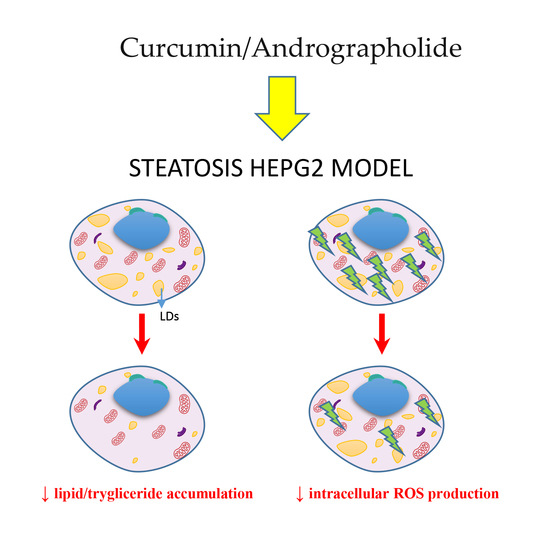
Curcumin and Andrographolide Co-Administration Safely Prevent Steatosis Induction and ROS Production in HepG2 Cell Line
Abstract
1. Introduction
2. Results
2.1. Andrographolide–Curcumin Association Prevents Cytotoxicity in Steatosis Hepatocyte Model
2.2. Andrographolide–Curcumin Association reverted Lipid and Trygliceride Accumulation in Steatosis Hepatocyte Model
2.3. Andrographolide–Curcumin Association Reduced Intracellular ROS Production
2.4. Effect of Curcumin–Andrographolide Association on Insulin and Adipokine Signaling, Metabolic Pathways, Inflammatory Response, and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Steatosis Induction and Cell Treatment
4.4. Cell Viability Assay
4.5. Oil Red O (ORO) Staining
4.6. Triglyceride Assay
4.7. Measurement of Intracellular ROS
4.8. RNA Isolation and Real-Time PCR Microarray
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADIPOR2 | Adiponectin receptor 2AKT1 |
| V-AKT | Murine thymoma viral oncogene homolog 1 |
| ANG | Andrographolide |
| APOC3 | Apolipoprotein C-III |
| CEBPB | CCAAT/enhancer binding protein (C/EBP), beta |
| CYP2E1 | Cytochrome P450, family, subfamily E, polypeptide 1 |
| CYP7A1 | Cytochrome P450, family 7, subfamily A, polypeptide 1 |
| DILI | Drug-induced liver injury |
| CUR | Curcumin |
| FABP1 | Fatty acid binding protein 1 |
| FAD | Flavin adenin dinucleotide |
| FFAs | Free fatty acids |
| FAS | Fas (TNF receptor superfamily, member 6 |
| FXR | Farnesoid X receptor |
| GK | Glycerol kinase |
| HCC | Hepatocellular carcinoma |
| IL 10 | Interleukin 10 |
| IR | Insulin resistance |
| LDs | Lipid droplets |
| MAPK8 | Mitogen-activated protein kinase 8 |
| NAD | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate reduced |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NF-κB | Nuclear factor kappa B |
| NR1H2 | Nuclear receptor subfamily 1, group H, member 2 |
| PIK3 | Phosphoinositide-3-kinase |
| PIK3CA | Phosphoinositide-3-kinase, catalytic, alpha polypeptide |
| PPAR | Peroxisome proliferator-activated receptor |
| PPARGC1A | Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RXRA | Retinoid X receptor, alpha |
| TGs | Triglycerides |
| TGF-β | Transforming grown factor beta |
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD Disease Burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the Period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Lazarus, J.V.; Newsome, P.N.; Serfaty, L.; Aghemo, A.; Augustin, S.; Tsochatzis, E.; de Ledinghen, V.; Bugianesi, E.; Romero-Gomez, M.; et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis in five European countries in 2018: A cost-of-illness analysis. Liver Int. 2021, 41, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Nonalcoholic Fat. Liver Dis. 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic Steatohepatitis Is the Most Rapidly Growing Indication for Liver Transplantation in Patients with Hepatocellular Carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. Emerg. Trends Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Maurice, J.; Manousou, P. Non-Alcoholic Fatty Liver Disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Steatohepatitis. Highlight Free. Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of Mitochondria in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Mann, J.P.; Raponi, M.; Nobili, V. Clinical Implications of Understanding the Association between Oxidative Stress and Pediatric NAFLD. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 321–350. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Chen, Y.; Li, X.; Lu, Y. The Role and Mechanism of Oxidative Stress and Nuclear Receptors in the Development of NAFLD. Oxid. Med. Cell. Longev. 2021, 2021, 6889533. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, W.; Zhang, C.; Chen, F.; Tan, H.Y.; Li, S.; Wang, N.; Feng, Y. Herbal Medicine in the Treatment of Non-Alcoholic Fatty Liver Diseases-Efficacy, Action Mechanism, and Clinical Application. Front. Pharmacol. 2020, 11, 601. [Google Scholar] [CrossRef]
- Sun, Q.; Niu, Q.; Guo, Y.; Zhuang, Y.; Li, X.; Liu, J.; Li, N.; Li, Z.; Huang, F.; Qiu, Z. Regulation on Citrate Influx and Metabolism through Inhibiting SLC13A5 and ACLY: A Novel Mechanism Mediating the Therapeutic Effects of Curcumin on NAFLD. J. Agric. Food Chem. 2021, 69, 8714–8725. [Google Scholar] [CrossRef]
- Lee, E.S.; Kwon, M.H.; Kim, H.M.; Woo, H.B.; Ahn, C.M.; Chung, C.H. Curcumin analog CUR5-8 ameliorates nonalcoholic fatty liver disease in mice with high-fat diet-induced obesity. Metabolism 2020, 103, 154015. [Google Scholar] [CrossRef]
- Cabrera, D.; Wree, A.; Povero, D.; Solís, N.; Hernandez, A.; Pizarro, M.; Moshage, H.; Torres, J.; Feldstein, A.E.; Cabello-Verrugio, C.; et al. Andrographolide Ameliorates Inflammation and Fibrogenesis and Attenuates Inflammasome Activation in Experimental Non-Alcoholic Steatohepatitis. Sci. Rep. 2017, 7, 3491. [Google Scholar] [CrossRef]
- Liang, W.-F.; Gong, Y.-X.; Li, H.-F.; Sun, F.-L.; Li, W.-L.; Chen, D.-Q.; Xie, D.-P.; Ren, C.-X.; Guo, X.-Y.; Wang, Z.-Y.; et al. Curcumin Activates ROS Signaling to Promote Pyroptosis in Hepatocellular Carcinoma HepG2 Cells. In Vivo 2021, 35, 249–257. [Google Scholar] [CrossRef]
- Shan, D.; Wang, J.; Di, Q.; Jang, Q.; Xu, Q. Steatosis induced by nonylphenol in HepG2 cells and the intervention effect of curcumin. Food Funct. 2022, 13, 327–343. [Google Scholar] [CrossRef]
- An, S.; Jang, E.; Lee, J.-H. Preclinical Evidence of Curcuma Longa and Its Noncurcuminoid Constituents against Hepatobiliary Diseases: A Review. Evid. Based Complement. Alternat. Med. 2020, 2020, 8761435. [Google Scholar] [CrossRef] [PubMed]
- Ngu, M.H.; Norhayati, M.N.; Rosnani, Z.; Zulkifli, M.M. Curcumin as adjuvant treatment in patients with non-alcoholic fatty liver (NAFLD) disease: A systematic review and meta-analysis. Complement. Ther. Med. 2022, 68, 102843. [Google Scholar] [CrossRef] [PubMed]
- White, C.M.; Lee, J.Y. The impact of turmeric or its curcumin extract on nonalcoholic fatty liver disease: A systematic review of clinical trials. Pharm. Pract. 2019, 17, 1350. [Google Scholar] [CrossRef]
- Jayakumar, T.; Hsieh, C.-Y.; Lee, J.-J.; Sheu, J.-R. Experimental and Clinical Pharmacology of Andrographis paniculata and Its Major Bioactive Phytoconstituent Andrographolide. Evid. Based Complement. Alternat. Med. 2013, 2013, 846740. [Google Scholar] [CrossRef] [PubMed]
- Toppo, E.; Darvin, S.S.; Esakkimuthu, S.; Nayak, M.K.; Balakrishna, K.; Sivasankaran, K.; Pandikumar, P.; Ignacimuthu, S.; Al-Dhabi, N.A. Effect of two andrographolide derivatives on cellular and rodent models of non-alcoholic fatty liver disease. Biomed. Pharmacother. 2017, 95, 402–411. [Google Scholar] [CrossRef]
- Chua, L.S. Review on Liver Inflammation and Antiinflammatory Activity of Andrographis paniculata for Hepatoprotection. Phytother. Res. 2014, 28, 1589–1598. [Google Scholar] [CrossRef]
- Campos-Espinosa, A.; Guzman, C. A model of experimental steatosis in vitro: Hepatocyte cell culture in lipid overload-conditioned medium. J. Vis. Exp. 2021, 171, e62543. [Google Scholar] [CrossRef]
- Jieliang, L.; Cheung, H.Y.; Zhiqiang, Z.; Chan, G.K.L.; Fong, W.F. Andrographolide induces cell cycle arrest at G2/M phase and cell death in HepG2 cells via alteration of reactive oxygen species. Eur. J. Phar. 2007, 568, 31–44. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023. ahead of print. [Google Scholar] [CrossRef]
- Francque, S.M.; Marchesini, G.; Kautz, A.; Walmsley, M.; Dorner, R.; Lazarus, J.V.; Zelber-Sagi, S.; Hallsworth, K.; Busetto, L.; Frühbeck, G.; et al. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep. 2021, 3, 100322. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef]
- Cong, T.; Hongfei, W.; Da, G.; Yanian, L.; Yuhan, F.; Jiahui, Z.; Wei, D. Effect of curcumin on the non-alcoholic steatohepatitis via inhibiting the M1 polarization of macrophages. Hum. Exp. Toxicol. 2021, 40 (Suppl. 12), S310–S317. [Google Scholar] [CrossRef]
- Sikora, E.; Scapagnini, G.; Barbagallo, M. Curcumin, inflammation, ageing and age-related diseases. Immun. Ageing 2010, 7, 1. [Google Scholar] [CrossRef]
- Sun, Y.K.; Zhang, Y.F.; Xie, L.; Rong, F.; Zhu, X.Y.; Xie, J.; Zhou, H.; Xu, T. Progress in the treatment of drug-induced liver injury with natural products. Pharmacol. Res. 2022, 183, 106361. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute liver injury following turmeric use in Tuscany: An analysis of the Italian Phytovigilance database and systematic review of case reports. Br. J. Clin. Pharmacol. 2021, 87, 741–753. [Google Scholar] [CrossRef]
- Fernández Bermejo, M.; Masa Caballero, A.; Lozano Lozano, Á. Curcumin and curcumoids: Hepatoprotection or hepatotoxicity? Rev. Esp. Enferm. Dig. 2022, 114, 304–305. [Google Scholar] [CrossRef]
- Venmathi Maran, B.A.; Iqbal, M.; Gangadaran, P.; Ahn, B.C.; Rao, P.V.; Shah, M.S. Hepatoprotective Potential of Malaysian Medicinal Plants: A Review on Phytochemicals, Oxidative Stress, and Antioxidant Mechanisms. Molecules 2022, 27, 1533. [Google Scholar] [CrossRef]
- Burgos, R.A.; Alarcón, P.; Quiroga, J.; Manosalva, C.; Hancke, J. Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism. Molecules 2021, 26, 5. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018, 8, 2039. [Google Scholar] [CrossRef]
- Wu, T.; Peng, Y.; Yan, S.; Li, N.; Chen, Y.; Lan, T. Andrographolide Ameliorates Atherosclerosis by Suppressing Pro-Inflammation and ROS Generation-Mediated Foam Cell Formation. Inflammation 2018, 4, 1681–1689. [Google Scholar] [CrossRef]
- Khalifa, O.; AL-Akl, N.S.; Errafii, K.; Arredouani, A. Exendin-4 alleviates steatosis in an in vitro cell model by lowering FABP1 and FOXA1 expression via the Wnt/-catenin signaling pathway. Sci. Rep. 2022, 12, 2226. [Google Scholar] [CrossRef]
- Pratelli, G.; Carlisi, D.; D’Anneo, A.; Maggio, A.; Emanuele, S.; Palumbo Piccionello, A.; Giuliano, M.; De Blasio, A.; Calvaruso, G.; Lauricella, M. Bio-Waste Products of Mangifera indica L. Reduce Adipogenesis and Exert Antioxidant Effects on 3T3-L1 Cells. Antioxidants 2022, 11, 363. [Google Scholar] [CrossRef]
- Abdulaziz, B.A.; Abdu, S.A.; Amin, A.M.; El Menyawi, A.K.A.H.; Ahmed, A.; Khalil, M.A.; Halim, W.A.A. Assessment of liver fatty acid binding protein (L-FABP) as a diagnostic marker in non-alcoholic fatty liver disease. Open J. Gastroenterol. 2019, 9, 113–124. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chang, C.C.; Wang, C.P.; Hung, W.C.; Tsai, I.T.; Tang, W.H.; Wu, C.C.; Wei, C.T.; Chung, F.M.; Lee, Y.J.; et al. Circulating fatty acid-binding protein 1 (FABP1) and nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Int. J. Med. Sci. 2020, 17, 182–190. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipitone, R.M.; Zito, R.; Lupo, G.; Javed, A.; La Mantia, C.; Di Maria, G.; Pratelli, G.; Di Salvo, F.; Fontana, S.; Pucci, M.; et al. Curcumin and Andrographolide Co-Administration Safely Prevent Steatosis Induction and ROS Production in HepG2 Cell Line. Molecules 2023, 28, 1261. https://doi.org/10.3390/molecules28031261
Pipitone RM, Zito R, Lupo G, Javed A, La Mantia C, Di Maria G, Pratelli G, Di Salvo F, Fontana S, Pucci M, et al. Curcumin and Andrographolide Co-Administration Safely Prevent Steatosis Induction and ROS Production in HepG2 Cell Line. Molecules. 2023; 28(3):1261. https://doi.org/10.3390/molecules28031261
Chicago/Turabian StylePipitone, Rosaria Maria, Rossella Zito, Giulia Lupo, Ayesha Javed, Claudia La Mantia, Gabriele Di Maria, Giovanni Pratelli, Francesca Di Salvo, Simona Fontana, Marzia Pucci, and et al. 2023. "Curcumin and Andrographolide Co-Administration Safely Prevent Steatosis Induction and ROS Production in HepG2 Cell Line" Molecules 28, no. 3: 1261. https://doi.org/10.3390/molecules28031261
APA StylePipitone, R. M., Zito, R., Lupo, G., Javed, A., La Mantia, C., Di Maria, G., Pratelli, G., Di Salvo, F., Fontana, S., Pucci, M., Carlisi, D., & Grimaudo, S. (2023). Curcumin and Andrographolide Co-Administration Safely Prevent Steatosis Induction and ROS Production in HepG2 Cell Line. Molecules, 28(3), 1261. https://doi.org/10.3390/molecules28031261