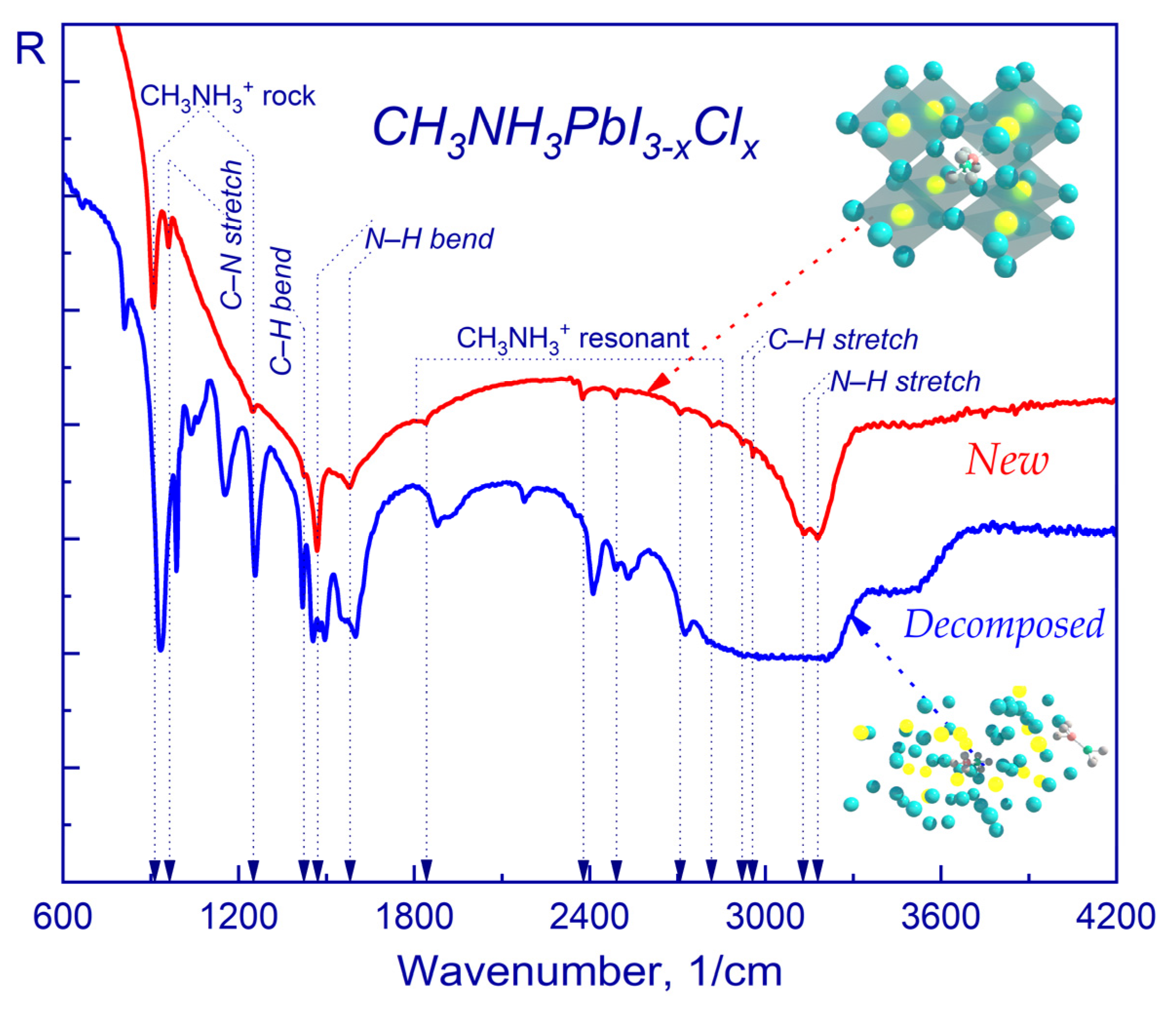
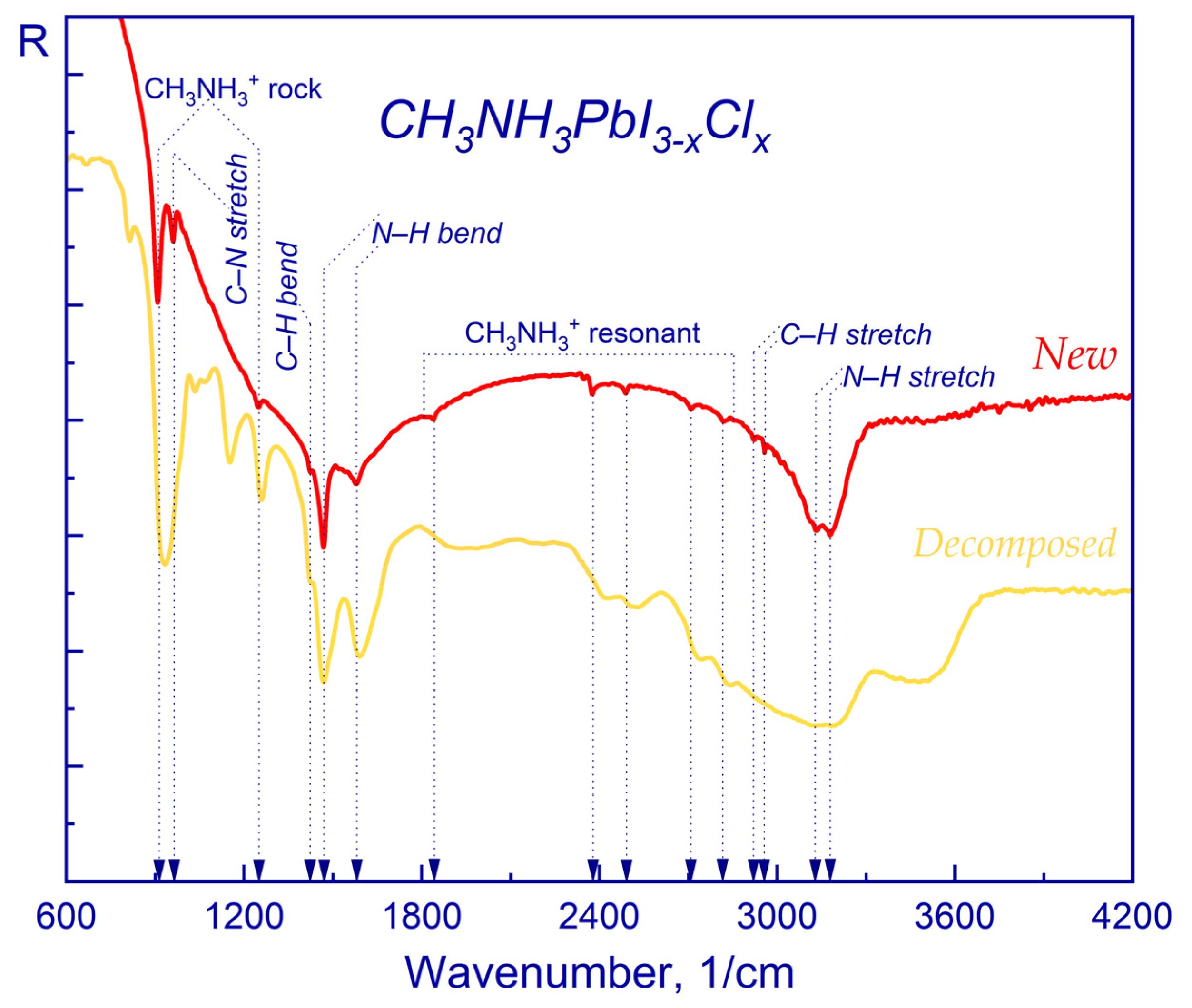
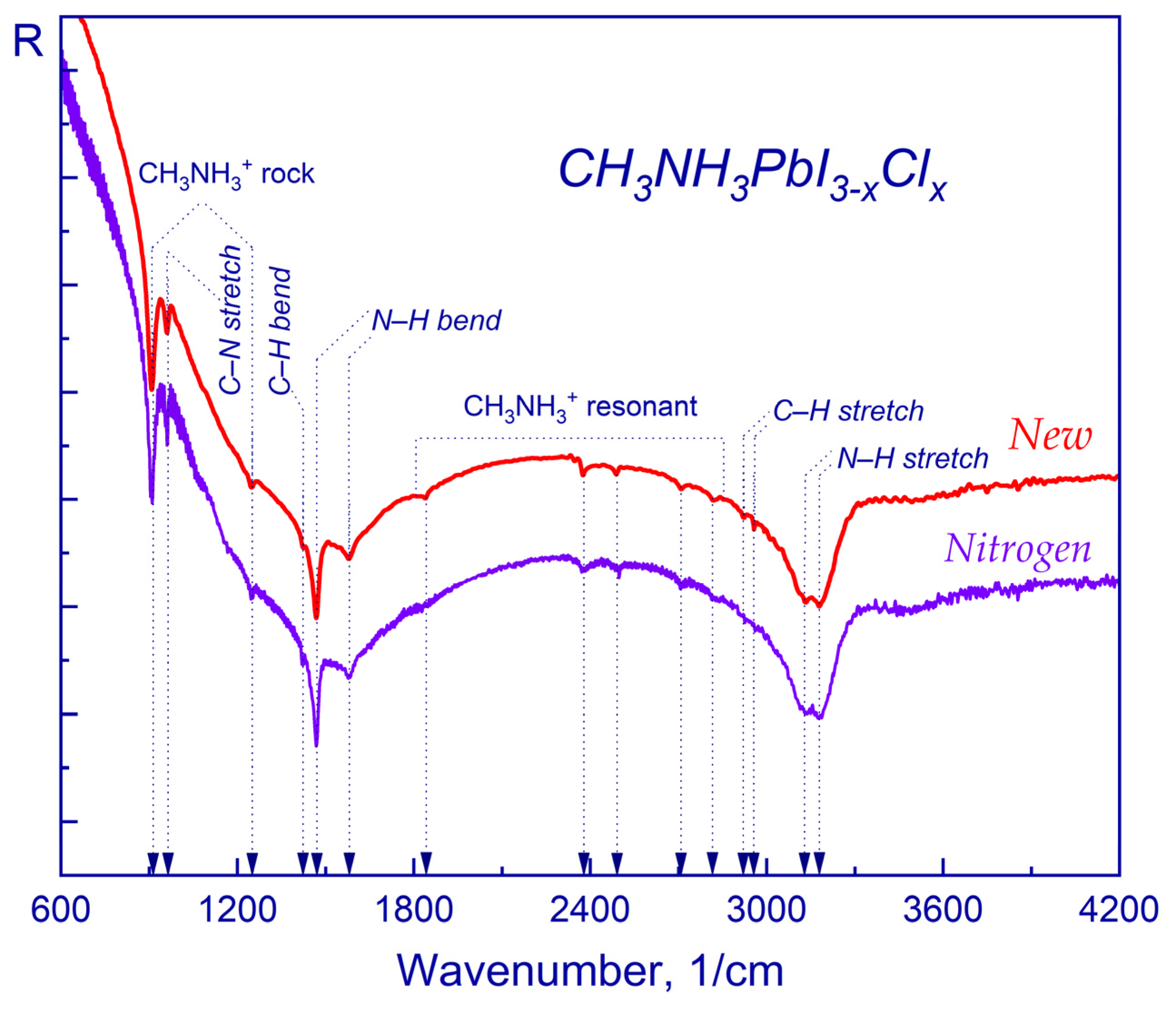
2.1. FTIR
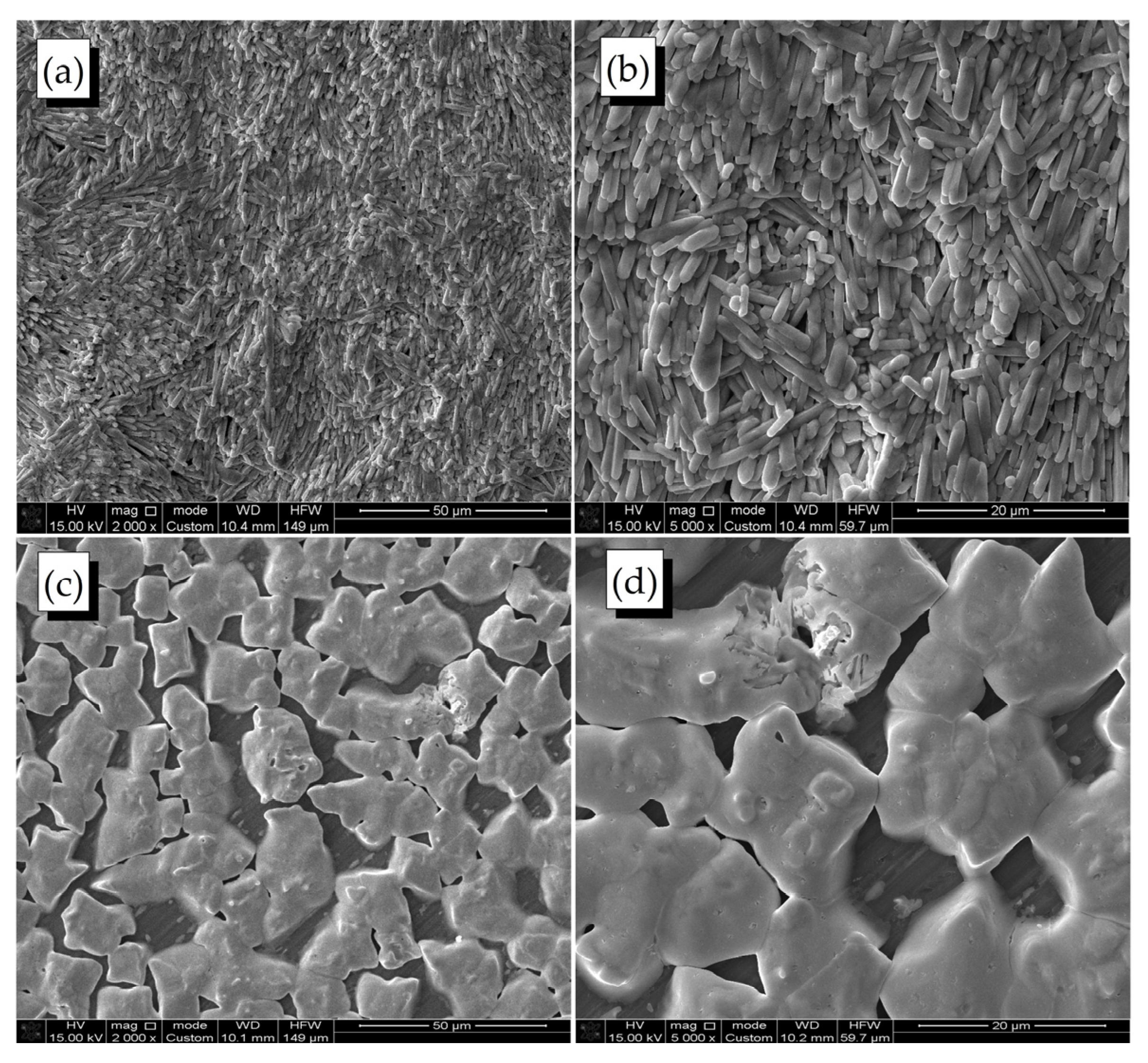
2.2. Surface Morphology
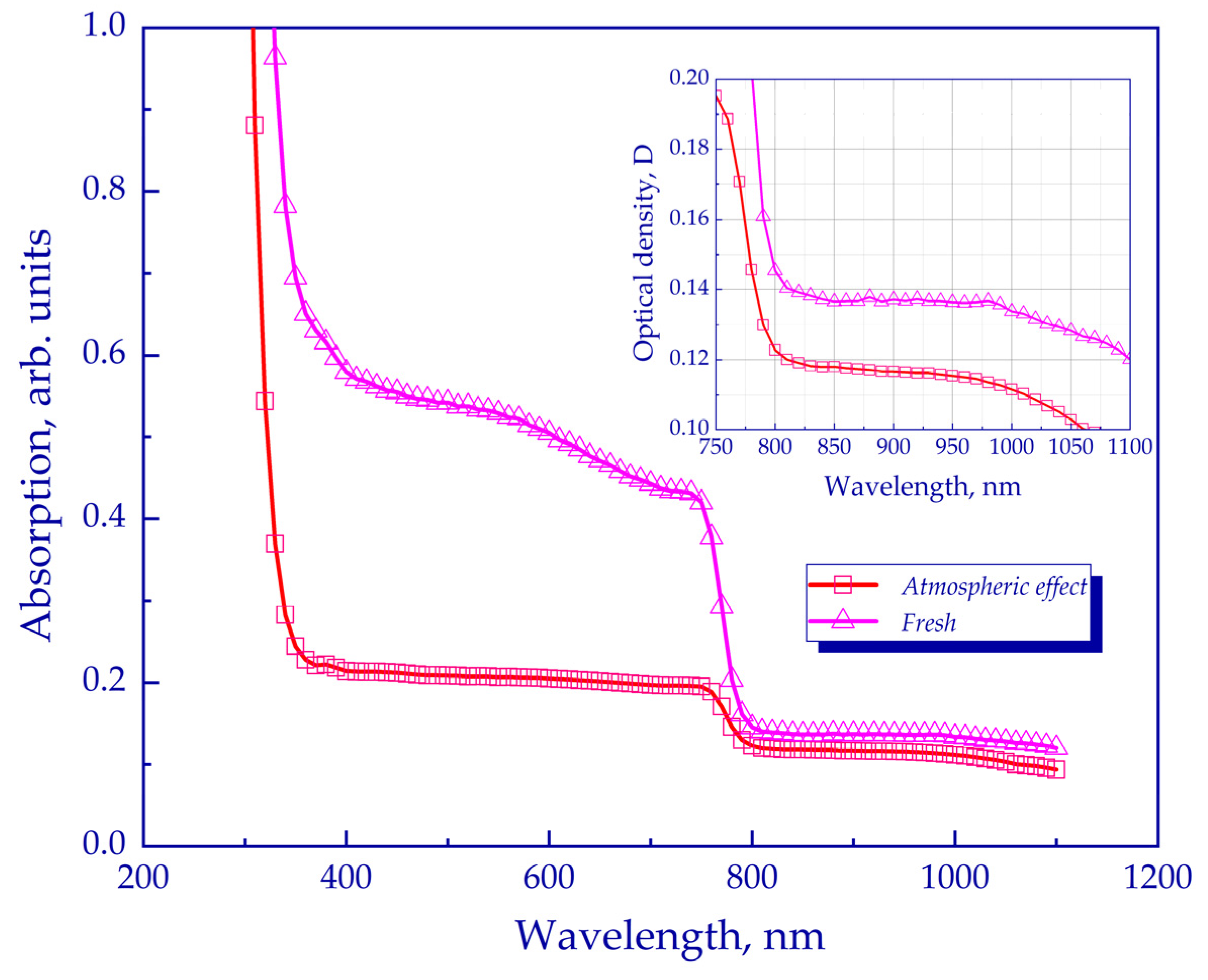
2.3. Optical Density
3. Materials and Methods
3.1. Materials
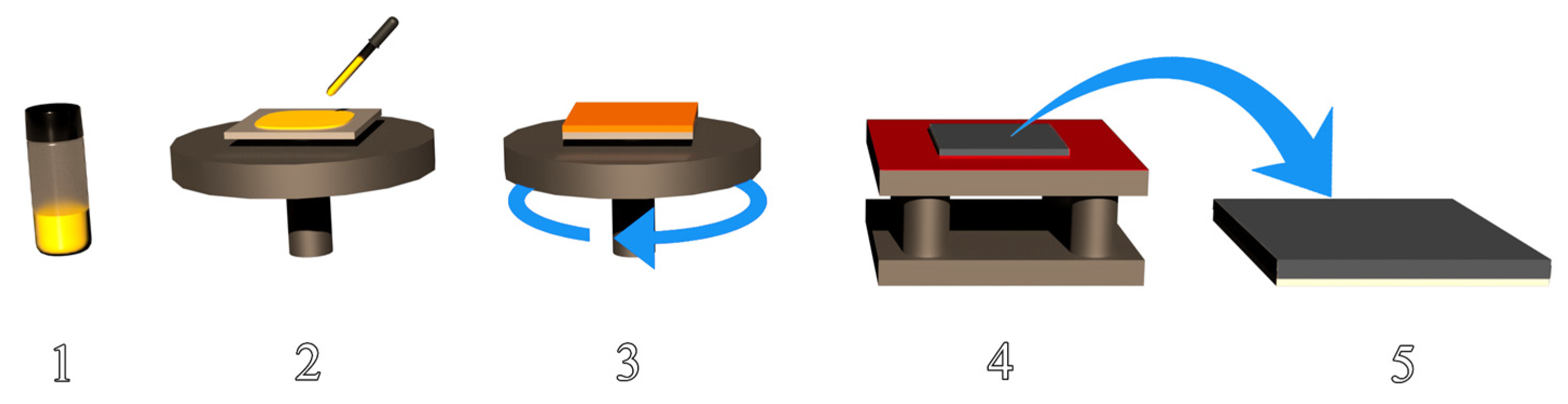
3.2. Preparation of CH3NH3PbI3-xClx
3.3. Film Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jefferson, M. Sustainable Energy Development: Performance and Prospects. Renew. Energy 2006, 31, 571–582. [Google Scholar] [CrossRef]
- Ahmad, T.; Zhang, D. A Critical Review of Comparative Global Historical Energy Consumption and Future Demand: The Story Told so Far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Jurasz, J.; Canales, F.A.; Kies, A.; Guezgouz, M.; Beluco, A. A Review on the Complementarity of Renewable Energy Sources: Concept, Metrics, Application and Future Research Directions. Sol. Energy 2020, 195, 703–724. [Google Scholar] [CrossRef]
- BARBIR, F.; VEZIROGLU, T.; PLASSJR, H. Environmental Damage Due to Fossil Fuels Use. Int. J. Hydrog. Energy 1990, 15, 739–749. [Google Scholar] [CrossRef]
- Hassan, A.; Ilyas, S.Z.; Jalil, A.; Ullah, Z. Monetization of the Environmental Damage Caused by Fossil Fuels. Environ. Sci. Pollut. Res. 2021, 28, 21204–21211. [Google Scholar] [CrossRef]
- Çakmak, E.E.; Acar, S. The Nexus between Economic Growth, Renewable Energy and Ecological Footprint: An Empirical Evidence from Most Oil-Producing Countries. J. Clean. Prod. 2022, 352, 131548. [Google Scholar] [CrossRef]
- El Chaar, L.; Lamont, L.A.; El Zein, N. Review of Photovoltaic Technologies. Renew. Sustain. Energy Rev. 2011, 15, 2165–2175. [Google Scholar] [CrossRef]
- Salameh, T.; Zhang, D.; Juaidi, A.; Alami, A.H.; Al-Hinti, I.; Olabi, A.G. Review of Solar Photovoltaic Cooling Systems Technologies with Environmental and Economical Assessment. J. Clean. Prod. 2021, 326, 129421. [Google Scholar] [CrossRef]
- Lowe, R.J.; Drummond, P. Solar, Wind and Logistic Substitution in Global Energy Supply to 2050–Barriers and Implications. Renew. Sustain. Energy Rev. 2022, 153, 111720. [Google Scholar] [CrossRef]
- Snaith, H.J. Present Status and Future Prospects of Perovskite Photovoltaics. Nat. Mater. 2018, 17, 372–376. [Google Scholar] [CrossRef]
- Degani, M.; An, Q.; Albaladejo-Siguan, M.; Hofstetter, Y.J.; Cho, C.; Paulus, F.; Grancini, G.; Vaynzof, Y. 23.7% Efficient Inverted Perovskite Solar Cells by Dual Interfacial Modification. Sci. Adv. 2021, 7, eabj7930. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Suresh Kumar, N.; Chandra Babu Naidu, K. A Review on Perovskite Solar Cells (PSCs), Materials and Applications. J. Mater. 2021, 7, 940–956. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Gong, O.Y.; Seo, M.K.; Choi, J.H.; Kim, S.-Y.; Kim, D.H.; Cho, I.S.; Park, N.-G.; Han, G.S.; Jung, H.S. High-Performing Laminated Perovskite Solar Cells by Surface Engineering of Perovskite Films. Appl. Surf. Sci. 2022, 591, 153148. [Google Scholar] [CrossRef]
- Wang, M.; Carmalt, C.J. Film Fabrication of Perovskites and Their Derivatives for Photovoltaic Applications via Chemical Vapor Deposition. ACS Appl. Energy Mater. 2022, 5, 5434–5448. [Google Scholar] [CrossRef]
- Kaur, J.; Chakraborty, S. Tuning Spin Texture and Spectroscopic Limited Maximum Efficiency through Chemical Composition Space in Double Halide Perovskites. ACS Appl. Energy Mater. 2022, 5, 5579–5588. [Google Scholar] [CrossRef]
- Cho, J.S.; Jang, W.; Wang, D.H. Gamma–Ray Irradiation of Lead Iodide Precursor for Enhanced Perovskite Crystalline Properties. Appl. Surf. Sci. 2022, 571, 151263. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Wang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A. Enhanced Performance of CH3NH3PbI3 Perovskite Solar Cells by Excess Halide Modification. Appl. Surf. Sci. 2021, 564, 150464. [Google Scholar] [CrossRef]
- Jiang, J.; Lin, W.; Liu, E.; Sha, J.; Ma, L. Efficient Passivation on Halide Perovskite by Tailoring the Organic Molecular Functional Groups: First-Principles Investigation. Appl. Surf. Sci. 2022, 597, 153716. [Google Scholar] [CrossRef]
- Maiti, A.; Pal, A.J. Carrier Recombination in CH3NH3PbI3: Why Is It a Slow Process? Rep. Prog. Phys. 2022, 85, 024501. [Google Scholar] [CrossRef]
- Oga, H.; Saeki, A.; Ogomi, Y.; Hayase, S.; Seki, S. Improved Understanding of the Electronic and Energetic Landscapes of Perovskite Solar Cells: High Local Charge Carrier Mobility, Reduced Recombination, and Extremely Shallow Traps. J. Am. Chem. Soc. 2014, 136, 13818–13825. [Google Scholar] [CrossRef]
- Tailor, N.K.; Yukta; Ranjan, R.; Ranjan, S.; Sharma, T.; Singh, A.; Garg, A.; Nalwa, K.S.; Gupta, R.K.; Satapathi, S. The Effect of Dimensionality on the Charge Carrier Mobility of Halide Perovskites. J. Mater. Chem. A 2021, 9, 21551–21575. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite Solar Cells with Atomically Coherent Interlayers on SnO2 Electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Park, S.Y.; Zhu, K. Advances in SnO2 for Efficient and Stable n–i–p Perovskite Solar Cells. Adv. Mater. 2022, 34, 2110438. [Google Scholar] [CrossRef]
- Hassan Kareem, S.; Harjan Elewi, M.; Muhson Naji, A.; Ahmed, D.S.; Mohammed, M.K.A. Efficient and Stable Pure α-Phase FAPbI3 Perovskite Solar Cells with a Dual Engineering Strategy: Additive and Dimensional Engineering Approaches. Chem. Eng. J. 2022, 443, 136469. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Wu, X.; Sheppard, S.A.; Zhang, S.; Gao, D.; Long, N.J.; Zhu, Z. Organometallic-Functionalized Interfaces for Highly Efficient Inverted Perovskite Solar Cells. Science 2022, 376, 416–420. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-Halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Omarova, Z.; Yerezhep, D.; Aldiyarov, A.; Tokmoldin, N. In Silico Investigation of the Impact of Hole-Transport Layers on the Performance of CH3NH3SnI3 Perovskite Photovoltaic Cells. Crystals 2022, 12, 699. [Google Scholar] [CrossRef]
- Haque, S.; Alexandre, M.; Baretzky, C.; Rossi, D.; De Rossi, F.; Vicente, A.T.; Brunetti, F.; Águas, H.; Ferreira, R.A.S.; Fortunato, E.; et al. Photonic-Structured Perovskite Solar Cells: Detailed Optoelectronic Analysis. ACS Photonics 2022, 9, 2408–2421. [Google Scholar] [CrossRef]
- Panigrahi, S.; Jana, S.; Calmeiro, T.; Nunes, D.; Deuermeier, J.; Martins, R.; Fortunato, E. Mapping the Space Charge Carrier Dynamics in Plasmon-Based Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 19811–19819. [Google Scholar] [CrossRef]
- Panigrahi, S.; Jana, S.; Calmeiro, T.; Nunes, D.; Martins, R.; Fortunato, E. Imaging the Anomalous Charge Distribution Inside CsPbBr3 Perovskite Quantum Dots Sensitized Solar Cells. ACS Nano 2017, 11, 10214–10221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Wang, S.; Chen, L.; Yin, Z.; Mei, S.; Zhong, Y.; Yao, Y.; Li, N.; Wang, J.; Song, W. Understanding Degradation Mechanisms of Perovskite Solar Cells Due to Electrochemical Metallization Effect. Sol. Energy Mater. Sol. Cells 2021, 230, 111278. [Google Scholar] [CrossRef]
- Mbumba, M.T.; Malouangou, D.M.; Tsiba, J.M.; Bai, L.; Yang, Y.; Guli, M. Degradation Mechanism and Addressing Techniques of Thermal Instability in Halide Perovskite Solar Cells. Sol. Energy 2021, 230, 954–978. [Google Scholar] [CrossRef]
- Liu, C.; Guo, M.; Su, H.; Zhai, P.; Xie, K.; Liu, Z.; Zhang, J.; Liu, L.; Fu, H. Highly Improved Efficiency and Stability of Planar Perovskite Solar Cells via Bifunctional Phytic Acid Dipotassium Anchored SnO2 Electron Transport Layer. Appl. Surf. Sci. 2022, 588, 152943. [Google Scholar] [CrossRef]
- Schutt, K.; Nayak, P.K.; Ramadan, A.J.; Wenger, B.; Lin, Y.; Snaith, H.J. Overcoming Zinc Oxide Interface Instability with a Methylammonium-Free Perovskite for High-Performance Solar Cells. Adv. Funct. Mater. 2019, 29, 1900466. [Google Scholar] [CrossRef]
- Jana, S.; Carlos, E.; Panigrahi, S.; Martins, R.; Fortunato, E. Toward Stable Solution-Processed High-Mobility p-Type Thin Film Transistors Based on Halide Perovskites. ACS Nano 2020, 14, 14790–14797. [Google Scholar] [CrossRef]
- Abiram, G.; Thanihaichelvan, M.; Ravirajan, P.; Velauthapillai, D. Review on Perovskite Semiconductor Field–Effect Transistors and Their Applications. Nanomaterials 2022, 12, 2396. [Google Scholar] [CrossRef]
- Walsh, A.; Stranks, S.D. Taking Control of Ion Transport in Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 1983–1990. [Google Scholar] [CrossRef]
- Chen, C.; Song, Z.; Xiao, C.; Awni, R.A.; Yao, C.; Shrestha, N.; Li, C.; Bista, S.S.; Zhang, Y.; Chen, L.; et al. Arylammonium-Assisted Reduction of the Open-Circuit Voltage Deficit in Wide-Bandgap Perovskite Solar Cells: The Role of Suppressed Ion Migration. ACS Energy Lett. 2020, 5, 2560–2568. [Google Scholar] [CrossRef]
- Tong, C.-J.; Cai, X.; Zhu, A.-Y.; Liu, L.-M.; Prezhdo, O.V. How Hole Injection Accelerates Both Ion Migration and Nonradiative Recombination in Metal Halide Perovskites. J. Am. Chem. Soc. 2022, 144, 6604–6612. [Google Scholar] [CrossRef]
- Yang, J.; Sheng, W.; Li, R.; Gong, L.; Li, Y.; Tan, L.; Lin, Q.; Chen, Y. Uncovering the Mechanism of Poly(Ionic-liquid)s Multiple Inhibition of Ion Migration for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2103652. [Google Scholar] [CrossRef]
- Zai, H.; Ma, Y.; Chen, Q.; Zhou, H. Ion Migration in Halide Perovskite Solar Cells: Mechanism, Characterization, Impact and Suppression. J. Energy Chem. 2021, 63, 528–549. [Google Scholar] [CrossRef]
- Aftab, A.; Md Ahmad, I. A Review of Stability and Progress in Tin Halide Perovskite Solar Cell. Sol. Energy 2021, 216, 26–47. [Google Scholar] [CrossRef]
- Bi, E.; Song, Z.; Li, C.; Wu, Z.; Yan, Y. Mitigating Ion Migration in Perovskite Solar Cells. Trends Chem. 2021, 3, 575–588. [Google Scholar] [CrossRef]
- Kim, B.; Seok, S. Il Molecular Aspects of Organic Cations Affecting the Humidity Stability of Perovskites. Energy Environ. Sci. 2020, 13, 805–820. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Duan, H.; Wang, H.; Fan, L.; Sun, Y.; Sui, Y.; Yang, J.; Wang, F.; Yang, L. Moisture-Preventing MAPbI3 Solar Cells with High Photovoltaic Performance via Multiple Ligand Engineering. Nano Res. 2022, 15, 1375–1382. [Google Scholar] [CrossRef]
- Zhou, C.; Tarasov, A.B.; Goodilin, E.A.; Chen, P.; Wang, H.; Chen, Q. Recent Strategies to Improve Moisture Stability in Metal Halide Perovskites Materials and Devices. J. Energy Chem. 2022, 65, 219–235. [Google Scholar] [CrossRef]
- Wu, C.; Wang, K.; Feng, X.; Jiang, Y.; Yang, D.; Hou, Y.; Yan, Y.; Sanghadasa, M.; Priya, S. Ultrahigh Durability Perovskite Solar Cells. Nano Lett. 2019, 19, 1251–1259. [Google Scholar] [CrossRef]
- Sutherland, L.J.; Weerasinghe, H.C.; Simon, G.P. A Review on Emerging Barrier Materials and Encapsulation Strategies for Flexible Perovskite and Organic Photovoltaics. Adv. Energy Mater. 2021, 11, 2101383. [Google Scholar] [CrossRef]
- Emery, Q.; Remec, M.; Paramasivam, G.; Janke, S.; Dagar, J.; Ulbrich, C.; Schlatmann, R.; Stannowski, B.; Unger, E.; Khenkin, M. Encapsulation and Outdoor Testing of Perovskite Solar Cells: Comparing Industrially Relevant Process with a Simplified Lab Procedure. ACS Appl. Mater. Interfaces 2022, 14, 5159–5167. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Song, J.; Dai, X.; Liu, Y.; Rudd, P.N.; Hong, X.; Huang, J. Synergistic Effect of Elevated Device Temperature and Excess Charge Carriers on the Rapid Light-Induced Degradation of Perovskite Solar Cells. Adv. Mater. 2019, 31, 1902413. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Pisoni, S.; Jeangros, Q.; Sastre-Pellicer, J.; Kawecki, M.; Paracchino, A.; Moser, T.; Werner, J.; Andres, C.; Duchêne, L.; et al. I 2 Vapor-Induced Degradation of Formamidinium Lead Iodide Based Perovskite Solar Cells under Heat–Light Soaking Conditions. Energy Environ. Sci. 2019, 12, 3074–3088. [Google Scholar] [CrossRef]
- Duan, L.; Uddin, A. Defects and Stability of Perovskite Solar Cells: A Critical Analysis. Mater. Chem. Front. 2022, 6, 400–417. [Google Scholar] [CrossRef]
- Domanski, K.; Alharbi, E.A.; Hagfeldt, A.; Grätzel, M.; Tress, W. Systematic Investigation of the Impact of Operation Conditions on the Degradation Behaviour of Perovskite Solar Cells. Nat. Energy 2018, 3, 61–67. [Google Scholar] [CrossRef]
- Sekimoto, T.; Uchida, R.; Hiraoka, M.; Matsui, T.; Kikuchi, R.; Nakamura, T.; Yamamoto, T.; Kawano, K.; Negami, T.; Kaneko, Y. Investigation of the Acceleration and Suppression of the Light-Induced Degradation of a Lead Halide Perovskite Solar Cell Using Hard X-Ray Photoelectron Spectroscopy. ACS Appl. Energy Mater. 2022, 5, 4125–4137. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Xie, L.; Wang, S.; Yang, C.; Fang, C.; Hao, F. Recent Advances and Perspectives of Photostability for Halide Perovskite Solar Cells. Adv. Opt. Mater. 2022, 10, 2101822. [Google Scholar] [CrossRef]
- Bracher, C.; Freestone, B.G.; Mohamad, D.K.; Smith, J.A.; Lidzey, D.G. Degradation of Inverted Architecture CH3NH3PbI3−xClx Perovskite Solar Cells Due to Trapped Moisture. Energy Sci. Eng. 2018, 6, 35–46. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Yan, H.-J.; Li, M.-H.; Sun, J.-K.; Chen, Y.-X.; Wang, D.; Hu, J.-S. Microscopic Investigations on the Surface-State Dependent Moisture Stability of a Hybrid Perovskite. Nanoscale 2020, 12, 7759–7765. [Google Scholar] [CrossRef]
- Busipalli, D.L.; Lin, K.-Y.; Nachimuthu, S.; Jiang, J.-C. Enhanced Moisture Stability of Cesium Lead Iodide Perovskite Solar Cells–a First-Principles Molecular Dynamics Study. Phys. Chem. Chem. Phys. 2020, 22, 5693–5701. [Google Scholar] [CrossRef]
- Li, J.; Xia, R.; Qi, W.; Zhou, X.; Cheng, J.; Chen, Y.; Hou, G.; Ding, Y.; Li, Y.; Zhao, Y.; et al. Encapsulation of Perovskite Solar Cells for Enhanced Stability: Structures, Materials and Characterization. J. Power Sources 2021, 485, 229313. [Google Scholar] [CrossRef]
- Raman, R.K.; Gurusamy Thangavelu, S.A.; Venkataraj, S.; Krishnamoorthy, A. Materials, Methods and Strategies for Encapsulation of Perovskite Solar Cells: From Past to Present. Renew. Sustain. Energy Rev. 2021, 151, 111608. [Google Scholar] [CrossRef]
- Wang, Y.; Ahmad, I.; Leung, T.; Lin, J.; Chen, W.; Liu, F.; Ng, A.M.C.; Zhang, Y.; Djurišić, A.B. Encapsulation and Stability Testing of Perovskite Solar Cells for Real Life Applications. ACS Mater. Au 2022, 2, 215–236. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, G.; Zhang, Y.; Yang, N.; Li, Y.; Chen, Q. Development of Encapsulation Strategies towards the Commercialization of Perovskite Solar Cells. Energy Environ. Sci. 2022, 15, 13–55. [Google Scholar] [CrossRef]
- Menda, U.D.; Ribeiro, G.; Nunes, D.; Calmeiro, T.; Águas, H.; Fortunato, E.; Martins, R.; Mendes, M.J. High-Performance Wide Bandgap Perovskite Solar Cells Fabricated in Ambient High-Humidity Conditions. Mater. Adv. 2021, 2, 6344–6355. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Rolston, N.; Harwood, D.; Bush, K.A.; Dauskardt, R.H.; McGehee, M.D. Design and Understanding of Encapsulated Perovskite Solar Cells to Withstand Temperature Cycling. Energy Environ. Sci. 2018, 11, 144–150. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, M.; Sheng, Y.; Yan, Z.; Meng, J.; Tong, C.; Li, D.; Wan, Z.; Ming, Y.; Mei, A.; et al. Encapsulation of Printable Mesoscopic Perovskite Solar Cells Enables High Temperature and Long-Term Outdoor Stability. Adv. Funct. Mater. 2019, 29, 1809129. [Google Scholar] [CrossRef]
- Ma, S.; Bai, Y.; Wang, H.; Zai, H.; Wu, J.; Li, L.; Xiang, S.; Liu, N.; Liu, L.; Zhu, C.; et al. 1000 h Operational Lifetime Perovskite Solar Cells by Ambient Melting Encapsulation. Adv. Energy Mater. 2020, 10, 1902472. [Google Scholar] [CrossRef]
- Chen, B.; Wang, S.; Song, Y.; Li, C.; Hao, F. A Critical Review on the Moisture Stability of Halide Perovskite Films and Solar Cells. Chem. Eng. J. 2022, 430, 132701. [Google Scholar] [CrossRef]
- Li, G.; Su, Z.; Li, M.; Lee, H.K.H.; Datt, R.; Hughes, D.; Wang, C.; Flatken, M.; Köbler, H.; Jerónimo-Rendon, J.J.; et al. Structure and Performance Evolution of Perovskite Solar Cells under Extreme Temperatures. Adv. Energy Mater. 2022, 12, 2202887. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S. Il Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.-B. Degradation Observations of Encapsulated Planar CH3NH3PbI3 Perovskite Solar Cells at High Temperatures and Humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, W. Progress on X-Ray Absorption Spectroscopy for the Characterization of Perovskite-Type Oxide Electrocatalysts. Energy Fuels 2021, 35, 5716–5737. [Google Scholar] [CrossRef]
- Meng, X.; Tian, X.; Zhang, S.; Zhou, J.; Zhang, Y.; Liu, Z.; Chen, W. In Situ Characterization for Understanding the Degradation in Perovskite Solar Cells. Sol. RRL 2022, 6, 2200280. [Google Scholar] [CrossRef]
- Béchu, S.; Ralaiarisoa, M.; Etcheberry, A.; Schulz, P. Photoemission Spectroscopy Characterization of Halide Perovskites. Adv. Energy Mater. 2020, 10, 1904007. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Thermal Degradation of Formamidinium Based Lead Halide Perovskites into Sym -Triazine and Hydrogen Cyanide Observed by Coupled Thermogravimetry-Mass Spectrometry Analysis. J. Mater. Chem. A 2019, 7, 16912–16919. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal Degradation of CH3NH3PbI3 Perovskite into NH3 and CH3I Gases Observed by Coupled Thermogravimetry–Mass Spectrometry Analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef]
- Liu, Y.; Lorenz, M.; Ievlev, A.V.; Ovchinnikova, O.S. Secondary Ion Mass Spectrometry (SIMS) for Chemical Characterization of Metal Halide Perovskites. Adv. Funct. Mater. 2020, 30, 2002201. [Google Scholar] [CrossRef]
- Shi, L.; Bucknall, M.P.; Young, T.L.; Zhang, M.; Hu, L.; Bing, J.; Lee, D.S.; Kim, J.; Wu, T.; Takamure, N.; et al. Gas Chromatography–Mass Spectrometry Analyses of Encapsulated Stable Perovskite Solar Cells. Science 2020, 368, eaba2412. [Google Scholar] [CrossRef]
- Manser, J.S.; Saidaminov, M.I.; Christians, J.A.; Bakr, O.M.; Kamat, P.V. Making and Breaking of Lead Halide Perovskites. Acc. Chem. Res. 2016, 49, 330–338. [Google Scholar] [CrossRef]
- Dong, Q.; Zhu, C.; Chen, M.; Jiang, C.; Guo, J.; Feng, Y.; Dai, Z.; Yadavalli, S.K.; Hu, M.; Cao, X.; et al. Interpenetrating Interfaces for Efficient Perovskite Solar Cells with High Operational Stability and Mechanical Robustness. Nat. Commun. 2021, 12, 973. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Jeon, M.-G.; Li, L.; Choi, J. Suppressed Phase and Structural Evolution of CH3NH3PbBr3 Microwires to (CH3)2NH2PbBr3 by Addition of Hydrazine Bromide. Appl. Surf. Sci. 2021, 566, 150691. [Google Scholar] [CrossRef]
- Abdelmageed, G.; Mackeen, C.; Hellier, K.; Jewell, L.; Seymour, L.; Tingwald, M.; Bridges, F.; Zhang, J.Z.; Carter, S. Effect of Temperature on Light Induced Degradation in Methylammonium Lead Iodide Perovskite Thin Films and Solar Cells. Sol. Energy Mater. Sol. Cells 2018, 174, 566–571. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, H.; Li, W.; Cao, D.; Xu, Y.; Wan, L.; Zhang, X.; Zhang, X.; Li, Y.; Ren, X.; et al. Bifunctional Ionic Liquid for Enhancing Efficiency and Stability of Carbon Counter Electrode-Based MAPbI3 Perovskites Solar Cells. Sol. Energy 2022, 231, 1048–1060. [Google Scholar] [CrossRef]
- Arobi, N.; Amir-Al Zumahi, S.M.; Ibrahim, K.; Rahman, M.M.; Hossain, M.K.; Rahman Bhuiyan, M.M.; Kabir, H.; Amri, A.; Hossain, M.A.; Ahmed, F. A Holistic Framework towards Understanding the Optical and Dielectric Behaviors of CH3NH3PbCl3 Perovskites/Graphene Oxide Hybrid Films for Light Absorbing Active Layer. J. Solid State Chem. 2021, 298, 122137. [Google Scholar] [CrossRef]
- Kalthoum, R.; Ben Bechir, M.; Ben Rhaiem, A.; Dhaou, M.H. Optical Properties of New Organic-Inorganic Hybrid Perovskites (CH3)2NH2CdCl3 And CH3NH3CdCl3 for Solar Cell Applications. Opt. Mater. 2022, 125, 112084. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, F.; Cao, Y.; Li, D.; Liu, Q.; Liu, H.; Li, S. Progress on the Stability and Encapsulation Techniques of Perovskite Solar Cells. Org. Electron. 2022, 106, 106515. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 Degradation Rates and Mechanisms in Controlled Humidity Environments Using in Situ Techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.; Long, G.; Tan, F.; Johnston, A.; Zhao, Y.; Voznyy, O.; et al. Suppression of Atomic Vacancies via Incorporation of Isovalent Small Ions to Increase the Stability of Halide Perovskite Solar Cells in Ambient Air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Yu, X.; Qin, Y.; Peng, Q. Probe Decomposition of Methylammonium Lead Iodide Perovskite in N2 and O2 by in Situ Infrared Spectroscopy. J. Phys. Chem. A 2017, 121, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Yu, Z.; Meng, M.; Xia, W.; Zhang, X. Hydration of Mixed Halide Perovskites Investigated by Fourier Transform Infrared Spectroscopy. APL Mater. 2019, 7, 031107. [Google Scholar] [CrossRef]
- Reese, M.O.; Gevorgyan, S.A.; Jørgensen, M.; Bundgaard, E.; Kurtz, S.R.; Ginley, D.S.; Olson, D.C.; Lloyd, M.T.; Morvillo, P.; Katz, E.A.; et al. Consensus Stability Testing Protocols for Organic Photovoltaic Materials and Devices. Sol. Energy Mater. Sol. Cells 2011, 95, 1253–1267. [Google Scholar] [CrossRef]
- Glaser, T.; Müller, C.; Sendner, M.; Krekeler, C.; Semonin, O.E.; Hull, T.D.; Yaffe, O.; Owen, J.S.; Kowalsky, W.; Pucci, A.; et al. Infrared Spectroscopic Study of Vibrational Modes in Methylammonium Lead Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Koushik, D.; Hazendonk, L.; Zardetto, V.; Vandalon, V.; Verheijen, M.A.; Kessels, W.M.M.; Creatore, M. Chemical Analysis of the Interface between Hybrid Organic–Inorganic Perovskite and Atomic Layer Deposited Al2O3. ACS Appl. Mater. Interfaces 2019, 11, 5526–5535. [Google Scholar] [CrossRef]
- Zhu, Z.; Hadjiev, V.G.; Rong, Y.; Guo, R.; Cao, B.; Tang, Z.; Qin, F.; Li, Y.; Wang, Y.; Hao, F.; et al. Interaction of Organic Cation with Water Molecule in Perovskite MAPbI 3: From Dynamic Orientational Disorder to Hydrogen Bonding. Chem. Mater. 2016, 28, 7385–7393. [Google Scholar] [CrossRef]
- Makableh, Y.F.; Dalal’ah, T.N. Investigation of the Effect of the Fabrication Temperature on the Optical, Morphological and Crystallinity Properties of Perovskites Composites. Micro Nanostruct. 2022, 167, 207259. [Google Scholar] [CrossRef]
- Amrollahi Bioki, H.; Moshaii, A.; Borhani Zarandi, M. Improved Morphology, Structure and Optical Properties of CH3NH3PbI3 Film via HQ Additive in PbI2 Precursor Solution for Efficient and Stable Mesoporous Perovskite Solar Cells. Synth. Met. 2022, 283, 116965. [Google Scholar] [CrossRef]
- Williams, A.E.; Holliman, P.J.; Carnie, M.J.; Davies, M.L.; Worsley, D.A.; Watson, T.M. Perovskite Processing for Photovoltaics: A Spectro-Thermal Evaluation. J. Mater. Chem. A 2014, 2, 19338–19346. [Google Scholar] [CrossRef]
- Choudhury, D.; Rajaraman, G.; Sarkar, S.K. Self Limiting Atomic Layer Deposition of Al2O3 on Perovskite Surfaces: A Reality? Nanoscale 2016, 8, 7459–7465. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Glaser, T.; Plogmeyer, M.; Sendner, M.; Döring, S.; Bakulin, A.A.; Brzuska, C.; Scheer, R.; Pshenichnikov, M.S.; Kowalsky, W.; et al. Water Infiltration in Methylammonium Lead Iodide Perovskite: Fast and Inconspicuous. Chem. Mater. 2015, 27, 7835–7841. [Google Scholar] [CrossRef]
- Hong, Q.-M.; Xu, R.-P.; Jin, T.-Y.; Tang, J.-X.; Li, Y.-Q. Unraveling the Light-Induced Degradation Mechanism of CH3NH3PbI3 Perovskite Films. Org. Electron. 2019, 67, 19–25. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and Thermal Decomposition in Methylammonium Halide Lead Perovskites and Inferred Design Principles to Increase Photovoltaic Device Stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Hong, L.; Hu, Y.; Mei, A.; Sheng, Y.; Jiang, P.; Tian, C.; Rong, Y.; Han, H. Improvement and Regeneration of Perovskite Solar Cells via Methylamine Gas Post-Treatment. Adv. Funct. Mater. 2017, 27, 1703060. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, D.H.; Lee, Y.-Y.; Shin, H.-W.; Han, G.S.; Hong, J.S.; Mahmood, K.; Ahn, T.K.; Joo, Y.-C.; Hong, K.S.; et al. Highly Efficient and Bending Durable Perovskite Solar Cells: Toward a Wearable Power Source. Energy Environ. Sci. 2015, 8, 916–921. [Google Scholar] [CrossRef]
- Hoch, L.B.; Szymanski, P.; Ghuman, K.K.; He, L.; Liao, K.; Qiao, Q.; Reyes, L.M.; Zhu, Y.; El-Sayed, M.A.; Singh, C.V.; et al. Carrier Dynamics and the Role of Surface Defects: Designing a Photocatalyst for Gas-Phase CO2 Reduction. Proc. Natl. Acad. Sci. 2016, 113, E8011–E8020. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect Passivation in Hybrid Perovskite Solar Cells Using Quaternary Ammonium Halide Anions and Cations. Nat. Energy 2017, 2, 17102. [Google Scholar] [CrossRef]
- Noel, N.K.; Abate, A.; Stranks, S.D.; Parrott, E.S.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Enhanced Photoluminescence and Solar Cell Performance via Lewis Base Passivation of Organic–Inorganic Lead Halide Perovskites. ACS Nano 2014, 8, 9815–9821. [Google Scholar] [CrossRef]
- Abdelmageed, G.; Jewell, L.; Hellier, K.; Seymour, L.; Luo, B.; Bridges, F.; Zhang, J.Z.; Carter, S. Mechanisms for Light Induced Degradation in MAPbI3 Perovskite Thin Films and Solar Cells. Appl. Phys. Lett. 2016, 109, 233905. [Google Scholar] [CrossRef]
- Si, H.; Zhang, S.; Ma, S.; Xiong, Z.; Kausar, A.; Liao, Q.; Zhang, Z.; Sattar, A.; Kang, Z.; Zhang, Y. Emerging Conductive Atomic Force Microscopy for Metal Halide Perovskite Materials and Solar Cells. Adv. Energy Mater. 2020, 10, 1903922. [Google Scholar] [CrossRef]
- Liu, Z.; Krückemeier, L.; Krogmeier, B.; Klingebiel, B.; Márquez, J.A.; Levcenko, S.; Öz, S.; Mathur, S.; Rau, U.; Unold, T.; et al. Open-Circuit Voltages Exceeding 1.26 V in Planar Methylammonium Lead Iodide Perovskite Solar Cells. ACS Energy Lett. 2019, 4, 110–117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerezhep, D.; Omarova, Z.; Aldiyarov, A.; Shinbayeva, A.; Tokmoldin, N. IR Spectroscopic Degradation Study of Thin Organometal Halide Perovskite Films. Molecules 2023, 28, 1288. https://doi.org/10.3390/molecules28031288
Yerezhep D, Omarova Z, Aldiyarov A, Shinbayeva A, Tokmoldin N. IR Spectroscopic Degradation Study of Thin Organometal Halide Perovskite Films. Molecules. 2023; 28(3):1288. https://doi.org/10.3390/molecules28031288
Chicago/Turabian StyleYerezhep, Darkhan, Zhansaya Omarova, Abdurakhman Aldiyarov, Ainura Shinbayeva, and Nurlan Tokmoldin. 2023. "IR Spectroscopic Degradation Study of Thin Organometal Halide Perovskite Films" Molecules 28, no. 3: 1288. https://doi.org/10.3390/molecules28031288
APA StyleYerezhep, D., Omarova, Z., Aldiyarov, A., Shinbayeva, A., & Tokmoldin, N. (2023). IR Spectroscopic Degradation Study of Thin Organometal Halide Perovskite Films. Molecules, 28(3), 1288. https://doi.org/10.3390/molecules28031288