Abstract
A simple and concise three-component synthesis of a key pyrrole framework was developed from the reaction between α-hydroxyketones, oxoacetonitriles, and anilines. The synthesis was used to obtain several pyrrole-based drug candidates, including COX-2 selective NSAID, antituberculosis lead candidates BM212, BM521, and BM533, as well as several analogues. This route has potential to obtain diverse libraries of these pyrrole candidates in a concise manner to develop optimum lead compounds.
1. Introduction
The practical synthesis of drug lead compounds in a concise manner is crucial for biological evaluation and the development of structure activity relationship (SAR) studies for further optimization. This becomes even more crucial when developing an industrial synthetic route for a specific drug. Several pyrrole-based drugs have already been introduced in the market with great success [1,2,3]. Additionally, many pyrrole-based lead compounds demonstrated positive bioactivities, such as anti-inflammatory [4,5], anti-bacterial [6], and anti-cancer [7] activities. For example, pyrrole-based compounds 1a–d have demonstrated selective cyclooxygenases (COX-2) inhibition with nonsteroidal anti-inflammatory activity [4,5,6,7,8], while pyrrole-based compounds 2a–e have demonstrated antituberculosis activities [9,10] (Figure 1).
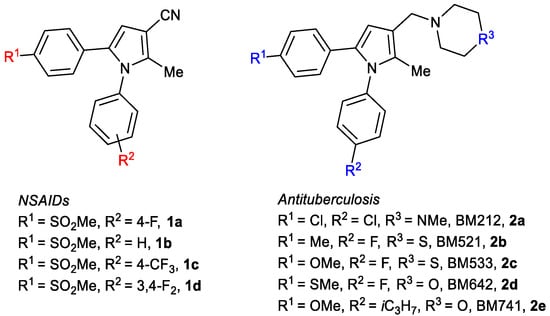
Figure 1.
Examples of pyrrole-based drug lead compounds and drug candidates.
Several procedures were reported to obtain pyrrole COX-2 selective NSAIDs. However, the reported syntheses are lengthy and gave low overall yields, making SAR studies difficult and synthesis at large scale less practical. Typical syntheses of pyrroles possessing COX-2 [4] and antituberculosis inhibitory activities [11] are shown in Scheme 1. Therefore, considering the problems associated with the current synthetic routes and the potential of pyrrole-based drugs, a practical and concise synthetic route is highly advantageous.
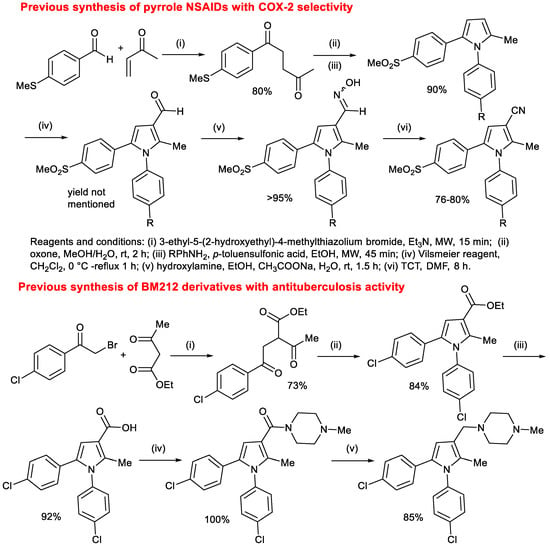
Scheme 1.
Examples of previous syntheses of pyrrole-based bioactive compounds [4,12].
Previously, we reported the selective synthesis of N-substituted 2,3,5-functionalized 3-cyanopyrroles via a one-step, three-component reaction between α-hydroxyketones, oxoacetonitriles, and primary amines [13]. The mild reaction conditions (AcOH as a catalyst, EtOH, 70 °C, 3 h), applicability on a large scale, and high atom efficiency (water is the only molecule lost during the reaction) warranted the application of this synthesis to important pyrrole-based lead drug candidates [13]. In this work, we report the synthesis of a key pyrrole framework and develop it further for the synthesis of several pyrrole lead drug candidates, including COX-2 selective inhibitor, antituberculosis lead candidates BM212 2a, BM521 2b, and BM533 2c, and several analogues.
2. Results and Discussions
The AcOH-catalyzed, one-pot, three-component reaction between 2-hydroxy-1-(4-(methylsulfonyl)phenyl)ethan-1-one 3, 3-oxobutanenitrile 4, and 4-fluoroaniline 5 gave the anticipated COX-2 selective NSAID pyrrole 9 in 53%. This one-step reaction is very powerful, since a library of analogues of pyrrole 1a can be prepared using the same procedure by changing the nitrile and amine substrates. For example, the reaction between 2-hydroxy-1-(4-(methylsulfonyl)phenyl)ethan-1-one 3, 3-oxobutanenitrile 4, and 2-phenylethylamine 6 gave pyrroles 10 in 76% yield, while the same reaction using 3-oxo-3-phenylpropanenitrile 7 and benzylamine 8 gave pyrrole 11 in 78% yield. (Scheme 2) Additionally, the cyano group can easily be transformed into aldehyde, alcohol, amide, and acid functional groups that act as handles for further structural modifications [8].
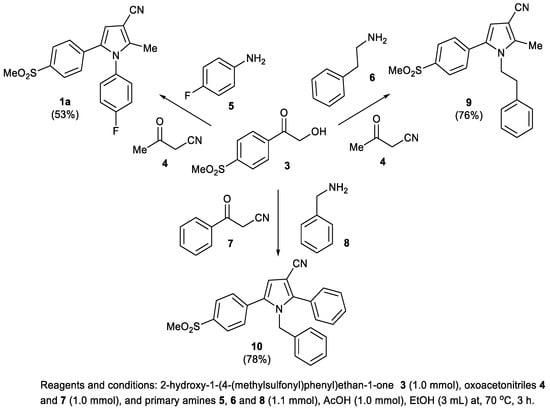
Scheme 2.
Synthesis of 5-(4-methylsulfonyl)phenyl-substituted pyrroles 1a, 9, and 10.
We adapted the same route to synthesize BM212 2a, BM521 2b, and BM533 2c framework. Thus, the reaction between phenacyl alcohols 11–13, 3-oxobutanenitrile 4, and anilines 5 and 14 gave pyrroles 15–17 in 60–64% yield. Pyrroles 15–17, which are analogues of pyrrole 1a, are precursors for BM212, BM521, and BM533, respectively (Scheme 3).
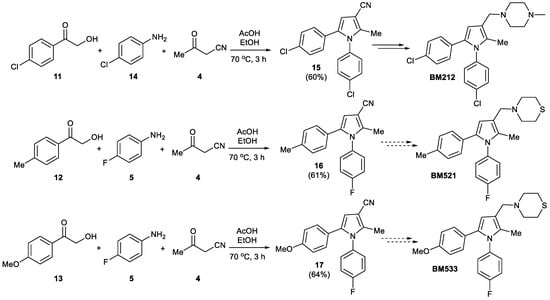
Scheme 3.
Synthesis of pyrroles 15–17.
To demonstrate the feasibility of converting compounds 15, 16, and 17 to BM212 2a, BM521 2b, and BM533 2c, respectively, and hence the power of the three-component reaction, we demonstrated the synthesis of BM212 2a from pyrrole 15. Hence, the cyano group of pyrrole 15 was reduced using diisobutylaluminium hydride (DIBAL-H) to give carbaldehyde 18 in 93% yield (Scheme 4) [14]. When carbaldehyde 18 was treated with 1-methylpiperazine in the presence of AcOH in DCM for 2 h, followed by the addition of NaBH(AcO)3 and stirring overnight, BM212 2a was obtained in 95% yield after silica gel column chromatographic purification [12]. BM521 2b and BM533 2c can be obtained in a similar fashion from pyrroles 16 and 17, respectively. α-Hydroxyketones 3 and 11–13 were synthesized from the corresponding substituted phenacyl bromide using sodium formate (General Procedure 1, experimental section and [13]).
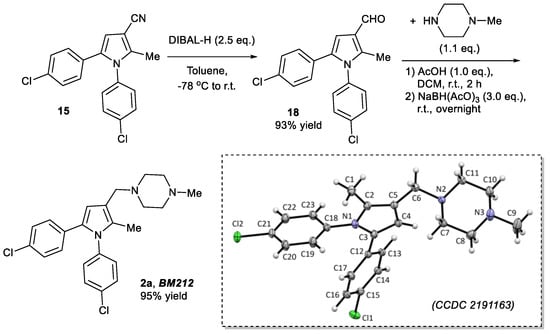
Scheme 4.
Synthesis of BM212 2a from pyrrole product 15, and X-ray single crystal structure of BM212 2a (CCDC 2191163) (displacement ellipsoids are drawn at the 50% probability level).
3. Materials and Methods
3.1. Materials and Methods
All chemicals and AR grade solvents were obtained from Sigma-Aldrich (Saint Louis, MO, USA), Merck (Lebanon, NJ, USA), or Alfa Aesar (Tewksbury, MA, USA) and were used as received without further purification. IR spectra were recorded using a Bruker MPA FT-IR machine (Karlsruhe, Germany). 1H NMR spectra were recorded at 400 MHz Bruker Avance III 400 (BBFO 400). 13C NMR spectra were recorded at 101 MHz Bruker Avance III 400 (BBFO 400). HRMS were measured using a hybrid Quadrupole Time-of-Flight (Q-TOF) on a Qstar XL MS/MS system (Milford, CT, USA). Single-crystal X-ray crystallographic analysis was done using Bruker D8 Quest (Karlsruhe, Germany). Analytical TLC was performed using Merck 60 F254 precoated silica gel plates (0.2 mm thickness) (Oakville, ON, Canada). The plates were visualized under UV (254 nm) or stained in ceric ammonium sulfate solution with heating to detect the reaction spots. Flash chromatography was performed using Merck silica gel 60 (230–400 mesh) (Oakville, ON, Canada). Copies of the 1H NMR, 13C NMR, and single-crystal X-ray data of pyrrole 20 can be found in the Supplementary Materials.
3.2. General Procedure 1: Preparation of Substituted Phenacyl Alcohols 3 and 11–13
A solution of the phenacyl bromide (20 mmol) and sodium formate (16 mmol) in an ethanol/water mixture (30 mL, EtOH: H2O = 9:1) was stirred at 90 °C for 12 h (Scheme 5). Once the reaction was completed (TLC), the mixture was allowed to cool to room temperature and ethanol was removed under vacuum. Water (30 mL) was added to the residue, and the resulting mixture was extracted with ethyl acetate (3 × 30 mL). The combined organic layers were dried over Mg2SO4 and the solvent was evaporated using under vacuum. The residue was then purified using column chromatography using EtOAc/Hexane as the eluent (2:3 for 3; 1:4 for 11–13).
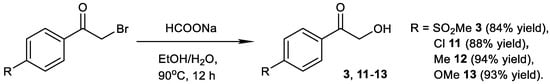
Scheme 5.
Preparation of phenacyl alcohols 3 and 11-13.
3.3. General Procedure 2: Synthesis of N-Substituted 1,2,3,5-Substituted Pyrroles 1a, 9–10 and 15–17
AcOH (1.0 eq.) was added dropwise to a stirred solution of substituted phenacyl alcohols 3 and 11–13 (1.0 eq.), oxoacetonitriles 4 and 7 (1.0 eq.), and primary amine 5, 6, 8, and 14 (1.1 eq.) in EtOH (3 mL) at room temperature. The resulting mixture was heated at 70 °C for 3 h (TLC). The reaction mixture was then evaporated to dryness under vacuum to give the crude product as a foam. The foam was purified using silica gel column chromatography with 5–35% EtOAc/Hexane as eluent t yield the pure products. All reactions were conducted using 1.0 mmol of the substrates 3 and 11–13. This general procedure was used to prepare N-substituted 2,3,5-functionalized pyrroles 1a, 9–10 and 15–17.
1-(4-Fluorophenyl)-2-methyl-5-(4-(methylsulfonyl)phenyl)-1H-pyrrole-3-carbonitrile 1a
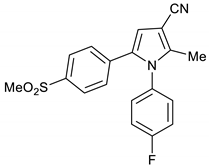
Obtained by General Procedure 2 using 2-hydroxy-1-(4-(methylsulfonyl)phenyl)ethan-1-one 3 (214 mg, 1.0 mmol), 3-oxobutanenitrile 4 (83 mg, 1.0 mmol), 4-fluoro aniline 5 (104 µL, 1.1 mmol), and AcOH (57 µL, 1.0 mmol). The reaction gave the desired 1a (334 mg, 53%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 2221, 1513, 1402, 1306, 1152 cm−1. 1H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 8.6 Hz, 2H), 7.21 (d, J = 8.6 Hz, 2H), 7.17 (dd, J = 6.3, 2.0 Hz, 4H), 6.71 (s, 1H), 3.04 (s, 3H), 2.31 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.8, 161.3, 143.7, 141.5, 138.9, 136.5, 133.1, 129.8, 129.7, 128.4, 127.6, 117.2, 116.9, 116.2, 112.3, 93.7, 44.4, 12.4. DEPT135 13C NMR (101 MHz, CDCl3) δ 129.8, 129.7, 128.4, 127.6, 120.0, 117.2, 116.9, 112.3, 44.4, 12.4. HRMS (ESI-TOF) m/z: [M+H]+ calculated for C19H16N2O2SF+ 355.0917; found 355.0907.
2-Methyl-5-(4-(methylsulfonyl)phenyl)-1-phenethyl-1H-pyrrole-3-carbonitrile 9
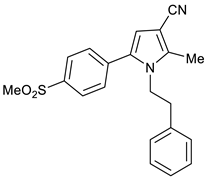
Obtained by General Procedure 2 using 2-hydroxy-1-(4-(methylsulfonyl)phenyl)ethan-1-one 3 (214 mg, 1.0 mmol), 3-oxobutanenitrile 4 (83 mg, 1.0 mmol), 2-phenylethylamine 6 (138 µL, 1.1 mmol), and AcOH (57 µL, 1.0 mmol). The reaction gave the desired 9 (277 mg, 76%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 2929, 2218, 1598, 1426, 1311, 1150, 1092 cm−1. 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 7.8 Hz, 2H), 7.39 (d, J = 7.8 Hz, 2H), 7.21 (d, J = 6.4 Hz, 3H), 6.78 (d, J = 6.7 Hz, 2H), 6.39 (s, 1H), 4.18 (t, J = 6.7 Hz, 2H), 3.12 (s, 3H), 2.74 (t, J = 6.6 Hz, 2H), 2.31 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 139.9, 139.7, 137.5, 136.6, 132.7, 129.7, 128.8, 128.6, 127.8, 127.2, 116.7, 111.9, 92.5, 46.4, 44.5, 36.8, 11.7. DEPT135 13C NMR (101 MHz, CDCl3) δ 129.7, 128.8, 128.6, 127.8, 127.2, 111.9, 46.4, 44.5, 36.8, 11.7. HRMS (ESI-TOF) m/z: [M+H]+ calculated for C21H21N2O2S+ 365.1324; found 365.1319.
1-Benzyl-5-(4-(methylsulfonyl)phenyl)-2-phenyl-1H-pyrrole-3-carbonitrile 10
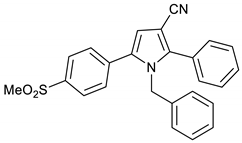
Obtained by General Procedure 2 using 2-hydroxy-1-(4-(methylsulfonyl)phenyl)ethan-1-one 3 (214 mg, 1.0 mmol), benzoylacetonitrile 7 (145 mg, 1.0 mmol), benzylamine 8 (120 µL, 1.1 mmol), and AcOH (57 µL, 1.0 mmol). The reaction gave the desired 10 (322 mg, 78%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 2222, 1602, 1403, 1313, 1151 cm−1. 1H NMR (400 MHz, CDCl3) δ 7.95–7.89 (m, 2H), 7.53–7.49 (m, 2H), 7.44 (s, 5H), 7.19 (dd, J = 4.2, 2.3 Hz, 3H), 6.71 (s, 1H), 6.64 (dd, J = 6.6, 2.9 Hz, 2H), 5.20 (s, 2H), 3.08 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 144.3, 140.0, 137.1, 136.9, 134.4, 129.8, 129.7, 129.6, 129.1, 129.0, 128.8, 127.8, 127.7, 125.7, 116.5, 113.6, 94.1, 49.5, 44.5. DEPT135 13C NMR (101 MHz, CDCl3) δ 129.8, 129.7, 129.6, 129.0, 128.8, 127.8, 127.8, 125.7, 113.6, 49.5, 44.5. HRMS (ESI-TOF) m/z: [M+H]+ calculated for C25H18N2O3F+ 413.1301; found 413.1281.
1,5-Bis(4-chlorophenyl)-2-methyl-1H-pyrrole-3-carbonitrile 15
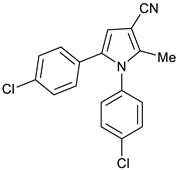
Obtained by General Procedure 2 using 1-(4-chlorophenyl)-2-hydroxyethan-1-one 11 (170 mg, 1.0 mmol), 3-oxobutanenitrile 4 (83 mg, 1.0 mmol), 4-chloro aniline 14 (141 mg, 1.1 mmol), and AcOH (57 µL, 1.0 mmol). The reaction gave the desired 15 (209 mg, 64%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 2218, 1651, 1496, 1400, 1094 cm−1. 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 8.6 Hz, 2H), 7.20 (d, J = 8.5 Hz, 2H), 7.08 (d, J = 8.6 Hz, 2H), 6.96 (d, J = 8.5 Hz, 2H), 6.55 (s, 1H), 2.29 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 140.1, 135.8, 135.0, 134.0, 133.5, 129.9, 129.6, 129.5, 129.3, 128.7, 116.6, 110.7, 93.2, 12.4. DEPT135 13C NMR (101 MHz, CDCl3) δ 129.9, 129.5, 129.3, 128.7, 110.7, 12.4. HRMS (ESI-TOF) m/z: [M+H]+ calculated for C18H13N2Cl2+ 327.0456; found 327.0451.
1-(4-Fluorophenyl)-2-methyl-5-(p-tolyl)-1H-pyrrole-3-carbonitrile 16
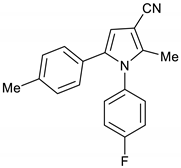
Obtained by General Procedure 2 using 2-hydroxy-1-(p-tolyl)ethan-1-one 12 (150 mg, 1.0 mmol), 3-oxobutanenitrile 4 (83 mg, 1.0 mmol), 4-fluoro aniline 5 (104 µL, 1.1 mmol), and AcOH (57 µL, 1.0 mmol). The reaction gave the desired 16 (174 mg, 60%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 2223, 1604, 1512, 1403, 1227 cm−1. 1H NMR (400 MHz, CDCl3) δ 7.16–7.08 (m, 4H), 7.02 (d, J = 8.0 Hz, 2H), 6.92 (d, J = 8.1 Hz, 2H), 6.51 (s, 1H), 2.30 (s, 3H), 2.28 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.5, 161.0, 139.6, 137.3, 135.4, 133.6, 129.9, 129.8, 129.0, 128.3, 127.3, 117.0, 116.6, 116.4, 109.8, 92.6, 21.1, 12.4. DEPT135 13C NMR (101 MHz, CDCl3) δ 129.9, 129.8, 129.0, 128.3, 116.6, 116.4, 109.8, 21.1, 12.4. HRMS (ESI-TOF) m/z: [M+Na]+ calculated for C19H15N2FNa+ 313.1117; found 313.1111.
1-(4-Fluorophenyl)-5-(4-methoxyphenyl)-2-methyl-1H-pyrrole-3-carbonitrile 17
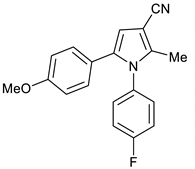
Obtained by General Procedure 2 using 2-hydroxy-1-(4-methoxyphenyl)ethan-1-one 13 (166 mg, 1.0 mmol), 3-oxobutanenitrile 4 (83 mg, 1.0 mmol), 4-fluoro aniline 5 (104 µL, 1.1 mmol), and AcOH (57 µL, 1.0 mmol). The reaction gave the desired 17 (187 mg, 61%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 2218, 1614, 1511, 1403, 1248 cm−1. 1H NMR (400 MHz, CDCl3) δ 7.12 (d, J = 6.3 Hz, 4H), 6.96 (d, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 2H), 6.47 (s, 1H), 3.78 (s, 3H), 2.27 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.4, 161.0, 158.9, 139.3, 135.2, 129.9, 129.9, 129.7, 128.6, 123.8, 116.6, 116.3, 114.1, 113.8, 109.4, 92.5, 55.2, 12.4. DEPT135 13C NMR (101 MHz, CDCl3) δ 129.9, 129.9, 129.7, 116.6, 116.4, 113.8, 109.4, 55.2, 12.4. HRMS (ESI-TOF) m/z: [M+Na]+ calculated for C19H15N2OFNa+ 329.1066; found 329.1069.
1,5-Bis(4-chlorophenyl)-2-methyl-1H-pyrrole-3-carbaldehyde 18
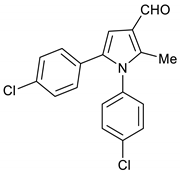
DIBAL-H (1.0 M solution in THF, 2.5 mL, 2.5 mmol) was added slowly to a solution of pyrrole 15 (326 mg, 1.0 mmol) in toluene (10 mL) at −78 °C under stirring. The reaction mixture was allowed to stir at room temperature for 2 h, quenched with saturated K2CO3 solution (15 mL) and then extracted with ethyl acetate (3 × 15 mL). The organic layers were dried using MgSO4 then evaporated under vacuum. This crude product was purified by column chromatography using Hexane/EtOAc (4:1) and gave 18 (307 mg, 93%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 1718, 1559, 1401, 1093 cm−1. 1H NMR (400 MHz, CDCl3) δ 9.99 (s, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.19 (d, J = 8.4 Hz, 2H), 7.11 (d, J = 8.4 Hz, 2H), 6.98 (d, J = 8.3 Hz, 2H), 6.80 (s, 1H), 2.42 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 185.6, 140.0, 135.7, 134.9, 134.6, 133.2, 130.1, 129.8, 129.5, 129.4, 128.6, 123.0, 109.0, 11.5. DEPT135 13C NMR (101 MHz, CDCl3) δ 185.6, 129.8, 129.5, 129.4, 128.6, 109.0, 11.5. HRMS (ESI-TOF) m/z: [M+H]+ calculated for C18H14NOCl2+ 330.0452; found 330.0457.
1-((1,5-Bis(4-chlorophenyl)-2-methyl-1H-pyrrol-3-yl)methyl)-4-methylpiperazine 2a
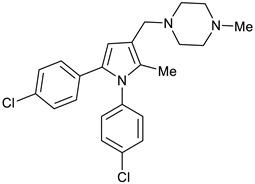
AcOH (57 µL, 1 mmol) and 1-methylpiperazine (122 µL, 1.1 mmol) were added to a stirring solution of pyrrole 18 (309 mg, 0.94 mmol) in DCM (10 mL) at room temperature. The mixture was allowed to stir at room temperature for 2 h before NaBH(AcO)3 (636 mg, 3 mmol) was added and the mixture was left to stir overnight. The reaction mixture was then quenched with 1M NaOH solution (15 mL), stirred for 30 min., diluted with water (10 mL) and extracted with DCM (2 X 10 mL). The organic layers were dried over Na2SO4 and evaporated under vacuum. The crude product was purified by silica gel column chromatography using EtOAc/MeOH (4:1) to give the desired BM212 2a (366 mg, 95%) as a hygroscopic pale-yellow solid. IR (KBr film) ѵmax = 2935, 2792, 1493, 1167, 1087, 1014 cm−1. 1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 8.6 Hz, 2H), 7.13 (d, J = 8.6 Hz, 2H), 7.07 (d, J = 8.6 Hz, 2H), 6.96 (d, J = 8.6 Hz, 2H), 6.37 (s, 1H), 3.48 (s, 2H), 2.52 (s, 8H), 2.32 (s, 3H), 2.08 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 137.8, 133.4, 131.9, 131.6, 131.5, 130.2, 129.7, 129.3, 128.8, 128.3, 116.9, 111.7, 55.1, 54.3, 52.7, 46.0, 11.2. DEPT135 13C NMR (101 MHz, CDCl3) δ 129.7, 129.3, 128.8, 128.3, 111.7, 55.1, 54.3, 52.7, 46.0, 11.2. HRMS (ESI-TOF) m/z: [M+H]+ calculated for C23H26N3Cl2+ 414.1504; found 414.1486.
4. Conclusions
A concise synthetic route for pyrrole-based drug candidates from α-hydroxyketones, 3-oxobutanenitrile, and anilines is presented. The route was demonstrated by the synthesis of several drugs, with high overall yields. This route provides an easy strategy to quickly obtain pyrrole-based drug candidates and has wider applications to the synthesis of analogue compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031265/s1. Copies of the 1H NMR, 13C NMR, and single-crystal X-ray data of pyrrole 20 are available online.
Author Contributions
Conceptualization, M.X., M.S. and Z.M.A.J.; methodology, M.X. and Z.M.A.J.; validation, M.X. and Z.M.A.J.; formal analysis, M.X., Z.M. and M.S.; investigation, M.X. and Z.M.; data curation, M.X.; writing—original draft preparation, M.X.; writing—review and editing, Z.M.A.J., Z.M.; M.S. and M.X.; supervision, Z.M.A.J.; project administration, Z.M.A.J.; funding acquisition, Z.M.A.J. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the College of Engineering (Startup Grant), Nanyang Technological University, Singapore, and by the United Arab Emirates University (UAEU), Al-Ain (Grant no. G00003918).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is published in the Supporting Material in this Journal under Supplementary Materials.
Acknowledgments
We thank Nanyang Technological University, Singapore (CoE, Start-up Grant) and UAEU (Grant no.G00003918) for financial support. We also thank the Department of Chemistry, Institut Teknologi Sepuluh Nopember, Indonesia for support through WCP-Like program.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Colhoun, H.; Thomason, M.; Mackness, M.; Maton, S.; Betteridge, D.; Durrington, P.; Hitman, G.; Neil, H.; Fuller, J. Collaborative AtoRvastatin Diabetes Study (CARDS)., Design of the Collaborative AtoRvastatin Diabetes Study (CARDS) in patients with type 2 diabetes. Diabet. Med. 2002, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, S.B.; Barrio, D.A.; Cortizo, A.M.; Williams, P.A.M. Three new vanadyl (IV) complexes with non-steroidal anti-inflammatory drugs (Ibuprofen, Naproxen and Tolmetin). Bioactivity on osteoblast-like cells in culture. J. Inorg. Biochem. 2002, 88, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Battilocchio, C.; Poce, G.; Alfonso, S.; Porretta, G.C.; Consalvi, S.; Sautebin, L.; Pace, S.; Rossi, A.; Ghelardini, C.; Mannelli, L.D.C. A class of pyrrole derivatives endowed with analgesic/anti-inflammatory activity. Bioorganic Med. Chem. 2013, 21, 3695–3701. [Google Scholar] [CrossRef] [PubMed]
- Fatahala, S.S.; Hasabelnaby, S.; Goudah, A.; Mahmoud, G.I.; Helmy Abd-El Hameed, R. Pyrrole and fused pyrrole compounds with bioactivity against inflammatory mediators. Molecules 2017, 22, 461. [Google Scholar] [CrossRef]
- Biava, M.; Porretta, G.; Manetti, F. New derivatives of BM212: A class of antimycobacterial compounds based on the pyrrole ring as a scaffold. Mini Rev. Med. Chem. 2007, 7, 65–78. [Google Scholar] [CrossRef]
- Pegklidou, K.; Papastavrou, N.; Gkizis, P.; Komiotis, D.; Balzarini, J.; Nicolaou, I. N-substituted pyrrole-based scaffolds as potential anticancer and antiviral lead structures. Med. Chem. 2015, 11, 602–608. [Google Scholar] [CrossRef]
- Khanna, I.K.; Weier, R.M.; Yu, Y.; Collins, P.W.; Miyashiro, J.M.; Koboldt, C.M.; Veenhuizen, A.W.; Currie, J.L.; Seibert, K.; Isakson, P.C. 1, 2-Diarylpyrroles as potent and selective inhibitors of cyclooxygenase-2. J. Med. Chem. 1997, 40, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Poce, G.; Bates, R.H.; Alfonso, S.; Cocozza, M.; Porretta, G.C.; Ballell, L.; Rullas, J.; Ortega, F.; De Logu, A.; Agus, E. Improved BM212 MmpL3 inhibitor analogue shows efficacy in acute murine model of tuberculosis infection. PLoS ONE 2013, 8, e56980. [Google Scholar] [CrossRef] [PubMed]
- Poce, G.; Consalvi, S.; Venditti, G.; Scarpecci, C.; Biava, M. Development of MmpL3 inhibitors for tuberculosis treatment. In Annual Reports in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 52, pp. 71–96. [Google Scholar]
- More, N.A.; Patil, M.D.; Garud, D.R.; Gajbhiye, J.M. An efficient synthesis of potent anti-tubercular drug candidate BM212. Rasayan J. Chem. 2016, 9, 806–811. [Google Scholar]
- Liu, P.; Yang, Y.; Ju, Y.; Tang, Y.; Sang, Z.; Chen, L.; Yang, T.; An, Q.; Zhang, T.; Luo, Y. Design, synthesis and biological evaluation of novel pyrrole derivatives as potential ClpP1P2 inhibitor against Mycobacterium tuberculosis. Bioorganic Chem. 2018, 80, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Moussa, Z.; Judeh, Z. Selective One-pot multicomponent synthesis of N-substituted 2,3,5-functionalized 3-cyanopyrroles via the reaction between α-hydroxyketones, oxoacetonitriles, and primary amines. Molecules 2022, 27, 5285. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; He, H.; Bertekap, R.; Westphal, R.; Lelas, S.; Newton, A.; Wallace, T.; Taber, M.; Davis, C.; Macor, J.E. Discovery of disubstituted piperidines and homopiperidines as potent dual NK1 receptor antagonists–serotonin reuptake transporter inhibitors for the treatment of depression. Bioorganic Med. Chem. 2013, 21, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).