Recent Advances in N-Heterocyclic Small Molecules for Synthesis and Application in Direct Fluorescence Cell Imaging
Abstract
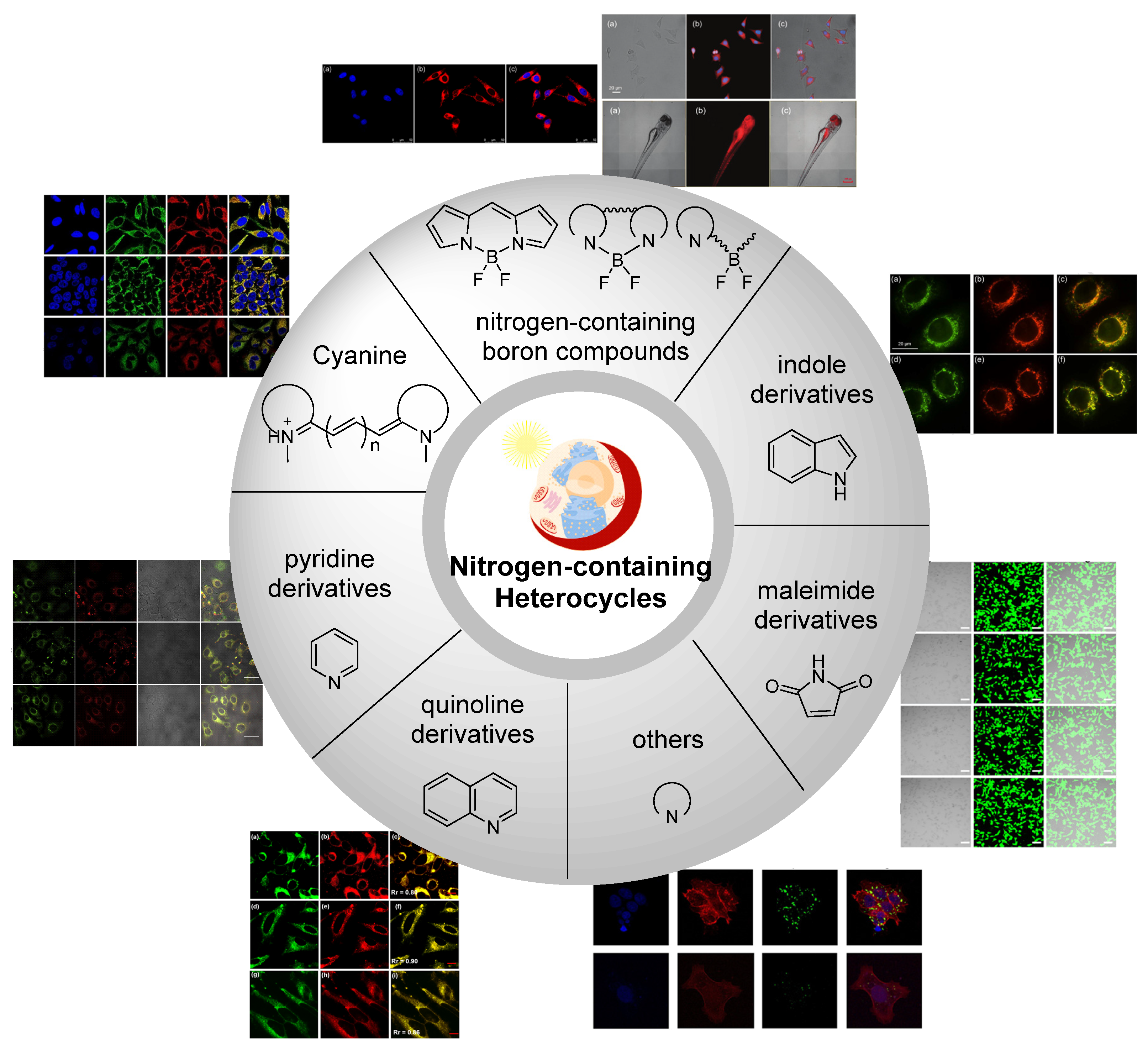
1. Introduction
2. Nitrogen-Containing Boron Compounds
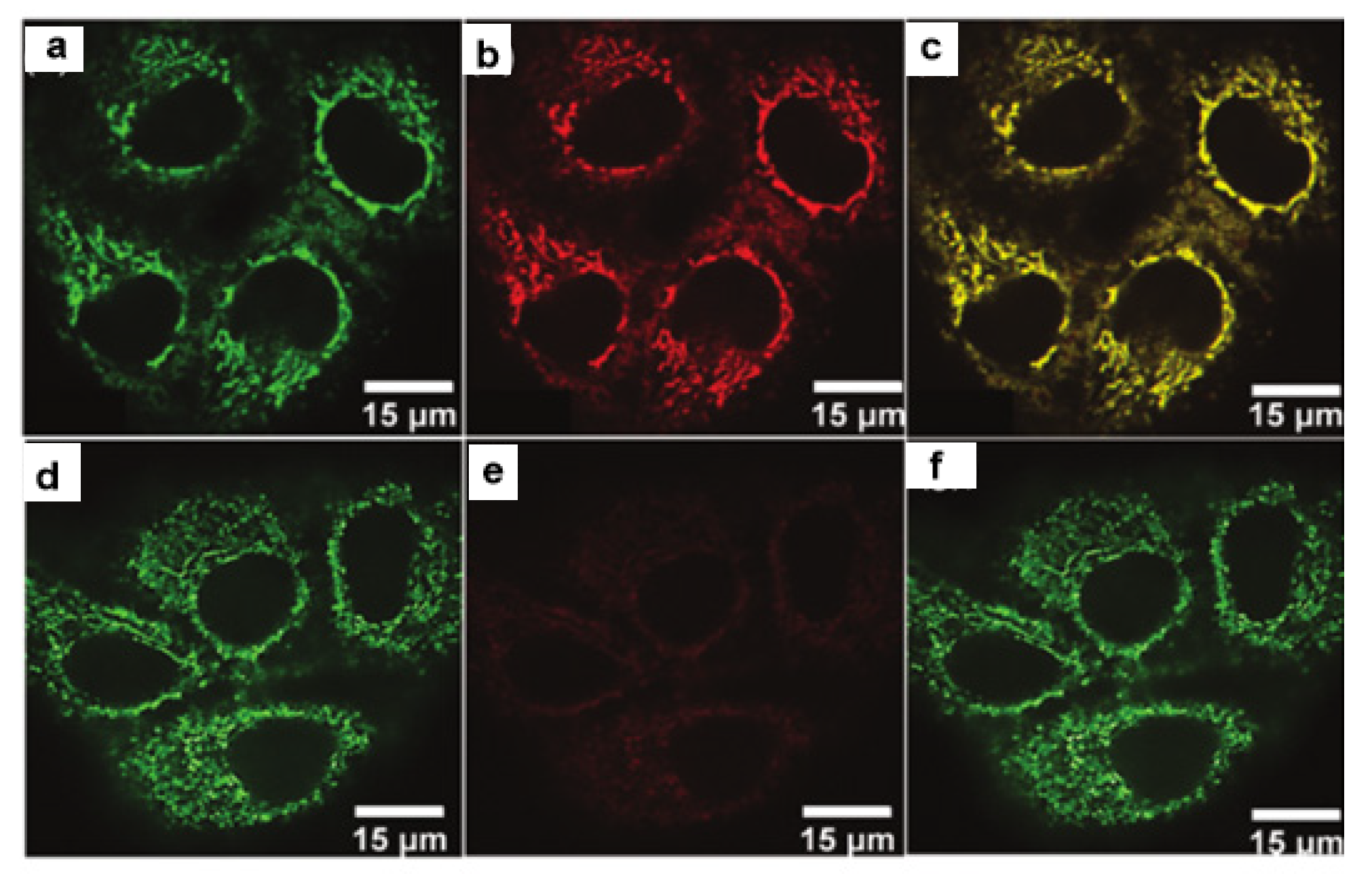
2.1. Boron Dipyrromethene (BODIPY) Derivates
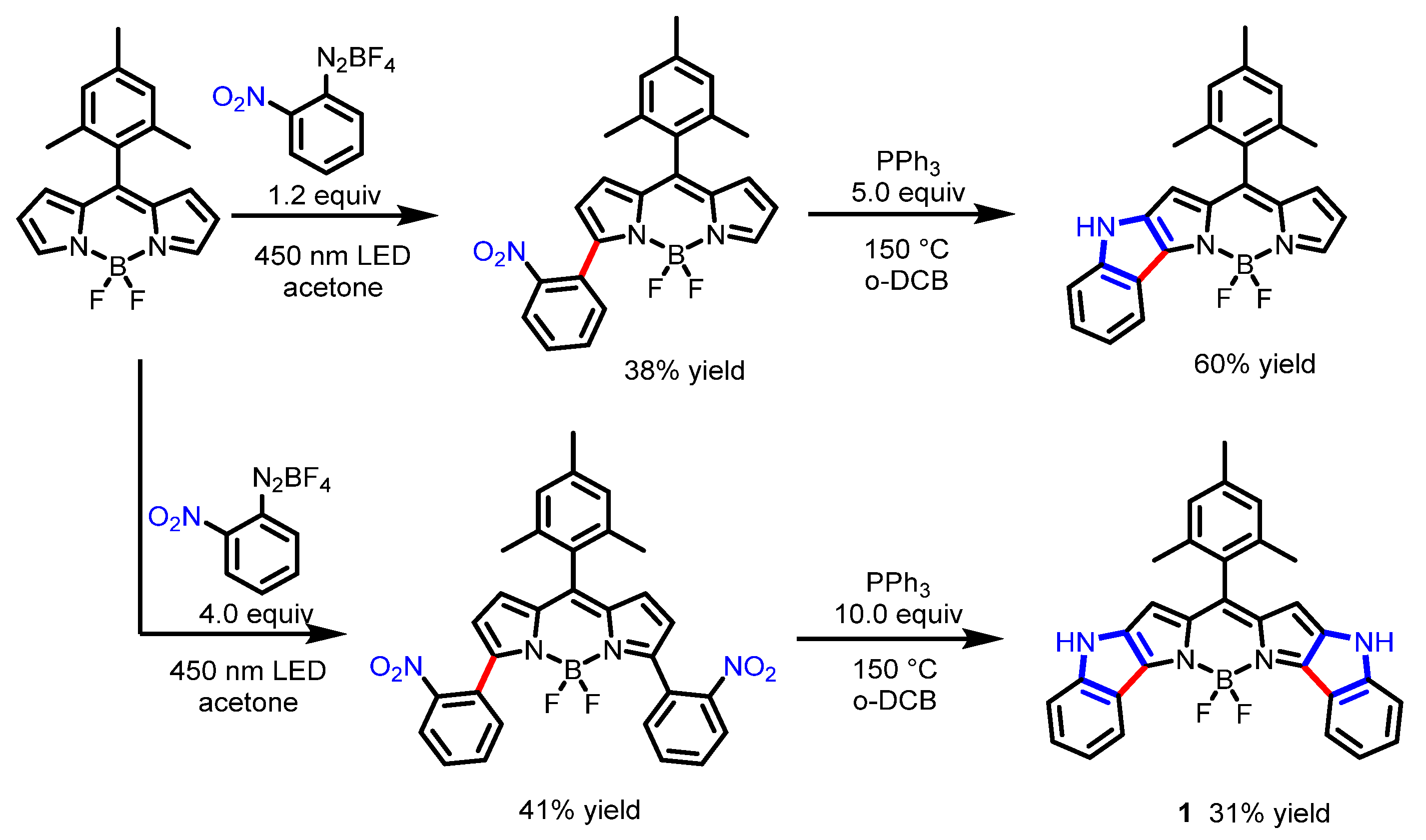
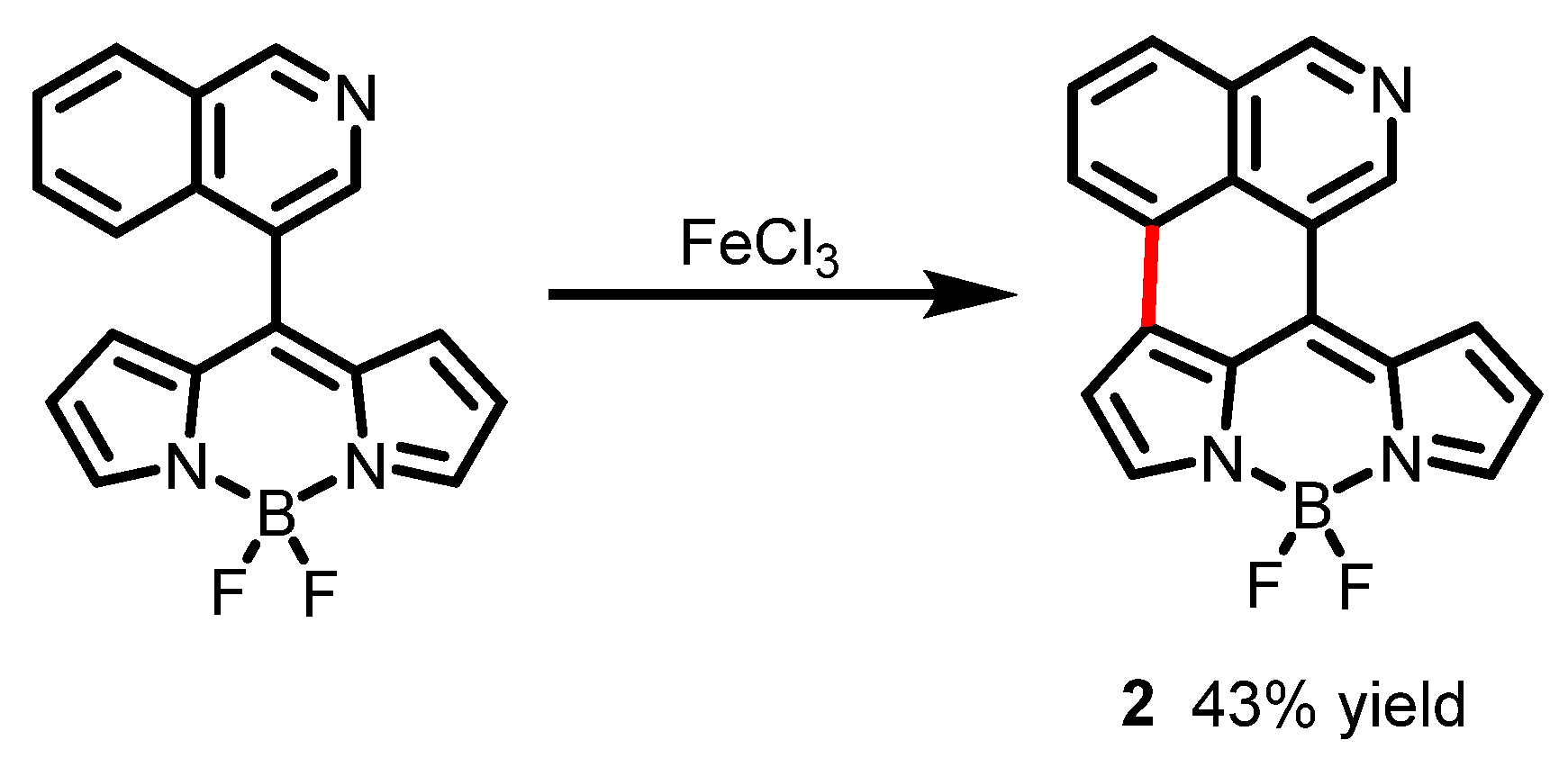
2.2. Other Nitrogen-Containing Boron Compounds
3. Cyanine
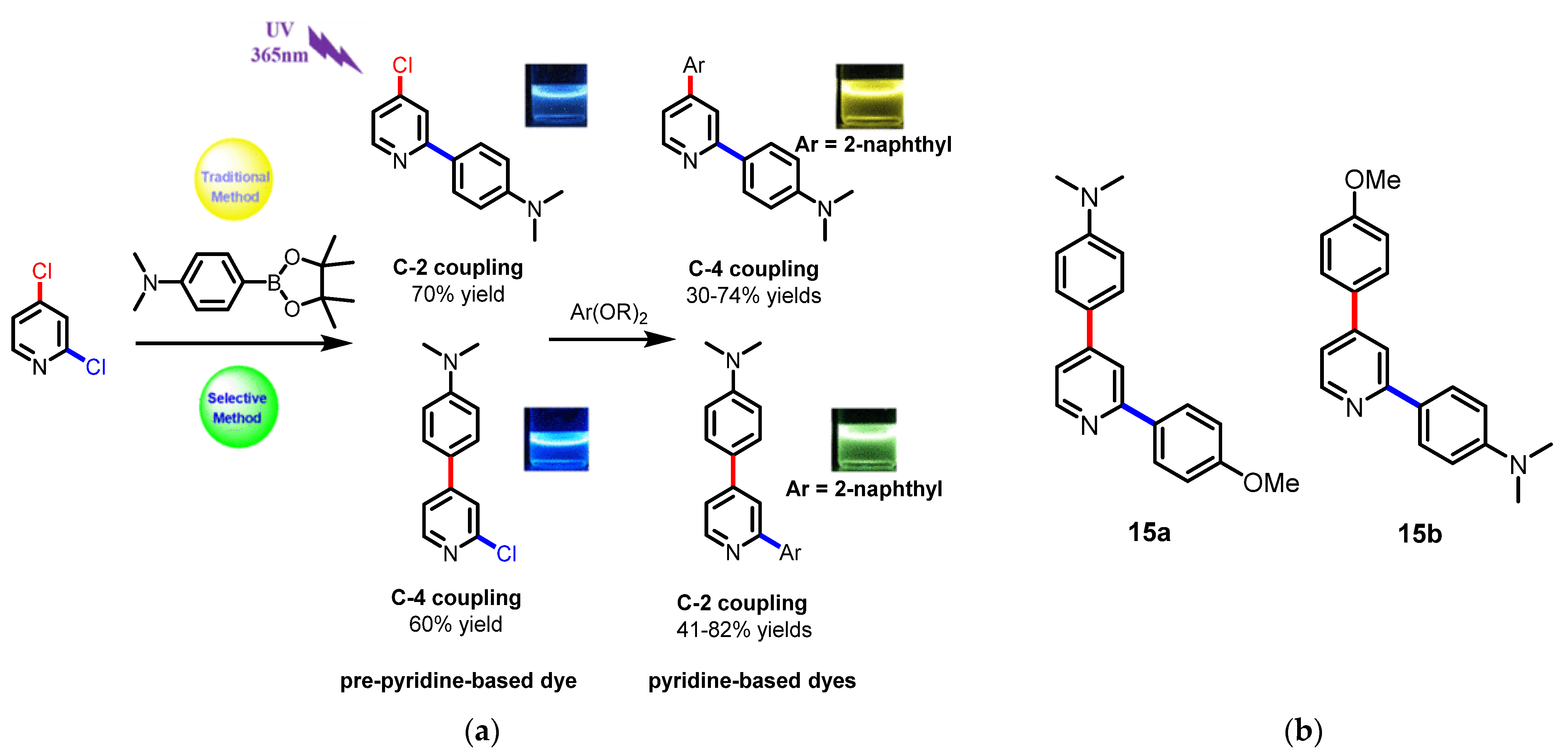
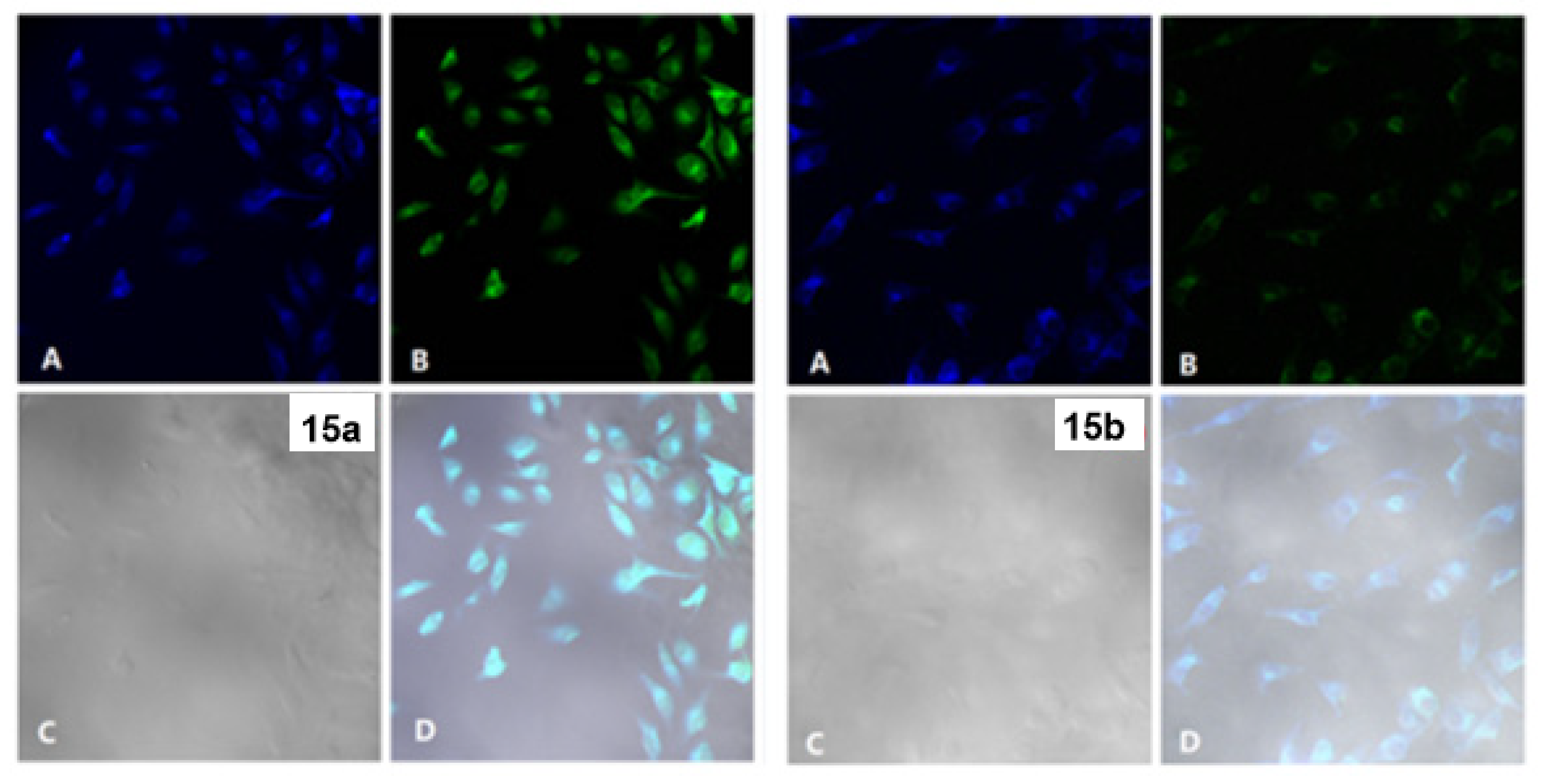
4. Pyridine Derivatives
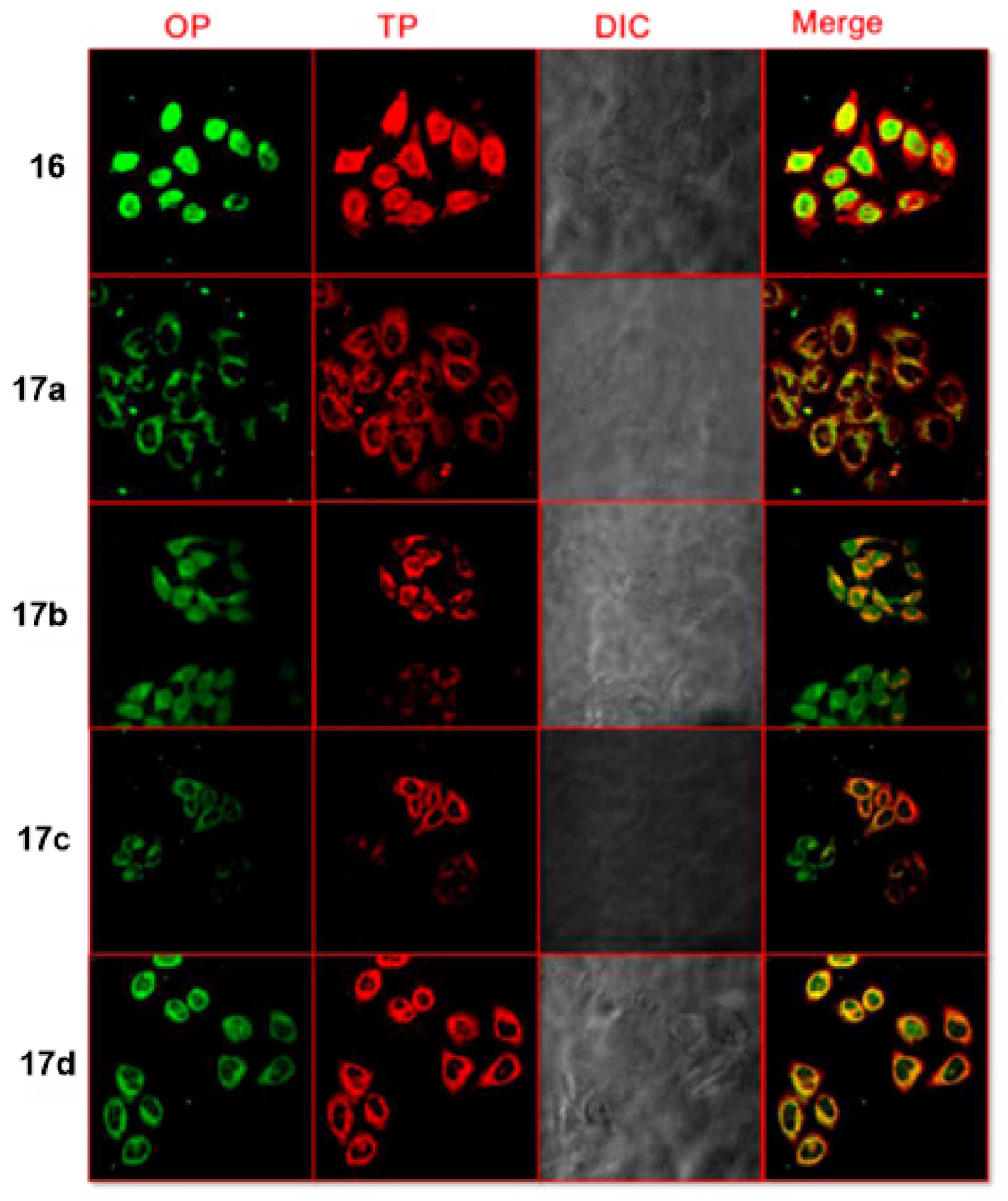
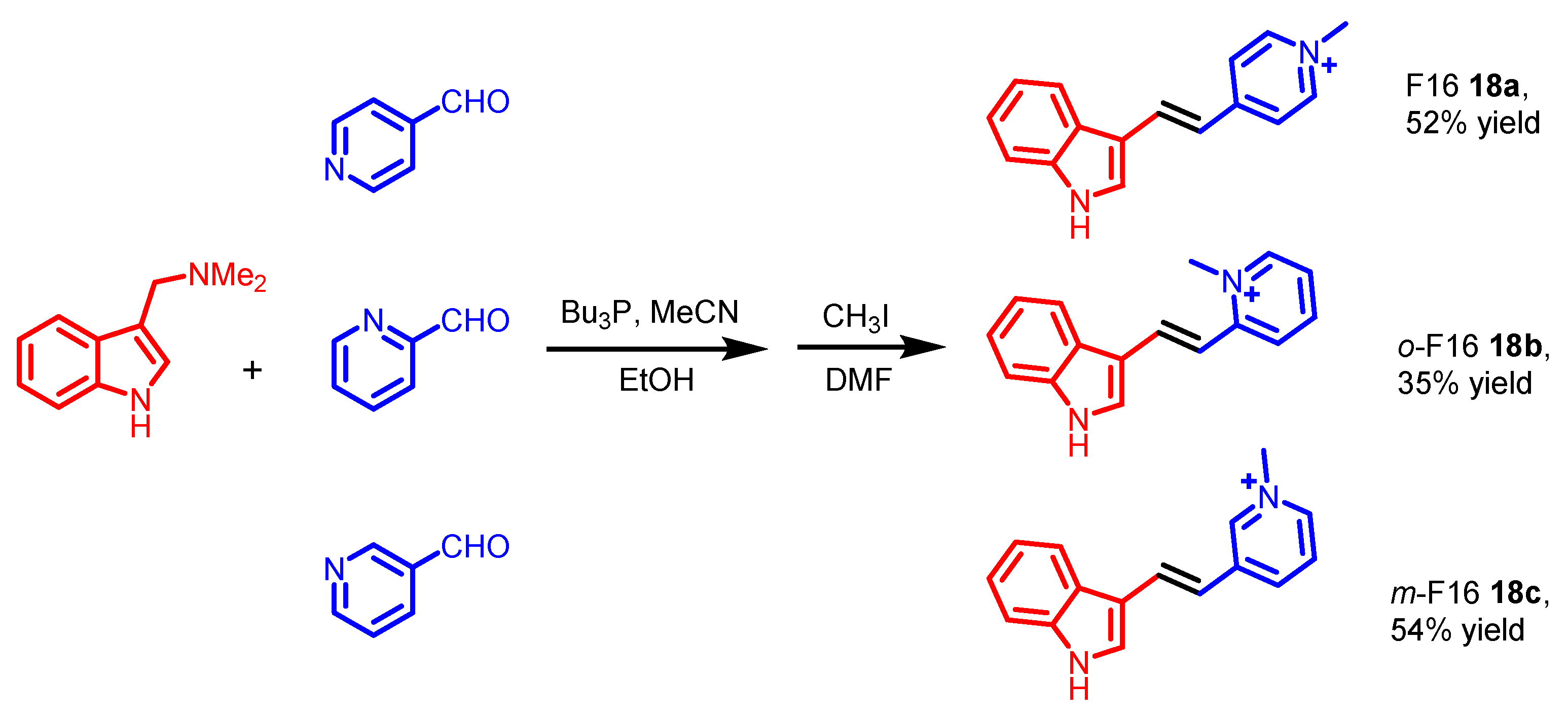
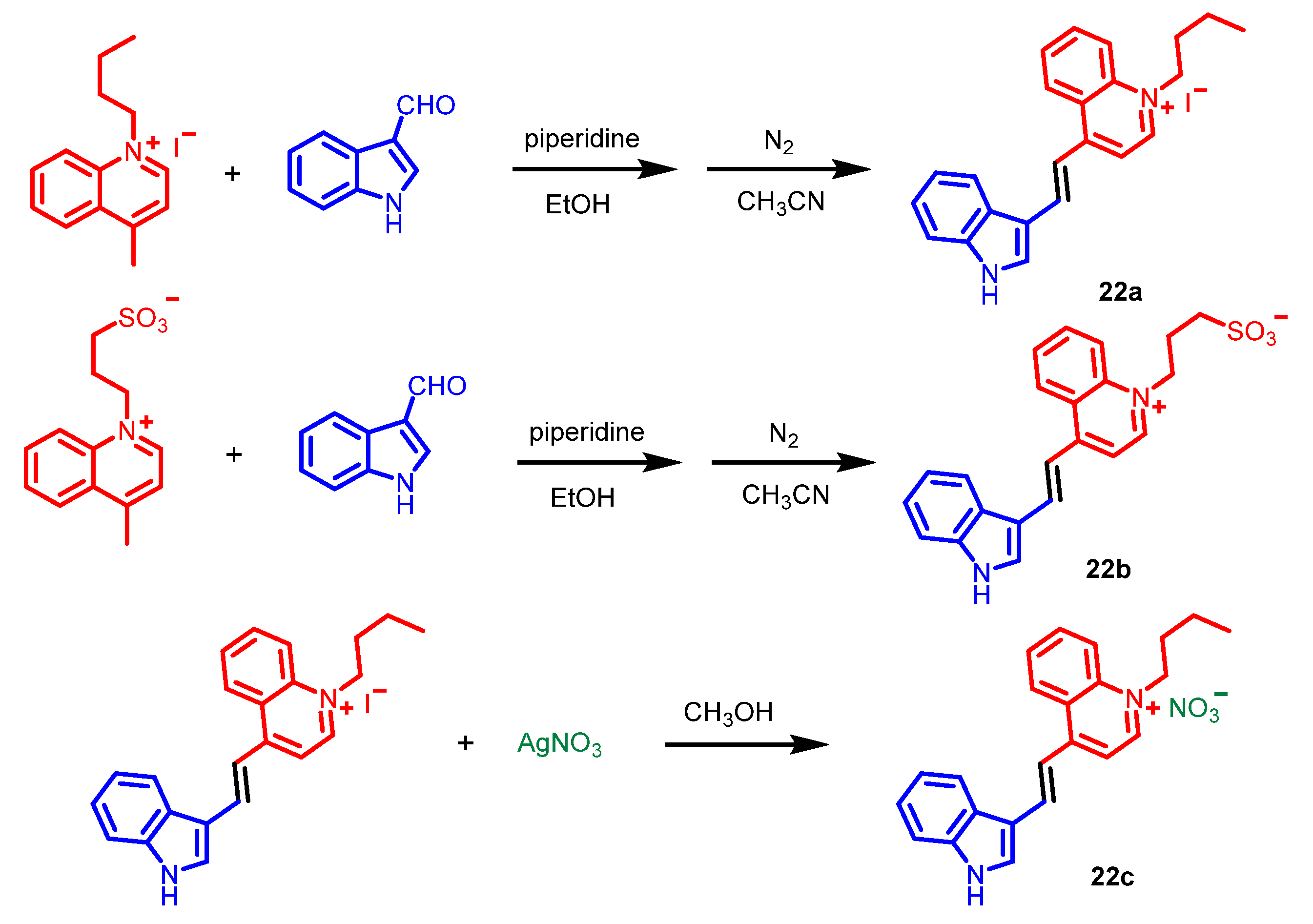
5. Indole Derivatives
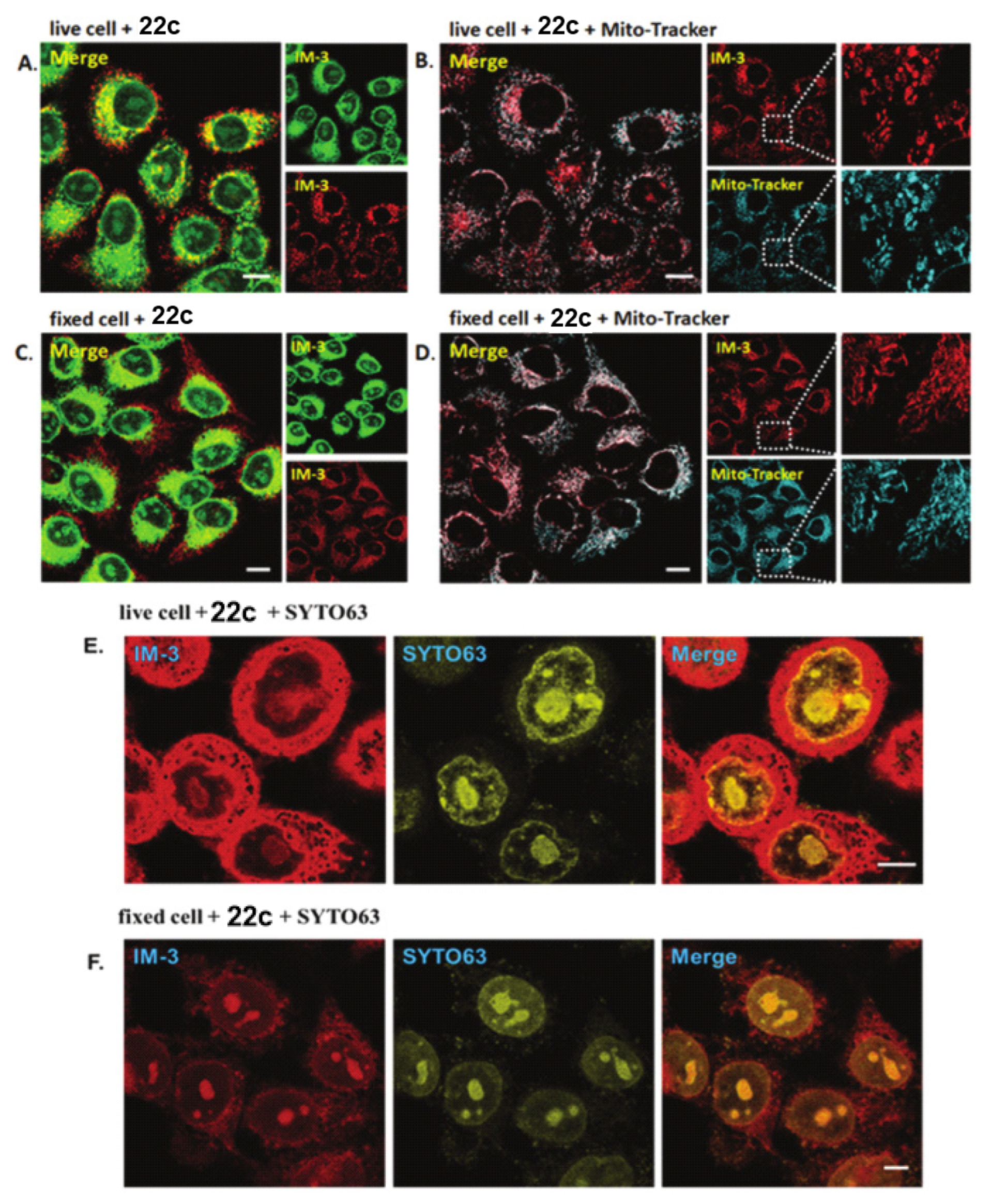
6. Quinoline Derivatives
7. Maleimide Derivatives
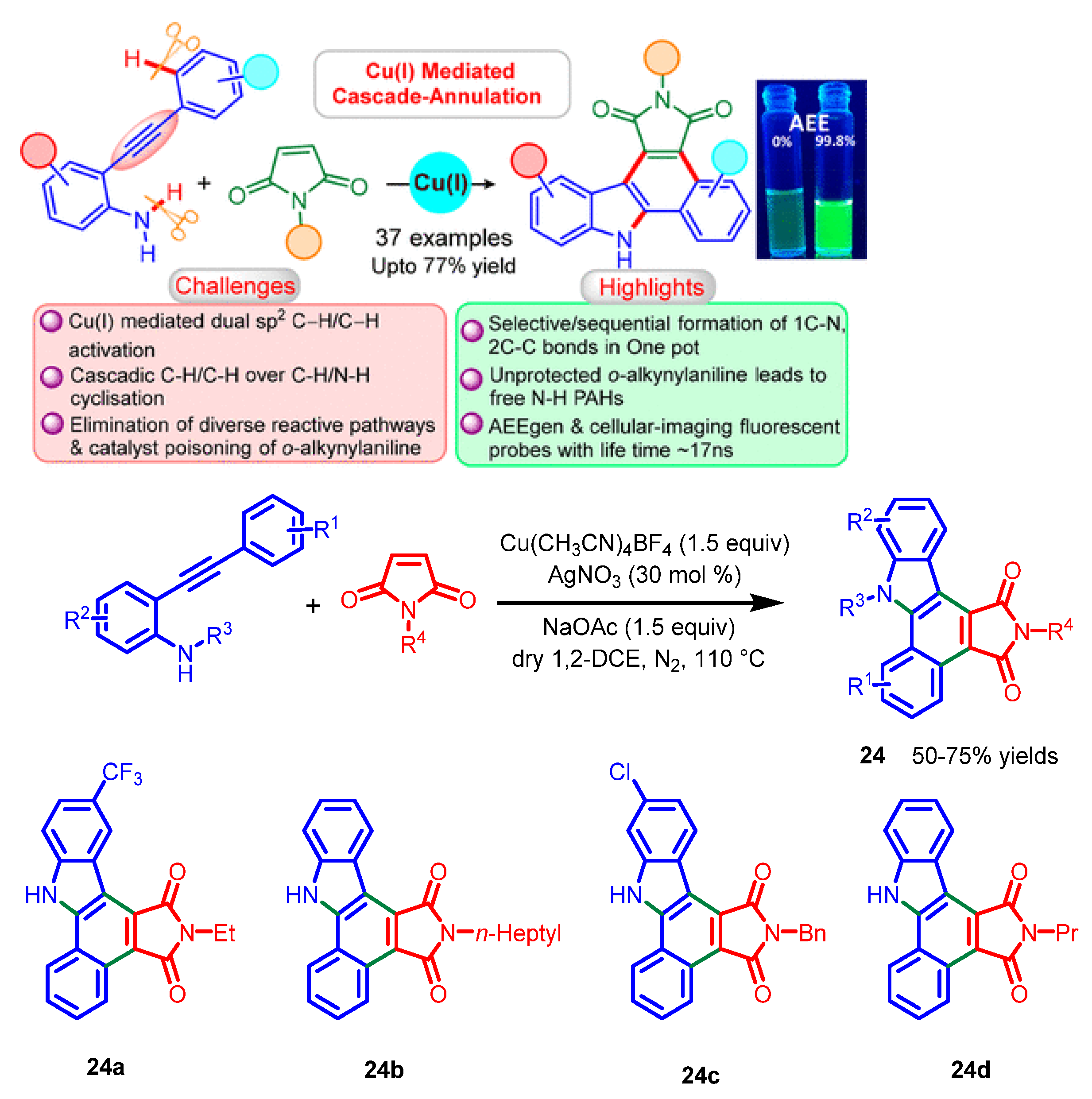
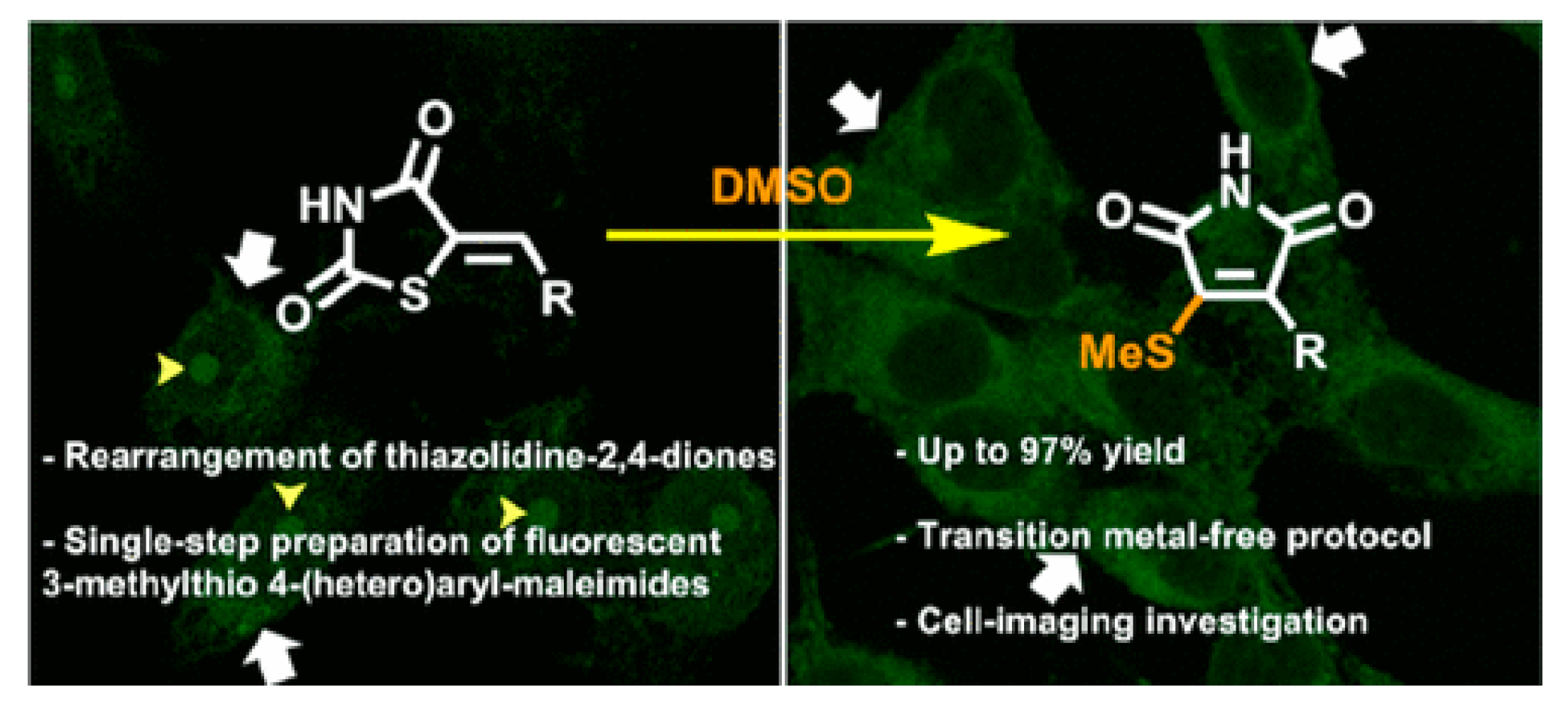
8. Others
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medway, A.M.; Sperry, J. Heterocycle construction using the biomass-derived building block itaconic acid. Green Chem. 2014, 16, 2084–2101. [Google Scholar] [CrossRef]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Varma, R.S. Aqueous N-heterocyclization of primary amines and hydrazines with dihalides: Microwave-assisted syntheses of N-azacycloalkanes, isoindole, pyrazole, pyrazolidine, and phthalazine derivatives. J. Org. Chem. 2006, 71, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Maddila, S.; Jonnalagadda, S.B. Design of carbon–carbon and carbon–heteroatom bond formation reactions under green conditions. Curr. Org. Chem. 2019, 23, 3156–3192. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Tran, T.N.; Henary, M. Synthesis and applications of nitrogen-containing heterocycles as antiviral agents. Molecules 2022, 27, 2700. [Google Scholar] [CrossRef]
- Cecchin, D.; Motta, R.; Zucchetta, P.; Bui, F.; Basso, S.M.M.; Lumachi, F. Imaging studies in hypercalcemia. Curr. Med. Chem. 2011, 18, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.S.; Antaris, A.L.; Dai, H.J. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 10. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Li, L.; Gong, C.; Wang, S.; Yang, F.; Hua, H.; Lin, H. New butanolide derivatives from the marine sponge-derived fungus aspergillus terreus. Bioorg. Med. Chem. Lett. 2018, 28, 315–318. [Google Scholar] [CrossRef]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, J.; Wang, S.; Du, M.; Hou, X.; Tian, T.; Qiao, X.; Tian, Z.; Stang, P.J.; Li, S.; et al. Self-assembled NIR-II fluorophores with ultralong blood circulation for cancer imaging and image-guided surgery. J. Med. Chem. 2022, 65, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Huang, L.; Wu, L.; Li, J.; James, T.D.; Lin, W. Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions. Chem. Soc. Rev. 2021, 50, 12098–12150. [Google Scholar] [CrossRef] [PubMed]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azer, F.; Frangioni, J.V. The flare™ intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, W.; Wang, R. Organic molecules: Desirable candidates for NIR-II window bioimaging. J. Phys. Conf. Ser. 2021, 1885, 032070. [Google Scholar] [CrossRef]
- Jean-Gérard, L.; Vasseur, W.; Scherninski, F.; Andrioletti, B. Recent advances in the synthesis of [a]-benzo-fused BODIPY fluorophores. Chem. Commun. 2018, 54, 12914–12929. [Google Scholar] [CrossRef]
- Treibs, A.; Kreuzer, F.H. Difluorboryl-komplexe von di-und tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent advances in the application of BODIPY in bioimaging and chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, C.; Wu, Q.; Wang, J.; Kang, Z.; Guo, X.; Wu, H.; Hao, E.; Jiao, L. Visible-light excitation of BODIPYs enables self-promoted radical arylation at their 3,5-positions with diazonium salts. Org. Lett. 2019, 21, 5121–5125. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Ma, C.; Tang, A. Quinoline-fused BODIPY with large stokes shift as near-infrared dye for cell imaging. Dyes Pigm. 2020, 173, 107981. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Gao, L.; Chen, J.; Zhou, T.; Liu, Y.; Jiang, F. A bright, red-emitting water-soluble BODIPY fluorophore as an alternative to the commercial mito tracker red for high-resolution mitochondrial imaging. J. Mater. Chem. B 2021, 9, 8639–8645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, J.; Hu, Y.; Liu, X.; Wen, L.; Zheng, K.; Zhang, N. A simple central seven-membered BOPYIN: Synthesis, structural, spectroscopic properties, and cellular imaging application. Chem. Eur. J. 2019, 25, 9266–9271. [Google Scholar] [CrossRef]
- Más-Montoya, M.; Montenegro, M.F.; Ferao, A.E.; Tárraga, A.; Rodríguez-López, J.N.; Curiel, D. Rigid π-extended boron difluoride complex with mega-stokes shift for bioimaging. Org. Lett. 2020, 22, 3356–3360. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, F.; Luo, H.; Liao, L.; Song, X.; Chen, W. Red-emitting boron difluoride complexes with a mega-large stokes shift and unexpectedly high fluorescence quantum yield. Chem. Commun. 2020, 56, 2159–2162. [Google Scholar] [CrossRef]
- Fang, M.; Xia, S.; Bi, J.; Wigstrom, T.; Valenzano, L.; Wang, J.; Mazi, W.; Tanasova, M.; Luo, F.; Liu, H. A cyanine-based fluorescent cassette with aggregation-induced emission for sensitive detection of pH changes in live cells. Chem. Commun. 2018, 54, 1133–1136. [Google Scholar] [CrossRef]
- Gui, L.; Yuan, Z.; Kassaye, H.; Zheng, J.; Yao, Y.; Wang, F.; He, Q.; Shen, Y.; Liang, L.; Chen, H. A tumor-targeting probe based on a mitophagy process for live imaging. Chem. Commun. 2018, 54, 9675–9678. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.; Noguchi, K.; Ishiyama, M.; Denny, W.A.; Jose, J. A mitochondria-selective near-infrared-emitting fluorescent dye for cellular imaging studies. Bioorg. Med. Chem. Lett. 2018, 28, 2013–2017. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Xiao, J.; Tan, X.; Zhu, Y.; Su, Y.; Cheng, T.; Shi, C. Sentinel lymph node mapping by a near-infrared fluorescent heptamethine dye. Biomaterials 2010, 31, 1911–1917. [Google Scholar] [CrossRef]
- Lydiard, R.B.; Gelenberg, A.J. Amoxapine—An antidepressant with some neuroleptic properties?: A review of its chemistry, animal pharmacology and toxicology, human pharmacology, and clinical efficacy. Pharmacotherapy 1981, 1, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, D.L.; Johnson, M.E. Development of near-infrared fluorophoric labels for the determination of fatty acids separated by capillary electrophoresis with diode laser induced fluorescence detection. Analyst 1999, 124, 1541–1546. [Google Scholar] [CrossRef]
- Braun, A.B.; Wehl, I.; Kolmel, D.K.; Schepers, U.; Brase, S. New polyfluorinated cyanine dyes for selective NIR staining of mitochondria. Chem. Eur. J. 2019, 25, 7998–8002. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Walsh, M.L.; Chen, L.B. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA 1980, 77, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, G.; Xu, Y.; Sun, R.; Ge, J. Near-infraredfluorescent probes based on a quinoxaline skeleton for imaging nucleic acids in mitochondria. Org. Biomol. Chem. 2022, 20, 5558–5565. [Google Scholar] [CrossRef] [PubMed]
- Boujut, M.; Chevalier, A.; Schapman, D.; Bénard, M.; Galas, L.; Gallavardin, T.; Franck, X. Indazole versus indole-based cationic merocyanines with red shifted in-cellulo emission for selective mitochondria imaging. Dyes Pigm. 2022, 198, 109988. [Google Scholar] [CrossRef]
- Anderson, T. Producte der trocknen Destillation thierischer Materien. Ann. Chem. Pharm. 1849, 70, 32–38. [Google Scholar] [CrossRef]
- Seferoglu, Z.; Ihmels, H.; Şahin, E. Synthesis and photophysical properties of fluorescent arylstyrylimidazo [1,2-a] pyridine-based donor-acceptor chromophores. Dyes Pigm. 2015, 113, 465–473. [Google Scholar] [CrossRef]
- Golden, J.H.; Facendola, J.W.D.; Sylvinson, M.R.; Baez, C.Q.; Djurovich, P.I.; Thompson, M.E. Boron dipyridylmethene (DIPYR) dyes: Shedding light on pyridine-based chromophores. J. Org. Chem. 2017, 82, 7215–7222. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.H.; Zhang, C.J.; Huang, J.; Sun, Q. A thiazolo [4, 5-b] pyridine-based fluorescent probe for detection of zinc ions and application for in vitro and in vivo bioimaging. Talanta 2018, 185, 396–404. [Google Scholar] [CrossRef]
- Xiang, M.H.; Huang, H.; Liu, X.J.; Tong, Z.X.; Zhang, C.X.; Wang, F.; Yu, R.Q.; Jiang, J.H. Mitochondrion-targeting fluorescence probe via reduction induced charge transfer for fast methionine sulfoxide reductases imaging. Anal. Chem. 2019, 91, 5489–5493. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H. Photophysical investigaions and the bioimaings of α-, β-, γ-pyridine-based terpyridine derivatives. J. Mol. Struct. 2018, 1157, 457. [Google Scholar] [CrossRef]
- Yang, M.; Chen, J.; He, C.; Hu, X.; Ding, Y.; Kuang, Y.; Liu, J.; Huang, Q. Palladium-catalyzed C-4 selective coupling of 2,4-dichloropyridines and synthesis of pyridine-based dyes for live-cell imaging. J. Org. Chem. 2020, 85, 6498–6508. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.; Wang, D.; Li, M.; Cai, H.; Kong, L.; Tian, Y.; Gan, X.; Zhou, H. Small molecules based benzothiazole-pyridinium salts with different anions: Two-photon fluorescence regulation and difference in cell imaging application. Dyes Pigm. 2021, 194, 109639. [Google Scholar] [CrossRef]
- Fischer, E.; Jourdan, F. Ueber die hydrazine der brenztraubensäure. Ber. Dtsch. Chem. Ges. 1883, 16, 2241–2245. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 10302. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Q.; Yang, G. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; He, H.; Zhou, L.J.; Liu, Y.Z.; Li, D.W.; Jiang, F.L.; Liu, Y. Pyridinium and indole orientation determines the mitochondrial uncoupling and anti-cancer efficiency of F16. Eur. J. Med. Chem. 2018, 154, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Feng, X.; Zhu, M.; Hoffmann, S.; Hsu, A.; Qian, K.; Huang, D.; Zhao, F.; Liu, W.; et al. Mitochondria-targetingfluorescent molecules for high efficiency cancer growth inhibition and imaging. Chem. Sci. 2019, 10, 7946–7951. [Google Scholar] [CrossRef]
- Beşer, B.M.; Altay, A.; Talaz, O.; Türkmenoğlu, B. A bis indole analog molecule fluorescent probe for living cell staining. J. Photoch. Photobio. A 2022, 424, 113613. [Google Scholar] [CrossRef]
- Runge, F.F. Ueber einige produkte der steinkohlendestillation. Ann. Phys. Chem. 1834, 31, 65–78. [Google Scholar] [CrossRef]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Patel, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Niu, W.; Fan, L.; Nan, M.; Li, Z.; Lu, D.; Wong, M.; Shuang, S.; Dong, C. Ratiometric emission fluorescent PH probe for imaging of living cells in extreme acidity. Anal. Chem. 2015, 87, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.V.; Petersson, E.J.; Chenoweth, D.M. Rational design and facile synthesis of a highly tunable quinoline-based fluorescent small-molecule scaffold for live cell imaging. J. Am. Chem. Soc. 2018, 140, 9486–9493. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Yang, L.; Jiang, J.; Bi, G.; Zhang, G.; Li, G.; Chen, X. Highly selective and efficient synthesis of 7-aminoquinolines and their applications as Golgi-localized probes. ACS Med. Chem. Lett. 2019, 10, 954–959. [Google Scholar] [CrossRef]
- Elhussin, I.E.H.; Zhang, S.; Liu, J.; Li, D.; Zhang, Q.; Li, S.; Tian, X.; Wu, J.; Tian, Y. A novel water-soluble quinoline-indole derivative as three-photon fluorescent probe for identifying nucleolus RNA and mitochondrial DNA. Chem. Commun. 2020, 56, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, X.; Wang, Y.; Xu, Y.; Sun, R.; Ge, J. Preparation of chromeno[b]quinoline derivatives and their application for lipid droplets markers. J. Org. Chem. 2022, 87, 10385–10389. [Google Scholar] [CrossRef]
- Cava, M.P.; Deana, A.A.; Muth, K.; Mitchell, M.J. N-Phenylmaleimide. Org. Synth. 1961, 41, 93. [Google Scholar]
- Wu, M.; Cheng, M.; Wang, B.; Yech, Y.; Lai, J.; Kuo, Y.; Yuan, G.; Chen, I. Maleimide and maleic anhydride derivatives from the mycelia of antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages. J. Nat. Prod. 2008, 71, 1258–1261. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Samanta, S.; Ghosh, P.; Neogi, S.; Hajra, A. Regioselective hydroarylation and arylation of maleimides with indazoles via a Rh(iii)-catalyzed C−H activation. Org. Biomol. Chem. 2020, 18, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yin, C.; Huo, F.; Li, J.; Chao, J.; Zhang, Y. A maleimide-based thiol fluorescent probe and its application for bioimaging. Sens. Actuators B Chem. 2014, 195, 246–251. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, M.; Tan, L.Y.F.; Hong, Q.; Pow, Z.L.; Owyong, T.C.; Ding, S.; Wong, W.W.H.; Hong, Y. A maleimide-functionalized tetraphenylethene for measuring and imaging unfolded proteins in cells. Chem. Asian J. 2019, 14, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Khandelia, T.; Ghosh, S.; Panigrahi, P.; Shome, R.; Ghosh, S.S.; Patel, B.K. Copper(I)-mediated cascade annulation via dual C−H/C−H activation: Access to benzo[a]carbazolic AEEgens. J. Org. Chem. 2021, 86, 16948–16964. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; de Castro, P.P.V.; Passos, S.T.A.; Carvalho, B.B.P.P.; Franco, C.H.J.; Correa, J.R.; Neto, B.A.D.; Amarante, G.W. Diverse 3-methylthio-4-substituted maleimides through a novel rearrangement reaction: Synthesis and selective cell imaging. J. Org. Chem. 2022, 87, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, K.; Li, L.; Chen, S.; Li, M.; Zhou, Q.; Wang, N.; Yu, X. Novel easily available purine-based AIEgens with colour tunability and applications in lipid droplet imaging. Chem. Sci. 2018, 9, 8969–8974. [Google Scholar] [CrossRef]
- Namba, K.; Osawa, A.; Ishizaka, S.; Kitamura, N.; Tanino, K. Direct synthesis of fluorescent 1,3a,6a-triazapentalene derivatives via click–cyclization–aromatization cascade reaction. J. Am. Chem. Soc. 2011, 133, 11466–11469. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, D.; Diharce, J.; Martinić, I.; Chopin, N.; Eliseeva, S.V.; Guillaumet, G.; Petoud, S.; Bonneta, P.; Suzenet, F. An original class of small sized molecules as versatile fluorescent probes for cellular imaging. Chem. Commun. 2019, 55, 7776–7779. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.G.; Kumar, V.; Kaur, K. Imidazole containing natural products as antimicrobial agents: A review. J. Nat. Prod. 2014, 4, 73–81. [Google Scholar]
- Zhang, L.; Peng, X.M.; Damu, G.L.V.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Majumder, L.; Pakrashy, S.; Srinath, R.; Mukherjee, K.; Mandal, C.; Banerji, B. Polysubstituted imidazoles as lysotracker molecules: Their synthesis via iodine/H2O and cell-imaging studies. ACS Omega 2020, 5, 14394–14407. [Google Scholar] [CrossRef]
- Hase, E.; Takanari, H.; Hoshi, K.; Okamoto, M.; Tabata, A.; Nagamune, H.; Minamikawa, T.; Yasui, T.; Yoshida, Y.; Minagawa, K.; et al. Two- and three-photon excitable quaternized imidazo[1,2-a]pyridines as mitochondrial imaging and potent cancer therapy agents. Org. Biomol. Chem. 2020, 18, 7571–7576. [Google Scholar] [CrossRef]
- Samanta, S.; Jana, S.; Mondal, S.; Monir, K.; Chandra, S.K.; Hajra, A. Switching the regioselectivity in the copper-catalyzed synthesis of iodoimidazo[1,2-a]pyridines. Org. Biomol. Chem. 2016, 14, 5073–5078. [Google Scholar] [CrossRef]
- Lima, M.L.S.O.; Braga, C.B.; Becher, T.B.; Odriozola-Gimeno, M.; Torrent-Sucarrat, M.; Rivilla, I.; Cossio, F.P.; Marsaioli, A.J.; Ornelas, C. Fluorescent imidazo[1,2-a]pyrimidine compounds as biocompatible organic photosensitizers that generate singlet oxygen: A potential tool for phototheranostics. Chem. Eur. J. 2021, 27, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Danylchuk, D.I.; Jouard, P.H.; Klymchenko, A.S. Targeted solvatochromic fluorescent probes for imaging lipid order in organelles under oxidative and mechanical stress. J. Am. Chem. Soc. 2021, 143, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Banala, S.; Tkachuk, A.N.; Patel, R.; Kumar, P.; Brown, T.A.; Lavis, L.D. 2,7-diaminobenzopyrylium dyes are live-cell mitochondrial stains. ACS Bio. Med. Chem. Au 2022, 2, 307–312. [Google Scholar] [CrossRef] [PubMed]


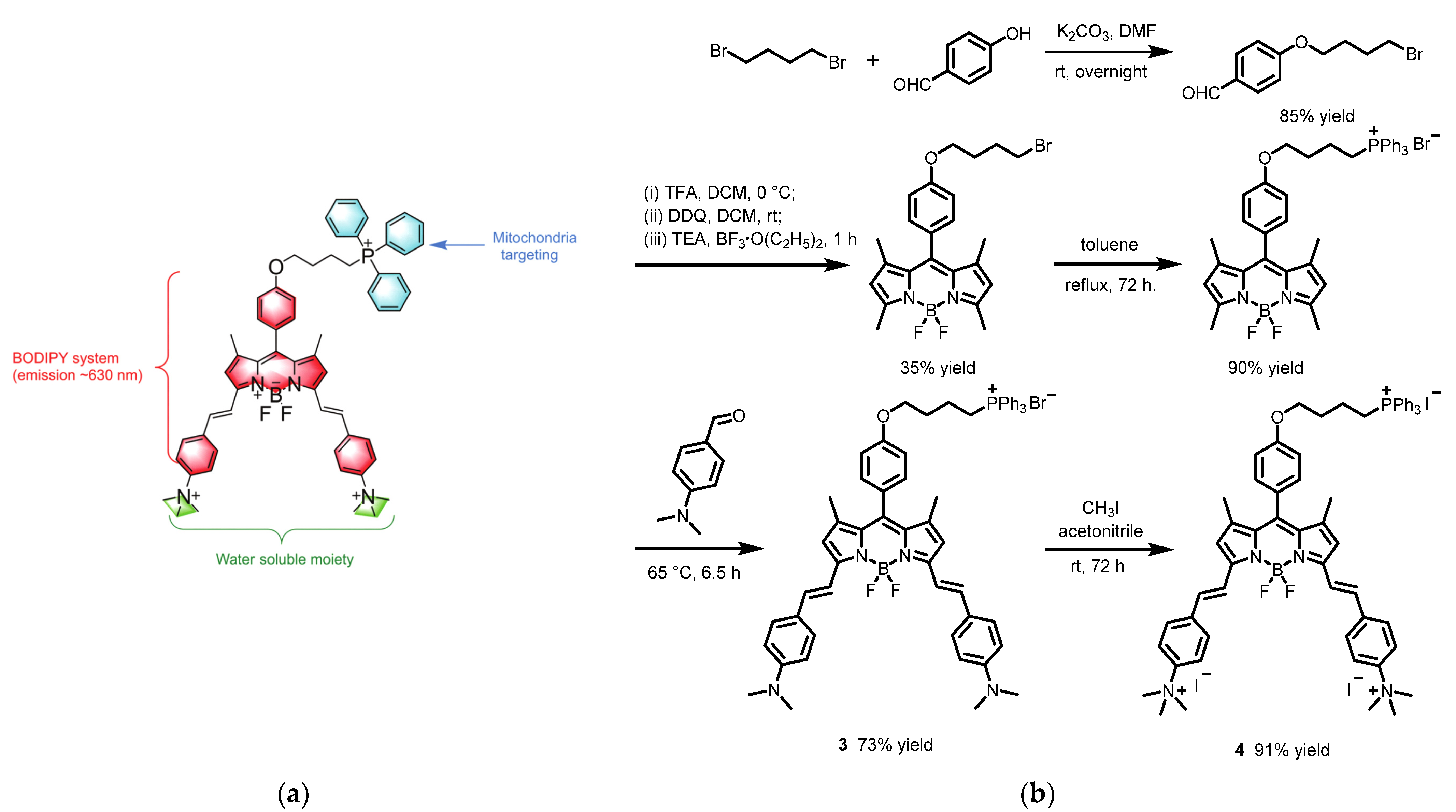
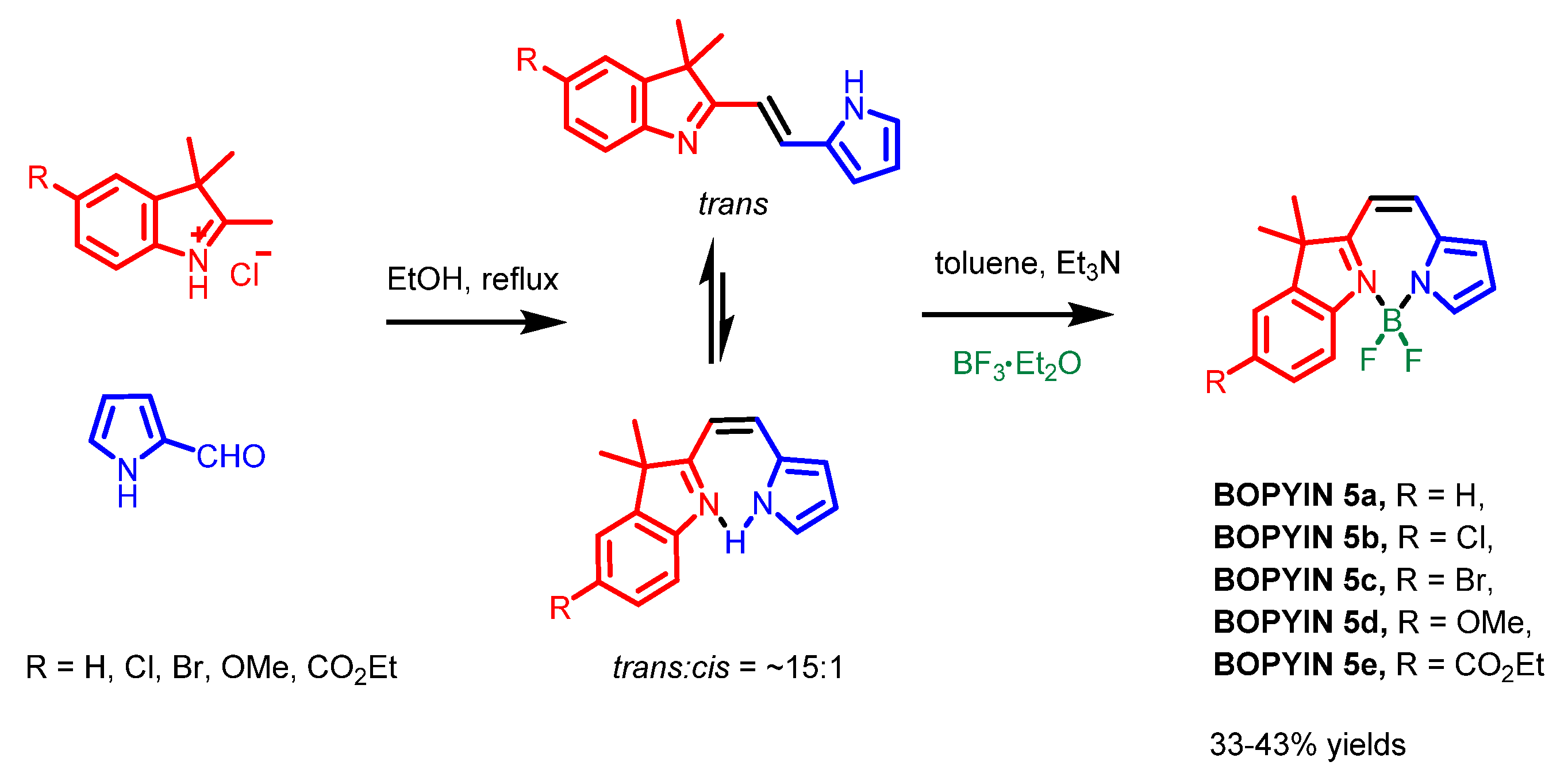

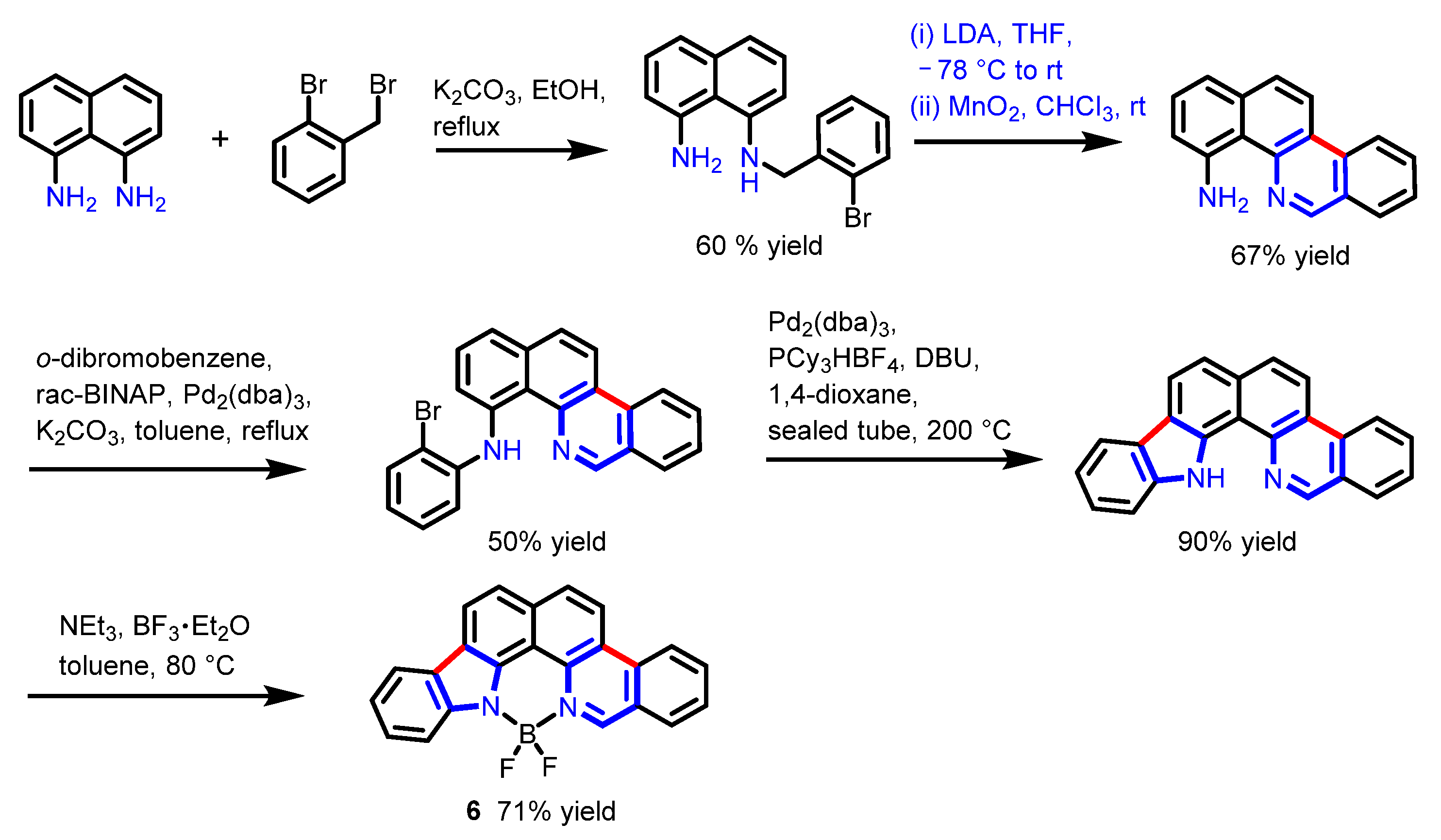
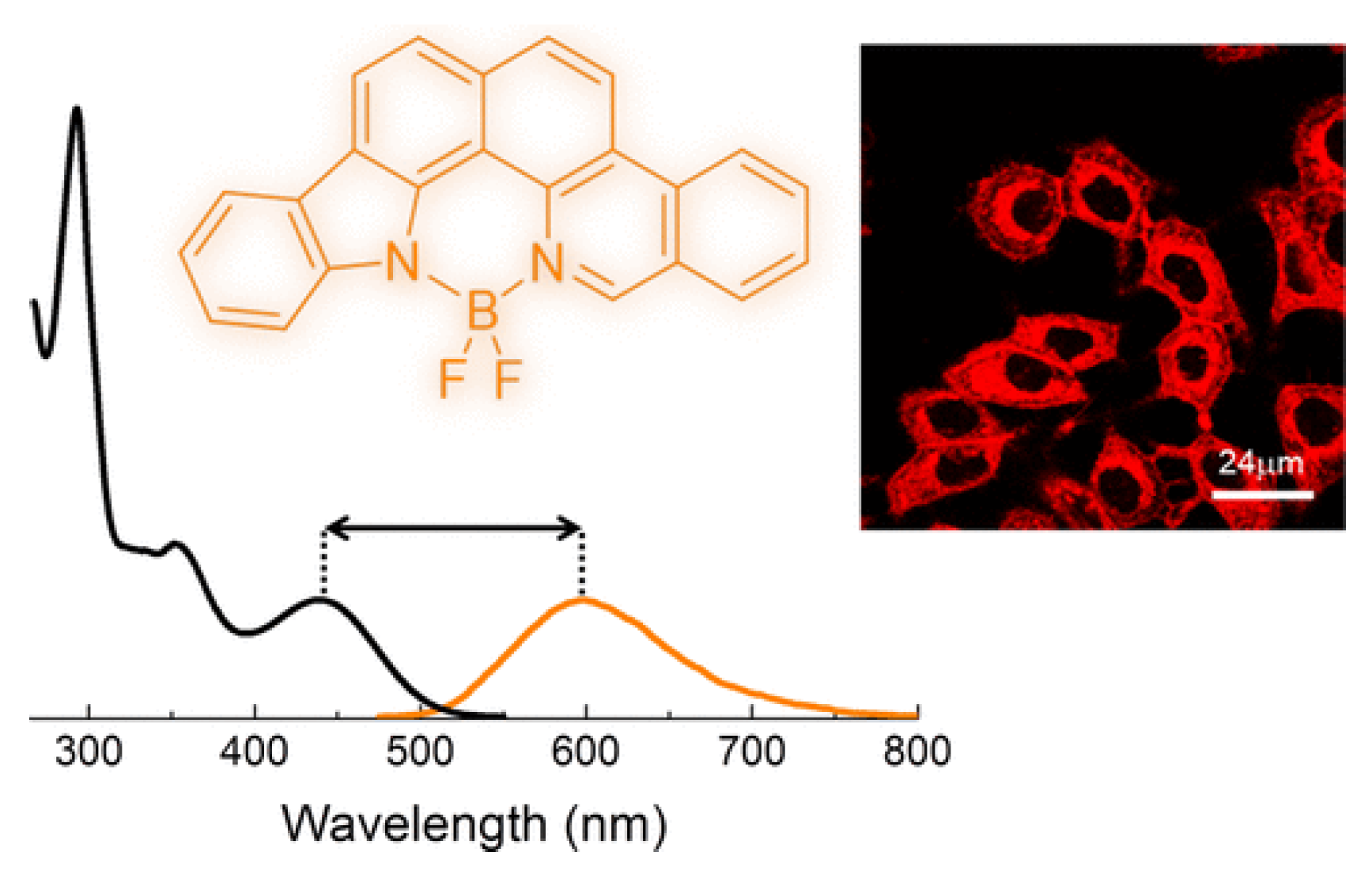
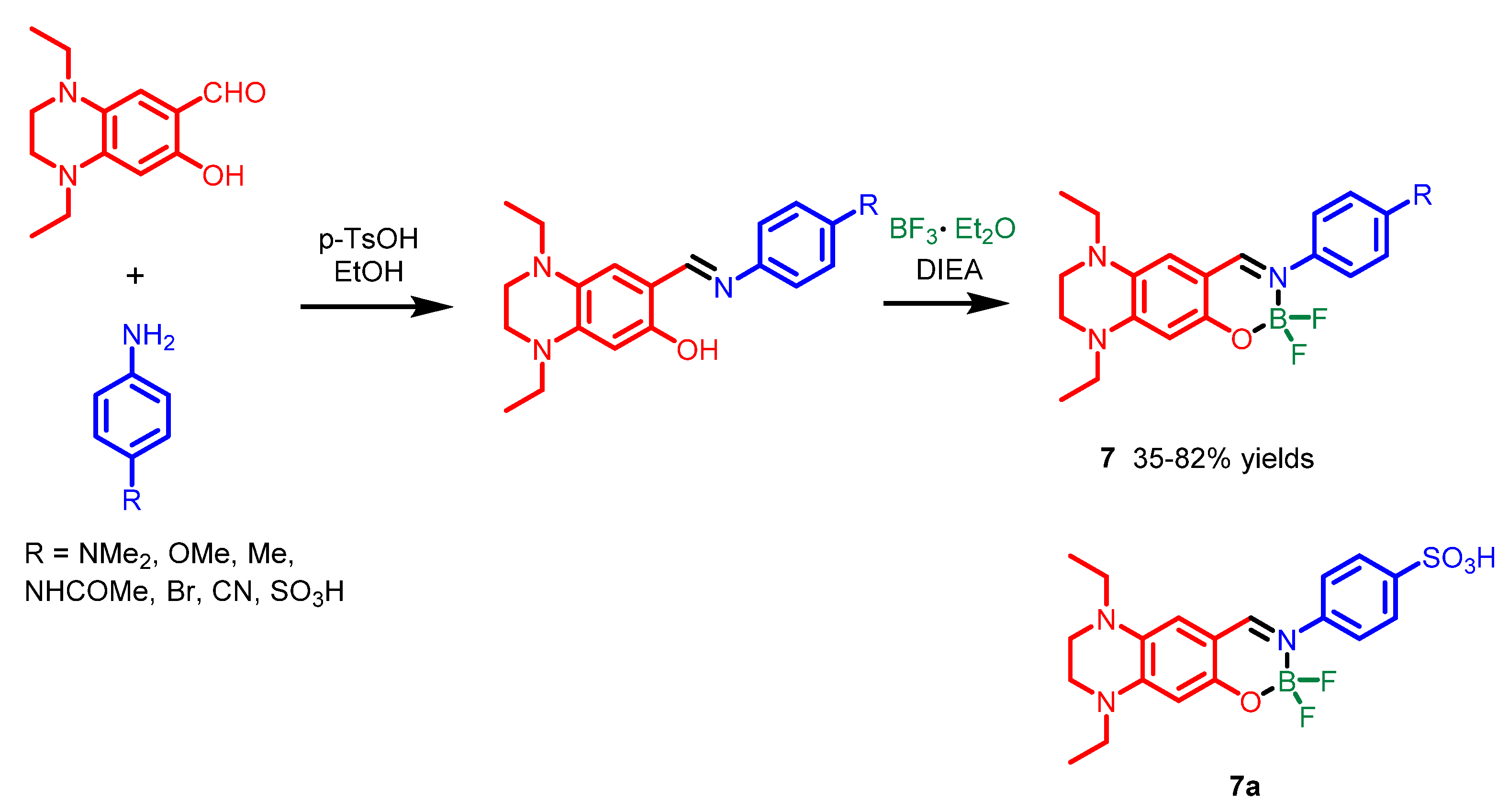
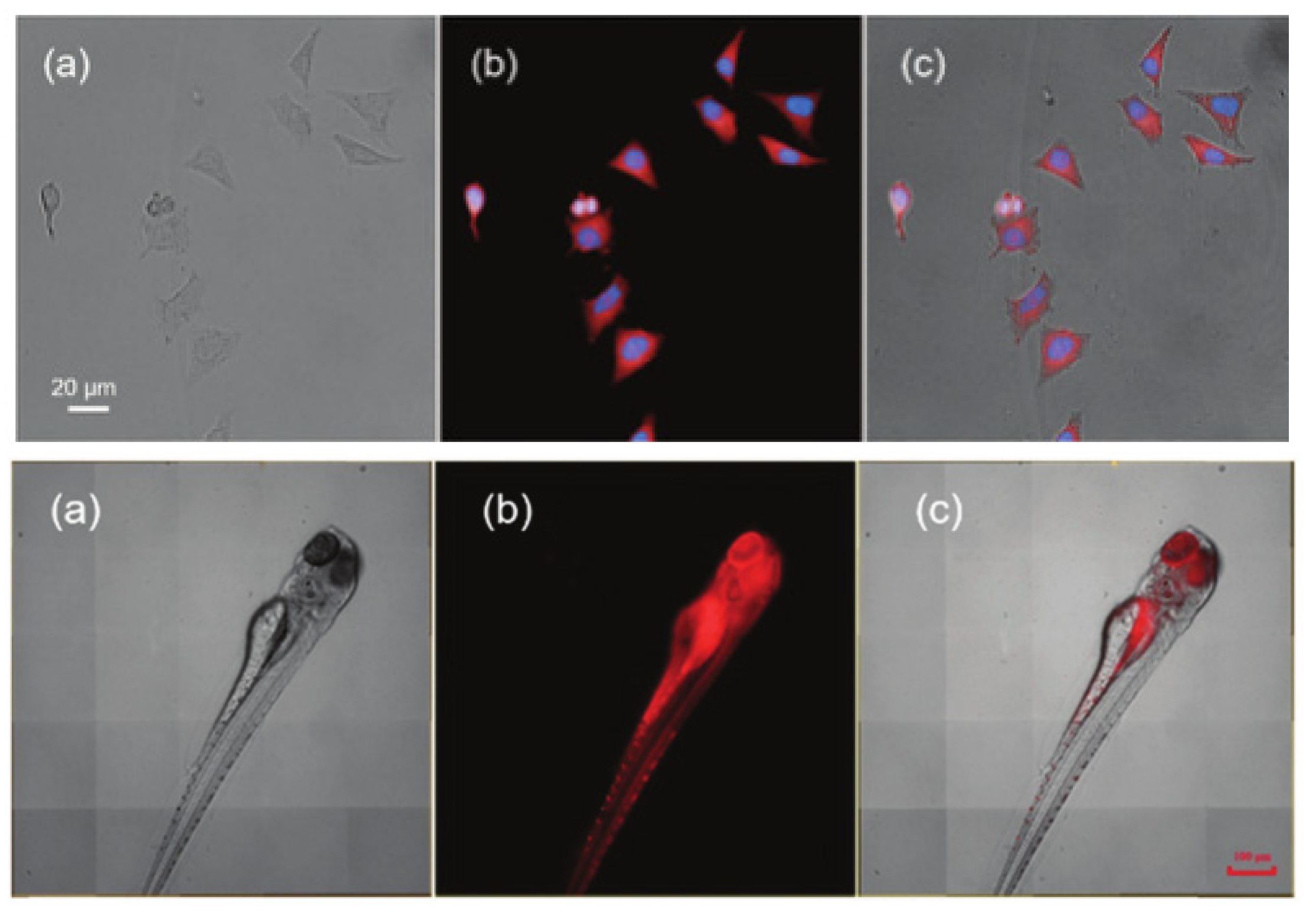

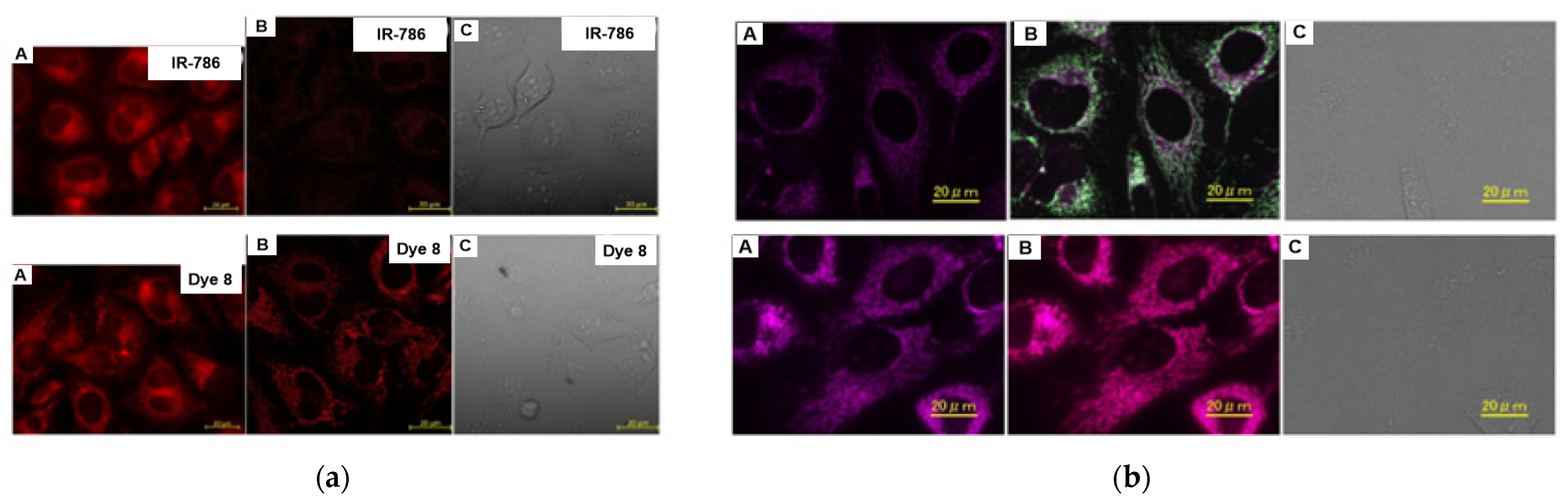

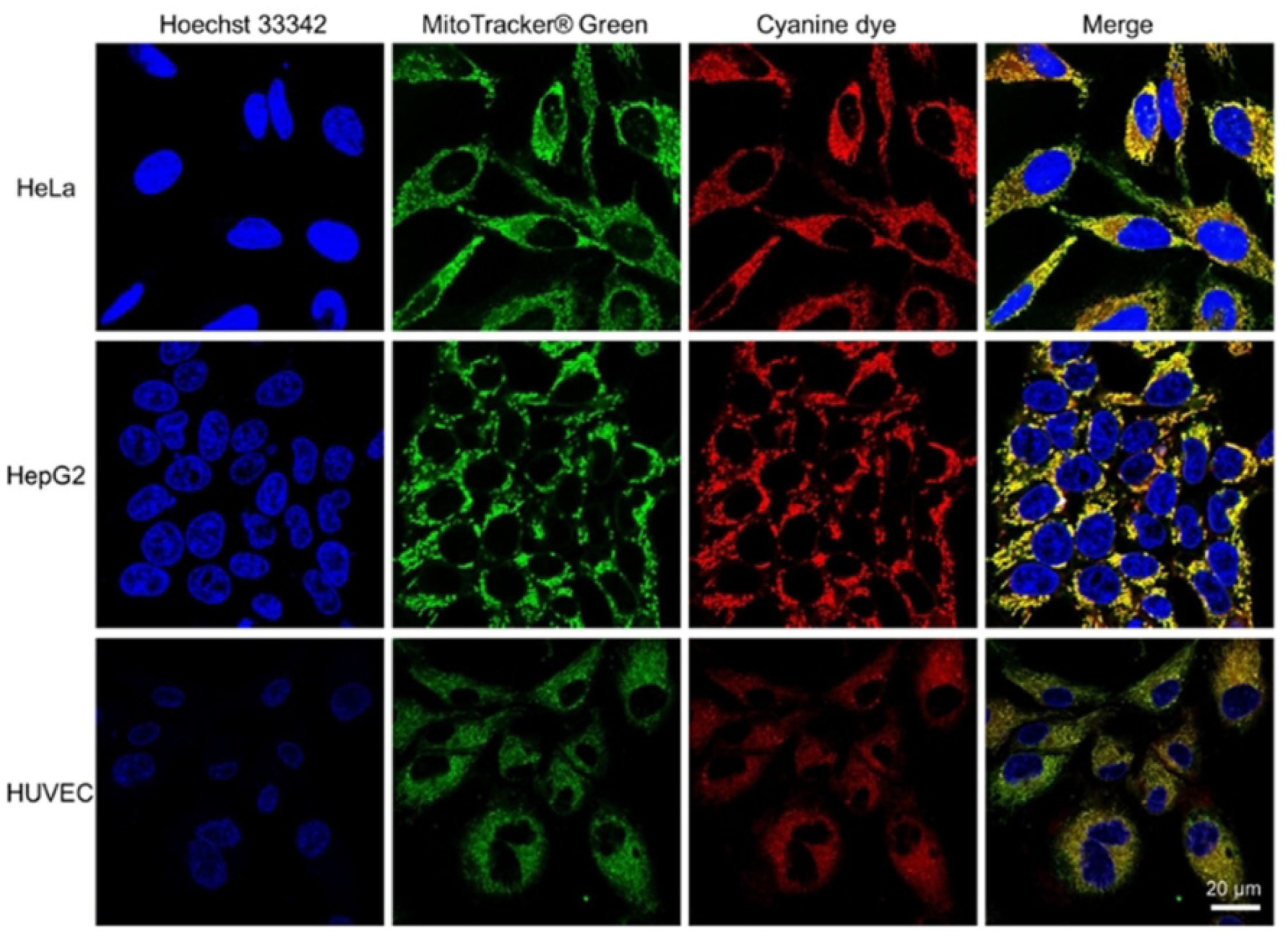
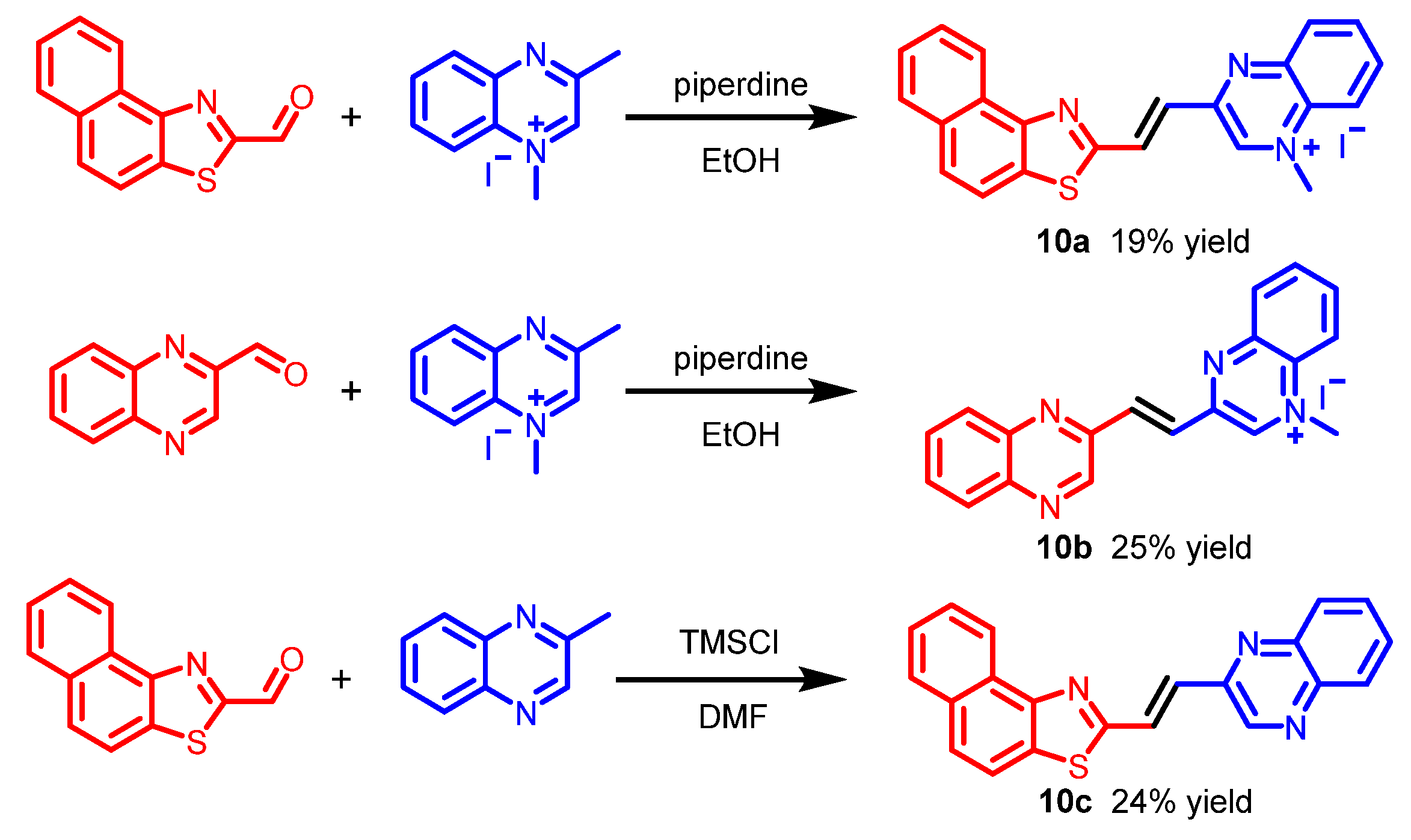
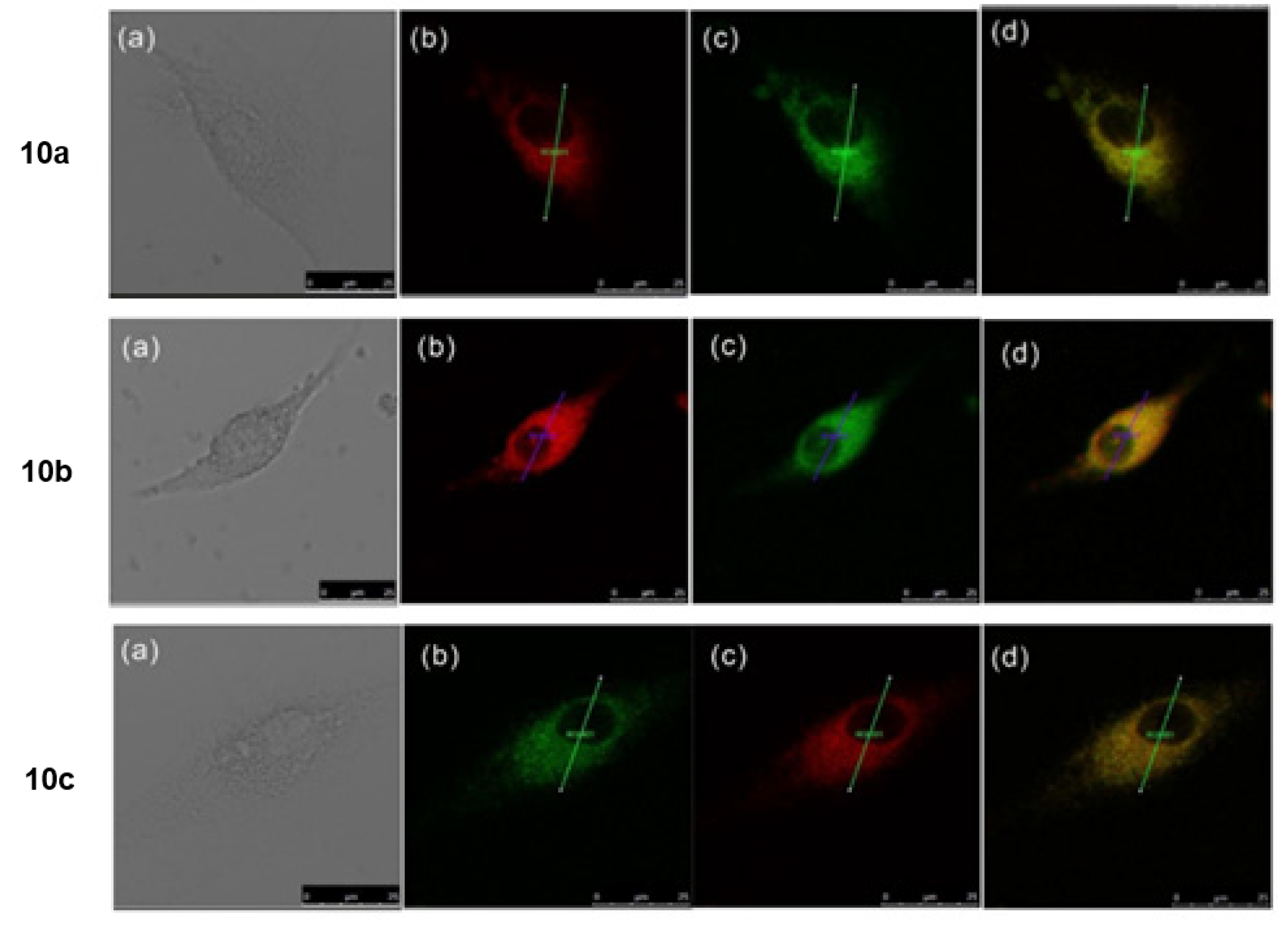

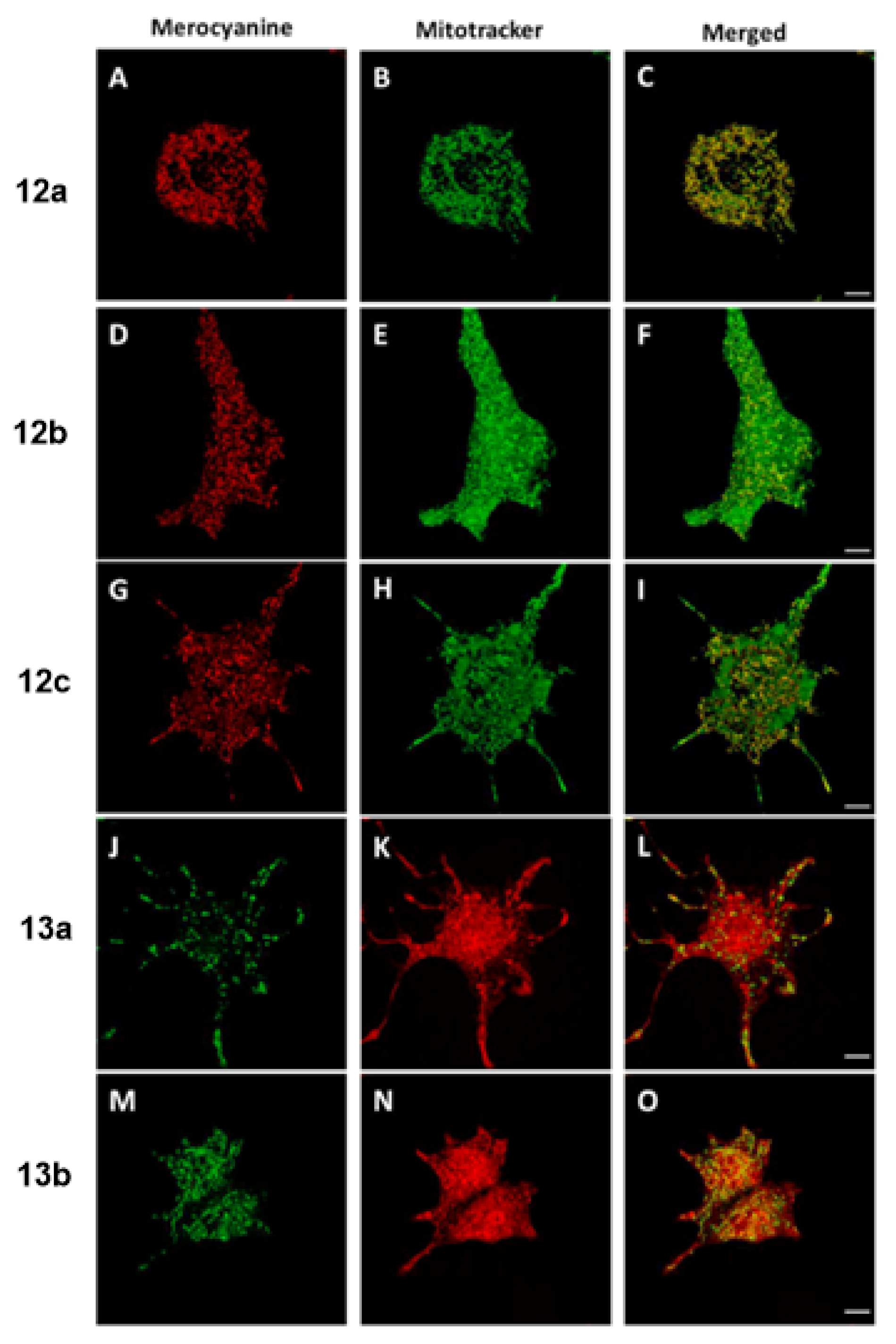
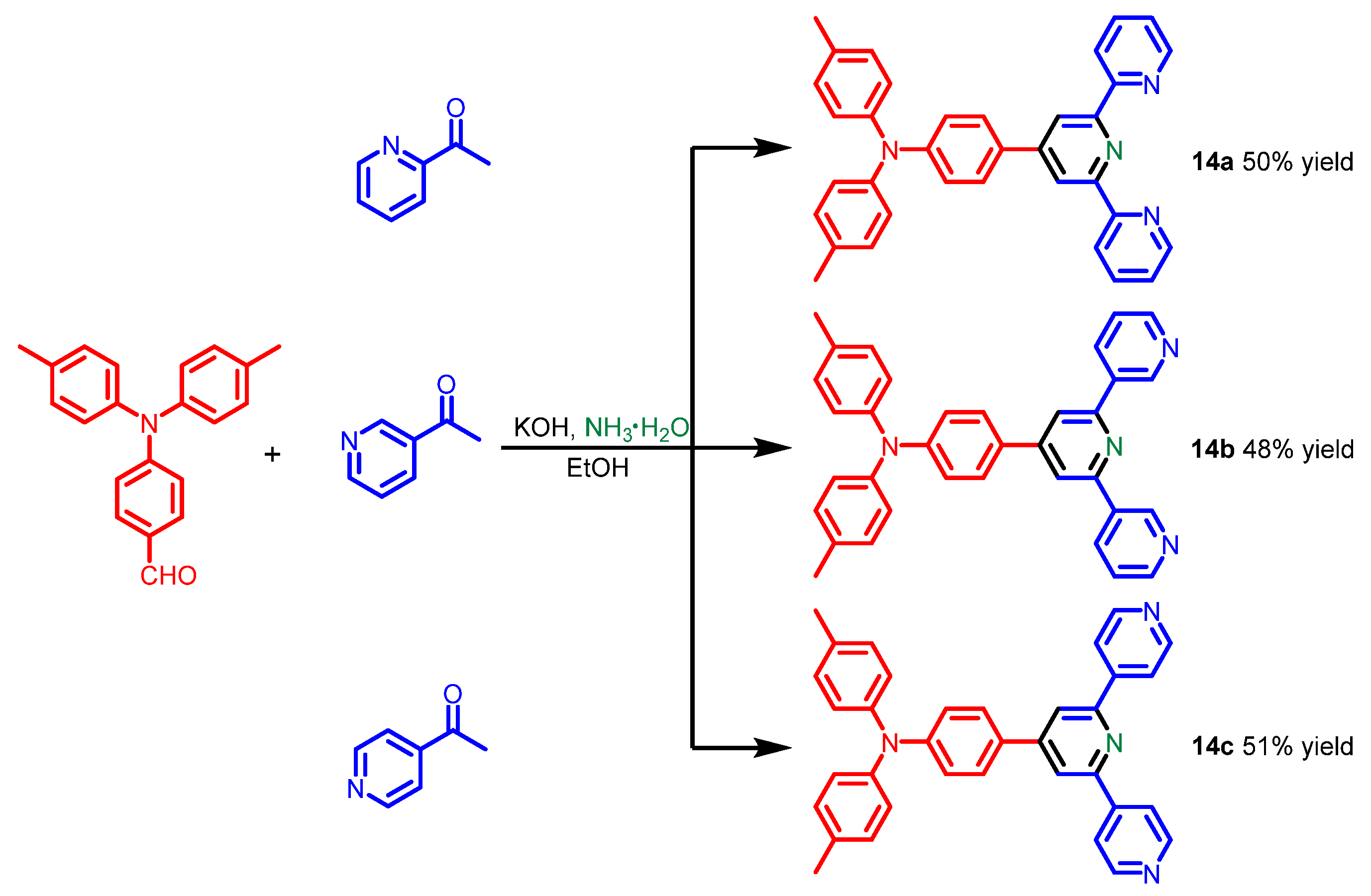








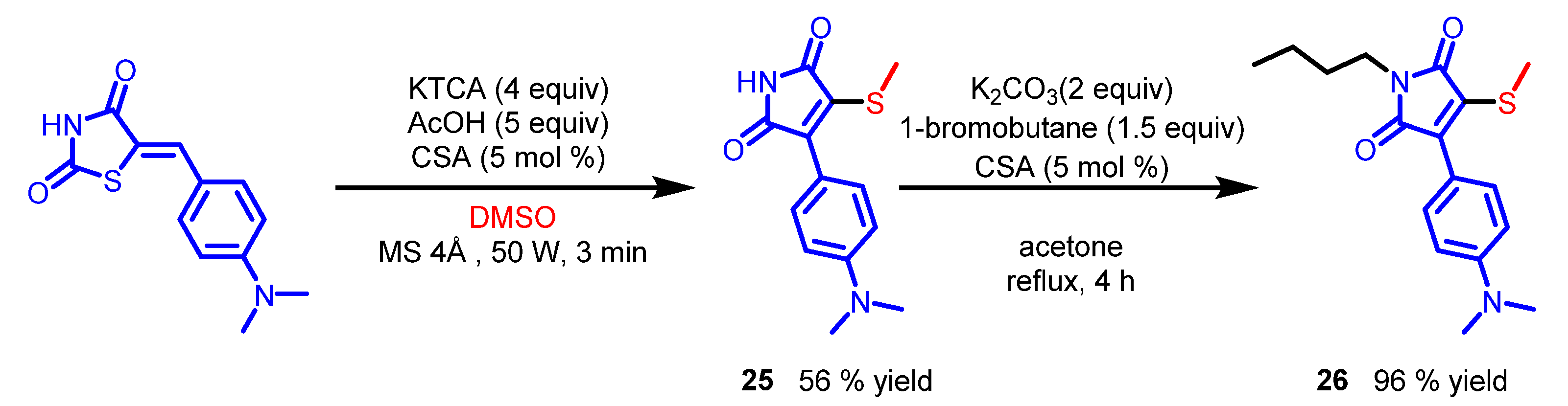
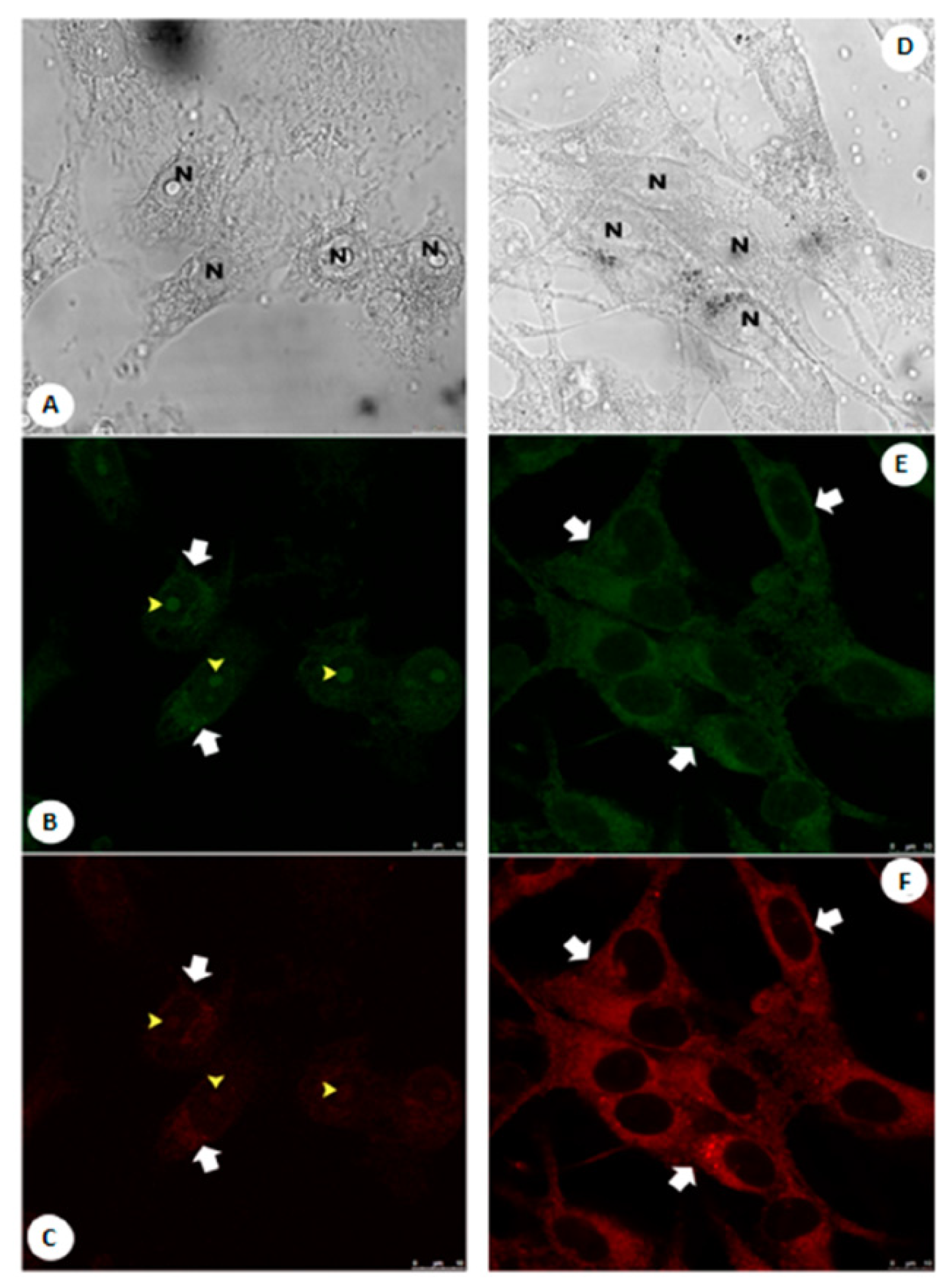
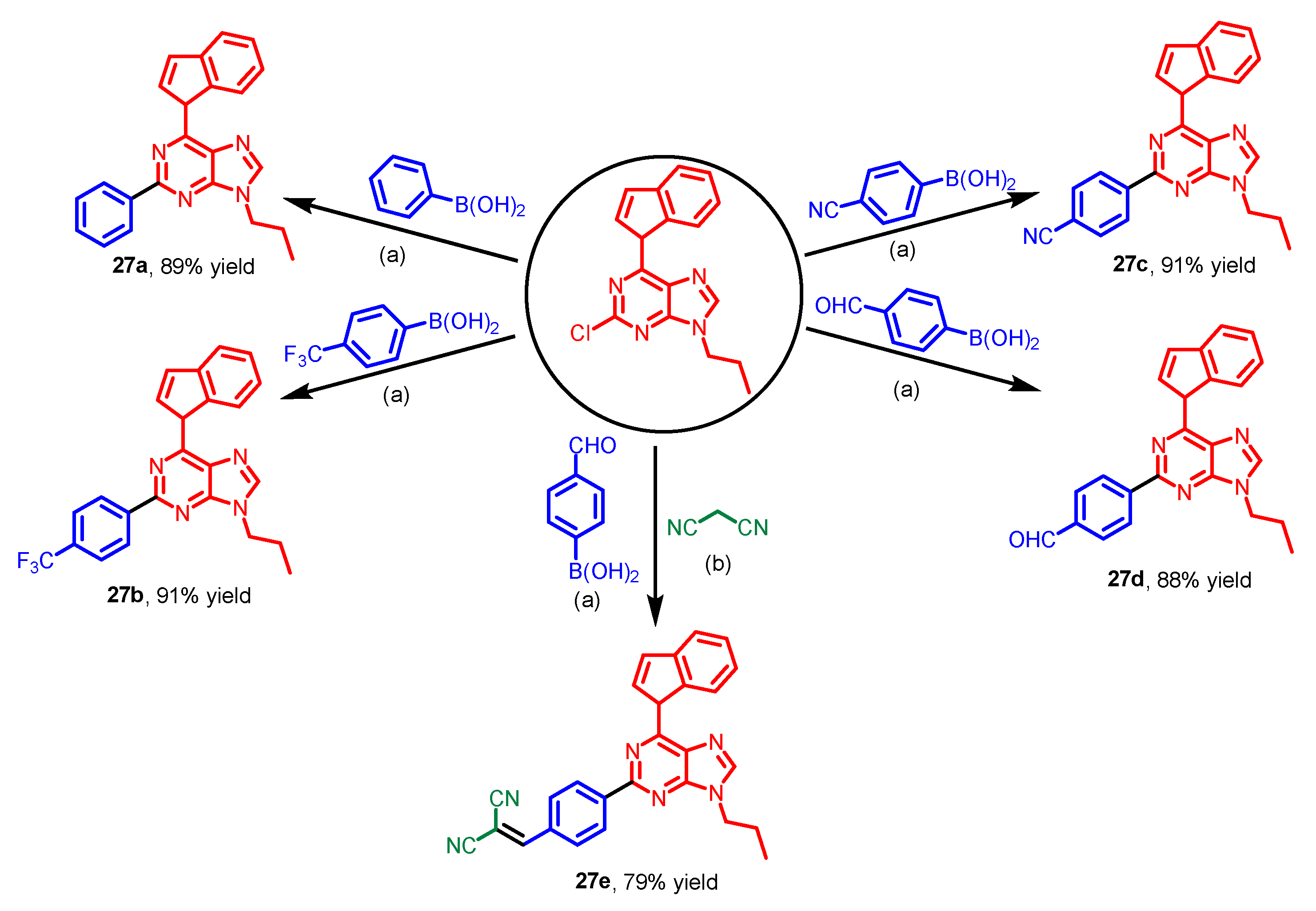

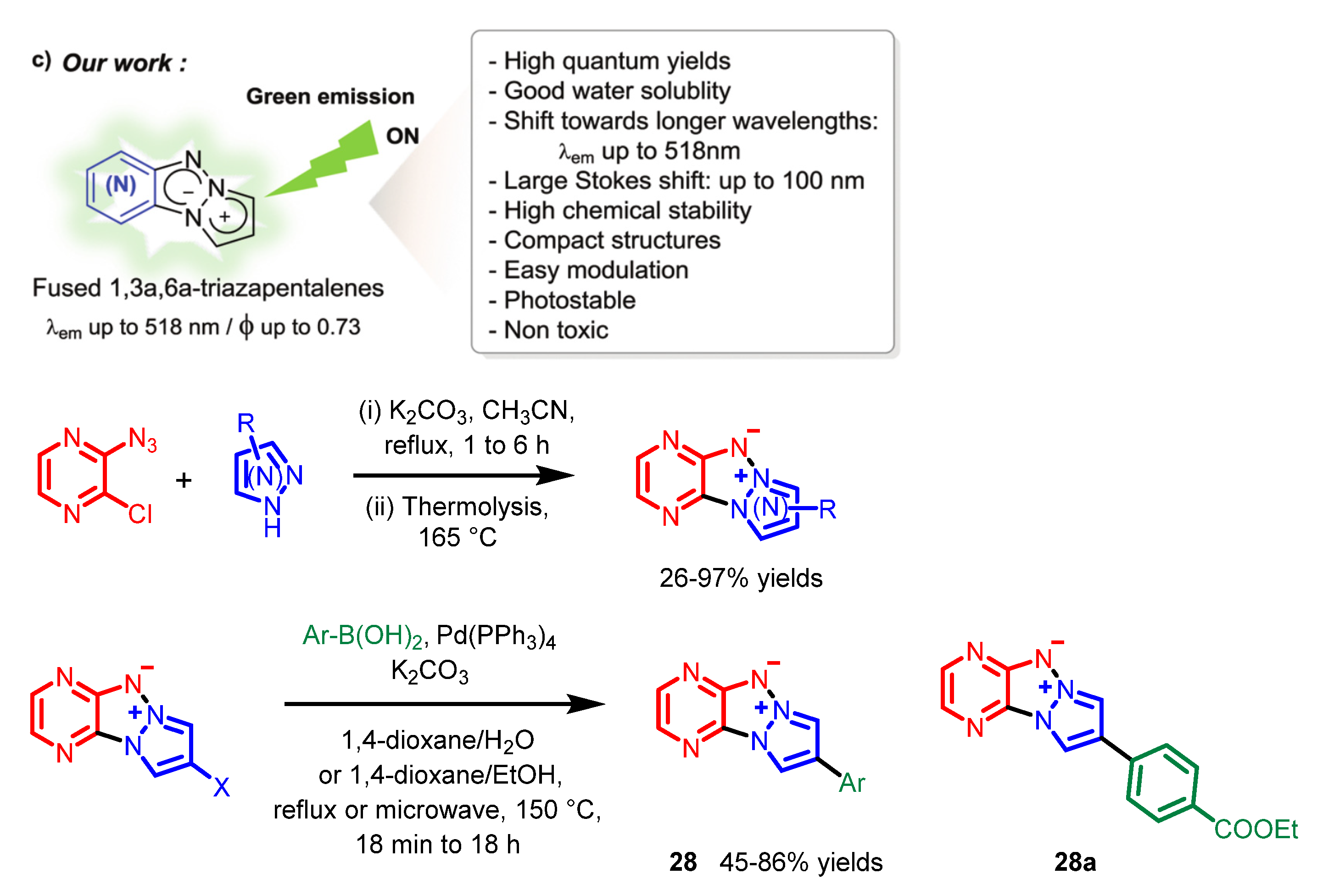
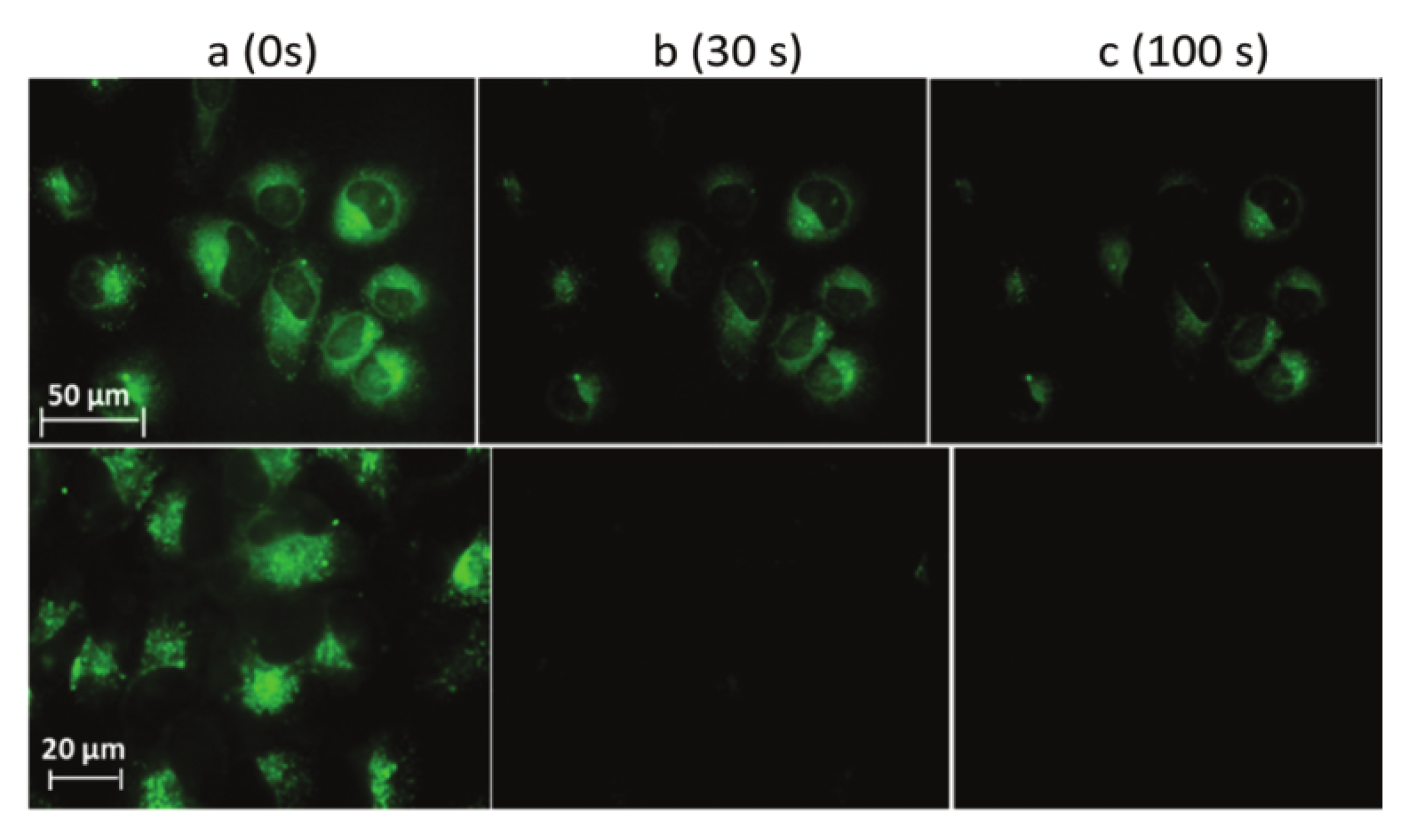



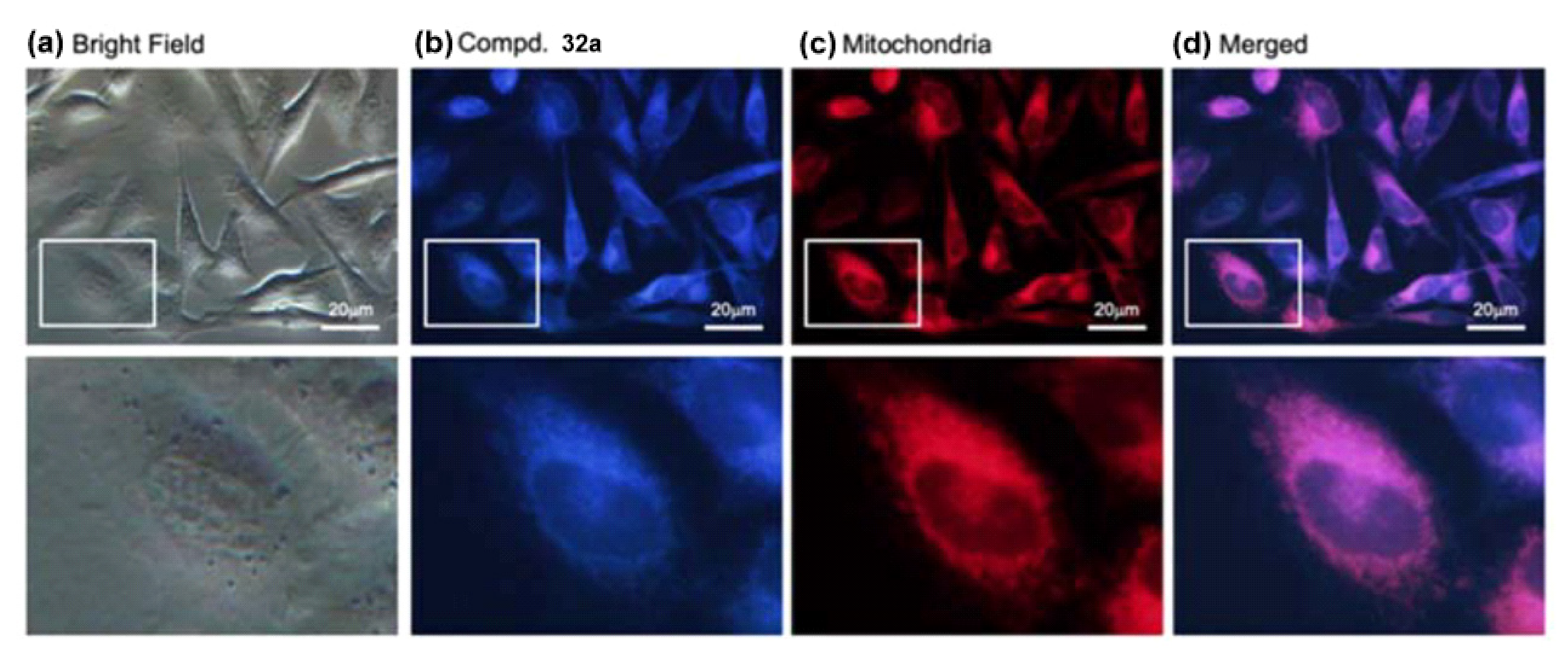
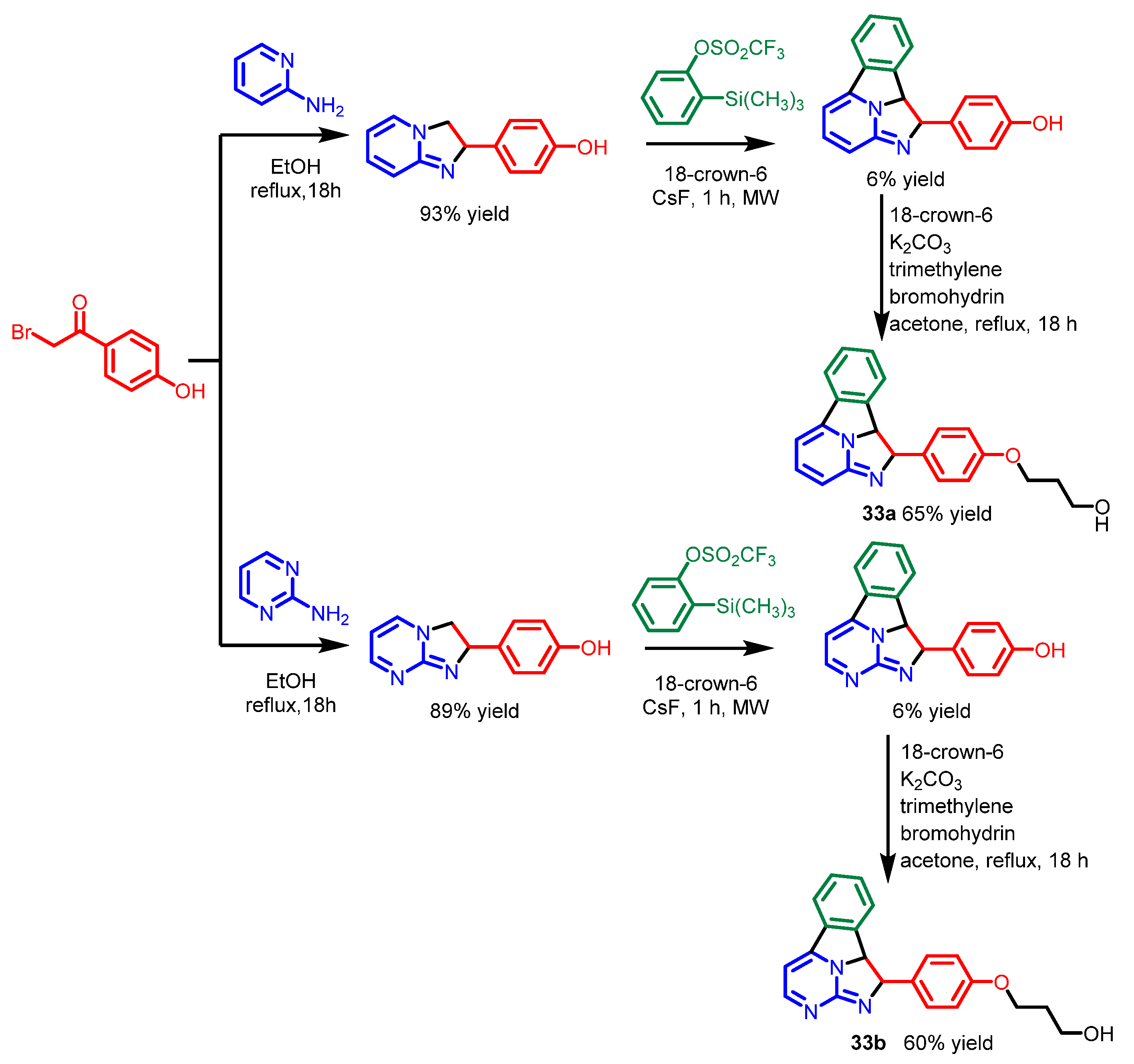
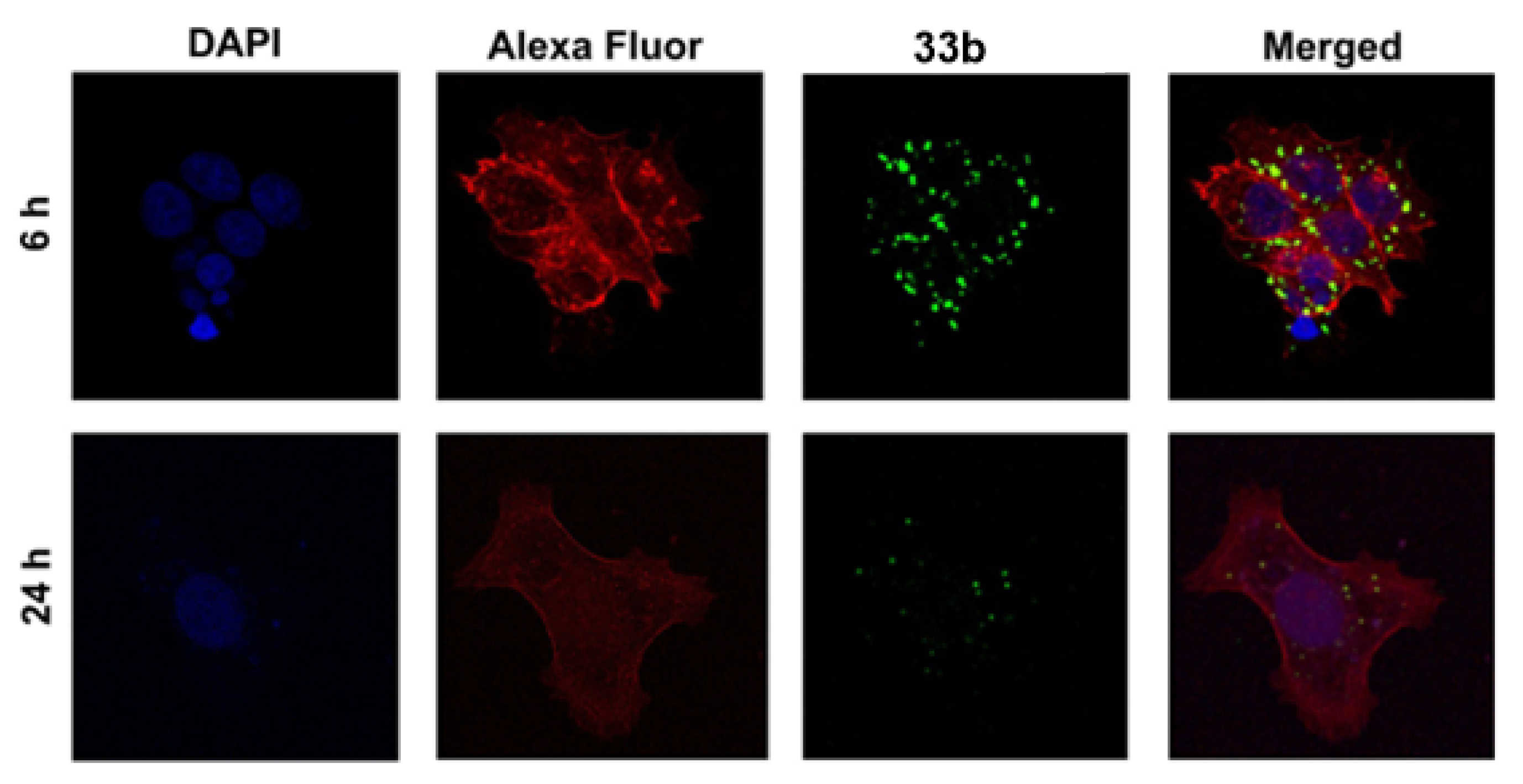
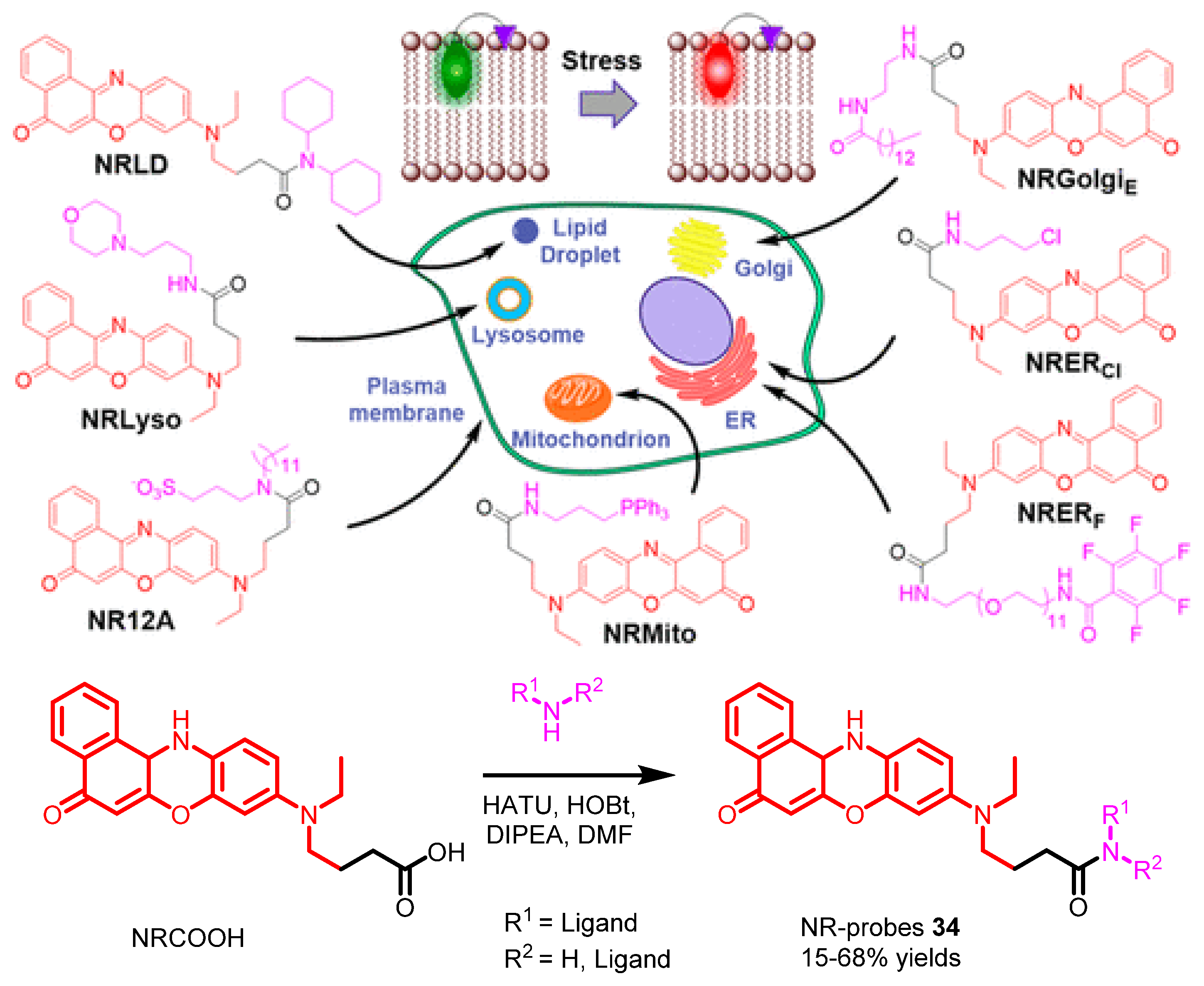

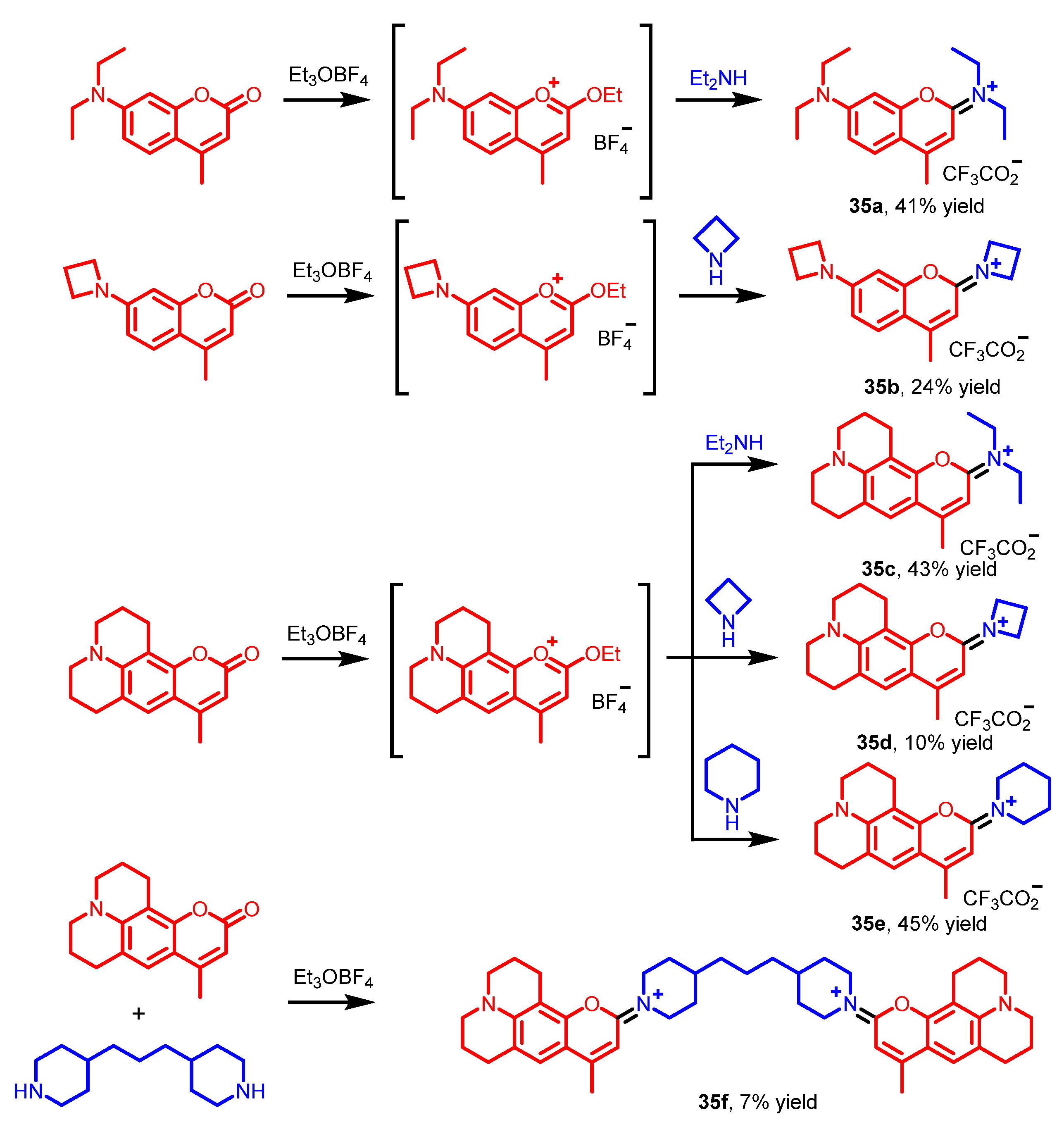

| Scaffolds | Compounds | ||
|---|---|---|---|
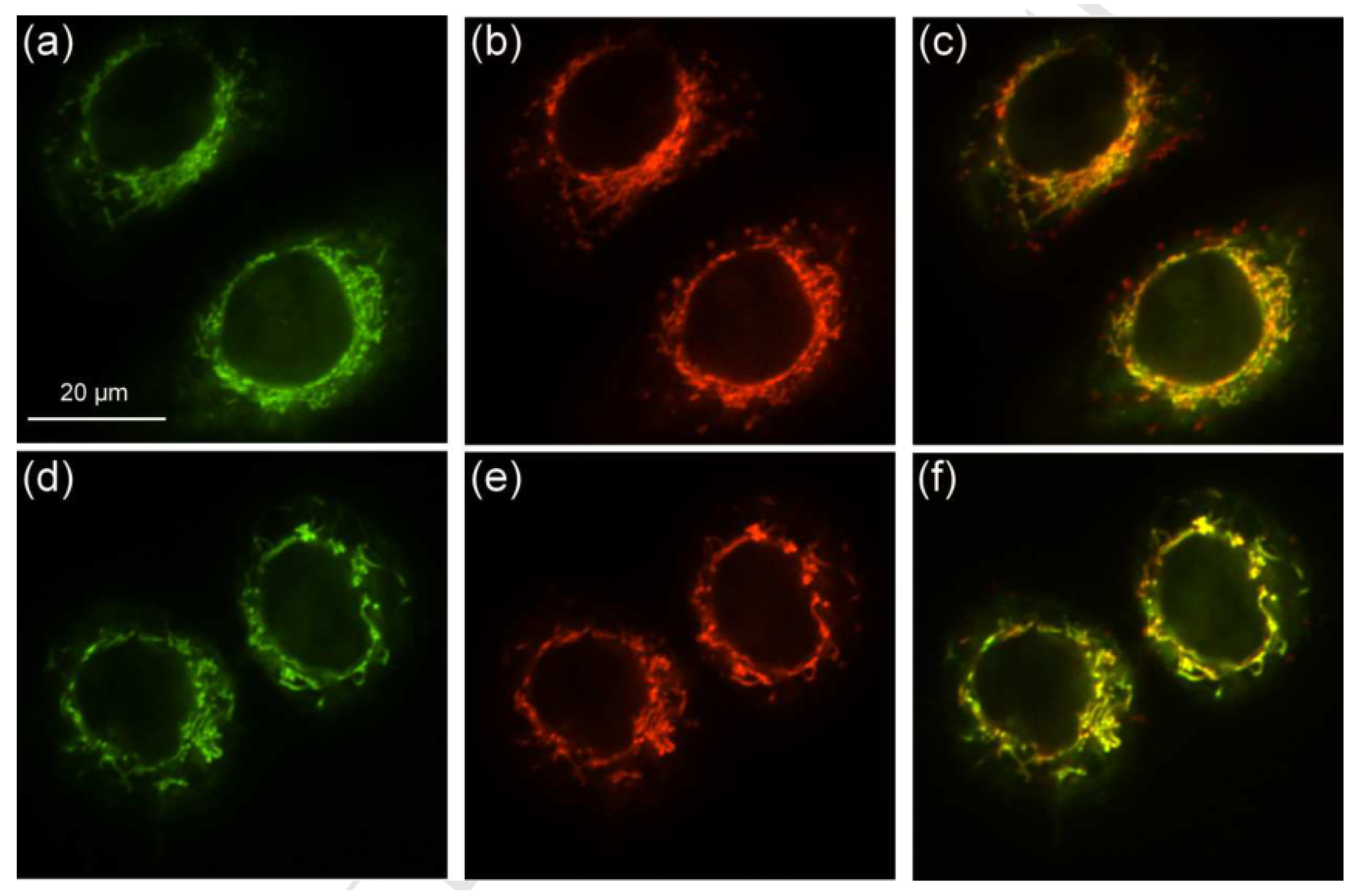
| Boron dipyrromethene (BODIPY) derivates | 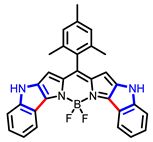 [19] | 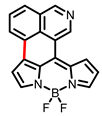 [20] | |
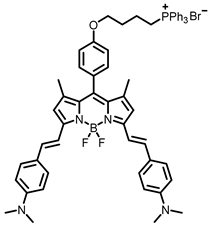 [21] | 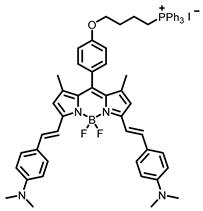 [21] | ||
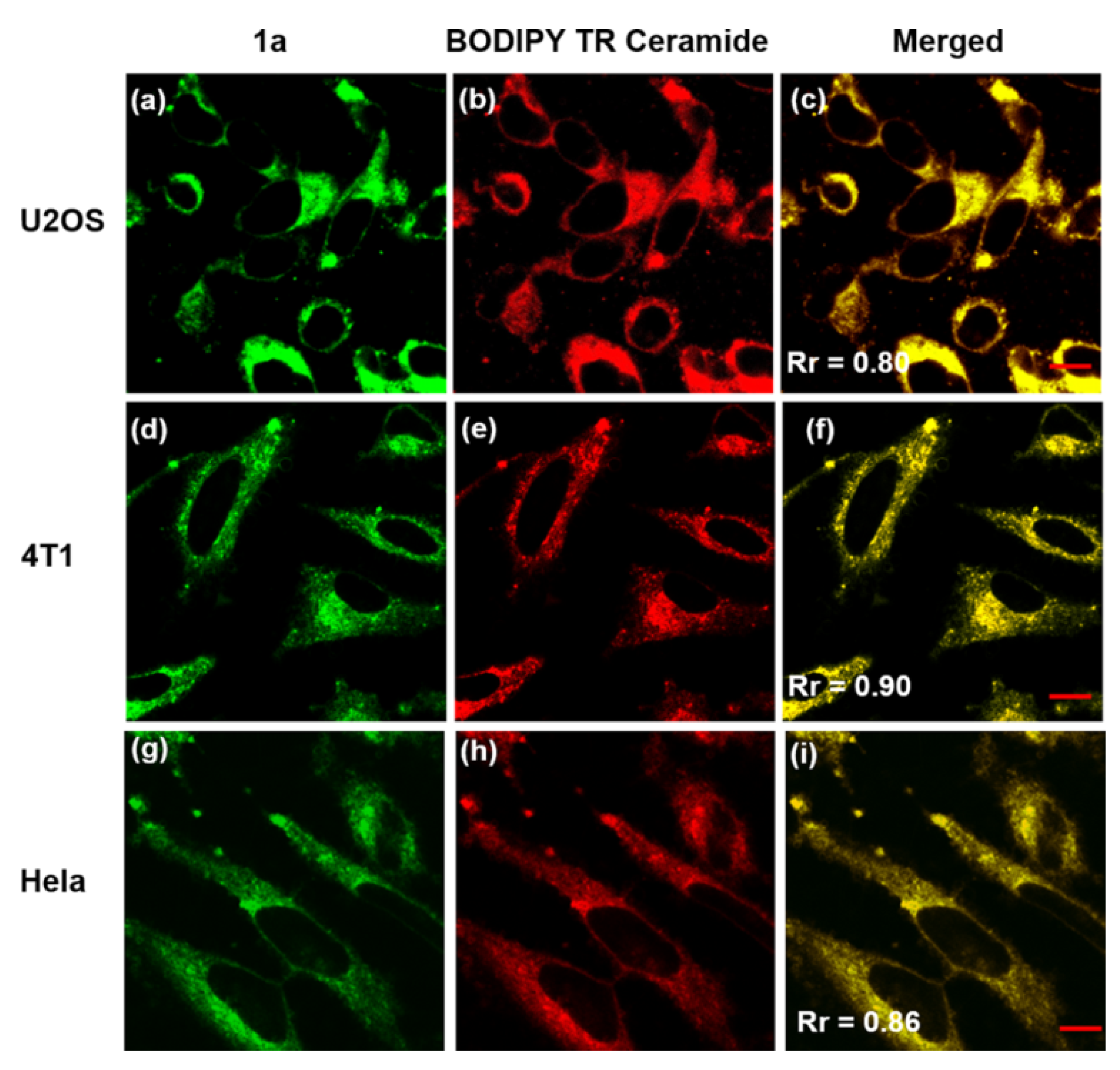
| Other nitrogen-containing boron compounds | 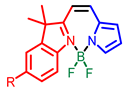 [22] | 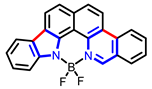 [23] |  [24] |
| Cyanine | 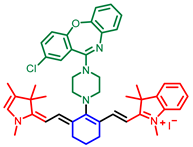 [27] | 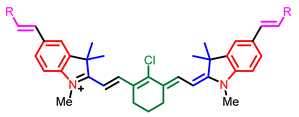 [31] | |
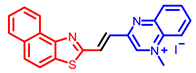 [33] | 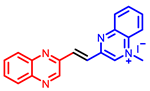 [33] | 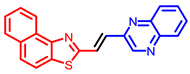 [33] | |
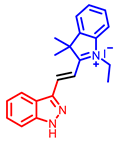 [34] | 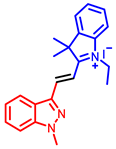 [34] | 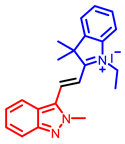 [34] | |
 [34] |  [34] | ||
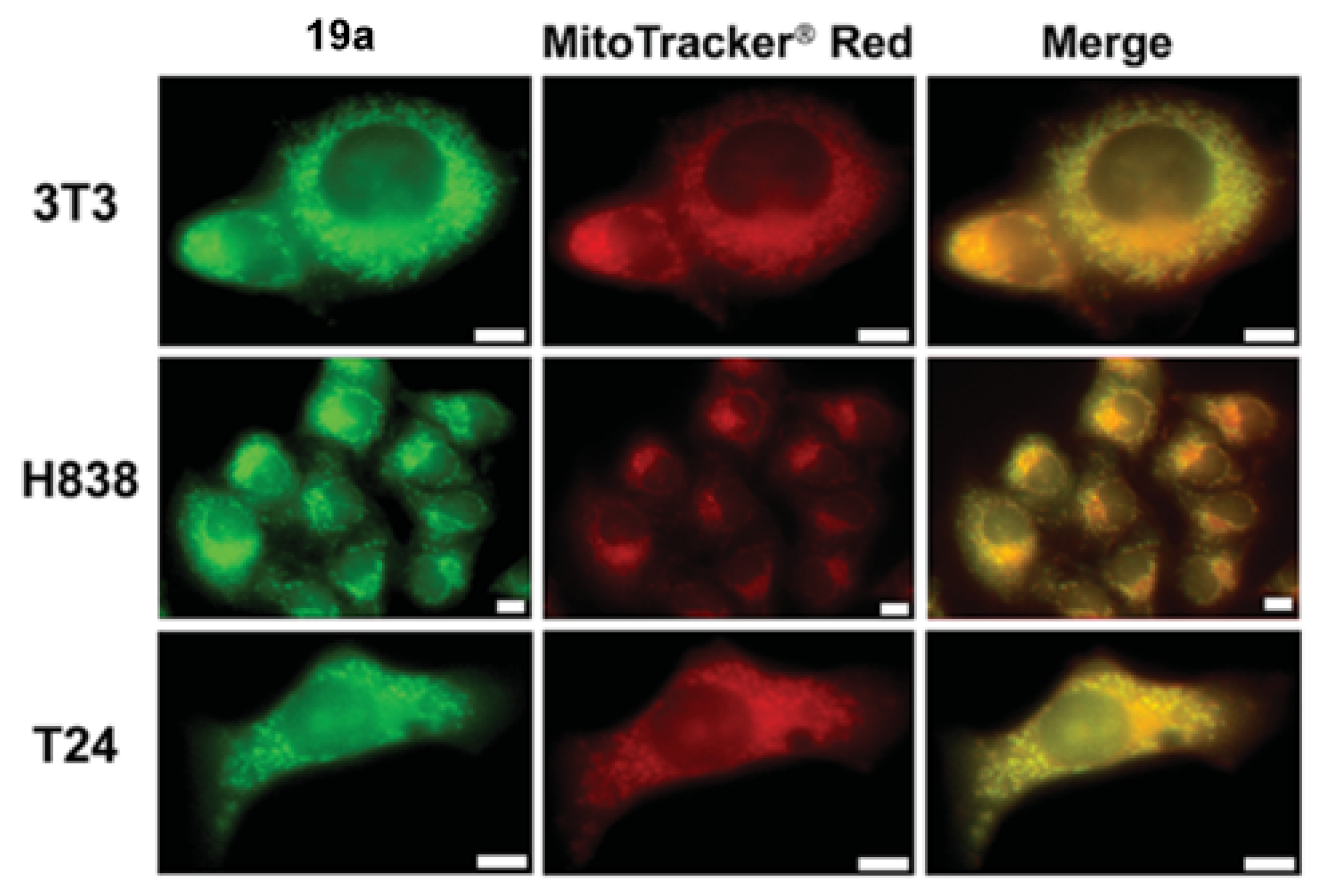
| Pyridine derivatives | 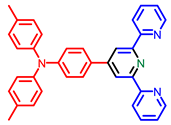 [40] | 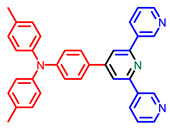 [40] |  [40] |
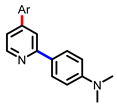 [41] | 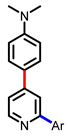 [41] | ||
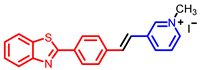 [42] | 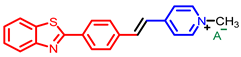 [42] | ||
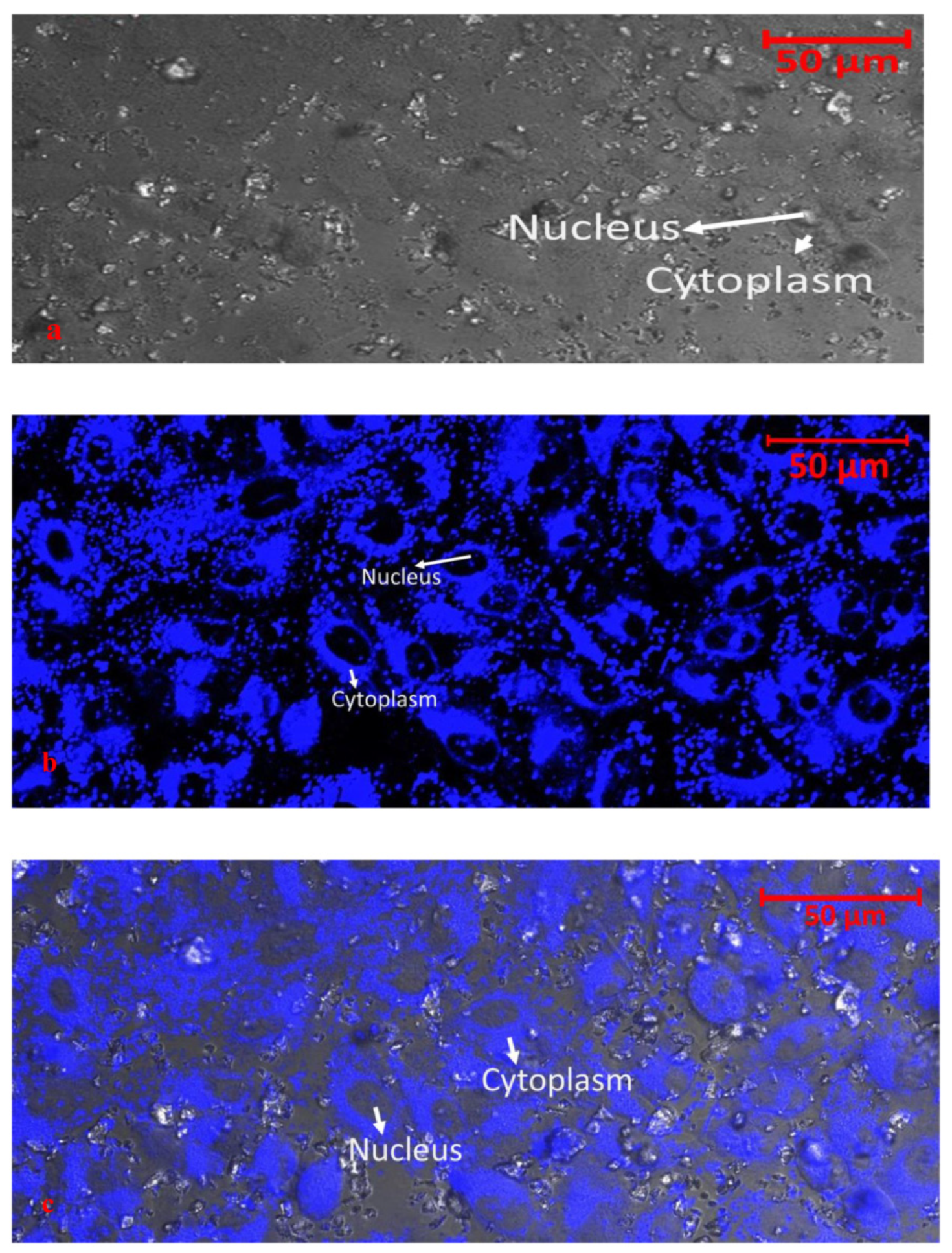
| Indole derivatives |  [46] |  [46] |  [46] |
 [47] | 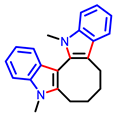 [48] | ||
| Quinoline derivatives |  [53] | 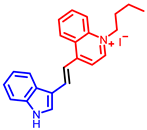 [54] | 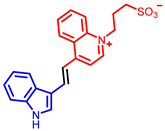 [54] |
 [54] |  [55] | ||
| Maleimide derivatives |  [61] | 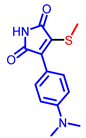 [62] | 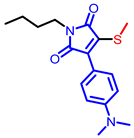 [62] |
| Others | 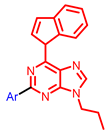 [63] | 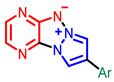 [65] | 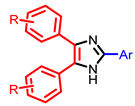 [68] |
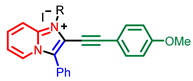 [69] |  [71] | 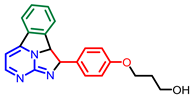 [71] | |
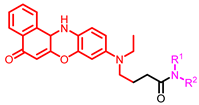 [72] | 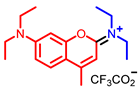 [73] | 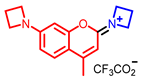 [73] | |
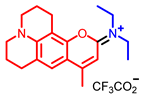 [73] |  [73] | 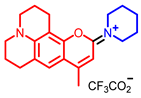 [73] | |
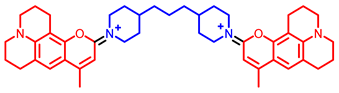 [73] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, T.; Sun, J. Recent Advances in N-Heterocyclic Small Molecules for Synthesis and Application in Direct Fluorescence Cell Imaging. Molecules 2023, 28, 733. https://doi.org/10.3390/molecules28020733
Li Y, Liu T, Sun J. Recent Advances in N-Heterocyclic Small Molecules for Synthesis and Application in Direct Fluorescence Cell Imaging. Molecules. 2023; 28(2):733. https://doi.org/10.3390/molecules28020733
Chicago/Turabian StyleLi, Yanan, Tao Liu, and Jianan Sun. 2023. "Recent Advances in N-Heterocyclic Small Molecules for Synthesis and Application in Direct Fluorescence Cell Imaging" Molecules 28, no. 2: 733. https://doi.org/10.3390/molecules28020733
APA StyleLi, Y., Liu, T., & Sun, J. (2023). Recent Advances in N-Heterocyclic Small Molecules for Synthesis and Application in Direct Fluorescence Cell Imaging. Molecules, 28(2), 733. https://doi.org/10.3390/molecules28020733






