Delphinidin-3-rutinoside from Blackcurrant Berries (Ribes nigrum): In Vitro Antiproliferative Activity and Interactions with Other Phenolic Compounds
Abstract
1. Introduction
2. Results
2.1. Antiproliferative Activity of Individual Compounds
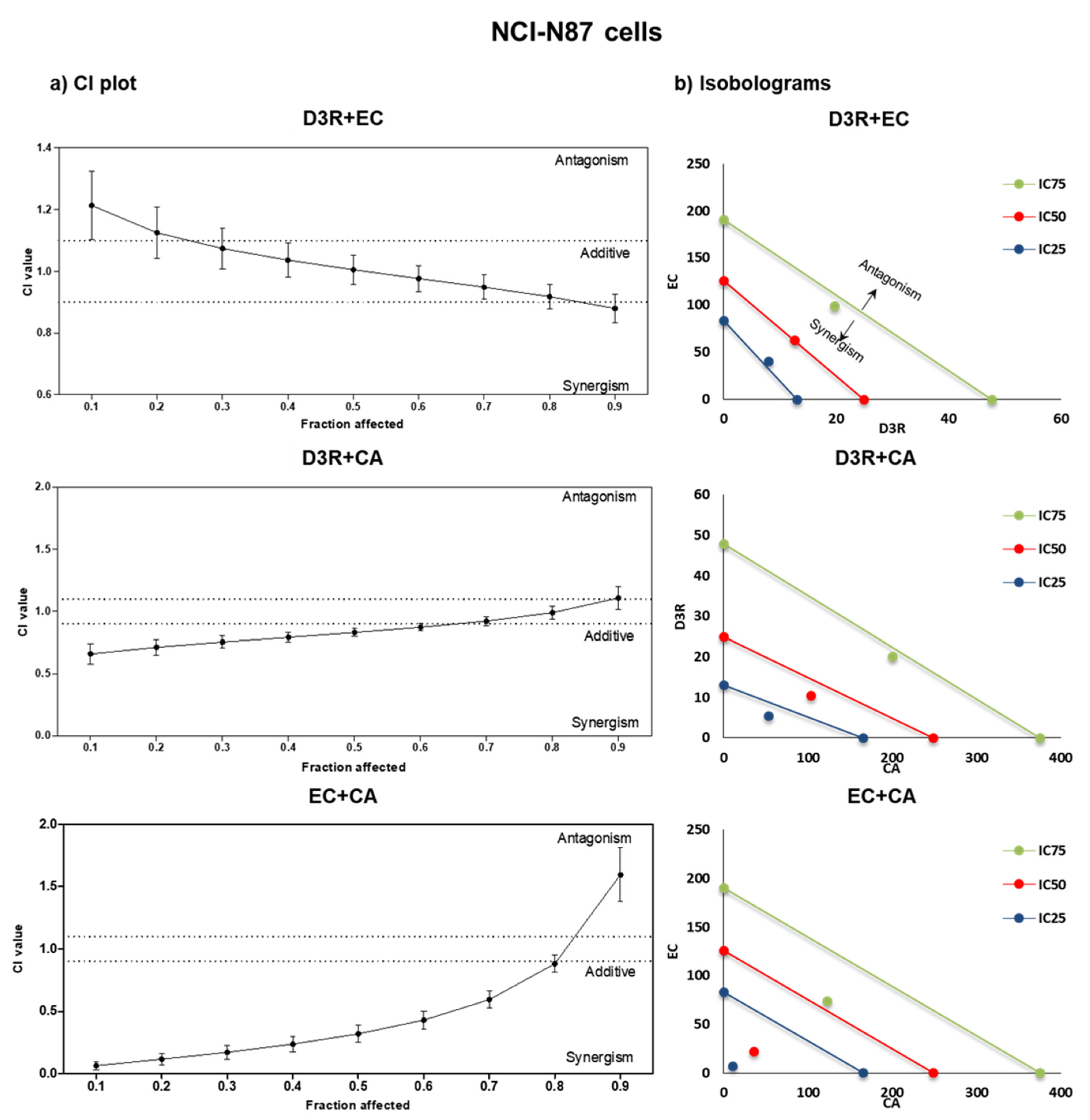
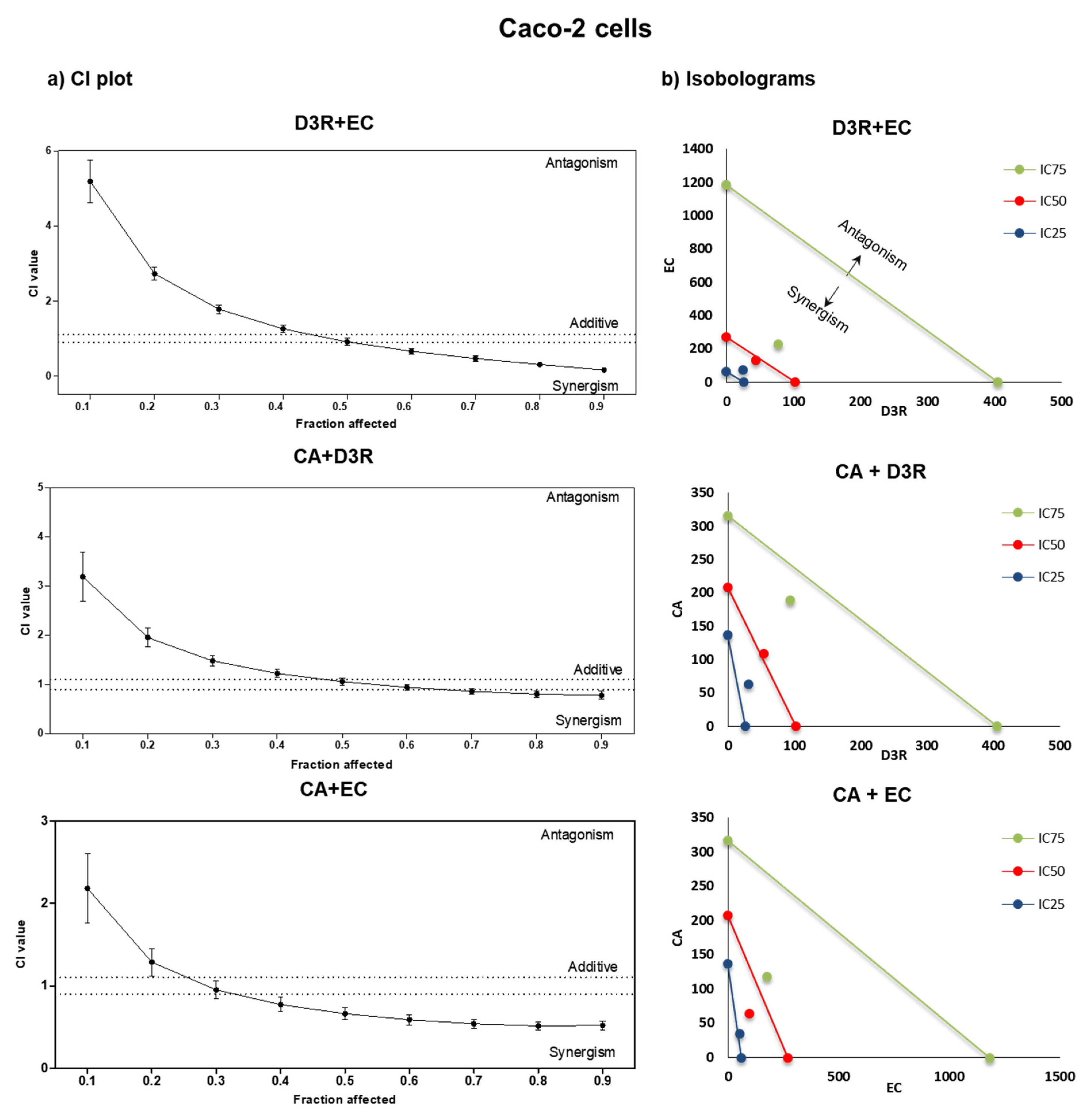
2.2. Antiproliferative Activity of Binary Combinations of D3R, EC, and CA
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Preparation of Standards Solutions
4.3. Cell Culture
4.4. Isolated and Combined Effects
4.5. Cell Viability Assay
4.6. Data Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA-Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Simon, K. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.; Wild, C. World Cancer Report; IARC: Lyon, France, 2014; ISBN 978-92-832-0443-5. [Google Scholar]
- Nielsen, I.L.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073s–2085s. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Prieto, M.A.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin prevents TNFα-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2012, 527, 113–118. [Google Scholar] [CrossRef]
- Seyed Fazel, N.; Silvia, T.; William, N.S.; Olga, G.; Antoni, S.; Nady, B.; Maria, D.; Azadeh, M.; Seyed Mohammad, N. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2017, 15, 471–479. [Google Scholar] [CrossRef]
- Miladinović, B.; Kostić, M.; Šavikin, K.; Đorđević, B.; Mihajilov-Krstev, T.; Živanović, S.; Kitić, D. Chemical profile and antioxidative and antimicrobial activity of juices and extracts of 4 black currants varieties (Ribes nigrum L.). J. Food Sci. 2014, 79, C301–C309. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Bishayee, A.; Mbimba, T.; Thoppil, R.J.; Háznagy-Radnai, E.; Sipos, P.; Darvesh, A.S.; Folkesson, H.G.; Hohmann, J. Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Biochem. 2011, 22, 1035–1046. [Google Scholar] [CrossRef]
- Jia, N.; Xiong, Y.L.; Kong, B.; Liu, Q.; Xia, X. Radical scavenging activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on gastric cancer cell proliferation via induction of apoptosis. J. Funct. Food. 2012, 4, 382–390. [Google Scholar] [CrossRef]
- van Breda, S.G.J.; de Kok, T.M.C.M. Smart Combinations of Bioactive Compounds in Fruits and Vegetables May Guide New Strategies for Personalized Prevention of Chronic Diseases. Mol. Nutr. Food Res. 2018, 62, 1700597. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Z. Black Currant (Ribes nigrum L.) Extract Induces Apoptosis of MKN-45 and TE-1 Cells Through MAPK- and PI3K/Akt-Mediated Mitochondrial Pathways. J. Med. Food 2016, 19, 365–373. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Bartoszek, A. The Relationship between Phytochemical Composition and Biological Activities of Differently Pigmented Varieties of Berry Fruits; Comparison between Embedded in Food Matrix and Isolated Anthocyanins. Foods 2019, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, K.; Stachniuk, A.; Szwajgier, D.; Trzpil, A. Polyphenols composition and the biological effects of six selected small dark fruits. Food Chem. 2022, 391, 133281. [Google Scholar] [CrossRef]
- Pott, D.M.; Durán-Soria, S.; Allwood, J.W.; Pont, S.; Gordon, S.L.; Jennings, N.; Austin, C.; Stewart, D.; Brennan, R.M.; Masny, A.; et al. Dissecting the impact of environment, season and genotype on blackcurrant fruit quality traits. Food Chem. 2023, 402, 134360. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Atnip, A.; Giusti, M.M.; Sigurdson, G.T.; Failla, M.L.; Chitchumroonchokchai, C.; Bomser, J.A. The NCI-N87 Cell Line as a Gastric Epithelial Model to Study Cellular Uptake, Trans-Epithelial Transport, and Gastric Anti-Inflammatory Properties of Anthocyanins. Nutr. Cancer 2020, 72, 686–695. [Google Scholar] [CrossRef]
- Guardamagna, I.; Lonati, L.; Savio, M.; Stivala, L.A.; Ottolenghi, A.; Baiocco, G. An Integrated Analysis of the Response of Colorectal Adenocarcinoma Caco-2 Cells to X-Ray Exposure. Front. Oncol. 2021, 11, 688919. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Chiba, M.; Nakano, M.; Maeda, H.; Nakamura, T. Anthocyanin-rich blackcurrant extract inhibits proliferation of the MCF10A healthy human breast epithelial cell line through induction of G0/G1 arrest and apoptosis. Mol. Med. Rep. 2017, 16, 6134–6141. [Google Scholar] [CrossRef]
- Majumdar, A.P.N.; Banerjee, S.; Nautiyal, J.; Patel, B.B.; Patel, V.; Du, J.; Yu, Y.; Elliott, A.A.; Levi, E.; Sarkar, F.H. Curcumin Synergizes With Resveratrol to Inhibit Colon Cancer. Nutr. Cancer 2009, 61, 544–553. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Lim, J.T.E.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)-Epigallocatechin Gallate and Polyphenon E Inhibit Growth and Activation of the Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor-2 Signaling Pathways in Human Colon Cancer Cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic Inhibitory Effects of Curcumin and 5-Fluorouracil on the Growth of the Human Colon Cancer Cell Line HT-29. Chemotherapy 2006, 52, 23–28. [Google Scholar] [CrossRef]
- Ramos, S.; Rodríguez-Ramiro, I.; Martín, M.A.; Goya, L.; Bravo, L. Dietary flavanols exert different effects on antioxidant defenses and apoptosis/proliferation in Caco-2 and SW480 colon cancer cells. Toxicol. Vitro 2011, 25, 1771–1781. [Google Scholar] [CrossRef]
- Yao, F.; Kausalya, J.P.; Sia, Y.Y.; Teo, A.S.; Lee, W.H.; Ong, A.G.; Zhang, Z.; Tan, J.H.; Li, G.; Bertrand, D.; et al. Recurrent Fusion Genes in Gastric Cancer: CLDN18-ARHGAP26 Induces Loss of Epithelial Integrity. Cell Rep. 2015, 12, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Fenger, J.A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
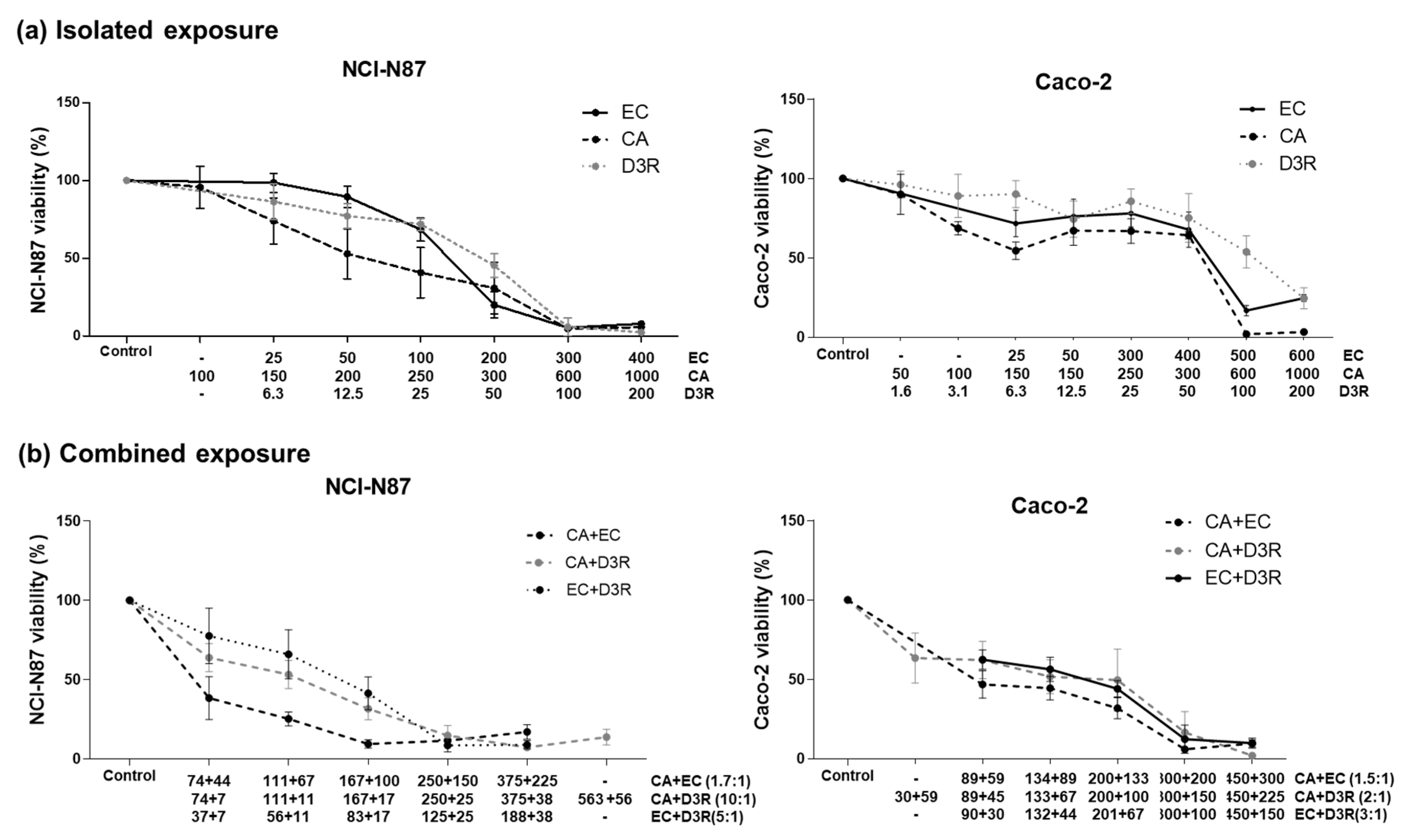
| (a) NCI N87 Cells | |||||
| r | m | IC25 | IC50 (Dm) | IC75 | |
| D3R | 0.96 | 1.69 | 13.0 | 24.9 | 47.8 |
| CA | 0.93 | 2.67 | 165.1 | 249.0 | 375.4 |
| EC | 0.98 | 2.65 | 83.3 | 126.1 | 190.8 |
| (b) Caco-2 cells | |||||
| r | m | IC25 | IC50 (Dm) | IC75 | |
| D3R | 0.86 | 0.79 | 25.9 | 102.5 | 405.3 |
| CA | 0.87 | 2.61 | 136.4 | 207.7 | 316.3 |
| EC | 0.79 | 0.74 | 61.6 | 270.2 | 1184 |
| NCI-N87 Cells | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination Ratio | r | m | IC25 | CI | DRI | IC50 | CI | DRI | IC75 | CI | DRI | |
| D3R | 10:1 | 0.93 | 1.66 | 5.3 | 0.73 | 3.1 | 10.3 | 0.83 | 2.4 | 20.0 | 0.93 | |
| CA | 53.5 | 2.4 | 103.5 | 2.4 | 200.0 | |||||||
| D3R | 5:1 | 0.93 | 2.43 | 8.0 | 1.09 | 12.6 | 1.0 | 19.8 | 0.95 | |||
| EC | 40.1 | 63.1 | 99.1 | |||||||||
| CA | 1.7:1 | 0.71 | 0.9 | 10.8 | 0.14 | 15.3 | 36.4 | 0.32 | 6.8 | 122.9 | 0.71 | 3.1 |
| EC | 6.48 | 12.9 | 21.9 | 5.8 | 73.7 | 2.6 | ||||||
| Caco-2 cells | ||||||||||||
| Combination ratio | r | m | IC25 | CI | DRI | IC50 | CI | DRI | IC75 | CI | DRI | |
| D3R | 2:1 | 0.84 | 2.0 | 31.5 | 1.68 | 54.6 | 1.05 | 94.4 | 0.83 | 1.7 | ||
| CA | 63.1 | 109.1 | 188.9 | 4.3 | ||||||||
| D3R | 3:1 | 0.93 | 1.95 | 24.9 | 2.17 | 43.7 | 0.91 | 76.8 | 0.38 | 5.1 | ||
| EC | 74.7 | 131.2 | 230.5 | 5.3 | ||||||||
| CA | 1.5:1 | 0.87 | 1.78 | 34.4 | 1.09 | 63.8 | 0.66 | 3.3 | 118.5 | 0.52 | 2.7 | |
| EC | 51.6 | 95.7 | 2.8 | 177.7 | 6.7 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miladinovic, B.; Faria, M.Â.; Ribeiro, M.; Sobral, M.M.C.; Ferreira, I.M.P.L.V.O. Delphinidin-3-rutinoside from Blackcurrant Berries (Ribes nigrum): In Vitro Antiproliferative Activity and Interactions with Other Phenolic Compounds. Molecules 2023, 28, 1286. https://doi.org/10.3390/molecules28031286
Miladinovic B, Faria MÂ, Ribeiro M, Sobral MMC, Ferreira IMPLVO. Delphinidin-3-rutinoside from Blackcurrant Berries (Ribes nigrum): In Vitro Antiproliferative Activity and Interactions with Other Phenolic Compounds. Molecules. 2023; 28(3):1286. https://doi.org/10.3390/molecules28031286
Chicago/Turabian StyleMiladinovic, Bojana, Miguel Ângelo Faria, Mafalda Ribeiro, Maria Madalena Costa Sobral, and Isabel M. P. L. V. O. Ferreira. 2023. "Delphinidin-3-rutinoside from Blackcurrant Berries (Ribes nigrum): In Vitro Antiproliferative Activity and Interactions with Other Phenolic Compounds" Molecules 28, no. 3: 1286. https://doi.org/10.3390/molecules28031286
APA StyleMiladinovic, B., Faria, M. Â., Ribeiro, M., Sobral, M. M. C., & Ferreira, I. M. P. L. V. O. (2023). Delphinidin-3-rutinoside from Blackcurrant Berries (Ribes nigrum): In Vitro Antiproliferative Activity and Interactions with Other Phenolic Compounds. Molecules, 28(3), 1286. https://doi.org/10.3390/molecules28031286








