Autoxidation Kinetics of Tetrahydrobiopterin—Giving Quinonoid Dihydrobiopterin the Consideration It Deserves
Abstract
1. Introduction
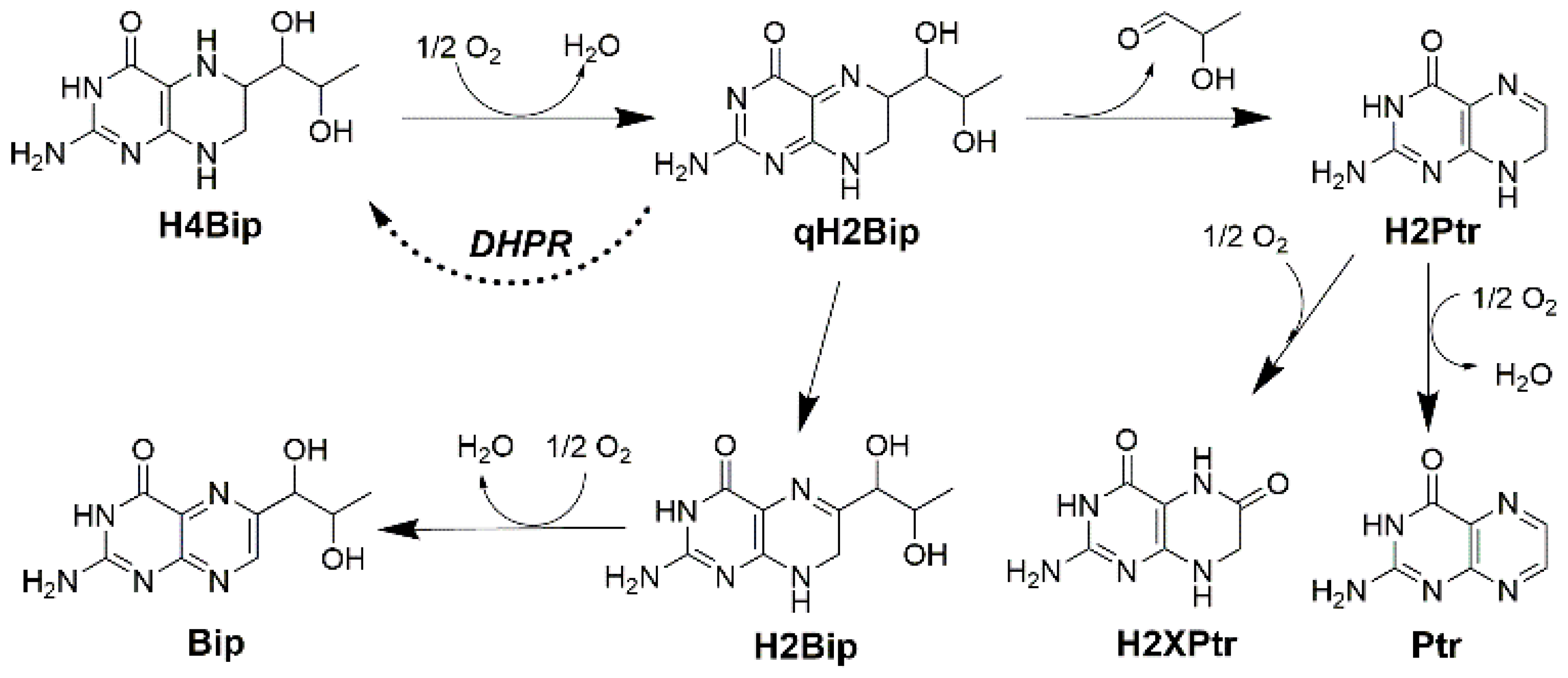
2. Results
2.1. LC-MS/MS Method Development
2.1.1. Optimization of MS/MS Conditions
2.1.2. Optimization of Chromatographic Conditions
2.1.3. Separating the Autoxidation Products
2.2. Autoxidation Kinetics
2.2.1. Selecting pH and Buffers
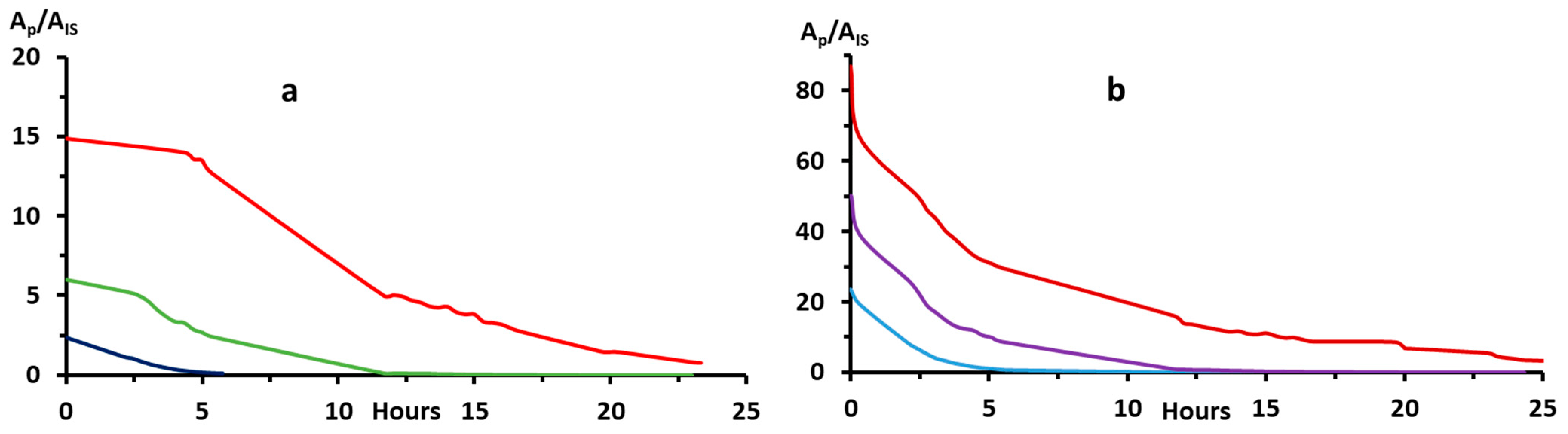
2.2.2. Bip and H2Bip Quantification
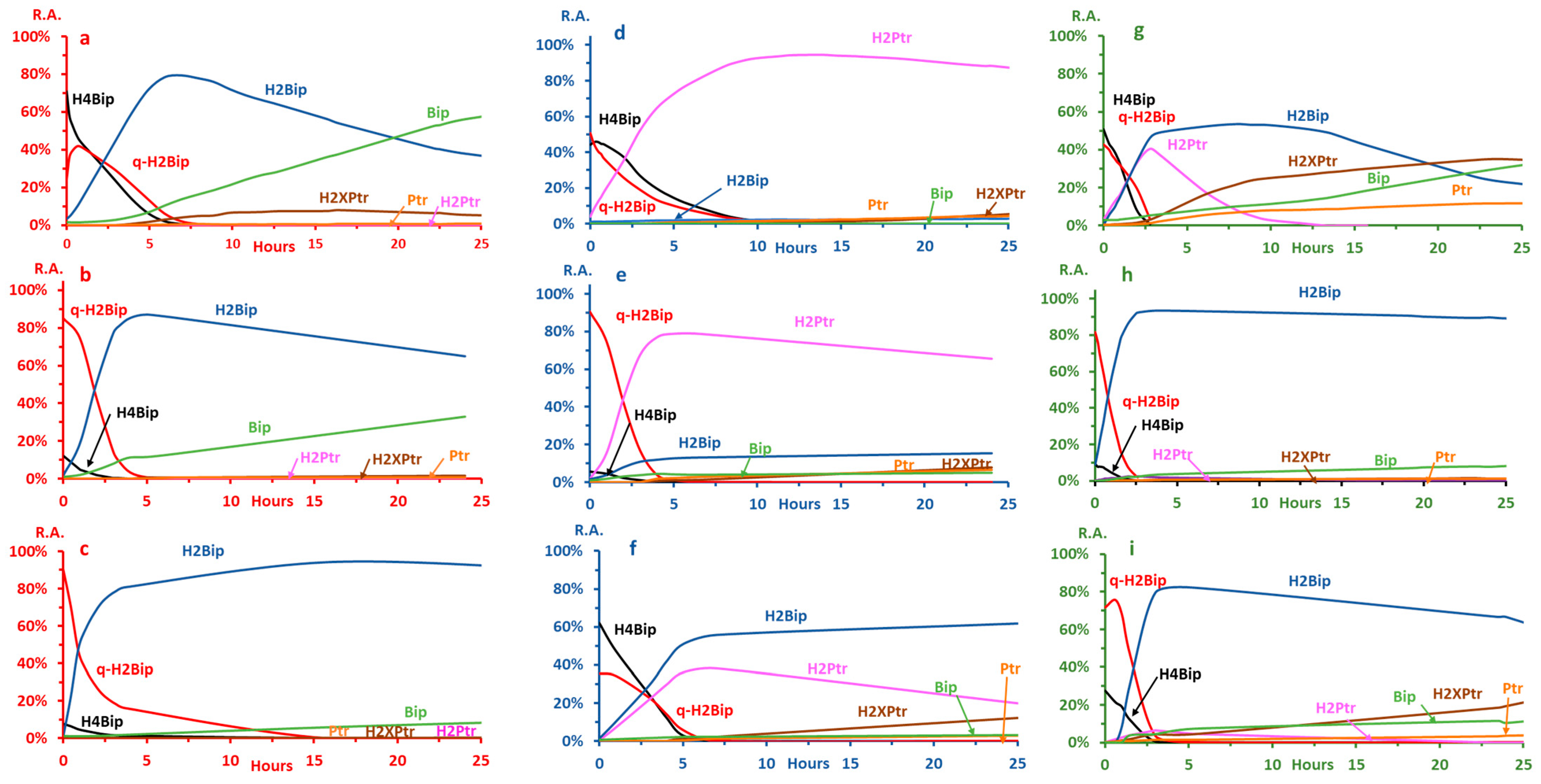
2.2.3. Autoxidation at pH ≤ 3.0
2.2.4. Autoxidation at pH 7.4
2.2.5. Autoxidation at pH 5.4
2.3. Effects of H4Bip Concentration
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Instrumental Analysis
4.2.1. Liquid Chromatography
4.2.2. Mass Spectrometry
4.3. Sample Preparation for Autoxidation Kinetic Studies
4.4. Autoxidation Reaction Order, Rate Constants and Half-Lives
4.5. Bip and H2Bip Quantification
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuda, T. Conversion of phenylalanine into tyrosine in the silkworm larva (Bombyx mori). Nature 1956, 177, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S. The structure of the phenylalanine-hydroxylation cofactor. Proc. Natl. Acad. Sci. USA 1963, 50, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, N.; Blau, N.; Thöny, B. Molecular and metabolic bases of tetrahydrobiopterin (BH4) deficiencies. Mol. Genet. Metab. 2021, 13, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Rilstone, J.J.; Alkhater, R.A.; Minassian, B.A. Brain dopamine-serotonin vesicular transport disease and its treatment. N. Engl. J. Med. 2013, 368, 543–550. [Google Scholar] [CrossRef]
- Telegina, T.A.; Lyudnikova, T.A.; Buglak, A.A.; Vechtomova, Y.L.; Biryukov, M.V.; Demin, V.V.; Kritsky, M.S. Transformation of 6-tetrahydrobiopterin in aqueous solutions under UV-irradiation. J. Photochem. Photobiol. A Chem. 2018, 354, 155–162. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Vechtomova, Y.L.; Kritsky, M.S. Autoxidation and photooxidation of tetrahydrobiopterin: A theoretical study. Free. Radic. Res. 2021, 55, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Cronin, S.J.F.; Seehus, C.; Weidinger, A.; Talbot, S.; Reissig, S.; Seifert, M.; Pierson, Y.; McNeill, E.; Longhi, M.S.; Turnes, B.L.; et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature 2018, 563, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wang, S.; Niu, Z.; Ye, Y.; Gao, L. Newly established LC-MS/MS method for measurement of plasma BH4 as a predictive biomarker for kidney injury in diabetes. Free. Radic. Biol. Med. 2022, 178, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.C.; Scrimgeour, K.G. Rearrangement of Quinonoid Dihydropteridines to 7,8-Dihydropteridines. Can. J. Biochem. 1970, 48, 278–287. [Google Scholar] [CrossRef]
- Kaufman, S.; Holtzman, N.A.; Milstien, S.; Butler, I.J.; Krumholz, A. Phenylketonuria Due to a Deficiency of Dihydropteridine Reductase. N. Engl. J. Med. 1975, 293, 785–790. [Google Scholar] [CrossRef]
- Koslow, S.H.; Butler, I.J. Biogenic amine synthesis defect in dihydropteridine reductase deficiency. Science 1977, 198, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Brennenstuhl, H.; Jung-Klawitter, S.; Assmann, B.; Opladen, T. Inherited Disorders of Neurotransmitters: Classification and Practical Approaches for Diagnosis and Treatment. Neuropediatrics 2019, 50, 002–014. [Google Scholar] [CrossRef] [PubMed]
- Jung-Klawitter, S.; Kuseyri Hübschmann, O. Analysis of Catecholamines and Pterins in Inborn Errors of Monoamine Neurotransmitter Metabolism—From Past to Future. Cells 2019, 8, 867. [Google Scholar] [CrossRef] [PubMed]
- International Working Group on Neurotransmitter Related Disorders. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies. Orphanet. J. Rare Dis. 2020, 15, 126. [Google Scholar] [CrossRef]
- Fukushima, T.; Nixon, J.C. Analysis of Reduced Forms of Biopterin in Biological Tissues and Fluids. Anal. Biochem. 1980, 102, 176–188. [Google Scholar] [CrossRef]
- Howells, D.W.; Hyland, K. Direct analysis of tetrahydrobiopterin in cerebrospinal fluid by high-performance liquid chromatography with redox electrochemistry: Prevention of autoxidation during storage and analysis. Clin. Chim. Acta 1987, 167, 23–30. [Google Scholar] [CrossRef]
- Guibal, P.; Lo, A.; Maitre, P.; Moussa, F. Pterin determination in cerebrospinal fluid: State of the art. Pteridines 2017, 28, 83–89. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Randles, D.; Taguchi, H. Peroxidase Catalysed Aerobic Degradation of 5,6,7,8-Tetrahydrobiopterin at Physiological PH. Eur. J. Biochem. 1983, 135, 393–403. [Google Scholar] [CrossRef]
- Benkovic, S.J.; Sammons, D.; Armarego, W.L.F.; Waring, P.; Inners, R. Tautomeric Nature of Quinonoid 6,7-Dimethyl-7,8-Dihydro-6H-Pterin in Aqueous Solution: A Nitrogen-15 NMR Study. J. Am. Chem. Soc. 1985, 107, 3706–3712. [Google Scholar] [CrossRef]
- Davis, M.D.; Kaufman, S.; Milstien, S. The Auto-Oxidation of Tetrahydrobiopterin. Eur. J. Biochem. 1988, 173, 345–351. [Google Scholar] [CrossRef]
- Kirsch, M.; Korth, H.-G.; Stenert, V.; Sustmann, R.; de Groot, H. The Autoxidation of Tetrahydrobiopterin Revisited: Proof of Superoxide Formation from Reaction Of Tetrahydrobiopterin With Molecular Oxygen. J. Biol. Chem. 2003, 278, 24481–24490. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.C.; Vonderschmitt, D.J.; Scrimgeour, K.G. Mechanism of Oxidation of Tetrahydropterins. Can. J. Biochem. 2011, 50, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Murata, S.; Sugimoto, T. Quinonoid dihydrobiopterin, an important metabolic intermediate of biopterin cofactor in the aromatic hydroxylation of amino acids. Tetrahedron Lett. 1986, 27, 585–588. [Google Scholar] [CrossRef]
- Haavik, J.; Flatmark, T. Isolation and characterization of tetrahydropterin oxidation products generated in the tyrosine 3-monooxygenase (tyrosine hydroxylase) reaction. Eur. J. Biochem. 1987, 168, 21–26. [Google Scholar] [CrossRef]
- Heales, S.; Hyland, K. Determination of Quinonoid Dihydrobiopterin by High-Performance Liquid Chromatography and Electrochemical Detection. J. Chromatogr. B Biomed. Sci. Appl. 1989, 494, 77–85. [Google Scholar] [CrossRef]
- Pearson, T.S.; Gupta, N.; San Sebastian, W.; Imamura-Ching, J.; Viehoever, A.; Grijalvo-Perez, A.; Fay, A.J.; Seth, N.; Lundy, S.M.; Seo, Y. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 2021, 12, 4251. [Google Scholar] [CrossRef]
- Batllori, M.; Molero-Luis, M.; Ormazabal, A.; Casado, M.; Sierra, C.; García-Cazorla, A.; Kurian, M.; Pope, S.; Heales, S.J.; Artuch, R. Analysis of Human Cerebrospinal Fluid Monoamines and Their Cofactors by HPLC. Nat. Protoc. 2017, 12, 2359–2366. [Google Scholar] [CrossRef]
- Fismen, L.; Eide, T.; Djurhuus, R.; Svardal, A.M. Simultaneous quantification of tetrahydrobiopterin, dihydrobiopterin, and biopterin by liquid chromatography coupled to electrospray tandem mass spectrometry. Anal. Biochem. 2012, 430, 163–170. [Google Scholar] [CrossRef]
- Allegri, G.; Costa Netto, H.J.B.; Ferreira Gomes, L.N.L.; Costa de Oliveira, M.L.; Scalco, F.B.; de Aquino Neto, F.R. Determination of Six Pterins in Urine by LC–MS/MS. Bioanalysis 2012, 4, 1739–1746. [Google Scholar] [CrossRef]
- Guibal, P.; Lévêque, N.; Doummar, D.; Giraud, N.; Roze, E.; Rodriguez, D.; Couderc, R.; Billette De Villemeur, T.; Moussa, F. Simultaneous Determination of All Forms of Biopterin and Neopterin in Cerebrospinal Fluid. ACS Chem. Neurosci. 2014, 5, 533–541. [Google Scholar] [CrossRef]
- Arning, E.; Bottiglieri, T. LC-MS/MS Analysis of Cerebrospinal Fluid Metabolites in the Pterin Biosynthetic Pathway. In JIMD Reports; Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V., Eds.; Springer: Berlin, Heidelberg, Germany, 2016; pp. 1–9. [Google Scholar] [CrossRef]
- Burton, C.; Shi, H.; Ma, Y. Development of a high-performance liquid chromatography–tandem mass spectrometry urinary pterinomics workflow. Anal. Chim. Acta 2016, 927, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, Y. Chromatographic behavior of 12 polar pteridines in hydrophilic interaction chromatography using five different HILIC columns coupled with tandem mass spectrometry. Talanta 2016, 150, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Guibal, P.; Doummar, D.; Rodriguez, D.; Hautem, J.-Y.; Couderc, R.; Billette De Villemeur, T.; Roze, E.; Chaminade, P.; Moussa, F. Single-Step Rapid Diagnosis of Dopamine and Serotonin Metabolism Disorders. ACS Omega 2017, 2, 5962–5972. [Google Scholar] [CrossRef] [PubMed]
- Galla, Z.; Rajda, C.; Rácz, G.; Grecsó, N.; Baráth, Á.; Vécsei, L.; Bereczki, C.; Monostori, P. Simultaneous determination of 30 neurologically and metabolically important molecules: A sensitive and selective way to measure tyrosine and tryptophan pathway metabolites and other biomarkers in human serum and cerebrospinal fluid. J. Chromatogr. A 2021, 1635, 461775. [Google Scholar] [CrossRef] [PubMed]
- Galla, Z.; Rácz, G.; Grecsó, N.; Baráth, Á.; Kósa, M.; Bereczki, C.; Monostori, P. Improved LC-MS/MS method for the determination of 42 neurologically and metabolically important molecules in urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 25, 122846. [Google Scholar] [CrossRef]
- Alhajji, E.; Boulghobra, A.; Bonose, M.; Berthias, F.; Moussa, F.; Maître, P. Multianalytical Approach for Deciphering the Specific MS/MS Transition and Overcoming the Challenge of the Separation of a Transient Intermediate, Quinonoid Dihydrobiopterin. Anal. Chem. 2022, 94, 12578–12585. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.B.; Nazarov, E.G.; Londry, F.; Vouros, P.; Covey, T.R. Differential mobility spectrometry/mass spectrometry history, theory, design optimization, simulations, and applications. Mass Spectrom. Rev. 2016, 35, 687–737. [Google Scholar] [CrossRef]
- Baird, M.A.; Shliaha, P.V.; Anderson, G.A.; Moskovets, E.; Laiko, V.; Makarov, A.A.; Jensen, O.N.; Shvartsburg, A.A. High-resolution differential ion mobility separations/orbitrap mass spectrometry without buffer gas limitations. Anal. Chem. 2019, 91, 6918–6925. [Google Scholar] [CrossRef]
- Sun, S.; Liu, W.; Yang, J.; Wang, H.; Qian, K. Nanoparticle-Assisted Cation Adduction and Fragmentation of Small Metabolites. Angew. Chem. Int. Ed. 2021, 60, 11310–11317. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Liu, C.; Pei, C.; Shu, W.; Zhang, C.; Liu, L.; Zhou, L.; Wan, J. Design of Multi-Shelled Hollow Cr2O3 Spheres for Metabolic Fingerprinting. Angew. Chem. Int. Ed. 2021, 60, 12504–12512. [Google Scholar] [CrossRef]
- Morand, K.; Talbo, G.; Mann, M. Oxidation of peptides during electrospray ionization. Rapid Commun. Mass Spectrom. 1993, 7, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-F.; Huang, H.-Q.; Gao, L.; Wang, S.-T.; Li, Y. A novel and reliable method for tetrahydrobiopterin quantification: Benzoyl chloride derivatization coupled with liquid chromatography-tandem mass spectrometry analysis. Free. Radic. Biol. Med. 2018, 118, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms; McGraw-Hill Primis Custom: New York, NY, USA, 2002. [Google Scholar]
- McNaught, A.D.; Wilkinson, A.; International Union of Pure and Applied Chemistry. Compendium of Chemical Terminology; IUPAC Recommendations; Blackwell Science: Oxford, UK, 1997. [Google Scholar]

| MRM Transitions | ||||||
|---|---|---|---|---|---|---|
| Analyte | Retention Time (min) | Parent Ion (m/z) | Fragment Ions (m/z) | Q1 Potential (V) | Collision Energy (V) | Q3 Potential (V) |
| H4Bip | 0.93 | 242 | 166 | −27.0 | −20.0 | −30.0 |
| 206 | −13.0 | −18.0 | −21.0 | |||
| qH2Bip | 1.05 | 240 | 166 | −27.0 | −15.0 | −16.0 |
| H2Ptr | 1.43 | 166 | 107 | −19.0 | −23.0 | −11.0 |
| 121 | −18.0 | −21.0 | −20.0 | |||
| N | 1.46 | 254 | 206 | −29.0 | −16.0 | −25.0 |
| 236 | −29.0 | −19.0 | −21.0 | |||
| H2Bip | 2.76 | 240 | 196 | −27.0 | −14.0 | −20.0 |
| 165 | −27.0 | −21.0 | −16.0 | |||
| H2Xptr | 2.88 | 182 | 154 | −14.0 | −19.0 | −28.0 |
| 137 | −14.0 | −22.0 | −25.0 | |||
| Bip | 3.12 | 238 | 220 | −27.0 | −16.0 | −23.0 |
| 178 | −27.0 | −21.0 | −19.0 | |||
| Ptr | 3.13 | 164 | 119 | −12.0 | −25.0 | −22.0 |
| 92 | −12.0 | −33.0 | −16.0 | |||
| Concentration (nM) | RSD (%) | |
|---|---|---|
| H2Bip | Bip | |
| 5 | 3.7 | |
| 50 | 0.6 | 1.7 |
| 100 | 0.3 | 0.9 |
| 500 | 0.8 | 2.4 |
| 1000 | 0.6 | 0.6 |
| 2000 | 0.9 | 0.9 |
| H4Bip | qH2Bip | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Ammonium Buffer | Half-Life (min) | Time Interval (hours) | Reaction Order | Rate Constant (h−1) | Half-Life (min) | Time Interval (hours) | Reaction Order | Rate Constant (h−1) |
| 2.8 | Formate ** | 87 | 0–3.4 | 1 | 0.5 | 106 | 0.8–3.9 | 1 | 0.4 |
| 38 | 3.4–8.7 | 1.3 | 38 | 3.9–9 | 1.1 | ||||
| 3.0 | Acetate | 25 | 0–4 | 1 | 1.7 | 34 | 0–6.6 | 1 | 1.2 |
| 2.8 | Citrate | 83 | 0–3.6 | 1 | 0.5 | 120 | 0–29.0 | 1 | 0.3 |
| 5.4 | Formate ** | 66 | 0–2.3 | 0 | 2.5 * | 79 | 0–2.7 | 0 | 1.8 * |
| 15 | 2.3–3.9 | 1 | 3.3 | 16 | 2.7–3.9 | 1 | 2.7 | ||
| Acetate | 22 | 0–3 | 1 | 1.9 | 20 | 0–3.5 | 1 | 2.1 | |
| Citrate | 23 | 0–3 | 1 | 1.8 | 29 | 0–3 | 1 | 1.4 | |
| 7.4 | Formate ** | 127 | 0.3–5.7 | 1 | 0.3 | 134 | 0.3–6.4 | 1 | 0.3 |
| 64 | 5.7–14.3 | 0.6 | 74 | 6.4–14.8 | 0.6 | ||||
| Acetate | 37 | 0–6 | 1 | 1.1 | 35 | 0–6.6 | 1 | 1.2 | |
| Citrate | 57 | 0–6 | 1 | 0.7 | 154 | 0–5 | 0 | 0.6 * | |
| H4Bip | qH2Bip | |||
|---|---|---|---|---|
| H4Bip Concentration (µM) | Half-Life (min) | Time Interval (hours) | Half-Life (min) | Time Interval (hours) |
| 0.5 | 85 | 0.3–3.4 | 66 | 0.3–5.8 |
| 53 | 3.4–5.8 | |||
| 1.0 | 193 | 0.7–5.4 | 129 | 0.7–14.3 |
| 107 | 5.4–14.3 | |||
| 2.0 | 299 | 3.4–12.0 | 406 | 3.4–23.3 |
| 245 | 12.0–23.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulghobra, A.; Bonose, M.; Alhajji, E.; Pallandre, A.; Flamand-Roze, E.; Baudin, B.; Menet, M.-C.; Moussa, F. Autoxidation Kinetics of Tetrahydrobiopterin—Giving Quinonoid Dihydrobiopterin the Consideration It Deserves. Molecules 2023, 28, 1267. https://doi.org/10.3390/molecules28031267
Boulghobra A, Bonose M, Alhajji E, Pallandre A, Flamand-Roze E, Baudin B, Menet M-C, Moussa F. Autoxidation Kinetics of Tetrahydrobiopterin—Giving Quinonoid Dihydrobiopterin the Consideration It Deserves. Molecules. 2023; 28(3):1267. https://doi.org/10.3390/molecules28031267
Chicago/Turabian StyleBoulghobra, Ayoub, Myriam Bonose, Eskandar Alhajji, Antoine Pallandre, Emmanuel Flamand-Roze, Bruno Baudin, Marie-Claude Menet, and Fathi Moussa. 2023. "Autoxidation Kinetics of Tetrahydrobiopterin—Giving Quinonoid Dihydrobiopterin the Consideration It Deserves" Molecules 28, no. 3: 1267. https://doi.org/10.3390/molecules28031267
APA StyleBoulghobra, A., Bonose, M., Alhajji, E., Pallandre, A., Flamand-Roze, E., Baudin, B., Menet, M.-C., & Moussa, F. (2023). Autoxidation Kinetics of Tetrahydrobiopterin—Giving Quinonoid Dihydrobiopterin the Consideration It Deserves. Molecules, 28(3), 1267. https://doi.org/10.3390/molecules28031267









