Aluminum Salen Complexes Modified with Unsaturated Alcohol: Synthesis, Characterization, and Their Activity towards Ring-Opening Polymerization of ε-Caprolactone and D,L-Lactide
Abstract
1. Introduction
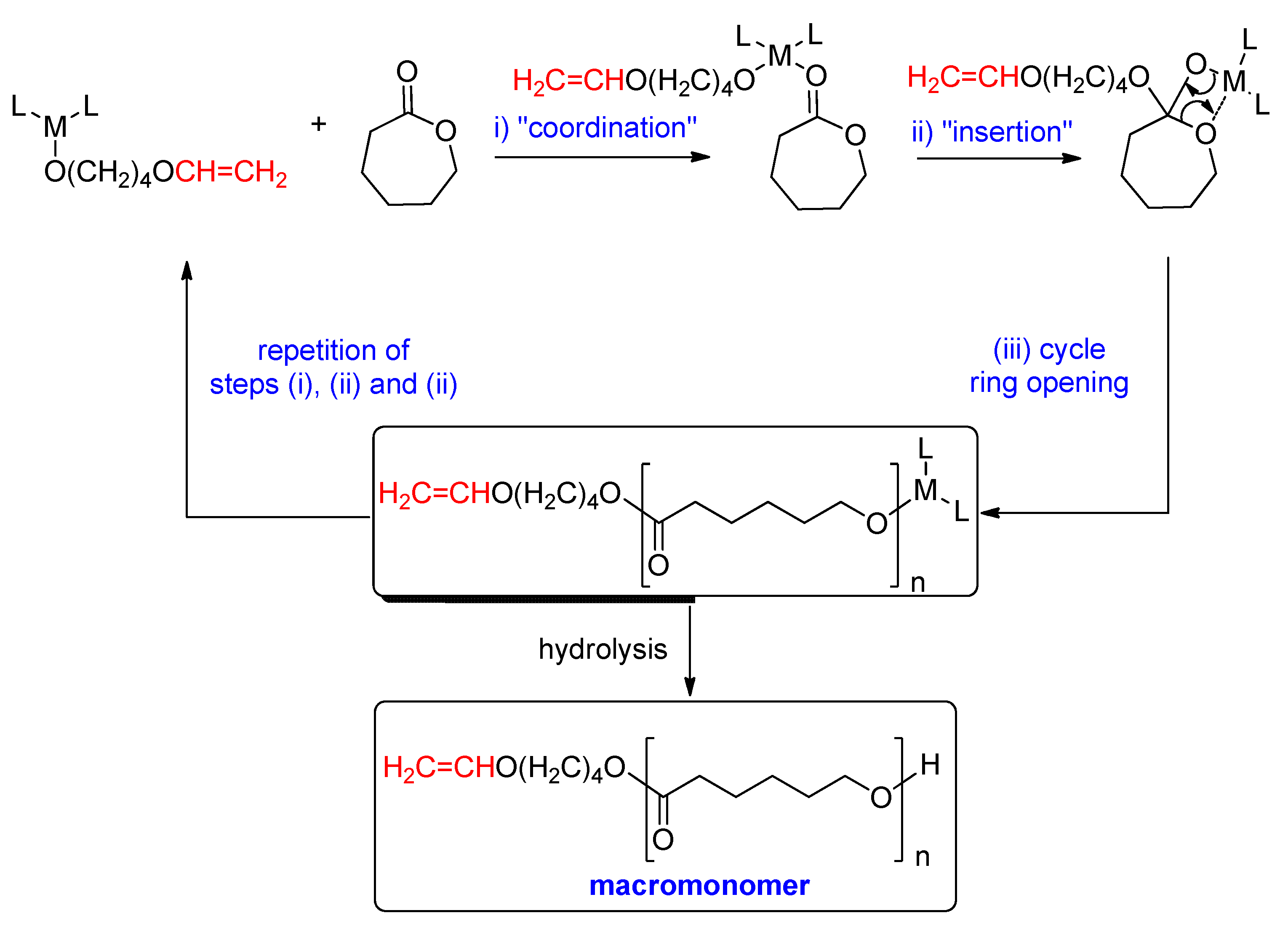
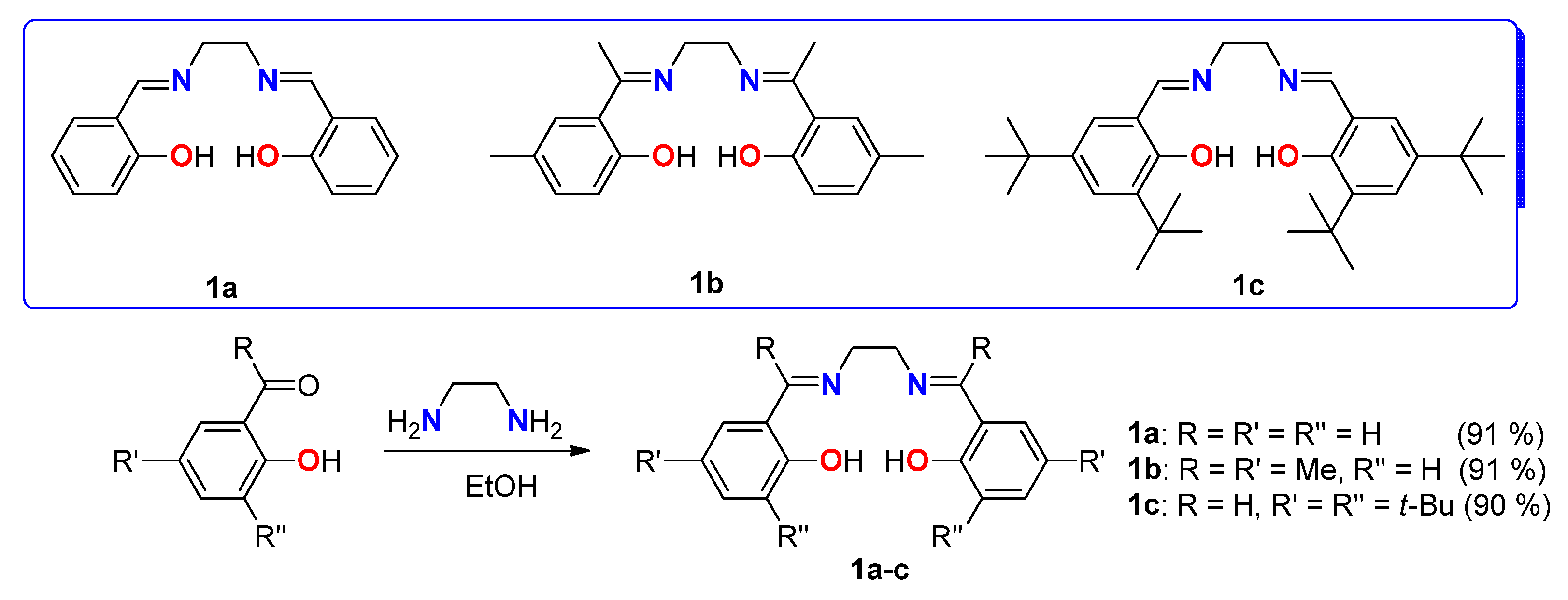
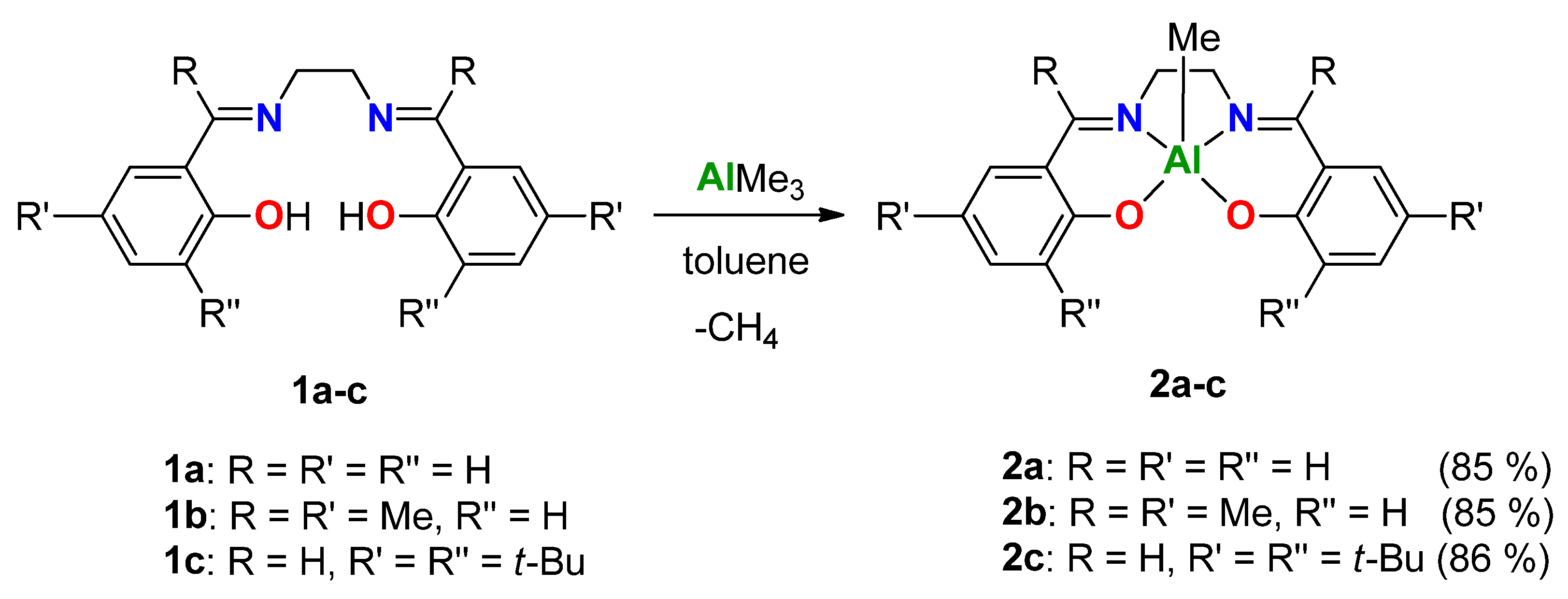
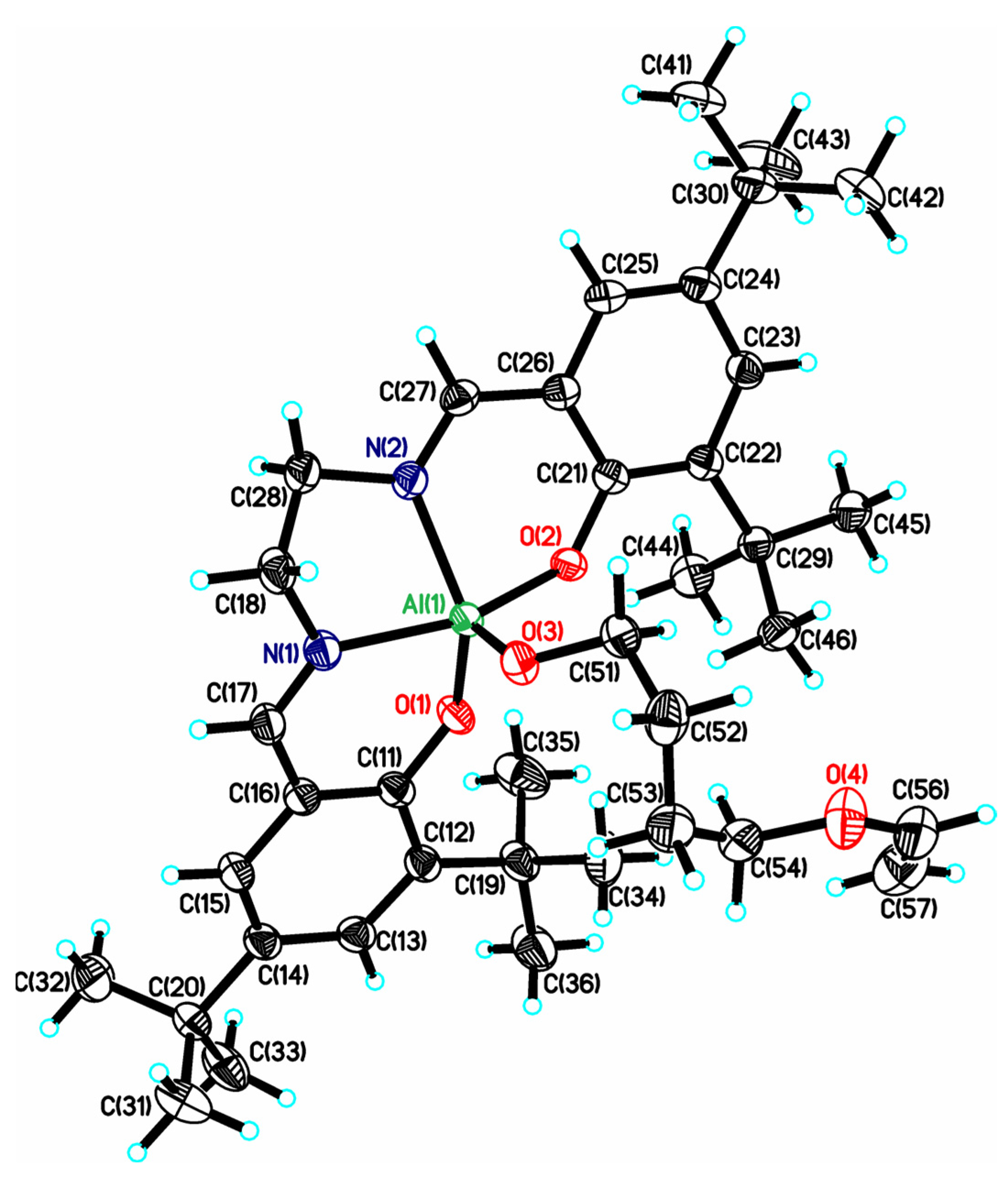
2. Results
2.1. Synthesis of Ligands and Complexes
2.2. X-ray Diffraction Analysis
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sigman, M.S.; Jacobsen, E.N. Enantioselective Addition of Hydrogen Cyanide to Imines Catalyzed by a Chiral (Salen)Al(III) Complex. J. Am. Chem. Soc. 1998, 120, 5315–5316. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef]
- Taylor, M.S.; Zalatan, D.N.; Lerchner, A.M.; Jacobsen, E.N. Highly Enantioselective Conjugate Additions to α,β-Unsaturated Ketones Catalyzed by a (Salen)Al Complex. J. Am. Chem. Soc. 2005, 127, 1313–1317. [Google Scholar] [CrossRef]
- Gualandi, A.; Calogero, F.; Potenti, S.; Cozzi, P.G. Al(Salen) Metal Complexes in Stereoselective Catalysis. Molecules 2019, 24, 1716. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, D.; Álvarez-Barcia, S.; Meisner, J.; Broghammer, F.; Klepp, J.; Garnier, D.; Frey, W.; Kästner, J.; Peters, R. Asymmetric Carboxycyanation of Aldehydes by Cooperative AlF/Onium Salt Catalysts: From Cyanoformate to KCN as Cyanide Source. Chem.–Eur. J. 2019, 25, 1515–1524. [Google Scholar] [CrossRef]
- Xia, Q.; Yuan, C.; Li, Y.; Cui, Y. Design and assembly of a chiral composite metal–organic framework for efficient asymmertric sequential transformation of alkenes to amino alcohols. Chem. Commun. 2019, 55, 9136–9139. [Google Scholar] [CrossRef]
- Lamb, J.R.; Hubbell, A.K.; MacMillan, S.N.; Coates, G.W. Carbonylative, Catalytic Deoxygenation of 2,3-Disubstituted Epoxides with Inversion of Stereochemistry: An Alternative Alkene Isomerization Method. J. Am. Chem. Soc. 2020, 142, 8029–8035. [Google Scholar] [CrossRef]
- Fish, H.; Hart, S.; Lamb, K.J.; North, M.; Quek, S.C.Z.; Whitwood, A.C.; Woods, B.; Wu, X. Structural analysis of five-coordinate aluminium(salen) complexes and its relationship to their catalytic activity. Dalton Trans. 2021, 50, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Sengoden, M.; North, M.; Whitwood, A.C. Synthesis of Oxazolidinones by using Carbon Dioxide as a C1 Building Block and an Aluminium-Based Catalyst. ChemSusChem 2019, 12, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Quek, S.C.Z.; Pridmore, N.E.; Whitwood, A.C.; Wu, X. Aluminum(salen) Complexes as Catalysts for the Kinetic Resolution of Terminal Epoxides via CO2 Coupling. ACS Catal. 2015, 5, 3398–3402. [Google Scholar] [CrossRef]
- Laiwattanapaisarn, N.; Virachotikul, A.; Phomphrai, K. Cycloaddition of carbon dioxide to epoxides by highly active constrained aluminum chloride complexes. Dalton Trans. 2021, 50, 11039–11048. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Oprunenko, Y.F.; Churakov, A.V.; Zaitseva, G.S.; Karlov, S.S. The reaction of Al(O-i-Pr)3 with the SalenH2 ligand: An unexpected product. Polyhedron 2014, 81, 312–315. [Google Scholar] [CrossRef]
- Mei, Y.; Borger, J.E.; Wu, D.-J.; Grützmacher, H. Salen supported Al–O–CP and Ga–PCO complexes. Dalton Trans. 2019, 48, 4370–4374. [Google Scholar] [CrossRef]
- Lichtenberger, R.; Schubert, U. Chemical modification of aluminium alkoxides for sol-gel processing. J. Mater. Chem. 2010, 20, 9287–9296. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Lee, M.H.; Jang, H.; Sung, Y.; Lee, J.S.; Kim, S.H.; Do, Y. Aluminium-salen luminophores as new hole-blocking materials for phosphorescent OLEDs. Dalton Trans. 2008, 14, 1818–1820. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Kim, H.; Lee, Y.S.; Lee, M.H.; Do, Y. Synthesis and Properties of Salen–Aluminum Complexes as a Novel Class of Color-Tunable Luminophores. Chem.–Eur. J. 2009, 15, 6478–6487. [Google Scholar] [CrossRef]
- Kwak, S.W.; Jin, H.; Shin, H.; Lee, J.H.; Hwang, H.; Lee, J.; Kim, M.; Chung, Y.; Kim, Y.; Lee, K.M.; et al. A salen–Al/carbazole dyad-based guest–host assembly: Enhancement of luminescence efficiency via intramolecular energy transfer. Chem. Commun. 2018, 54, 4712–4715. [Google Scholar] [CrossRef]
- Kireenko, M.M.; Kuchuk, E.A.; Zaitsev, K.V.; Tafeenko, V.A.; Oprunenko, Y.F.; Churakov, A.V.; Lermontova, E.K.; Zaitseva, G.S.; Karlov, S.S. Aluminum complexes based on pyridine substituted alcohols: Synthesis, structure, and catalytic application in ROP. Dalton Trans. 2015, 44, 11963–11976. [Google Scholar] [CrossRef]
- Hormnirun, P.; Marshall, E.L.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Remarkable Stereocontrol in the Polymerization of Racemic Lactide Using Aluminum Initiators Supported by Tetradentate Aminophenoxide Ligands. J. Am. Chem. Soc. 2004, 126, 2688–2689. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereoselective Ring-Opening Polymerization of meso-Lactide: Synthesis of Syndiotactic Poly(lactic acid). J. Am. Chem. Soc. 1999, 121, 4072–4073. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Billodeaux, D.R. Aluminum Salen Complexes and Tetrabutylammonium Salts: A Binary Catalytic System for Production of Polycarbonates from CO2 and Cyclohexene Oxide. Inorg. Chem. 2005, 44, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Marlier, E.E.; Macaranas, J.A.; Marell, D.J.; Dunbar, C.R.; Johnson, M.A.; DePorre, Y.; Miranda, M.O.; Neisen, B.D.; Cramer, C.J.; Hillmyer, M.A.; et al. Mechanistic Studies of ε-Caprolactone Polymerization by (salen)AlOR Complexes and a Predictive Model for Cyclic Ester Polymerizations. ACS Catal. 2016, 6, 1215–1224. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Kuchuk, E.A.; Mankaev, B.N.; Churakov, A.V.; Zaitseva, G.S.; Lemenovskii, D.A.; Karlov, S.S. Synthesis, structure, and catalytic activity of new aluminum complexes formed with sterically bulky ligands. Russ. Chem. Bull. 2014, 63, 2630–2634. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef]
- Dijkstra, P.J.; Du, H.; Feijen, J. Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym. Chem. 2011, 2, 520–527. [Google Scholar] [CrossRef]
- Guillaume, S.M.; Kirillov, E.; Sarazin, Y.; Carpentier, J.-F. Beyond Stereoselectivity, Switchable Catalysis: Some of the Last Frontier Challenges in Ring-Opening Polymerization of Cyclic Esters. Chem.–Eur. J. 2015, 21, 7988–8003. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Cherepakhin, V.S.; Zherebker, A.; Kononikhin, A.; Nikolaev, E.; Churakov, A.V. Aluminum Complexes Based on Tridentate Amidoalkoxide NNO-Ligands: Synthesis, Structure, and Properties. J. Organomet. Chem. 2018, 875, 11–23. [Google Scholar] [CrossRef]
- Fedulin, A.I.; Churakov, A.V.; Zaitsev, K.V. Methyl aluminum complexes based on tridentate 2,6-bis(mercapto)pyridinyl SNS-ligands. Mendeleev Commun. 2021, 31, 847–849. [Google Scholar] [CrossRef]
- Moon, S.I.; Lee, C.W.; Miyamoto, M.; Kimura, Y. Melt polycondensation of L-lactic acid with Sn(II) catalysts activated by various proton acids: A direct manufacturing route to high molecular weight Poly(L-lactic acid). J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1673–1679. [Google Scholar] [CrossRef]
- Marques, D.A.S.; Jarmelo, S.; G. Baptista, C.M.S.; Gil, M.H. Poly(lactic acid) Synthesis in Solution Polymerization. Macromol. Symp. 2010, 296, 63–71. [Google Scholar] [CrossRef]
- Spassky, N.; Wisniewski, M.; Pluta, C.; Le Borgne, A. Highly stereoelective polymerization of rac-(D,L)-lactide with a chiral Schiff’s base/aluminium alkoxide initiator. Macromol. Chem. Phys. 1996, 197, 2627–2637. [Google Scholar] [CrossRef]
- Miranda, M.O.; DePorre, Y.; Vazquez-Lima, H.; Johnson, M.A.; Marell, D.J.; Cramer, C.J.; Tolman, W.B. Understanding the Mechanism of Polymerization of ε-Caprolactone Catalyzed by Aluminum Salen Complexes. Inorg. Chem. 2013, 52, 13692–13701. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, M.H.; Patmore, N.J.; Zhou, Z. Concerning the relative importance of enantiomorphic site vs. chain end control in the stereoselective polymerization of lactides: Reactions of (R,R-salen)- and (S,S-salen)-aluminium alkoxides LAlOCH2R complexes (R = CH3 and S-CHMeCl). Chem. Commun. 2005, 1, 127–129. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Hermsen, I.; Noordzij, G.J.; Duchateau, R. Molecular Structure–Catalytic Activity Relationship in the Ring-Opening Polymerization of (Macro)lactones. Macromolecules 2016, 49, 796–806. [Google Scholar] [CrossRef]
- Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Stereoselective Ring-Opening Polymerization of a Racemic Lactide by Using Achiral Salen– and Homosalen–Aluminum Complexes. Chem.–Eur. J. 2007, 13, 4433–4451. [Google Scholar] [CrossRef]
- Majerska, K.; Duda, A. Stereocontrolled Polymerization of Racemic Lactide with Chiral Initiator: Combining Stereoelection and Chiral Ligand-Exchange Mechanism. J. Am. Chem. Soc. 2004, 126, 1026–1027. [Google Scholar] [CrossRef]
- Breteler, M.R.T.; Zhong, Z.; Dijkstra, P.J.; Palmans, A.R.A.; Peeters, J.; Feijen, J. Ring-opening polymerization of substituted ε-caprolactones with a chiral (salen) AlOiPr complex. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 429–436. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. Controlled and Stereoselective Polymerization of Lactide: Kinetics, Selectivity, and Microstructures. J. Am. Chem. Soc. 2003, 125, 11291–11298. [Google Scholar] [CrossRef]
- van der Meulen, I.; Gubbels, E.; Huijser, S.; Sablong, R.; Koning, C.E.; Heise, A.; Duchateau, R. Catalytic Ring-Opening Polymerization of Renewable Macrolactones to High Molecular Weight Polyethylene-like Polymers. Macromolecules 2011, 44, 4301–4305. [Google Scholar] [CrossRef]
- Cross, E.D.; Allan, L.E.N.; Decken, A.; Shaver, M.P. Aluminum salen and salan complexes in the ring-opening polymerization of cyclic esters: Controlled immortal and copolymerization of rac-β-butyrolactone and rac-lactide. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1137–1146. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Bouyahyi, M.; Heise, A.; Duchateau, R. Kinetic Investigation on the Catalytic Ring-Opening (Co)Polymerization of (Macro)Lactones Using Aluminum Salen Catalysts. Macromolecules 2013, 46, 4324–4334. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Gallucci, J.C.; Quisenberry, K.T.; Zhou, Z. Complexities in the Ring-Opening Polymerization of Lactide by Chiral Salen Aluminum Initiators. Inorg. Chem. 2008, 47, 2613–2624. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. [(salen)Al]-Mediated, Controlled and Stereoselective Ring-Opening Polymerization of Lactide in Solution and without Solvent: Synthesis of Highly Isotactic Polylactide Stereocopolymers from Racemic D,L-Lactide. Angew. Chem. Int. Ed. 2002, 41, 4510–4513. [Google Scholar] [CrossRef]
- Pang, X.; Duan, R.; Li, X.; Hu, C.; Wang, X.; Chen, X. Breaking the Paradox between Catalytic Activity and Stereoselectivity: Rac-Lactide Polymerization by Trinuclear Salen–Al Complexes. Macromolecules 2018, 51, 906–913. [Google Scholar] [CrossRef]
- Gaston, A.J.; Navickaite, G.; Nichol, G.S.; Shaver, M.P.; Garden, J.A. Electron rich salen-AlCl catalysts as efficient initiators for the ring-opening polymerisation of rac-lactide. Eur. Polym. J. 2019, 119, 507–513. [Google Scholar] [CrossRef]
- Macaranas, J.A.; Luke, A.M.; Mandal, M.; Neisen, B.D.; Marell, D.J.; Cramer, C.J.; Tolman, W.B. Sterically Induced Ligand Framework Distortion Effects on Catalytic Cyclic Ester Polymerizations. Inorg. Chem. 2018, 57, 3451–3457. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Karroonnirun, O. Stereoselective Ring-Opening Polymerization of rac-Lactides Catalyzed by Chiral and Achiral Aluminum Half-Salen Complexes. Organometallics 2010, 29, 5627–5634. [Google Scholar] [CrossRef]
- Nomura, N.; Akita, A.; Ishii, R.; Mizuno, M. Random Copolymerization of ε-Caprolactone with Lactide Using a Homosalen−Al Complex. J. Am. Chem. Soc. 2010, 132, 1750–1751. [Google Scholar] [CrossRef]
- Chen, H.-L.; Dutta, S.; Huang, P.-Y.; Lin, C.-C. Preparation and Characterization of Aluminum Alkoxides Coordinated on salen-Type Ligands: Highly Stereoselective Ring-Opening Polymerization of rac-Lactide. Organometallics 2012, 31, 2016–2025. [Google Scholar] [CrossRef]
- Jianming, R.; Anguo, X.; Hongwei, W.; Hailin, Y. Review–recent development of ring-opening polymerization of cyclic esters using aluminum complexes. Des. Monomers Polym. 2014, 17, 345–355. [Google Scholar] [CrossRef]
- Gao, B.; Li, D.; Li, Y.; Duan, Q.; Duan, R.; Pang, X. Ring-opening polymerization of lactide using chiral salen aluminum complexes as initiators: High productivity and stereoselectivity. New J. Chem. 2015, 39, 4670–4675. [Google Scholar] [CrossRef]
- Strianese, M.; Pappalardo, D.; Mazzeo, M.; Lamberti, M.; Pellecchia, C. Salen-type aluminum and zinc complexes as two-faced Janus compounds: Contribution to molecular sensing and polymerization catalysis. Dalton Trans. 2020, 49, 16533–16550. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Swisher, J.H.; Meyer, T.Y.; Coates, G.W. Chirality-Directed Regioselectivity: An Approach for the Synthesis of Alternating Poly(Lactic-co-Glycolic Acid). J. Am. Chem. Soc. 2021, 143, 4119–4124. [Google Scholar] [CrossRef]
- Haque, F.M.; Ishibashi, J.S.A.; Lidston, C.A.L.; Shao, H.; Bates, F.S.; Chang, A.B.; Coates, G.W.; Cramer, C.J.; Dauenhauer, P.J.; Dichtel, W.R.; et al. Defining the Macromolecules of Tomorrow through Synergistic Sustainable Polymer Research. Chem. Rev. 2022, 122, 6322–6373. [Google Scholar] [CrossRef]
- Butala, R.R.; Parkin, S.; Walrod, J.H.; Atwood, D.A. Synthesis, Characterization, and Stability of Dealkylated Salen-Supported Aluminum Phosphates. Inorg. Chem. 2021, 60, 4456–4462. [Google Scholar] [CrossRef] [PubMed]
- Piskun, Y.A.; Vasilenko, I.V.; Zaitsev, K.V.; Oprunenko, Y.F.; Kostjuk, S.V. Synthesis of Functional Poly(ε-caprolactone)s via Living Ring-Opening Polymerization of ε-Caprolactone Using Functionalized Aluminum Alkoxides as Initiators. Macromol. Chem. Phys. 2017, 218, 1600580. [Google Scholar] [CrossRef]
- Zhang, M.; Müller, A.H.E. Cylindrical polymer brushes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3461–3481. [Google Scholar] [CrossRef]
- Lee, H.-i.; Pietrasik, J.; Sheiko, S.S.; Matyjaszewski, K. Stimuli-responsive molecular brushes. Prog. Polym. Sci. 2010, 35, 24–44. [Google Scholar] [CrossRef]
- Ishizu, K.; Satoh, J. Synthesis of isopropenylbenzyl-terminated macromonomers and preparation of polymer brushes by anionic homopolymerization. J. Appl. Polym. Sci. 2003, 87, 1790–1793. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic Polyester Block Polymers: Renewable, Degradable, and Sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, G.; Guo, R. Multiple morphologies from amphiphilic graft copolymers based on chitooligosaccharides as backbones and polycaprolactones as branches. Chem. Commun. 2005, 28, 3591–3593. [Google Scholar] [CrossRef]
- Lemmouchi, Y.; Perry, M.C.; Amass, A.J.; Chakraborty, K.; Schacht, E. Novel synthesis of biodegradable amphiphilic linear and star block copolymers based on poly(ε-caprolactone) and poly(ethylene glycol). J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3975–3985. [Google Scholar] [CrossRef]
- Li, B.; Chen, G.; Meng, F.; Li, T.; Yue, J.; Jing, X.; Huang, Y. A novel amphiphilic copolymer poly(ethylene oxide-co-allyl glycidyl ether)-graft-poly(ε-caprolactone): Synthesis, self-assembly, and protein encapsulation behavior. Polym. Chem. 2012, 3, 2421–2429. [Google Scholar] [CrossRef]
- Nouri, S.; Dubois, C.; Lafleur, P.G. Synthesis and characterization of polylactides with different branched architectures. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 522–531. [Google Scholar] [CrossRef]
- Liu, Y.; Schulze, M.; Albertsson, A.C. α-Methacryloyl-ω-Hydroxyl-Poly(ϵ-Caprolactone) Macromonomer: Synthesis, Characterization, and Copolymerization. J. Macromol. Sci. Part A 1998, 35, 207–232. [Google Scholar] [CrossRef]
- Lang, M.; Chu, C.-C. Functionalized multiarm poly(ϵ-caprolactone)s: Synthesis, structure analysis, and network formation. J. Appl. Polym. Sci. 2002, 86, 2296–2306. [Google Scholar] [CrossRef]
- Fu, Q.; Ren, J.M.; Qiao, G.G. Synthesis of novel cylindrical bottlebrush polypseudorotaxane via inclusion complexation of high density poly(ε-caprolactone) bottlebrush polymer and [small alpha]-cyclodextrins. Polym. Chem. 2012, 3, 343–351. [Google Scholar] [CrossRef]
- Eguiburu, J.L.; Fernandez-Berridi, M.J.; Cossío, F.P.; Román, J.S. Ring-Opening Polymerization of l-Lactide Initiated by (2-Methacryloxy)ethyloxy−Aluminum Trialkoxides. 1. Kinetics. Macromolecules 1999, 32, 8252–8258. [Google Scholar] [CrossRef]
- Tian, D.; Dubois, P.; Jerome, R.; Teyssie, P. Macromolecular Engineering of Polylactones and Polylactides. 18. Synthesis of Star-Branched Aliphatic Polyesters Bearing Various Functional End Groups. Macromolecules 1994, 27, 4134–4144. [Google Scholar] [CrossRef]
- Dubois, P.; Ropson, N.; Jérôme, R.; Teyssié, P. Macromolecular Engineering of Polylactones and Polylactides. 19. Kinetics of Ring-Opening Polymerization of ε-Caprolactone Initiated with Functional Aluminum Alkoxides. Macromolecules 1996, 29, 1965–1975. [Google Scholar] [CrossRef]
- Rooney, T.R.; Monyatsi, O.; Hutchinson, R.A. Polyester Macromonomer Syntheses and Radical Copolymerization Kinetics with Styrene. Macromolecules 2017, 50, 784–795. [Google Scholar] [CrossRef]
- Pearce, A.K.; Vasey, C.E.; Anane-Adjei, A.B.; Sodano, F.; Crucitti, V.C.; Irvine, D.J.; Howdle, S.M.; Alexander, C.; Taresco, V. Versatile, Highly Controlled Synthesis of Hybrid (Meth)acrylate–Polyester–Carbonates and their Exploitation in Tandem Post-Polymerization–Functionalization. Macromol. Chem. Phys. 2019, 220, 1900270. [Google Scholar] [CrossRef]
- Luo, S.H.; Xiao, Y.; Lin, J.Y.; Chen, Z.H.; Lin, S.T.; Wang, Z.Y. Preparation, characterization and application of maleic anhydride-modified polylactic acid macromonomer based on direct melt polymerization. Mater. Today Chem. 2022, 25, 100986. [Google Scholar] [CrossRef]
- Fuoco, T.; Chen, M.; Jain, S.; Wang, X.V.; Wang, L.; Finne-Wistrand, A. Hydrogel Polyester Scaffolds via Direct-Ink-Writing of Ad Hoc Designed Photocurable Macromonomer. Polymers 2022, 14, 711. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Xu, Y.; Wang, J.; Shi, Y.; Niu, W.; Song, W.; Liu, R.; Yu, C.-Y.; Wei, H. Facile synthesis and self-assembly behaviors of biodegradable amphiphilic hyperbranched copolymers with reducible poly(caprolactone) grafts. Polym. Chem. 2022, 13, 6162–6170. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, C.; Wu, S.; An, Z. A highly efficient macromonomer approach to core cross-linked star (CCS) polymers via one-step RAFT emulsion polymerization. Polym. Chem. 2014, 5, 4277–4284. [Google Scholar] [CrossRef]
- Rooney, T.R.; Moscatelli, D.; Hutchinson, R.A. Polylactic acid macromonomer radical propagation kinetics and degradation behaviour. React. Chem. Eng. 2017, 2, 487–497. [Google Scholar] [CrossRef]
- Fuoco, T.; Nguyen, T.T.; Kivijärvi, T.; Finne-Wistrand, A. Organocatalytic strategy to telechelic oligo(ε-caprolactone-co-p-dioxanone): Photocurable macromonomers for polyester networks. Eur. Polym. J. 2020, 141, 110098. [Google Scholar] [CrossRef]
- Kongprayoon, A.; Ross, G.; Limpeanchob, N.; Mahasaranon, S.; Punyodom, W.; Topham, P.D.; Ross, S. Bio-derived and biocompatible poly(lactic acid)/silk sericin nanogels and their incorporation within poly(lactide-co-glycolide) electrospun nanofibers. Polym. Chem. 2022, 13, 3343–3357. [Google Scholar] [CrossRef]
- Ma, H.; Ha, S.; Jeong, J.; Wang, V.; Kim, K.T. Synthesis of discrete bottlebrush polymers via the iterative convergent growth technique and post-functionalization. Polym. Chem. 2022, 13, 3689–3695. [Google Scholar] [CrossRef]
- Ksendzov, E.A.; Nikishau, P.A.; Zurina, I.M.; Presniakova, V.S.; Timashev, P.; Rochev, Y.A.; Kotova, S.; Kostjuk, S.V. Graft Copolymers of N-Isopropylacrylamide with Poly(d,l-lactide) or Poly(ε-caprolactone) Macromonomers: A Promising Class of Thermoresponsive Polymers with a Tunable LCST. ACS Appl. Polym. Mater. 2022, 4, 1344–1357. [Google Scholar] [CrossRef]
- Kocen, A.L.; Cui, S.; Lin, T.-W.; LaPointe, A.M.; Coates, G.W. Chemically Recyclable Ester-Linked Polypropylene. J. Am. Chem. Soc. 2022, 144, 12613–12618. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Piskun, Y.A.; Vasilenko, I.V.; Zaitsev, K.V.; Karlov, S.S.; Zaitseva, G.S.; Gaponik, L.V.; Kostjuk, S.V. Controlled homo- and copolymerization of ε-caprolactone and D,L-lactide in the presence of TiIV complexes. Russ. Chem. Bull. 2015, 64, 181–188. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Piskun, Y.A.; Oprunenko, Y.F.; Karlov, S.S.; Zaitseva, G.S.; Vasilenko, I.V.; Churakov, A.V.; Kostjuk, S.V. Controlled ring-opening homo- and copolymerization of ɛ-caprolactone and d,l-lactide by iminophenolate aluminum complexes: An efficient approach toward well-defined macromonomers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1237–1250. [Google Scholar] [CrossRef]
- Iojoiu, C.; Cade, D.; Fessi, H.; Hamaide, T. Synthesis of oligocaprolactone vinyl ether macromonomers and their use for indomethacin encapsulation in polymer nanoparticles based on polycaprolactone macromonomer–maleic anhydride–N-vinyl pyrrolidone terpolymers. Polym. Int. 2006, 55, 222–228. [Google Scholar] [CrossRef]
- Li, P.; Zerroukhi, A.; Chen, J.; Chalamet, Y.; Jeanmaire, T.; Xia, Z. Synthesis, kinetic study, and application of Ti[O(CH2)4OCH=CH2]4 in ring-opening polymerization of ε-caprolactone and radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7773–7784. [Google Scholar] [CrossRef]
- Pantiru, M.; Iojoiu, C.; Hamaide, T.; Delolme, F. Influence of the chemical structure of transfer agents in coordinated anionic ring-opening polymerization: Application to one-step functional oligomerization of ε-caprolactone. Polym. Int. 2004, 53, 506–514. [Google Scholar] [CrossRef]
- Dzugan, S.J.; Goedken, V.L. Factors affecting aluminum-carbon bond reactivity of tetradentate Schiff-base organoaluminum complexes. Inorg. Chem. 1986, 25, 2858–2864. [Google Scholar] [CrossRef]
- Atwood, D.A.; Jegier, J.A.; Rutherford, D. The First Structurally Characterized Salen-Indium Complexes. Bull. Chem. Soc. Jpn. 1997, 70, 2093–2100. [Google Scholar] [CrossRef]
- Atwood, D.A.; Jegier, J.A.; Rutherford, D. A New Class of Aluminum Cations Based upon Tetradentate (N2O2) Chelating Ligands. Inorg. Chem. 1996, 35, 63–70. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Atwood, D.A.; Harvey, M.J. Group 13 Compounds Incorporating Salen Ligands. Chem. Rev. 2001, 101, 37–52. [Google Scholar] [CrossRef]
- Atwood, D.A.; Hill, M.S.; Jegier, J.A.; Rutherford, D. The Use of Five-Coordinate Aluminum Alkyls to Prepare Molecules Containing a Single Al−O−Si Linkage. Organometallics 1997, 16, 2659–2664. [Google Scholar] [CrossRef]
- Munoz-Hernandez, M.-A.; Keizer, T.S.; Wei, P.; Parkin, S.; Atwood, D.A. Reactivity and Derivatization of Five-Coordinate, Chelated Aluminum. Inorg. Chem. 2001, 40, 6782–6787. [Google Scholar] [CrossRef]
- Gurian, P.L.; Cheatham, L.K.; Ziller, J.W.; Barron, A.R. Aluminium complexes of N,N-ethylenebis(salicylideneimine)(H2salen). X-Ray crystal structures of [{Al(salen)}2(.mu.-O)]*MeCN and [Al(OC6H2Me3-2,4,6)(salen)]. J. Chem. Soc. Dalton Trans. 1991, 6, 1449–1456. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Kuchuk, E.A.; Karlov, S.S.; Zaitseva, G.S.; Churakov, A.V. {2,2′-[Ethane-1,2-diylbis(nitrilomethanylylidene)]diphenolato}(isopropanolato)aluminium dichloromethane hemisolvate. Acta Crystallogr. Sect. E 2013, 69, m631–m632. [Google Scholar] [CrossRef]
- Amgoune, A.; Lavanant, L.; Thomas, C.M.; Chi, Y.; Welter, R.; Dagorne, S.; Carpentier, J.F. An aluminum complex supported by a fluorous diamino-dialkoxide ligand for the highly productive ring-opening polymerization of epsilon-caprolactone. Organometallics 2005, 24, 6279–6282. [Google Scholar] [CrossRef]
- Muñoz-Hernandez, M.-A.; Keizer, T.S.; Parkin, S.; Zhang, Y.; Atwood, D.A. Chelated aluminum alkoxides. J. Chem. Crystallogr. 2000, 30, 219–222. [Google Scholar] [CrossRef]
- Pappalardo, D.; Annunziata, L.; Pellecchia, C. Living Ring-Opening Homo- and Copolymerization of ε-Caprolactone and L- and D,L-Lactides by Dimethyl(salicylaldiminato)aluminum Compounds. Macromolecules 2009, 42, 6056–6062. [Google Scholar] [CrossRef]
- Iwasa, N.; Katao, S.; Liu, J.; Fujiki, M.; Furukawa, Y.; Nomura, K. Notable Effect of Fluoro Substituents in the Imino Group in Ring-Opening Polymerization of ε-Caprolactone by Al Complexes Containing Phenoxyimine Ligands. Organometallics 2009, 28, 2179–2187. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Kostjuk, S.V.; Ganachaud, F. Cationic polymerization of isobutyl vinyl ether in aqueous media: Physical chemistry tricks to fight against thermal runaway. Polym. Chem. 2013, 4, 1883–1892. [Google Scholar] [CrossRef]
- Larrow, J.F.; Jacobsen, E.N.; Gao, Y.; Hong, Y.; Nie, X.; Zepp, C.M. A Practical Method for the Large-Scale Preparation of [N,N′-Bis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato(2-)]manganese(III) chloride, a Highly Enantioselective Epoxidation Catalyst. J. Org. Chem. 1994, 59, 1939–1942. [Google Scholar] [CrossRef]
- Kemp, D.S.; Hanson, G. New protective groups for peptide synthesis. 4. Chromone-derived protection for amine and carboxyl functions. J. Org. Chem. 1981, 46, 4971–4975. [Google Scholar] [CrossRef]
- Koley, M.K.; Manoharan, P.T.; Koley, A.P. Synthesis and characterization of a stable paramagnetic hexacoordinated oxochromium(IV) complex with dianionic tetradentate Schiff base ligand salen. Inorg. Chim. Acta 2010, 363, 3798–3802. [Google Scholar] [CrossRef]
- Chen, F.-X.; Liu, X.; Qin, B.; Zhou, H.; Feng, X.; Zhang, G. Highly Efficient Double-Activation Catalysts for the Synthesis of Ketone Cyanohydrins. Synthesis 2004, 2004, 2266–2272. [Google Scholar] [CrossRef]


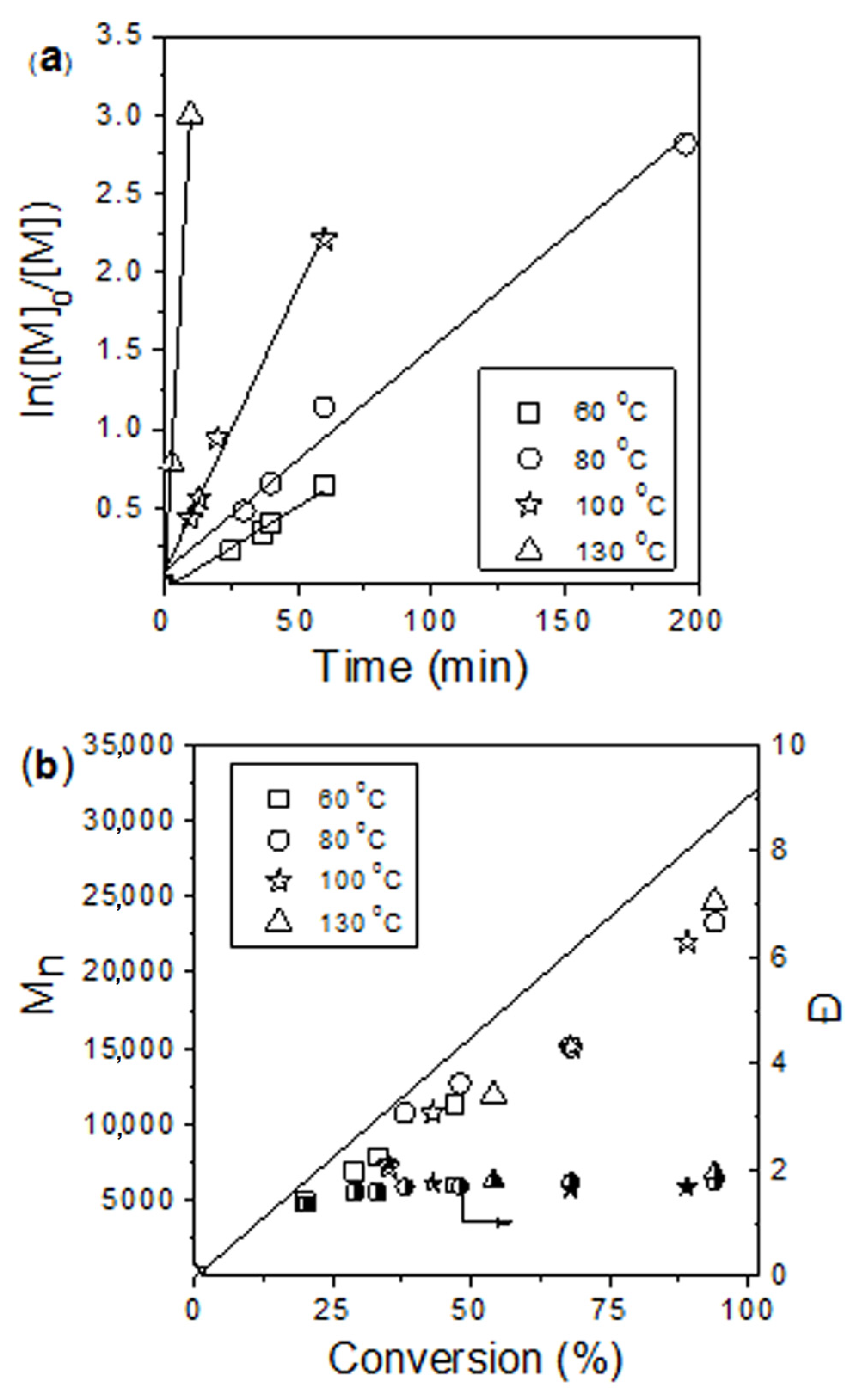
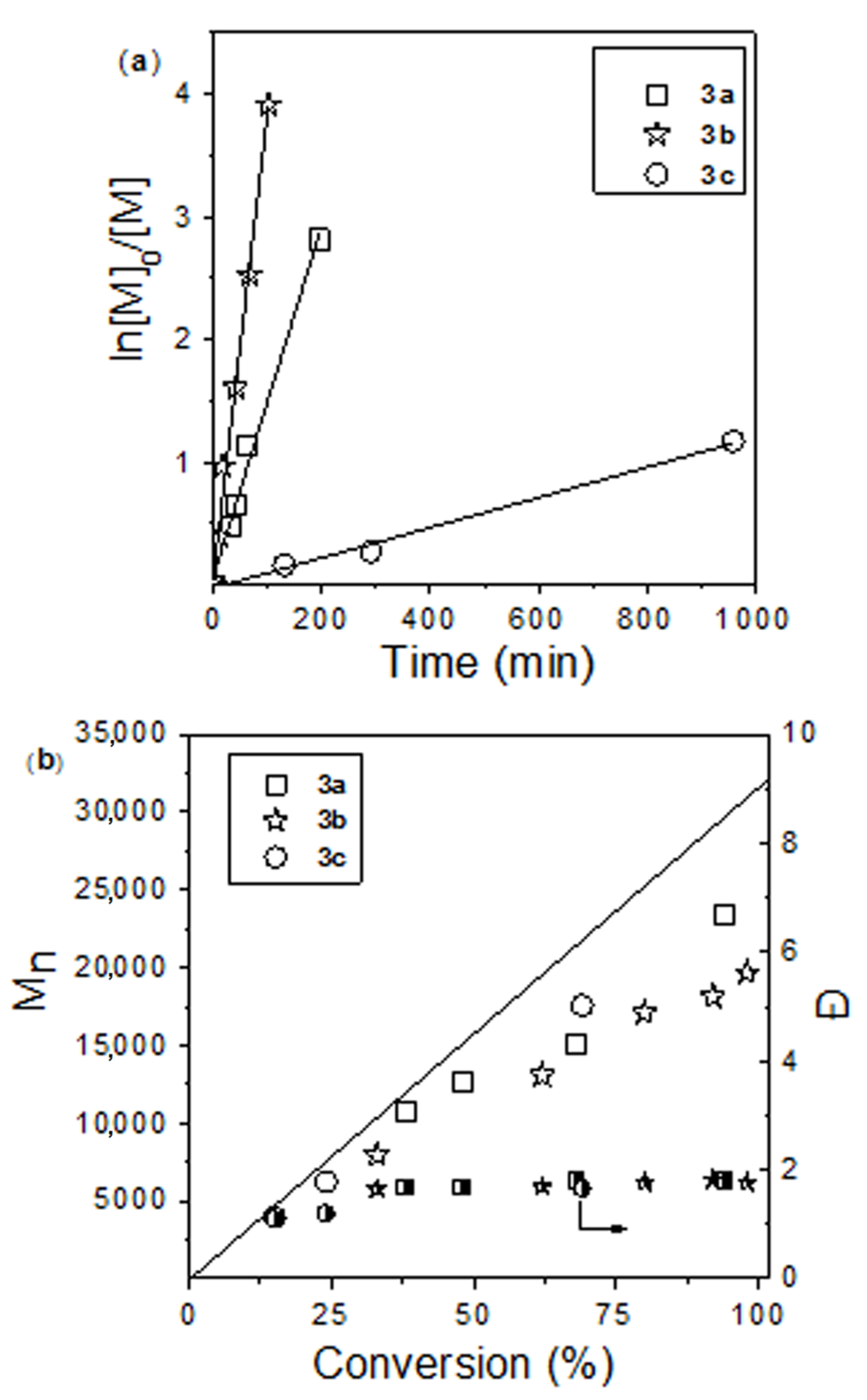

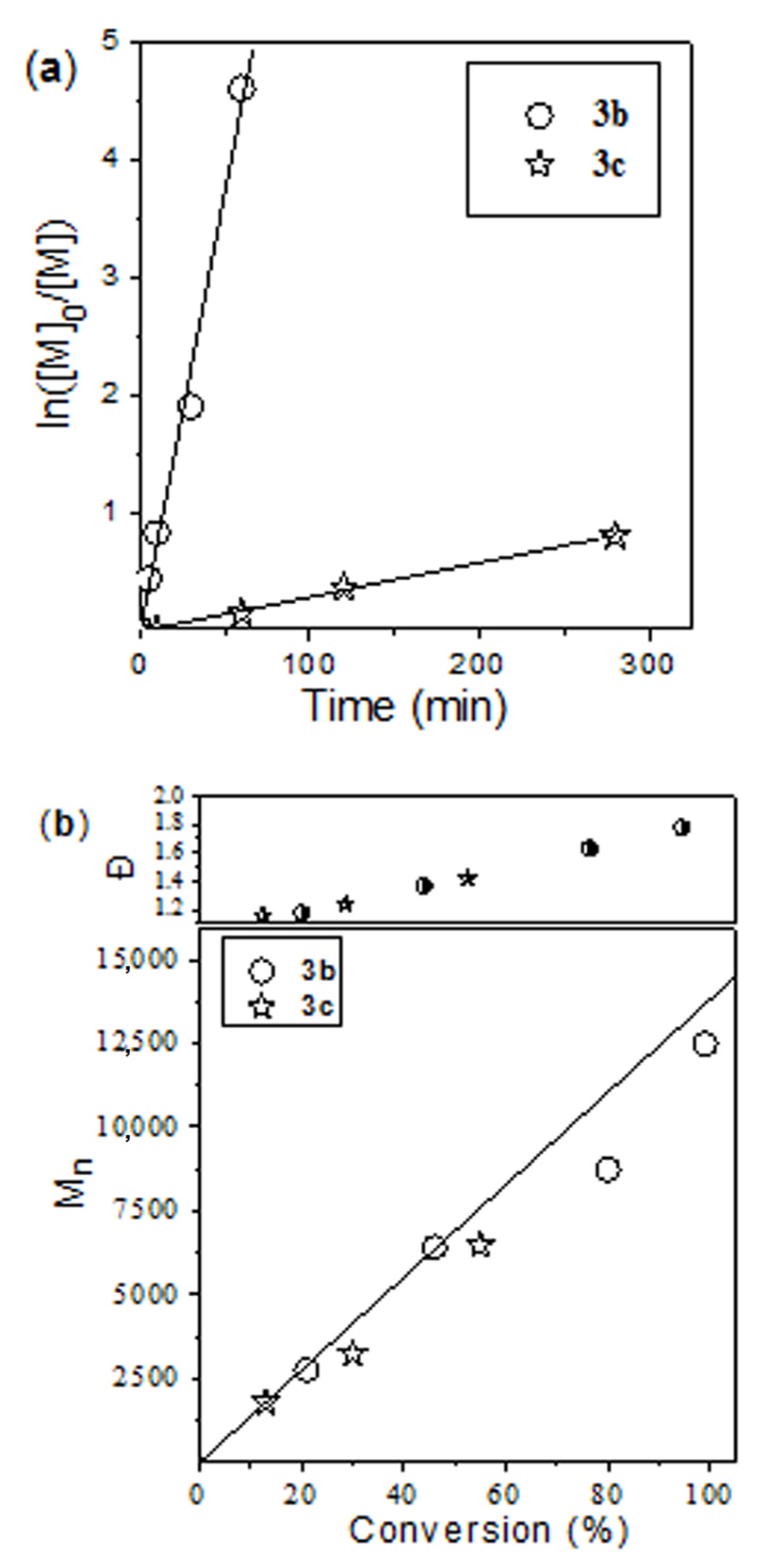
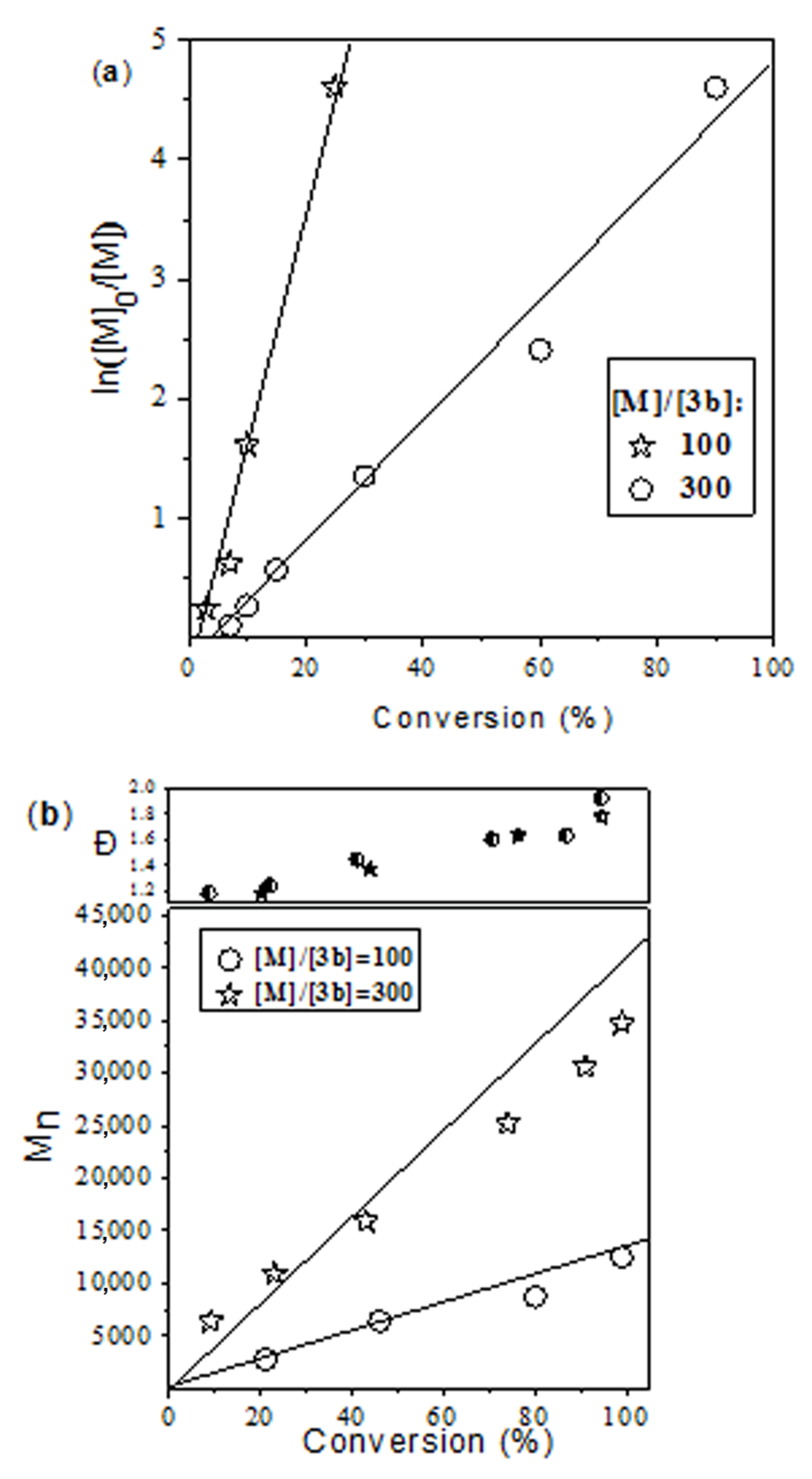
| Catalyst | [M]/[cat] | Time (h) | Conversion (%) | Mn (theor) (g mol−1) 2 | Mn (g mol−1) 3 | Ð | Fn (%) 4 |
|---|---|---|---|---|---|---|---|
| 3a | 300 | 3.2 | 94 | 32,150 | 23,400 | 1.70 | 38 |
| 3b | 300 | 1.7 | 100 | 34,200 | 19,600 | 1.77 | 63 |
| 100 | 0.25 | 100 | 11,500 | 10,500 | 1.71 | 79 | |
| 3c | 300 | 16 | 69 | 23,600 | 17,600 | 1.71 | 68 |
| 100 | 3.2 | 100 | 11,500 | 13,100 | 1.65 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, K.V.; Trubachev, A.D.; Oprunenko, Y.F.; Piskun, Y.A.; Vasilenko, I.V.; Churakov, A.V.; Kostjuk, S.V. Aluminum Salen Complexes Modified with Unsaturated Alcohol: Synthesis, Characterization, and Their Activity towards Ring-Opening Polymerization of ε-Caprolactone and D,L-Lactide. Molecules 2023, 28, 1262. https://doi.org/10.3390/molecules28031262
Zaitsev KV, Trubachev AD, Oprunenko YF, Piskun YA, Vasilenko IV, Churakov AV, Kostjuk SV. Aluminum Salen Complexes Modified with Unsaturated Alcohol: Synthesis, Characterization, and Their Activity towards Ring-Opening Polymerization of ε-Caprolactone and D,L-Lactide. Molecules. 2023; 28(3):1262. https://doi.org/10.3390/molecules28031262
Chicago/Turabian StyleZaitsev, Kirill V., Andrey D. Trubachev, Yuri F. Oprunenko, Yuliya A. Piskun, Irina V. Vasilenko, Andrei V. Churakov, and Sergei V. Kostjuk. 2023. "Aluminum Salen Complexes Modified with Unsaturated Alcohol: Synthesis, Characterization, and Their Activity towards Ring-Opening Polymerization of ε-Caprolactone and D,L-Lactide" Molecules 28, no. 3: 1262. https://doi.org/10.3390/molecules28031262
APA StyleZaitsev, K. V., Trubachev, A. D., Oprunenko, Y. F., Piskun, Y. A., Vasilenko, I. V., Churakov, A. V., & Kostjuk, S. V. (2023). Aluminum Salen Complexes Modified with Unsaturated Alcohol: Synthesis, Characterization, and Their Activity towards Ring-Opening Polymerization of ε-Caprolactone and D,L-Lactide. Molecules, 28(3), 1262. https://doi.org/10.3390/molecules28031262









