Polysaccharides from Nitraria retusa Fruit: Extraction, Purification, Structural Characterization, and Antioxidant Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Model Fitting
2.2. Model Validation
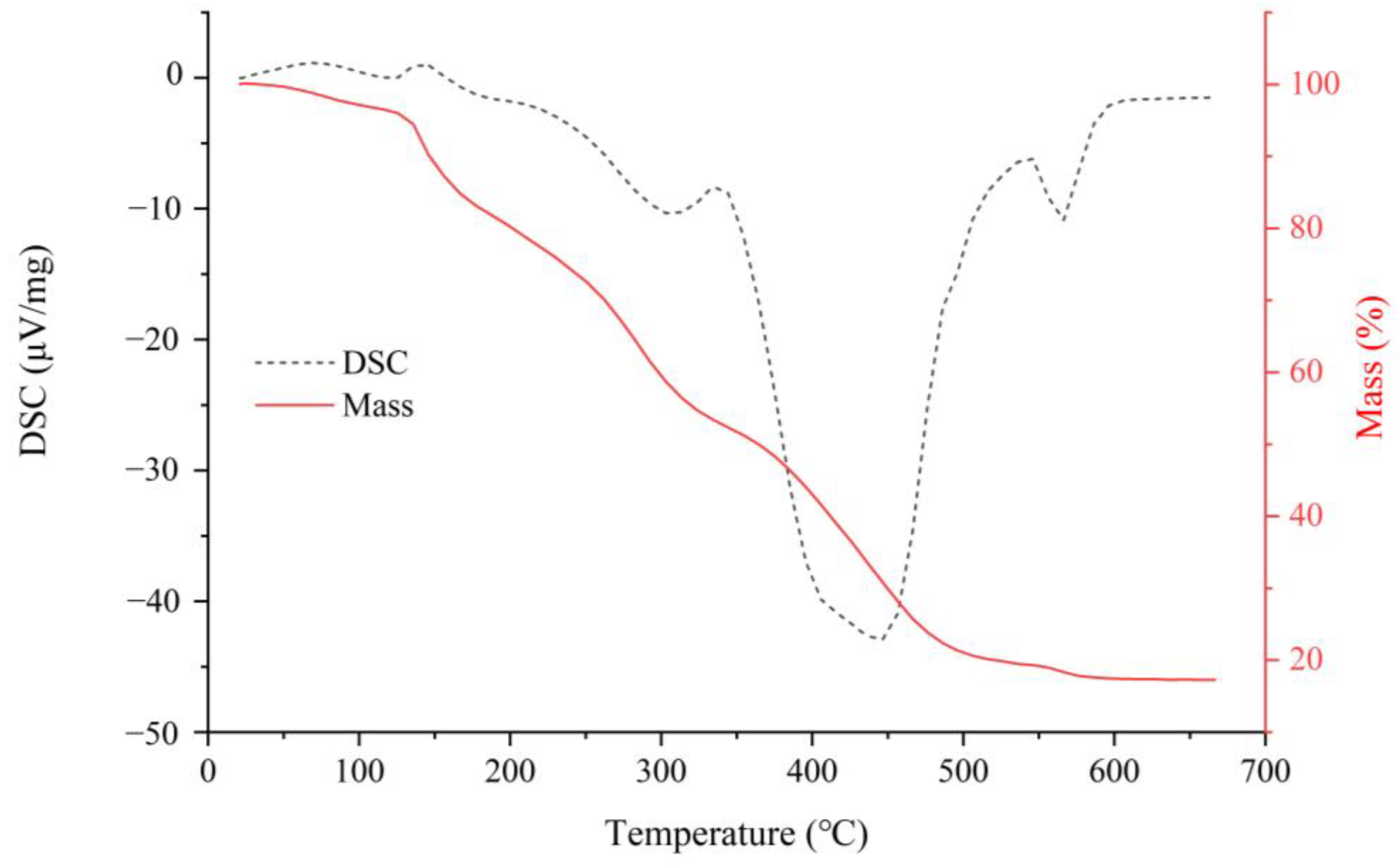
2.3. Thermal Characteristic of Crude NRFP
2.4. Chemical Composition of Crude NRFP
2.5. Structural Characterization of NRFPs
2.5.1. Fractions Purification
2.5.2. Molecular Weight and Zeta Potential of NRFPs
2.5.3. Monosaccharide Composition of NRFPs
2.5.4. FT-IR Spectrum Analysis of NRFPs
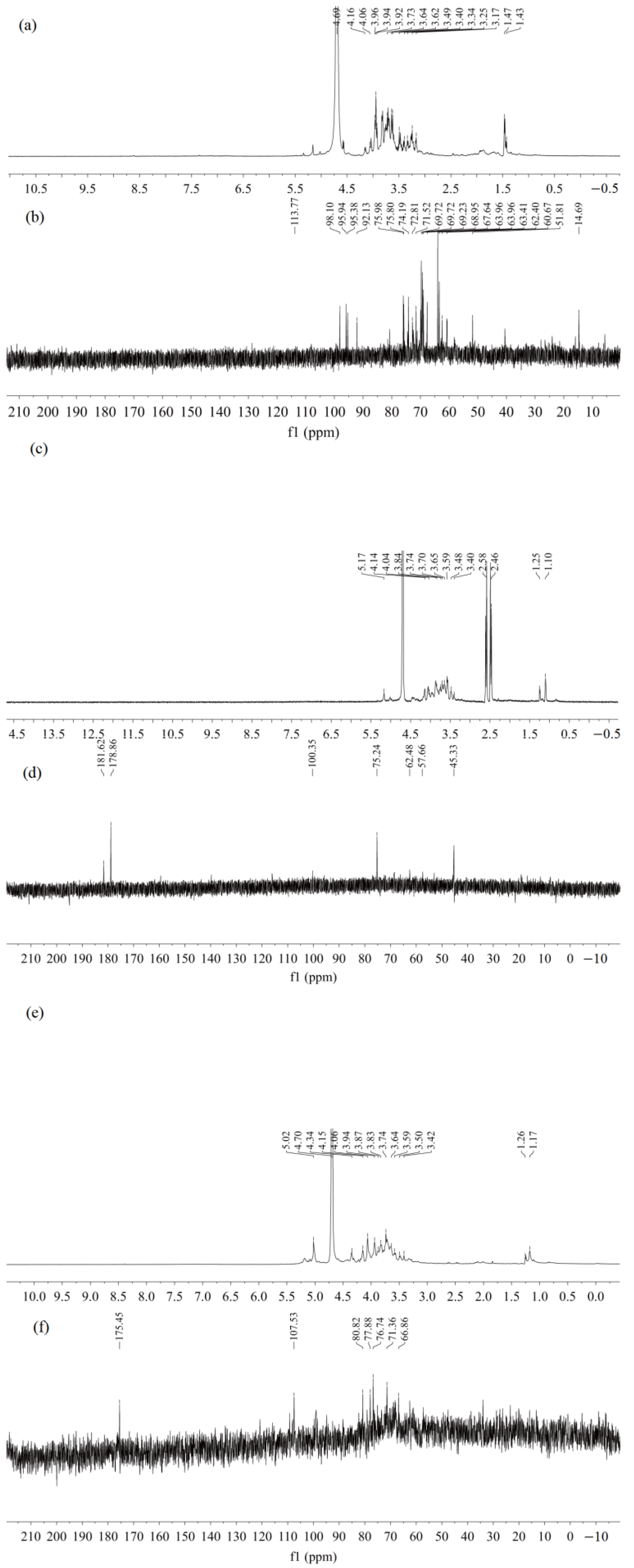
2.5.5. NMR Spectrum Analysis of NRFPs
2.5.6. SEM Analysis of NRFPs
2.6. Antioxidant Activity of NRFPs
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction Procedure
3.2.1. UAE Extraction
3.2.2. Process Optimization
3.3. Chemical Composition Analysis
3.4. Isolation and Purification
3.5. Thermal Analysis
3.6. Structural Analysis
3.6.1. Monosaccharide Composition
3.6.2. Molecular Weight Analysis
3.6.3. Fourier Transform Infrared Spectrometer (FT-IR) Analysis
3.6.4. Nuclear Magnetic Resonance (NMR) Analysis
3.6.5. Determination of Zeta-Potential
3.6.6. Scanning Electron Microscopy (SEM) Analysis
3.7. Antioxidant Activities Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Q.B.; Xia, X.; Ji, C.M.; Chen, D.F.; Lu, Y. Optimized flash extraction and UPLC-MS analysis on antioxidant compositions of Nitraria sibirica fruit. J. Pharmaceu. Biomed. 2019, 172, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Turghun, C.; Bakri, M.; Abdulla, R.; Ma, Q.L.; Aisa, H.A. Comprehensive characterization of phenolics from Nitraria sibirica leaf extracts by UHPLC-quadrupole-orbitrap-MS and evaluation of their anti-hypertensive activity. J. Ethnopharmacol. 2020, 261, 113019. [Google Scholar] [CrossRef] [PubMed]
- Rjeibi, I.; Feriani, A.; Hentati, F.; Hfaiedh, N.; Michaud, P.; Pierre, G. Structural characterization of water-soluble polysaccharides from Nitraria retusa fruits and their antioxidant and hypolipidemic activities. Int. J. Biol. Macromol. 2019, 129, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Abuduwaili, A.; Mutailifu, P.; Nuerxiati, R.; Gao, Y.H.; Ais, H.A.; Yili, A. Structure and biological activity of polysaccharides from Nitraria sibirica pall fruit. Food Biosci. 2021, 40, 100903. [Google Scholar] [CrossRef]
- Zhang, T.H.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C.H. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.S.; Yu, X.J.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
- Li, J.; Niu, D.B.; Zhang, Y.; Zeng, X.A. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef]
- Cao, Z.H.; Guo, Y.; Liu, Z.H.; Zhang, H.X.; Zhou, H.Z.; Shang, H.M. Ultrasonic enzyme-assisted extraction of comfrey (Symphytum officinale L.) polysaccharides and their digestion and fermentation behaviors in vitro. Process Biochem. 2022, 112, 98–111. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, J.C.; Liu, X.F.; Zhang, X.; Xu, Y.; Leng, F.F.; Avwenagbiku, M.O. Kinetic modeling of the ultrasonic-assisted extraction of polysaccharide from Nostoc commune and physicochemical properties analysis. Int. J. Biol. Macromol. 2019, 128, 421–428. [Google Scholar] [CrossRef]
- Fang, C.C.; Chen, G.J.; Kan, J.Q. Comparison on characterization and biological activities of Mentha haplocalyx polysaccharides at different solvent extractions. Int. J. Biol. Macromol. 2020, 154, 916–928. [Google Scholar] [CrossRef]
- Chen, S.L.; Shang, H.M.; Yang, J.Y.; Li, R.; Wu, H.X. Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Ind. Crop. Prod. 2018, 121, 18–25. [Google Scholar] [CrossRef]
- Lei, J.; Li, W.; Fu, M.X.; Wang, A.Q.; Wu, D.T.; Guo, H.; Hu, Y.C.; Gan, R.Y.; Zou, L.; Liu, Y. Pressurized hot water extraction, structural properties, biological effects, and in vitro microbial fermentation characteristics of sweet tea polysaccharide. Int. J. Biol. Macromol. 2022, 222, 3215–3228. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Fu, M.X.; Zhao, Y.X.; Li, H.; Li, H.B.; Wu, D.T.; Gan, R.Y. The chemical, structural, and biological properties of crude polysaccharides from sweet tea (Lithocarpus litseifolius (Hance) Chun) based on different extraction. Trends Food Sci. Tech. 2021, 10, 1779. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, F.; Wang, P.; Liu, X.; He, J.J.; Xian, M.L.; Zhao, L.; Qin, W.; Gan, R.Y.; Wu, D.T. Effects of drying methods on the physicochemical characteristics and bioactivities of polyphenolic-protein-polysaccharide conjugates from Hovenia dulcis. Int. J. Biol. Macromol. 2020, 148, 1211–1221. [Google Scholar] [CrossRef]
- Song, Y.R.; Sung, S.K.; Jang, M.; Lim, T.G.; Cho, C.W.; Han, C.J.; Hong, H.D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer). Int. J. Biol. Macromol. 2018, 116, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Cai, X.B.; Ai, J.X.; Zhang, C.; Gao, W. Extraction, structures, bioactivities and structure-function analysis of the polysaccharides from Safflower (Carthamus tinctorius L.). Front. Pharmacol. 2021, 12, 767947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Gao, H.Q.; Ling, L.J.; Cong, M.; Lu, Y.; Chen, D.F. Crude polysaccharides extracted from three characteristic plants in Qinghai province and their activities. Nat. Prod. Res. 2017, 29, 34–39. [Google Scholar]
- Zhao, B.T.; Liu, J.; Chen, X.; Wang, J.L. Purification, structure, and anti-oxidation of polysaccharides from the fruit of Nitraria tangutorum Bobr. RSC Adv. 2018, 8, 11731–11743. [Google Scholar] [CrossRef]
- Zhu, K.X.; Zhang, Y.J.; Nie, S.P.; Xu, F.; He, S.Z.; Gong, D.M.; Wu, G.; Tan, L.H. Physicochemical properties and in vitro antioxidant activities of polysaccharide from Artocarpus heterophyllus Lam. Pulp. Carbohydr. Polym. 2017, 155, 354–361. [Google Scholar] [CrossRef]
- Du, Q.H.; Xin, H.L.; Peng, C. Pharmacology and phytochemistry of the Nitraria genus. Mol. Med. Rep. 2015, 11, 11–20. [Google Scholar] [CrossRef]
- Ji, X.L.; Liu, F.; Peng, Q.; Wang, M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus Jujuba cv Muzao. Food Chem. 2018, 245, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, J.Y.; Liu, Y.Q.; Wu, D.T.; Pan, Y.J. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide isolated from Botrychium ternatum. Carbohydr. Polym. 2017, 171, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.H.; Wu, J.J.; Shen, L.; Chen, G.W.; Jin, L.; Yan, M.X.; Wan, H.T.; He, Y. Microwave-assisted extraction, characterization of a polysaccharide from Salvia miltiorrhiza Bunge and its antioxidant effects via ferroptosis-mediated activation of the Nrf2/HO-1 pathway. Int. J. Biol. Macromol. 2022, 215, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Sun, R.G.; Zhang, J.; Chen, Y.Y.; Liu, N.N. Structure and antioxidant activity of polysaccharide POJ-U1a extracted by ultrasound from Ophiopogon japonicus. Fitoterapia 2012, 83, 1576–1584. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.J.; Zhang, R.F.; Dong, L.H.; Liu, L.; Ma, Y.X.; Jia, X.C.; Wang, G.J.; Zhang, M.W. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, Z.Y.; Lv, M.S.; Li, X.Y.; Wang, J.H.; Yue, Z.R.; Shi, J.M.; Zhang, G.C.; Sui, G.C. Comparative study of structural properties and biological activities of polysaccharides extracted from Chroogomphus rutilus by four different approaches. Int. J. Biol. Macromol. 2021, 188, 215–225. [Google Scholar] [CrossRef]
- Liao, D.W.; Cheng, C.; Liu, J.P.; Huang, D.C.; Chen, G.T. Characterization and antitumor activities of polysaccharides obtained from ginger (Zingiber officinale) by different extraction methods. Int. J. Biol. Macromol. 2020, 152, 894–903. [Google Scholar] [CrossRef]
- Zhi, F.; Yang, T.L.; Wang, Q.; Jiang, B.; Wang, Z.P.; Zhang, J.; Chen, Y.Z. Isolation, structure and activity of a novel water-soluble polysaccharide from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2019, 133, 1201–1209. [Google Scholar] [CrossRef]
- Zhao, T.; Mao, G.H.; Feng, W.W.; Mao, R.W.; Gu, X.Y.; Li, T.; Li, Q.; Bao, Y.T.; Yang, L.Q.; Wu, X.Y. Isolation, characterization and antioxidant activity of polysaccharide from Schisandra sphenanthera. Carbohydr. Polym. 2014, 105, 26–33. [Google Scholar] [CrossRef]
- Chen, W.B.; Zhu, X.L.; Ma, J.J.; Zhang, M.M.; Wu, H. Structural elucidation of a novel pectin polysaccharide from the petal of Saussurea laniceps and the mechanism of its anti-HBV activity. Carbohydr. Polym. 2019, 223, 115077. [Google Scholar] [CrossRef]
- Xiao, Z.Q.; Zhang, Q.; Dai, J.; Wang, X.L.; Yang, Q.Q.; Cai, C.G.; Mao, J.W.; Ge, Q. Structural characterization, antioxidant and antimicrobial activity of water-soluble polysaccharides from bamboo (Phyllostachys pubescens Mazel) leaves. Int. J. Biol. Macromol. 2020, 142, 432–442. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, W.C.; Wu, Z.Y.; Wang, H.Y.; Hui, A.L.; Meng, L.; Chen, P.P.; Xian, Z.J.; He, Y.W.; Li, H.H.; et al. Preparation, characterization, and improvement in the intestinal function of polysaccharide fractions from okra. J. Funct. Foods 2018, 50, 147–157. [Google Scholar] [CrossRef]
- Ma, F.Y.; Wang, D.K.; Li, M.J.; Qing, W.X.; Tikkanen-Kaukanen, C.; Liu, X.H.; Bell, A.E. Characterization of the mucilage polysaccharides from Dioscorea opposite Thunb. with enzymatic hydrolysis. Food Chem. 2018, 245, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Guo, J.H.; Ding, D.Q.; Gao, J.; Hao, L.R.; Guo, X.D.; Liu, Y.Q. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J. Food Meas. Charact. 2022, 16, 2191–2200. [Google Scholar] [CrossRef]
- Barbosa, J.R.; dos Santos Freitas, M.M.; da Silva Martins, L.H.; de Carvalho, R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohyd. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar]
- Liu, H.F.; Guo, S.X.; Lin, Y.Y.; Lai, F.R.; Liu, J.G.; Li, Z.L.; Ren, H.; Zong, Y.M.; Ma, L.K. The relationship between the structure and function of Dendrobium polysaccharides: A review. Mod. Food Sci. Technol. 2021, 37, 308–338. [Google Scholar]
- Lu, J.H.; He, R.J.; Sun, P.L.; Zhang, F.M.; Robert, J.L.; Zhang, A.Q. Molecular mechanisms of bioactive polysaccharides from Ganoderma Lucidum (Lingzhi), A review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Hu, J.L.; Liu, Y.; Cheng, L.M.; Shi, R.J.; Qayum, A.; Bilawal, A.; Gantumur, M.A.; Hussain, M.A.; Jiang, Z.M.; Tian, B. Comparison in bioactivity and characteristics of Ginkgo biloba seed polysaccharides from four extract pathways. Int. J. Biol. Macromol. 2020, 159, 1156–1164. [Google Scholar] [CrossRef]
- Cheong, K.L.; Yu, B.; Chen, J.; Zhong, S.A. Comprehensive review of the cardioprotective effect of marine algae polysaccharide on the gut microbiota. Foods 2022, 11, 3550. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, G.J.; Wang, Z.R.; Kan, J.Q. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Song, L.J.; Liu, P.Z.; Yan, Y.Z.; Bai, B.Y.; Hou, X.J.; Zang, L. Supercritical CO2 fluid extraction of flavonoid compounds from Xinjiang jujube (Ziziphus jujuba Mill.) leaves and associated biological activities and flavonoid compositions. Int. Crop. Prod. 2019, 139, 111508. [Google Scholar] [CrossRef]
- Xiong, G.Y.; Ma, L.S.; Zhang, H.; Li, Y.P.; Zou, W.S.; Wang, X.F.; Xu, Q.S.; Xiong, J.T.; Hu, Y.P.; Wang, X.Y. Physicochemical properties, antioxidant activities, and α-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol. 2022, 194, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Fang, C.C.; Ran, C.X.; Tan, Y.; Yu, Q.Q.; Kan, J.Q. Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2019, 130, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Zhang, F.; Zhang, R.; Liu, F.; Peng, Q.; Wang, M. An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: Purification and structural characterization. Food Chem. 2019, 274, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.L.; Li, J.H.; Yan, L.F.; Li, S.; Ye, X.Q.; Liu, D.H.; Ding, T.; Linhardt, R.J.; Orfila, C.; et al. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocolloid. 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Wang, L.; Ruan, Y.; Ge, M.H.; Qian, Y.Y.; Ma, B.J. Physicochemical properties and biological activities of polysaccharides from the peel of Dioscorea opposita Thunb. extracted by four different methods. Food Sci. Hum. Wellness 2023, 12, 130–139. [Google Scholar] [CrossRef]
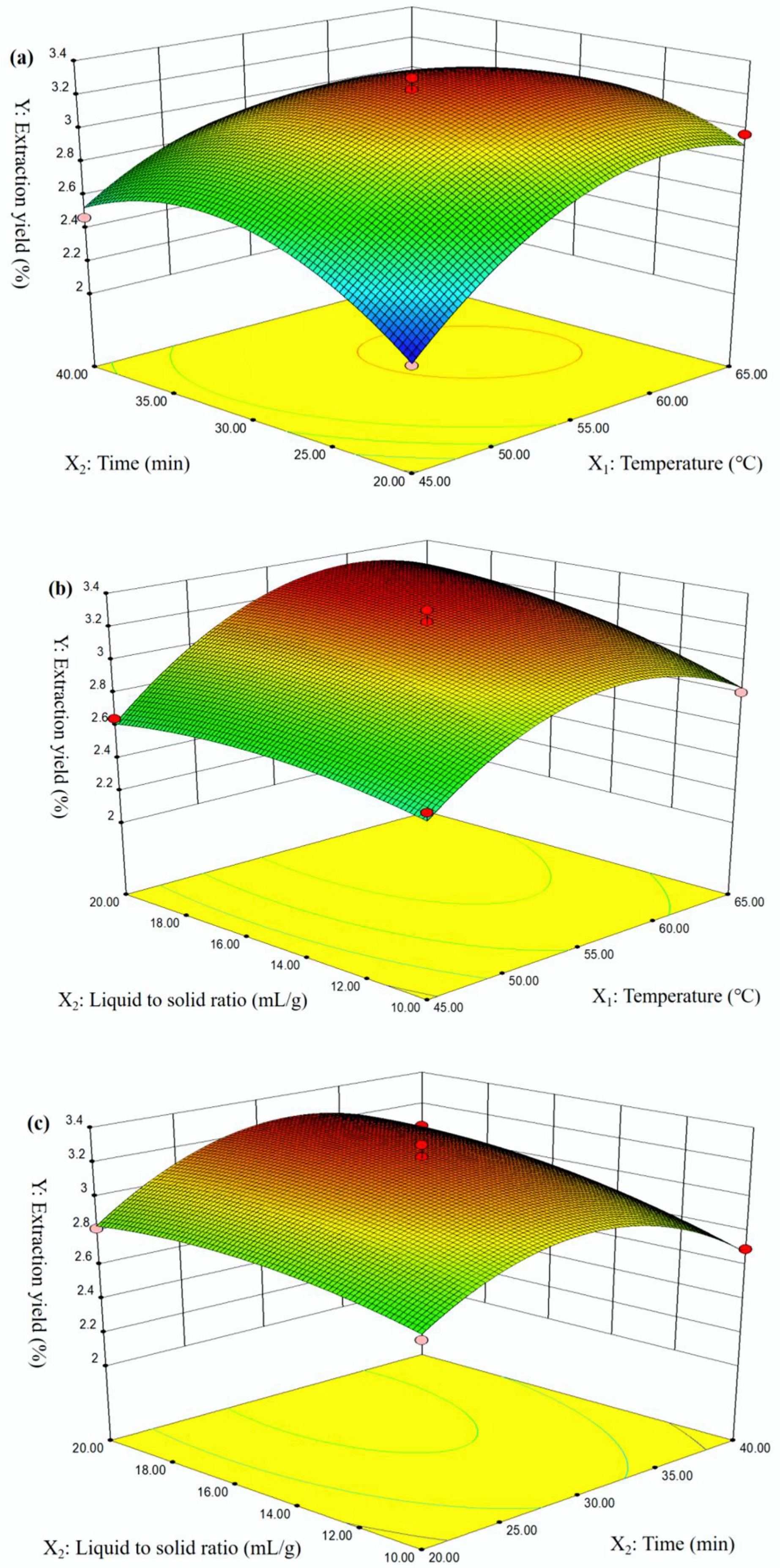



| No. | X1: Temperature (°C) | X2: Time (min) | X3: Liquid-to-Solid Ratio (mL/g) | Y: Extraction Yield (%) |
|---|---|---|---|---|
| 1 | 65 | 30 | 20 | 3.20 |
| 2 | 55 | 40 | 10 | 2.70 |
| 3 | 55 | 30 | 15 | 3.30 |
| 4 | 55 | 20 | 20 | 2.82 |
| 5 | 45 | 30 | 10 | 2.60 |
| 6 | 55 | 30 | 15 | 3.20 |
| 7 | 65 | 20 | 15 | 2.97 |
| 8 | 45 | 30 | 20 | 2.65 |
| 9 | 65 | 40 | 15 | 2.71 |
| 10 | 55 | 30 | 15 | 3.23 |
| 11 | 55 | 30 | 15 | 3.20 |
| 12 | 45 | 20 | 15 | 2.15 |
| 13 | 45 | 40 | 15 | 2.47 |
| 14 | 65 | 30 | 10 | 2.81 |
| 15 | 55 | 40 | 20 | 3.06 |
| 16 | 55 | 30 | 15 | 3.20 |
| 17 | 55 | 20 | 10 | 2.68 |
| Source | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Model | 1.67 | 9 | 0.19 | 53.53 | <0.0001 |
| X1 | 0.41 | 1 | 0.41 | 119.67 | <0.0001 |
| X2 | 0.013 | 1 | 0.013 | 3.7 | 0.0959 |
| X3 | 0.11 | 1 | 0.11 | 31.92 | 0.0008 |
| X1X2 | 0.084 | 1 | 0.084 | 24.31 | 0.0017 |
| X1X3 | 0.029 | 1 | 0.029 | 8.35 | 0.0233 |
| X2X3 | 0.012 | 1 | 0.012 | 3.5 | 0.1037 |
| X12 | 0.45 | 1 | 0.45 | 128.93 | <0.0001 |
| X22 | 0.45 | 1 | 0.45 | 128.93 | <0.0001 |
| X32 | 0.031 | 1 | 0.031 | 8.9 | 0.0204 |
| Residual | 0.024 | 7 | 3.46 × 10−3 | ||
| Lack of fit | 0.017 | 3 | 5.57 × 10−3 | 2.96 | 0.1608 |
| Pure error | 7.52 × 10−3 | 4 | 1.88 × 10−3 | ||
| Cor. total | 1.69 | 16 | |||
| Adeq precision | 1.67 | 9 | 0.19 | 53.53 | <0.0001 |
| C.V% | 0.41 | 1 | 0.41 | 119.67 | <0.0001 |
| R2 = 0.9719, Adj-R2 = 0.9673, Pred-R2 = 0.8350 | |||||
| Total Sugar Content (%) | Uronic Acid Content (%) | Protein Content (%) | Total Polyphenol Content (mg GAE/g) |
|---|---|---|---|
| 72.58 ± 2.68 | 18.98 ± 1.50 | 8.69 ± 1.66 | 6.32 ± 0.86 |
| Samples | MW (kDa) | Mn (kDa) | Mp (kDa) | PDI (MW/Mn) | Zeta Potential |
|---|---|---|---|---|---|
| NRFP-1 | 20.01 | 8.75 | 12.15 | 1.39 | −9.27 |
| NRFP-2 | 28.96 | 12.46 | 16.24 | 1.30 | −10.9 |
| NRFP-3 | 67.45 | 48.97 | 55.69 | 1.76 | −19.8 |
| NRFPs | Man | Rib | Rha | GluA | GalA | Glc | Gal | Xyl | Ara | Fuc |
|---|---|---|---|---|---|---|---|---|---|---|
| NRFP-1 | 3.73 ± 0.21 | 2.01 ± 0.22 | 0.46 ± 0.10 | 2.97 ± 0.26 | 0.54 ± 0.07 | 47.22 ± 0.86 | 15.97 ± 0.65 | 4.06 ± 0.19 | 21.28 ± 0.72 | 1.51 ± 0.10 |
| NRFP-2 | 0.90 ± 0.13 | 0.47 ± 0.11 | 1.24 ± 0.11 | 3.93 ± 0.23 | 3.16 ± 0.16 | 8.05 ± 0.37 | 43.07 ± 1.02 | 3.03 ± 0.21 | 36.05 ± 0.77 | 0.10 ± 0.03 |
| NRFP-3 | 6.96 ± 0.42 | ND | 3.41 ± 0.23 | 3.40 ± 0.28 | 9.26 ± 0.41 | 6.10 ± 0.39 | 21.30 ± 0.79 | 2.95 ± 0.18 | 46.33 ± 0.98 | 0.30 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Liu, S.; Zhang, L.; Pan, L.; Xu, L. Polysaccharides from Nitraria retusa Fruit: Extraction, Purification, Structural Characterization, and Antioxidant Activities. Molecules 2023, 28, 1266. https://doi.org/10.3390/molecules28031266
Song L, Liu S, Zhang L, Pan L, Xu L. Polysaccharides from Nitraria retusa Fruit: Extraction, Purification, Structural Characterization, and Antioxidant Activities. Molecules. 2023; 28(3):1266. https://doi.org/10.3390/molecules28031266
Chicago/Turabian StyleSong, Lijun, Shiqi Liu, Li Zhang, Leiqing Pan, and Long Xu. 2023. "Polysaccharides from Nitraria retusa Fruit: Extraction, Purification, Structural Characterization, and Antioxidant Activities" Molecules 28, no. 3: 1266. https://doi.org/10.3390/molecules28031266
APA StyleSong, L., Liu, S., Zhang, L., Pan, L., & Xu, L. (2023). Polysaccharides from Nitraria retusa Fruit: Extraction, Purification, Structural Characterization, and Antioxidant Activities. Molecules, 28(3), 1266. https://doi.org/10.3390/molecules28031266