Catechol-O-methyltransferase Inhibitors from Calendula officinalis Leaf
Abstract
1. Introduction
2. Results and Discussion
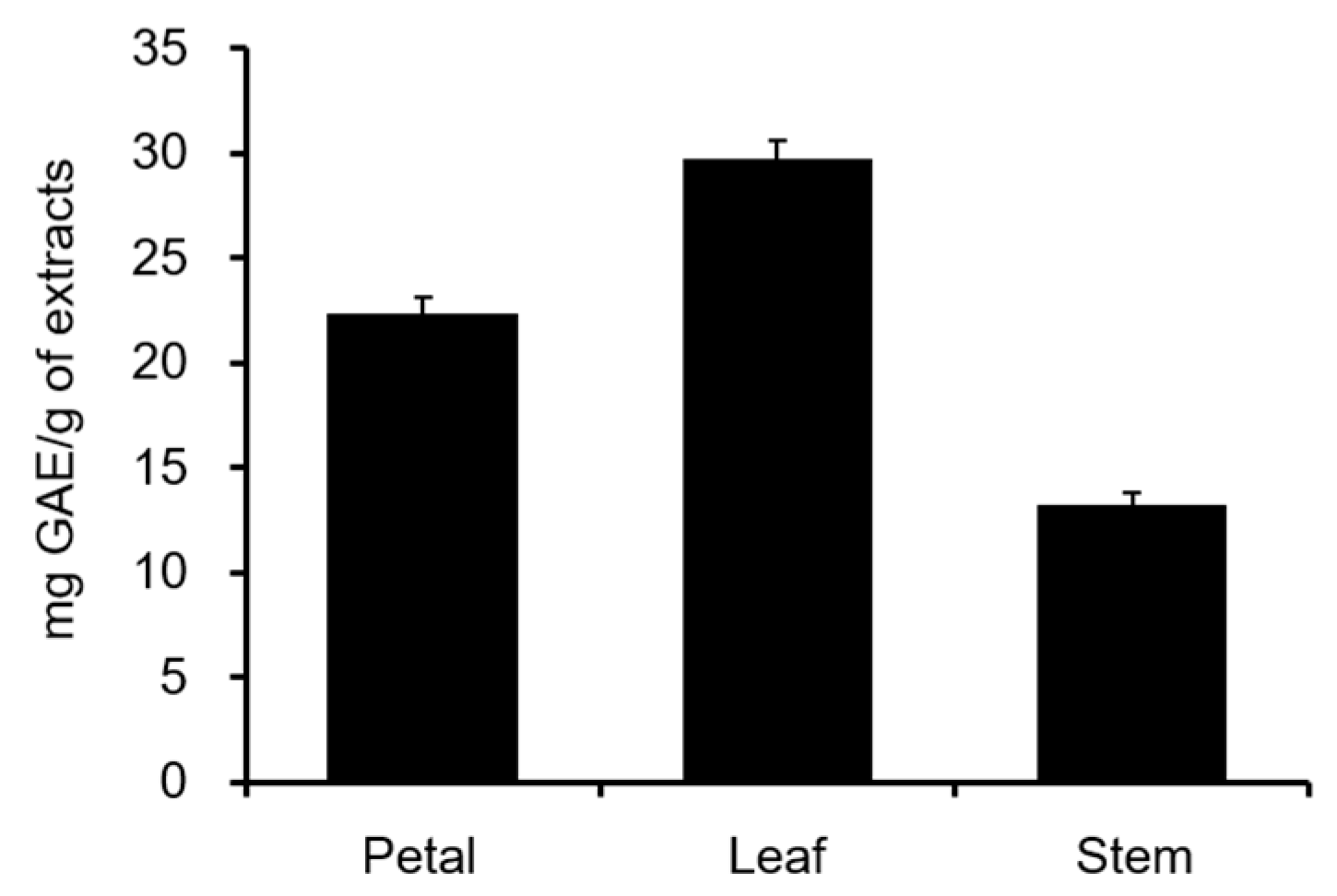
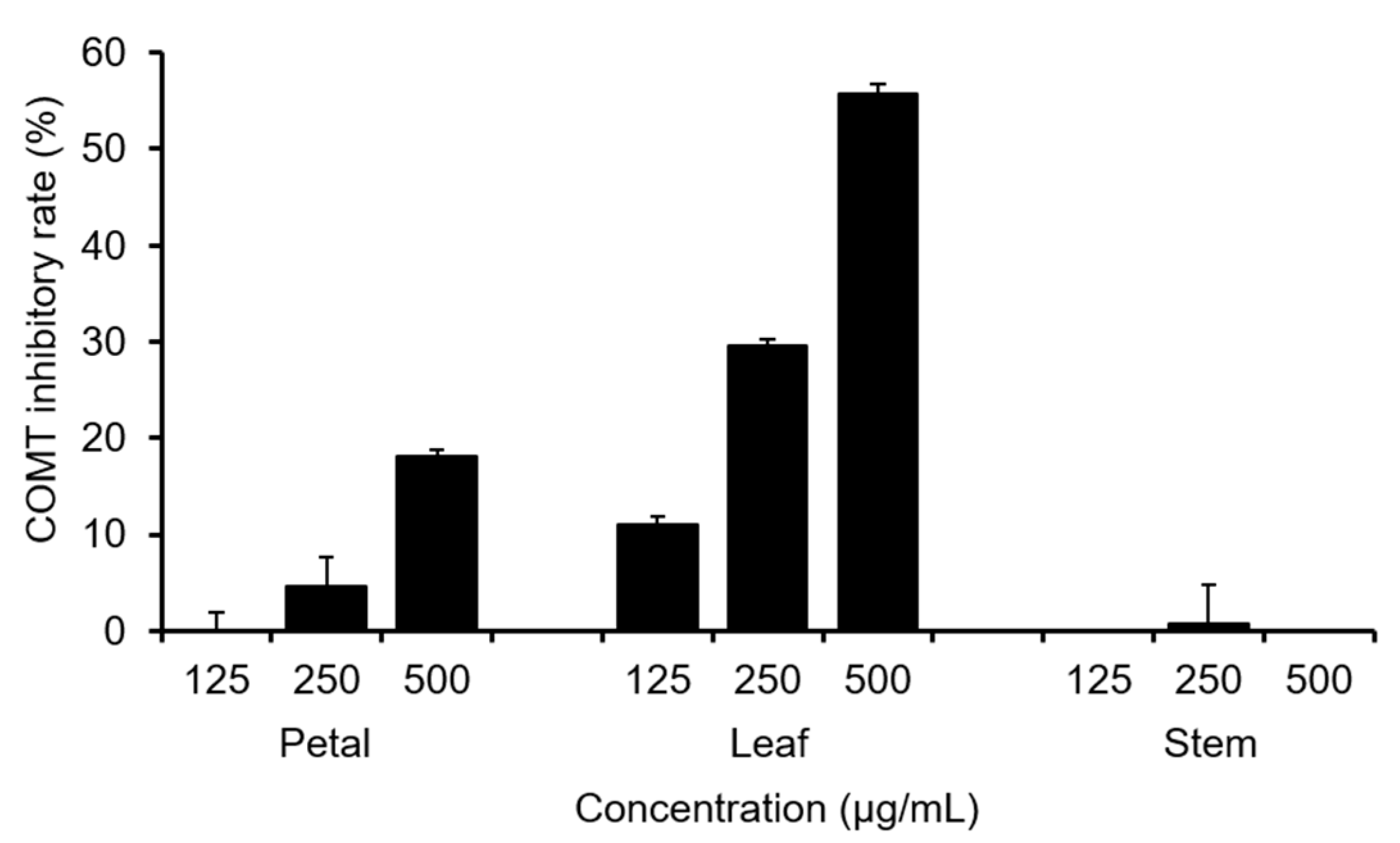
2.1. Total Polyphenol Content and COMT Inhibitory Activity of C. officinalis Leaves
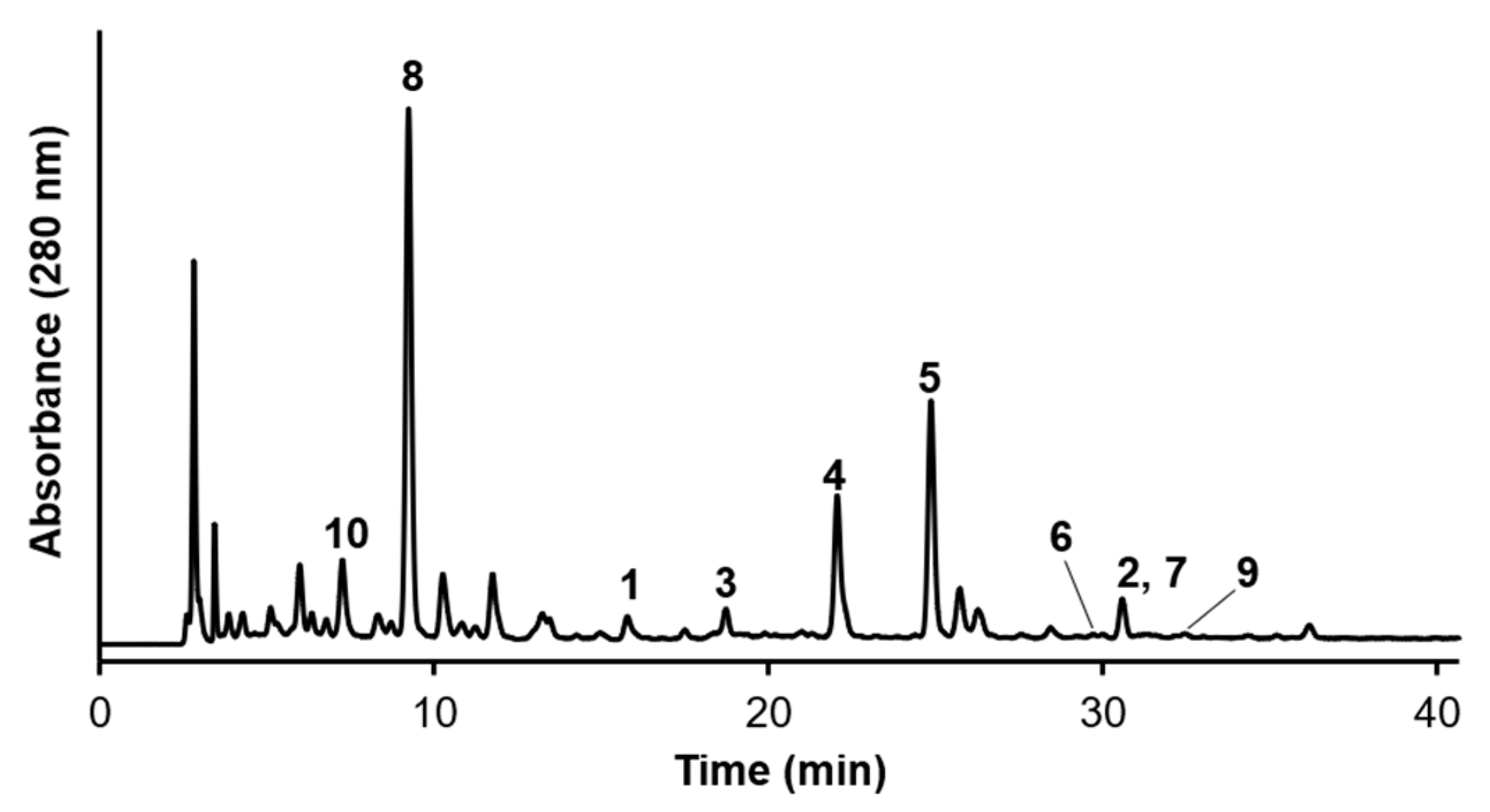
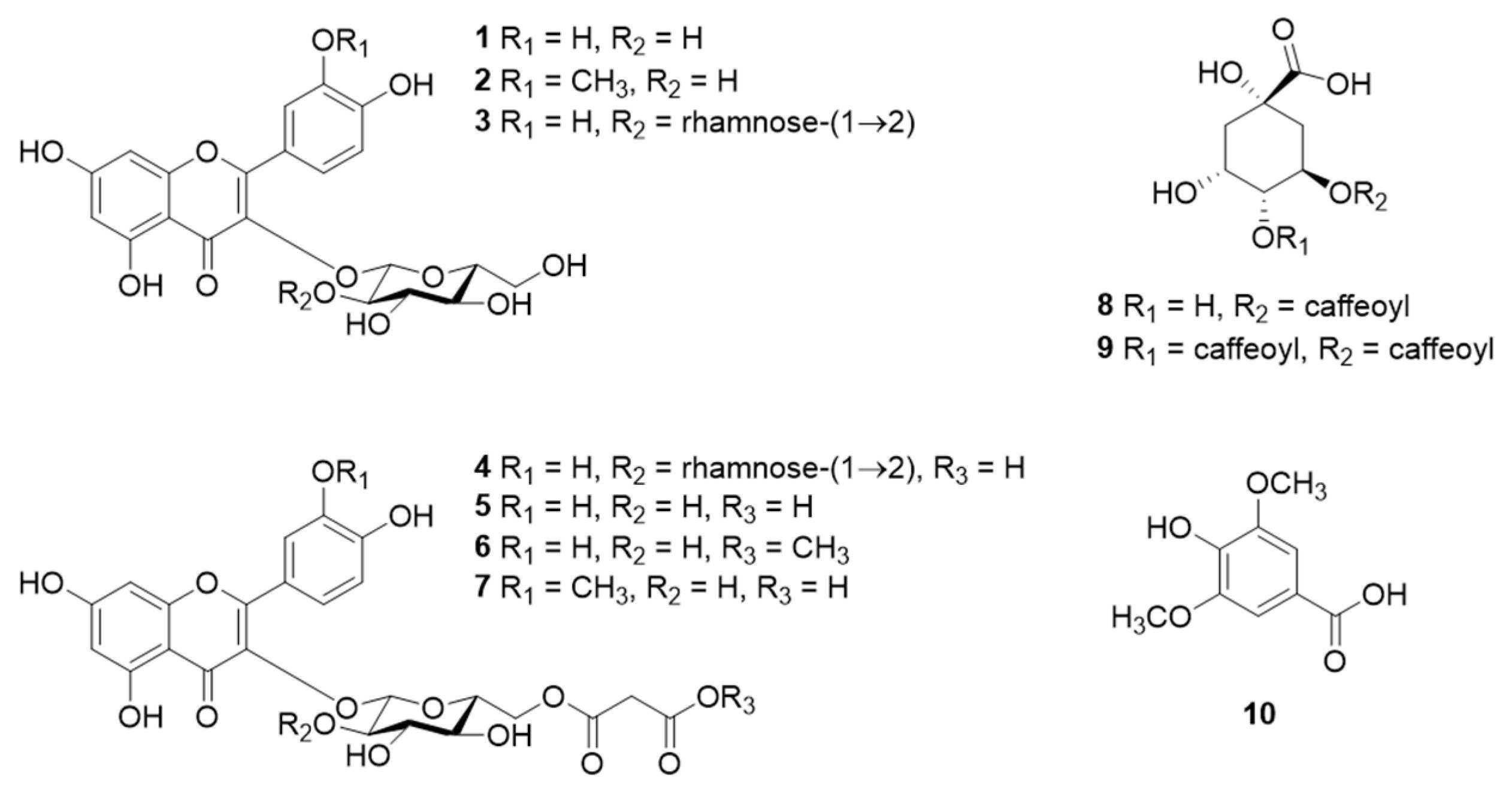
2.2. Component Analysis of the Leaves of C. officinalis
2.3. COMT Inhibitory Activities of 1–10
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Folin–Ciocalteu Colorimetric Method
3.4. Extraction and Isolation of Compounds in Calendula Leaves
3.5. COMT Inhibitory Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Muley, B.P.; Khadabadi, S.S.; Banarase, N.B. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): A review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179–187. [Google Scholar] [CrossRef]
- Frankič, T.; Salobir, K.; Salobir, J. The comparison of in vivo antigenotoxic and antioxidative capacity of two propylene glycol extracts of Calendula officinalis (marigold) and vitamin E in young growing pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2009, 93, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory, anti-tumor-promoting, and cytotoxic activities of constituents of marigold (Calendula officinalis) flowers. J. Nat. Prod. 2006, 69, 1692–1696. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Kishi, A.; Kageura, T.; Matsuda, H. Medicinal flowers. III. marigold. (1): Hypoglycemic, gastric emptying inhibitory, and gastroprotective principles and new oleanane-Type triterpene oligoglycosides, calendasaponins A, B, C, and D, from Egyptian Calendula officinalis. Chem. Pharm. Bull. 2001, 49, 863–870. [Google Scholar] [CrossRef]
- Śliwowski, J.; Dziewanowska, K.; Kasprzyk, Z. Ursadiol: A new triterpene diol from Calendula officinalis flowers. Phytochemistry 1973, 12, 157–160. [Google Scholar] [CrossRef]
- Wilkomirski, B.; Kasprzyk, Z. Free and ester-bound triterpene alcohols and sterols in cellular subfractions of Calendula officinalis flowers. Phytochemistry 1979, 18, 253–255. [Google Scholar] [CrossRef]
- Bakó, E.; Deli, J.; Tóth, G. HPLC study on the carotenoid composition of Calendula products. J. Biochem. Biophys. Methods 2002, 53, 241–250. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Diniz, R.M.; Suarez, M.A.M.; Figueiredo, C.S.S.S.; Zagmignan, A.; Grisotto, M.A.G.; Fernandes, E.S.; da Silva, L.C.N. Use of some Asteraceae plants for the treatment of wounds: From ethnopharmacological studies to scientific evidences. Front. Pharmacol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Final report of the cosmetic ingredient review expert panel amended safety assessment of Calendula officinalis-derived cosmetic ingredients. Int. J. Toxicol. 2010, 29, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Mitrea, L.; Calinoiu, L.F.; Teleky, B.E.; Martau, G.A.; Plamada, D.; Pascuta, M.S.; Nemes, S.A.; Varvara, R.A.; Vodnar, D.C. Natural polyphenol recovery from apple-, cereal-, and tomato-processing by-products and related health-promoting properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of agro-industrial waste as potential source of bioactive compounds for aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Tunbridge, E.M.; Harrison, P.J.; Weinberger, D.R. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol. Psychiatry 2006, 60, 141–151. [Google Scholar] [CrossRef]
- Bilder, R.M.; Volavka, J.; Lachman, H.M.; Grace, A.A. The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 2004, 29, 1943–1961. [Google Scholar] [CrossRef]
- Silva, T.B.; Borges, F.; Serrão, M.P.; Soares-da-Silva, P. Liver says no: The ongoing search for safe catechol O-methyltransferase inhibitors to replace tolcapone. Drug Discov. Today 2020, 25, 1846–1854. [Google Scholar] [CrossRef]
- Jaramillo, K.; Dawid, C.; Hofmann, T.; Fujimoto, Y.; Osorio, C. Identification of antioxidative flavonols and anthocyanins in Sicana odorifera fruit peel. J. Agric. Food Chem. 2011, 59, 975–983. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Wald, B.; Wray, V.; Galensa, R.; Herrmann, K. Malonated flavonol glycosides and 3,5-dicaffeoylquinic acid from pears. Phytochemistry 1989, 28, 663–664. [Google Scholar] [CrossRef]
- Jin, H.G.; Kim, A.R.; Ko, H.J.; Woo, E.R. A new megastigmane glycoside from Akebia quinata. Arch. Pharm. Res. 2015, 38, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cho, J.Y.; Ma, Y.K.; Park, K.Y.; Lee, S.H.; Ham, K.S.; Lee, H.J.; Park, K.H.; Moon, J.H. Dicaffeoylquinic acid derivatives and flavonoid glucosides from glasswort (Salicornia herbacea L.) and their antioxidative activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Xiao, C.; Dai, H.; Liu, H.; Wang, Y.; Tang, H. Revealing the metabonomic variation of rosemary extracts using 1H NMR spectroscopy and multivariate data analysis. J. Agric. Food Chem. 2008, 56, 10142–10153. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I. New Isorhamnetin glycosides and other phenolic compounds from Calendula officinalis. Chem. Nat. Compd. 2013, 49, 833–840. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Z.-J.; Jiang, H.-D.; Chen, J.-Z. Computational studies of the regioselectivities of COMT-catalyzed Meta-/Para-O methylations of luteolin and quercetin. J. Phys. Chem. B 2014, 118, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-F.; Fan, Y.-F.; Yu, H.-N.; Hou, F.-B.; Xiang, Y.-W.; Wang, P.; Ge, G.-B.; Yang, L.; Xu, J.-G. Discovery and characterization of flavonoids in vine tea as catechol-O-methyltransferase inhibitors. Fitoterapia 2021, 152, 104913. [Google Scholar] [CrossRef]
- Ikeda, M.; Iijima, H.; Shinoda, I.; Iwamoto, H.; Takeda, Y. Inhibitory effect of bovine lactoferrin on catechol-O-methyltransferase. Molecules 2017, 22, 1373. [Google Scholar] [CrossRef]
- Olanow, C.W. Tolcapone and hepatotoxic effects. Arch. Neurol. 2000, 57, 263–267. [Google Scholar] [CrossRef]
- Fabbri, M.; Ferreira, J.J.; Lees, A.; Stocchi, F.; Poewe, W.; Tolosa, E.; Rascol, O. Opicapone for the treatment of Parkinson’s disease: A review of a new licensed medicine. Mov. Disord. 2018, 33, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, Y.-L.; Zou, L.-W.; Qian, X.-K.; Dou, T.-Y.; Jin, Q.; Li, S.-Y.; Yu, Y.; Wang, D.-D.; Luo, Q.; et al. An optimized two-photon fluorescent probe for biological sensing and imaging of catechol-O-methyltransferase. Chem. Eur. J. 2017, 23, 10800–10807. [Google Scholar] [CrossRef]
- Miyata, R.; Motoyama, T.; Nakano, S.; Ito, S.; Mukaide, K.; Vongsak, B.; Kumazawa, S. Catechol-O-methyltransferase inhibitors isolated from Thai propolis. Nat. Prod. Commun. 2021, 16, 1–5. [Google Scholar] [CrossRef]
- Miyata, R.; Hoshino, S.; Ahn, M.R.; Kumazawa, S. Chemical profiles of Korean bee pollens and their catechol-O-methyltransferase inhibitory activities. J. Agric. Food Chem. 2022, 70, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
| Compound | IC50 (µM) |
|---|---|
| 1 | 50 |
| 2 | 55 |
| 3 | 71 |
| 4 | 65 |
| 5 | 59 |
| 6 | 42 |
| 7 | >100 |
| 8 | 100 |
| 9 | 21 |
| 10 | >100 |
| Tolcapone | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadowaki, W.; Miyata, R.; Fujinami, M.; Sato, Y.; Kumazawa, S. Catechol-O-methyltransferase Inhibitors from Calendula officinalis Leaf. Molecules 2023, 28, 1333. https://doi.org/10.3390/molecules28031333
Kadowaki W, Miyata R, Fujinami M, Sato Y, Kumazawa S. Catechol-O-methyltransferase Inhibitors from Calendula officinalis Leaf. Molecules. 2023; 28(3):1333. https://doi.org/10.3390/molecules28031333
Chicago/Turabian StyleKadowaki, Wataru, Ryo Miyata, Misa Fujinami, Yoshizumi Sato, and Shigenori Kumazawa. 2023. "Catechol-O-methyltransferase Inhibitors from Calendula officinalis Leaf" Molecules 28, no. 3: 1333. https://doi.org/10.3390/molecules28031333
APA StyleKadowaki, W., Miyata, R., Fujinami, M., Sato, Y., & Kumazawa, S. (2023). Catechol-O-methyltransferase Inhibitors from Calendula officinalis Leaf. Molecules, 28(3), 1333. https://doi.org/10.3390/molecules28031333








