Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture
Abstract
1. Introduction
2. Purification and Identification of Bioactive Peptides from Insect Protein Hydrolysates
3. Applications in Human Health Management
4. Applications in Farm Animal Health Management
5. Applications in Plant Health Management
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Feehan, J.; Kaczmarek, K.; Matsoukas, J.M.; Paredes Lopez, O.; Saviano, M.; Skwarczynski, M.; Smith-Carpenter, J.; et al. New advances in short peptides: Looking forward. Molecules 2022, 27, 3635. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.-T.; Ee, K.Y.; Kumar, D.T.; Manan, F.A.; Wong, F.-C. Plant bioactive peptides: Current status and prospects towards use on human health. Protein Pept. Lett. 2020, 28, 623–642. [Google Scholar]
- Jakubczyk, A.; Karas, M.; Rybczynska-Tkaczyk, K.; Zielinska, E.; Zielinski, D. Current trends of bioactive peptides—New sources and therapeutic effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A global review on short peptides: Frontiers and perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Fields, K.; Falla, T.J.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S. Edible insects—A new trend in functional food science. Funct. Food Sci. 2022, 2, 157–162. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Correia, P.; Coelho, C.; Costa, C.A. The role of edible insects to mitigate challenges for sustainability. Open Agric. 2021, 6, 24–36. [Google Scholar] [CrossRef]
- Mlcek, J.; Borkovcova, M.; Bednarova, M. Biologically active substances of edible insects and their use in agriculture, veterinary and human medicine—A review. J. Cent. Eur. Agric. 2014, 15, 225–237. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliam. Off. J. Eur. Union 2015, 327, 1–22. [Google Scholar]
- EFSA Panel on Nutrition, N.F.; Allergens, F.; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef] [PubMed]
- Bullard, B.; Linke, W.A.; Leonard, K. Varieties of elastic protein in invertebrate muscles. J. Muscle Res. Cell Motil. 2002, 23, 435–447. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef]
- Jongema, Y. Available online: http://www.wur.nl/en/Expertise-Services/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm (accessed on 29 June 2017).
- Halloran, A.; Vantomme, P.; Hanboonsong, Y.; Ekesi, S. Regulating edible insects: The challenge of addressing food security, nature conservation, and the erosion of traditional food culture. Food Secur. 2015, 7, 739–746. [Google Scholar] [CrossRef]
- Bergier, E. Peuples Entomophages et Insectes Comestibles: Etude sur les Moeurs de L’homme et de L’insecte; Imprimerie Rulliere Freres: Avignon, France, 1941. [Google Scholar]
- Bodenheimer, F.S. Insects as Human Food: A Chapter of the Ecology of Man; Springer: Dordrecht, The Netherlands, 1951. [Google Scholar]
- Vercruysse, L.; Smagghe, G.; Herregods, G.; Van Camp, J. ACE inhibitory activity in enzymatic hydrolysates of insect protein. J. Agric. Food Chem. 2005, 53, 5207–5211. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Reyes, A.; Rosell, C.M.; Rodrigo, D.; Ibarra-Herrera, C.C. Benefits and challenges in the incorporation of insects in food products. Front. Nutr. 2021, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Nakamura, Y. Edible insects as future food: Chances and challenges. J. Future Foods 2021, 1, 38–46. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect antimicrobial peptides, a mini review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Khammuang, S.; Sarnthima, R.; Sanachai, K. Purification and identification of novel antioxidant peptides from silkworm pupae (Bombyx mori) protein hydrolysate and molecular docking study. Biocatal. Agric. Biotechnol. 2022, 42, 102367. [Google Scholar] [CrossRef]
- Tao, M.; Wang, C.; Liao, D.; Liu, H.; Zhao, Z.; Zhao, Z. Purification, modification and inhibition mechanism of angiotensin I-converting enzyme inhibitory peptide from silkworm pupa (Bombyx mori) protein hydrolysate. Process Biochem. 2017, 54, 172–179. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, J.; Yan, H.; Du, J.; Gui, Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhu, Z.; Li, X.; Sun, S.; Wang, W.; Sadiq, F.A. Identification and characterization of two novel antioxidant peptides from silkworm pupae protein hydrolysates. Eur. Food Res. Technol. 2021, 247, 343–352. [Google Scholar] [CrossRef]
- Cho, H.-R.; Lee, S.-O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef]
- Pattarayingsakul, W.; Nilavongse, A.; Reamtong, O.; Chittavanich, P.; Mungsantisuk, I.; Mathong, Y.; Prasitwuttisak, W.; Panbangred, W. Angiotensin-converting enzyme inhibitory and antioxidant peptides from digestion of larvae and pupae of Asian weaver ant, Oecophylla smaragdina, Fabricius. J. Sci. Food Agric. 2017, 97, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Choi, Y.-J.; Tang, Y.; Kim, J.H.; Kim, B.-g.; Lee, B.; Bae, S.M.; Kim, E.-K. AGL9: A novel hepatoprotective peptide from the larvae of edible insects alleviates obesity-induced hepatic inflammation by regulating AMPK/Nrf2 signaling. Foods 2021, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.M.; Fan, M.; Choi, Y.-J.; Tang, Y.; Jeong, G.; Myung, K.; Kim, B.-g.; Kim, E.-K. Exploring the role of a novel peptide from Allomyrina dichotoma larvae in ameliorating lipid metabolism in obesity. Int. J. Mol. Sci. 2020, 21, 8537. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, T.-K.; Yong, H.I.; Cha, J.Y.; Song, K.-M.; Lee, H.G.; Je, J.-G.; Kang, M.-C.; Choi, Y.-S. Peptides inhibiting angiotensin-I-converting enzyme: Isolation from flavourzyme hydrolysate of Protaetia brevitarsis larva protein and identification. Food Chem. 2022, 399, 133897. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yang, J.; Zhou, X.; Hamdy, A.M.; Zhang, X.; Suo, H.; Zhang, Y.; Li, N.; Song, J. Tenebrio molitor proteins-derived DPP-4 inhibitory peptides: Preparation, identification, and molecular binding mechanism. Foods 2022, 11, 3626. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lee, J.-H.; Yong, H.I.; Kang, M.-C.; Cha, J.Y.; Chun, J.Y.; Choi, Y.-S. Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae. Foods 2022, 11, 1400. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2020, 49, D437–D451. [Google Scholar] [CrossRef]
- Prasasty, V.D.; Istyastono, E.P. Data of small peptides in SMILES and three-dimensional formats for virtual screening campaigns. Data Brief 2019, 27, 104607. [Google Scholar] [CrossRef]
- Wong, F.-C.; Ong, J.-H.; Chai, T.-T. Identification of putative cell-entry-inhibitory peptides against SARS-CoV-2 from edible insects: An in silico study. eFood 2020, 1, 357–368. [Google Scholar] [CrossRef]
- Matemu, A.; Nakamura, S.; Katayama, S. Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score. Antioxidants 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B.; Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol. 2020, 246, 1361–1369. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Mudd, N.; Martin-Gonzalez, F.S.; Ferruzzi, M.; Liceaga, A.M. In vivo antioxidant effect of edible cricket (Gryllodes sigillatus) peptides using a Caenorhabditis elegans model. Food Hydrocoll. Health 2022, 2, 100083. [Google Scholar] [CrossRef]
- Amada44. Schistocerca gregaria—0061.jpg (Creative Commons Attribution-Share Alike 3.0 Unported license). Available online: https://commons.wikimedia.org/wiki/File:Schistocerca_gregaria_-_0061.jpg (accessed on 16 December 2022).
- Woo, B. Gryllodes sigillatus (Permission Granted by Image Owner via Email). Available online: https://theincorrigibleentomologist.files.wordpress.com/2018/03/img_7249.jpg?w=768 (accessed on 16 December 2022).
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification and characterization of a novel immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated silkworm (Bombyx mori) pupa protein. J. Asia-Pac. Entomol. 2019, 22, 633–637. [Google Scholar] [CrossRef]
- Vercruysse, L.; Van Camp, J.; Morel, N.; Rougé, P.; Herregods, G.; Smagghe, G. Ala-Val-Phe and Val-Phe: ACE inhibitory peptides derived from insect protein with antihypertensive activity in spontaneously hypertensive rats. Peptides 2010, 31, 482–488. [Google Scholar] [CrossRef]
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and characterization of edible cricket peptides on hypertensive and glycemic in vitro inhibition and their anti-inflammatory activity on RAW 264.7 macrophage cells. Nutrients 2020, 12, 3588. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, H.; Lu, Y.; Chen, W.; Huang, G. Identification and in silico analysis of antithrombotic peptides from the enzymatic hydrolysates of Tenebrio molitor larvae. Eur. Food Res. Technol. 2019, 245, 2687–2695. [Google Scholar] [CrossRef]
- Montiel-Aguilar, L.J.; Torres-Castillo, J.A.; Rodríguez-Servin, R.; López-Flores, A.B.; Aguirre-Arzola, V.E.; Méndez-Zamora, G.; Sinagawa-García, S.R. Nutraceutical effects of bioactive peptides obtained from Pterophylla beltrani (Bolivar & Bolivar) protein isolates. J. Asia-Pac. Entomol. 2020, 23, 756–761. [Google Scholar]
- Mendoza-Salazar, A.; Santiago-López, L.; Torres-Llanez, M.J.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. In vitro antioxidant and antihypertensive activity of edible insects flours (mealworm and grasshopper) fermented with Lactococcus lactis strains. Fermentation 2021, 7, 153. [Google Scholar] [CrossRef]
- Hypertension; World Health Organization: Geneva, Switzerland, 2021.
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-H.; Le, G.-W.; Shi, Y.-H.; Shrestha, S. Angiotensin I–converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Cheung, B.M.Y.; Li, C. Diabetes and hypertension: Is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B. DPP-4 inhibition and the path to clinical proof. Front. Endocrinol. 2019, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef] [PubMed]
- ElHeineken. Allomyrina dichotoma L3 Larva.JPG (Creative Commons Attribution 3.0 Unported license). Available online: https://commons.wikimedia.org/wiki/File:Allomyrina_dichotoma_L3_Larva.JPG (accessed on 16 December 2022).
- Xiong, Y.; Chen, Z.H.; Zhang, F.L.; Yu, Z.Y.; Liu, B.; Zhang, C.; Zhao, L.N. A specific selenium-chelating peptide isolated from the protein hydrolysate of Grifola frondosa. RSC Adv. 2021, 11, 10272–10284. [Google Scholar] [CrossRef]
- Sonklin, C.; Alashi, A.M.; Laohakunjit, N.; Aluko, R.E. Functional characterization of mung bean meal protein-derived antioxidant peptides. Molecules 2021, 26, 1515. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mnolf. Tenebrio molitor Larvae (GFDL & CC ShareAlike 2.0 License). Available online: https://commons.wikimedia.org/wiki/File:Tenebrio_molitor_larvae.jpg (accessed on 16 December 2022).
- Józefiak, A.; Engberg, R.M. Insect proteins as a potential source of antimicrobial peptides in livestock production. A review. J. Anim. Feed. Sci. 2017, 26, 87–99. [Google Scholar] [CrossRef]
- Veldkamp, T.; Dong, L.; Paul, A.; Govers, C. Bioactive properties of insect products for monogastric animals—A review. J. Insects Food Feed. 2022, 8, 1027–1040. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The FAO Action Plan on Antimicrobial Resistance 2016–2020; FAO: Rome, Italy, 2016. [Google Scholar]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO; OIE. Antimicrobial Resistance and the United Nations Sustainable Development Cooperation Framework: Guidance for United Nations Country Teams; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Józefiak, D.; Kierończyk, B.; Juśkiewicz, J.; Zduńczyk, Z.; Rawski, M.; Długosz, J.; Sip, A.; Højberg, O. Dietary nisin modulates the gastrointestinal microbial ecology and enhances growth performance of the broiler chickens. PLoS ONE 2013, 8, e85347. [Google Scholar] [CrossRef] [PubMed]
- Kierończyk, B.; Pruszyńska-Oszmałek, E.; Światkiewicz, S.; Rawski, M.; Długosz, J.; Engberg, R.M.; Józefiak, D. The nisin improves broiler chicken growth performance and interacts with salinomycin in terms of gastrointestinal tract microbiota composition. J. Anim. Feed. Sci. 2016, 25, 309–316. [Google Scholar] [CrossRef]
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M.; et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2009, 101, 998–1005. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lee, S.H.; Park, Y.K.; Lee, S.C.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation of synthetic antimicrobial peptide-A3 and P5 on growth performance, apparent total tract digestibility of nutrients, fecal and intestinal microflora and intestinal morphology in weanling pigs. Livest. Sci. 2014, 159, 53–60. [Google Scholar] [CrossRef]
- Mohideen, H.S.; Louis, H.P. Insect antimicrobial peptides—Therapeutic and agriculture perspective. J. Appl. Biotechnol. Rep. 2021, 8, 193–202. [Google Scholar]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef]
- Dai, J.; Ou, W.; Yu, G.; Ai, Q.; Zhang, W.; Mai, K.; Zhang, Y. The antimicrobial peptide cecropin ad supplement alleviated soybean meal-induced intestinal inflammation, barrier damage, and microbial dysbiosis in juvenile turbot, Scophthalmus maximus. Front. Mar. Sci. 2020, 7, 584482. [Google Scholar] [CrossRef]
- Wen, L.F.; He, J.G. Dose-response effects of an antimicrobial peptide, a cecropin hybrid, on growth performance, nutrient utilisation, bacterial counts in the digesta and intestinal morphology in broilers. Br. J. Nutr. 2012, 108, 1756–1763. [Google Scholar] [CrossRef]
- Duclohier, H. How do channel- and pore-forming helical peptides interact with lipid membranes and how does this account for their antimicrobial activity? Mini Rev. Med. Chem. 2002, 2, 331–342. [Google Scholar] [CrossRef]
- Nicolas, P. Multifunctional host defense peptides: Intracellular-targeting antimicrobial peptides. FEBS J. 2009, 276, 6483–6496. [Google Scholar] [CrossRef]
- Wang, B.; Xie, N.; Li, B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: A review. J. Food Biochem. 2019, 43, e12571. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli O157:H7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Peng, Y.; Gao, X.; Song, Q.; Zhang, H.; Elhag, O.; Cai, M.; Zheng, L.; Yu, Z.; et al. Structural and functional characterizations and heterogenous expression of the antimicrobial peptides, Hidefensins, from black soldier fly, Hermetia illucens (L.). Protein Expr. Purif. 2022, 192, 106032. [Google Scholar] [CrossRef]
- Cociancich, S.; Ghazi, A.; Hetru, C.; Hoffmann, J.A.; Letellier, L. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J. Biol. Chem. 1993, 268, 19239–19245. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, S.; Gordya, N.; Suborova, T. Insect antimicrobial peptide complexes prevent resistance development in bacteria. PLoS ONE 2015, 10, e0130788. [Google Scholar] [CrossRef] [PubMed]
- Mouithys-Mickalad, A.; Schmitt, E.; Dalim, M.; Franck, T.; Tome, N.M.; van Spankeren, M.; Serteyn, D.; Paul, A. Black soldier fly (Hermetia illucens) larvae protein derivatives: Potential to promote animal health. Animals 2020, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Chu, X.; Li, M.; Wang, G.; Wang, K.; Shang, R.; Wang, Z.; Li, L. Evaluation of the low inclusion of full-fatted Hermetia illucens larvae meal for layer chickens: Growth performance, nutrient digestibility, and gut health. Front. Vet. Sci. 2020, 7, 585843. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Ekengren, S.; Hultmark, D. Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol. 1999, 29, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.; Kogel, K.-H. Insect antimicrobial peptides as new weapons against plant pathogens. In Insect Biotechnology; Vilcinskas, A., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 123–144. [Google Scholar]
- Casteels, P.; Ampe, C.; Jacobs, F.; Vaeck, M.; Tempst, P. Apidaecins: Antibacterial peptides from honeybees. EMBO J. 1989, 8, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Jan, P.S.; Huang, H.Y.; Chen, H.M. Expression of a synthesized gene encoding cationic peptide cecropin B in transgenic tomato plants protects against bacterial diseases. Appl. Environ. Microbiol. 2010, 76, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Yevtushenko, D.P.; Romero, R.; Forward, B.S.; Hancock, R.E.; Kay, W.W.; Misra, S. Pathogen-induced expression of a cecropin A-melittin antimicrobial peptide gene confers antifungal resistance in transgenic tobacco. J. Exp. Bot. 2005, 56, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Khademi, M.; Varasteh-Shams, M.; Nazarian-Firouzabadi, F.; Ismaili, A. New recombinant antimicrobial peptides confer resistance to fungal pathogens in tobacco plants. Front. Plant Sci. 2020, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Goldsworthy, G.J. Insect peptide hormones: A selective review of their physiology and potential application for pest control. Pest Manag. Sci. 2003, 59, 1063–1075. [Google Scholar] [CrossRef]
- Audsley, N.; Weaver, R.J.; Edwards, J.P. In vivo effects of Manduca sexta allatostatin and allatotropin on larvae of the tomato moth, Lacanobia oleracea. Physiol. Entomol. 2001, 26, 181–188. [Google Scholar] [CrossRef]
- Bendena, W.G.; Donly, B.C.; Tobe, S.S. Allatostatins: A growing family of neuropeptides with structural and functional diversity. Ann. N. Y. Acad. Sci. 1999, 897, 311–329. [Google Scholar] [CrossRef]
- Chong, Y.; Hayes, J.L.; Sollod, B.; Wen, S.; Wilson, D.T.; Hains, P.G.; Hodgson, W.C.; Broady, K.W.; King, G.F.; Nicholson, G.M. The ω-atracotoxins: Selective blockers of insect M-LVA and HVA calcium channels. Biochem. Pharmacol. 2007, 74, 623–638. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Smith, R.; O’Donoghue, S.I.; Nilges, M.; Connor, M.; Howden, M.E.H.; Christie, M.J.; King, G.F. The structure of a novel insecticidal neurotoxin, ω-atracotoxin-HV1, from the venom of an Australian funnel web spider. Nat. Struct. Biol. 1997, 4, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Zafar, Y.; Briddon, R.W.; Malik, K.A.; Mukhtar, Z. Spider venom toxin protects plants from insect attack. Transgenic Res. 2006, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Pagano, E.M.; Meijles, N.D.; Pagano, J.P. NOX inhibitors & therapies: Rational design of peptidic and small molecule inhibitors. Curr. Pharm. Des. 2015, 21, 6032–6035. [Google Scholar]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.-J. Effects of plant-derived protein hydrolysates on yield, quality, and nitrogen use efficiency of greenhouse grown lettuce and tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]


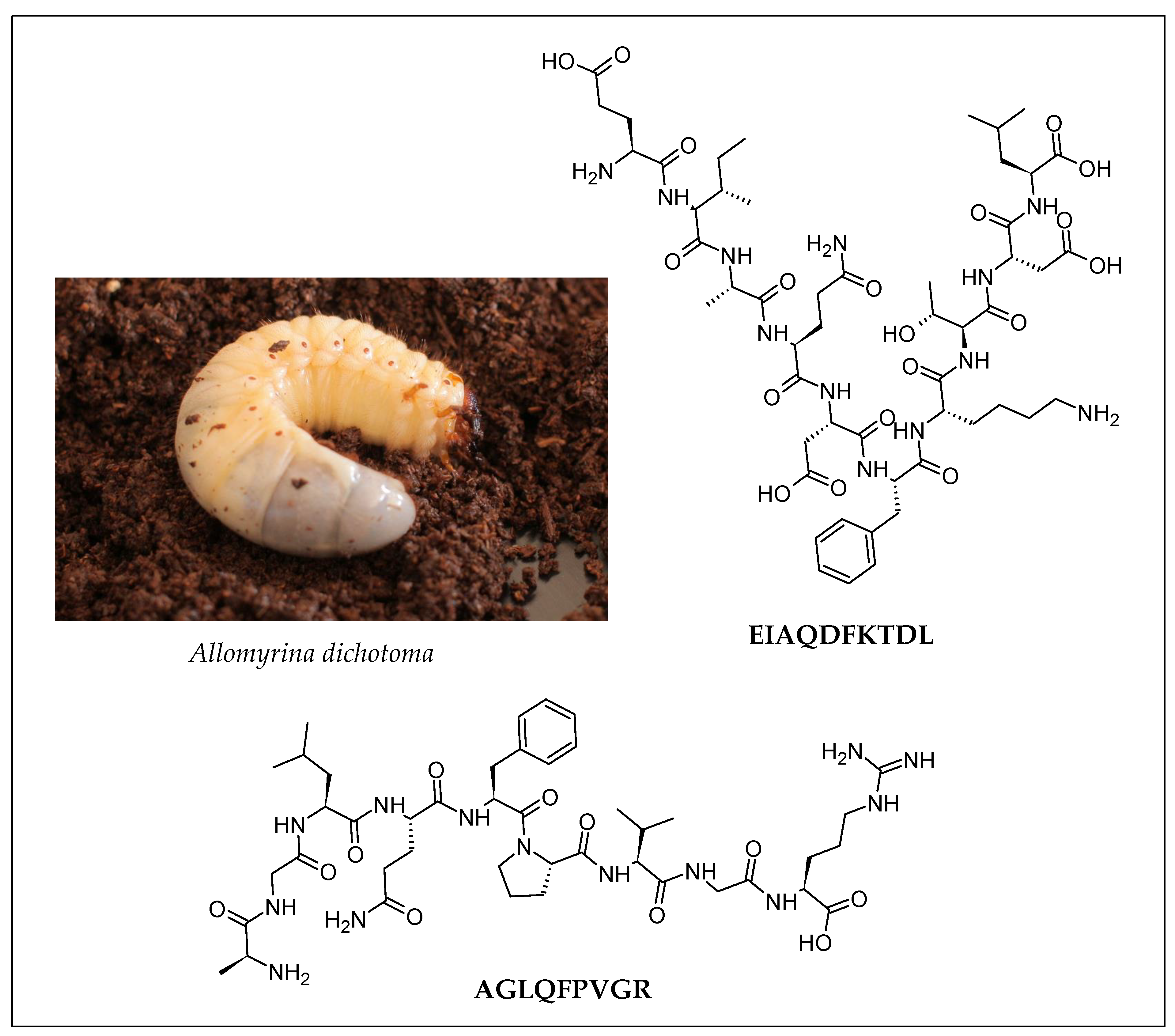
| Insect | Peptide Sequence (Validated Activity) | Enzymatic Hydrolysis | Peptide Purification Strategy | Peptide Identification | Reference |
|---|---|---|---|---|---|
| Larva of the Japanese rhinoceros beetle (Allomyrina dichotoma) | EIAQDFKTDL (Anti-obesity) AGLQFPVGR (Hepatoprotective) | Promod 278P *, pepsin, trypsin, protease NP, pancreatin, alphalase NP, alkaline protease, alcalase, neutrase, protamex |
|
| [36,37] |
| Larva of the white-spotted flower chafer (Protaetia brevitarsis) | SY, PF, YPY, WI (Anti-ACE) | Flavourzyme |
|
| [38] |
| Mealworm (Tenebrio molitor) | LPDQWDWR, APPDGGFWEWGD (Anti-DPP-IV) | Flavourzyme *, alcalase, papain, trypsin |
|
| [39] |
| Mealworm (Tenebrio molitor) | LE, AKKHKE (Hepatoprotective) | Alcalase *, flavourzyme, neutrase |
|
| [34] |
| Asian weaver ant larva and pupa mixture (Oecophylla smaragdina) | FFGT, LSRVP (Anti-ACE) CTKKHKPNC (Antioxidant) | SGD (Pepsin and trypsin) |
|
| [35] |
| Silkworm pupa (Bombyx mori) | AAEYPA, AKPGVY (Antioxidant) | Alcalase *, papain, trypsin |
|
| [30] |
| Silkworm pupa (Bombyx mori) | SWFVTPF, NDVLEF (Antioxidant) | Alcalase *, Prolyve, Flavourzyme, Brewers Clarex |
|
| [29] |
| Silkworm pupa (Bombyx mori) | FKGPACA, SVLGTGC (Antioxidant) | Acidic protease, followed by neutral protease |
|
| [33] |
| Silkworm pupa (Bombyx mori) | ASL (Anti-ACE) | SGD (pepsin, trypsin, and α-chymotrypsin) |
|
| [32] |
| Silkworm pupa (Bombyx mori) | GNPWM (Anti-ACE) | Neutral protease |
|
| [31] |
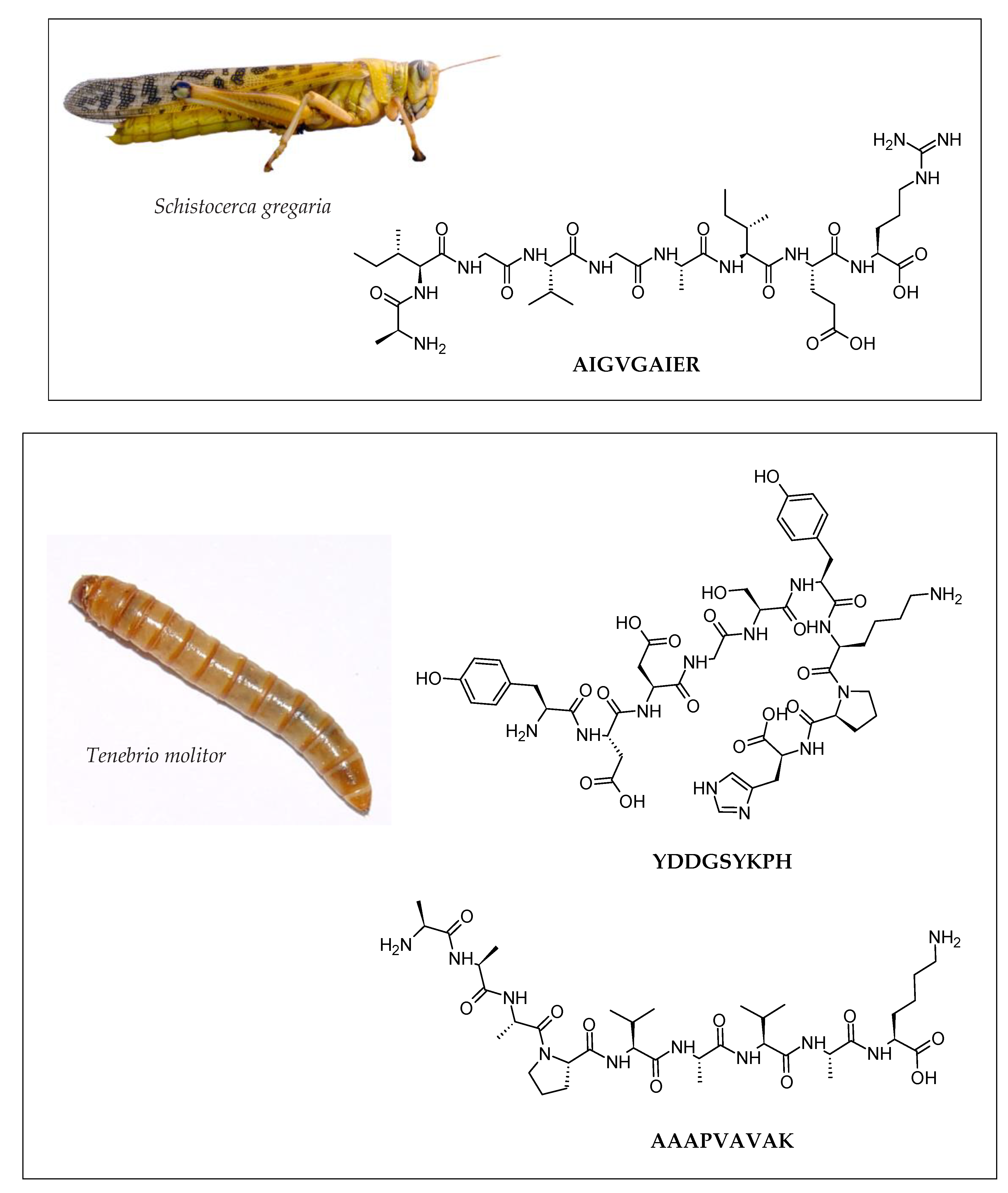
| Insect | Peptide/Hydrolysate | Bioactivity * | Potential Application | References |
|---|---|---|---|---|
| Cricket (Gryllodes sigillatus) | IIAPPER |
| Anti-hypertension, antidiabetic, weight control, antioxidant, and anti-inflammation | [47,48] |
| LAPSTIK |
| |||
| VAPEEHPV |
| |||
| KVEGDLK |
| |||
| Mealworm (Tenebrio molitor) | NYVADGLG |
| ||
| AAAPVAVAK |
| |||
| YDDGSYKPH |
| |||
| AGDDAPR |
| |||
| Locust (Schistocerca gregaria) | GKDAVIV |
| ||
| AIGVGAIER |
| |||
| FDPFPK |
| |||
| YETGNGIK |
| |||
| Silkworm pupa (Bombyx mori) | AAEYPA |
| Antioxidant | [30] |
| AKPGVY |
| |||
| Silkworm pupa (Bombyx mori) | SWFVTPF NDVLFF |
| Antioxidant | [29] |
| Silkworm pupa (Bombyx mori) | FKGPACA SVLGTGC |
| Antioxidant | [33] |
| Silkworm pupa (Bombyx mori) | ASL |
| Anti-hypertension | [32] |
| Silkworm pupa (Bombyx mori) | GNPWM WW |
| Anti-hypertension | [31] |
| Silkworm pupa (Bombyx mori) | PNPNTN |
| Immunomodulation | [52] |
| Asian weaver ant (Oecophylla smaragdina) | FFGT LSRVP |
| Anti-hypertension | [35] |
| CTKKHKPNC |
| Antioxidant | ||
| Mealworm (Tenebrio molitor) | LPDQWDWR APPDGGFWEWGD |
| Antidiabetic | [39] |
| Larva of the Japanese rhinoceros beetle (Allomyrina dichotoma) | EIAQDFKTDL | In vivo model: HFD mouse model
| Anti-obesity, weight control | [37] |
| Larva of the Japanese rhinoceros beetle (Allomyrina dichotoma) | AGLQFPVGR | In vivo model: HFD mouse model
| Anti-obesity, weight control, hepatoprotective | [36] |
| Cotton leafworm (Spodoptera littoralis) | VF AVF | In vivo model: SHR rat model
| Anti-hypertensive | [53] |
| Egyptian cotton leafworm (Spodoptera littoralis) | SGD hydrolysate | In vivo model: Caenorhabditis elegans
| Antioxidant | [49] |
| Cricket (Gryllodes sigillatus) | Cationic peptide fraction from sequential alcalase and SGD hydrolysates |
| Antidiabetic and anti-hypertension | [54] |
| Yellow mealworms (Tenebrio molitor) | RP-HPLC fraction of pepsin and trypsin hydrolysate |
| Antithrombotic | [55] |
| Mexican katydid (Pterophylla beltrani) | SGD hydrolysate |
| Anti-hypertension | [56] |
| <3 kDa fraction of SGD hydrolysate |
| Antidiabetic, Anti-hypertension, |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quah, Y.; Tong, S.-R.; Bojarska, J.; Giller, K.; Tan, S.-A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.-T. Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture. Molecules 2023, 28, 1233. https://doi.org/10.3390/molecules28031233
Quah Y, Tong S-R, Bojarska J, Giller K, Tan S-A, Ziora ZM, Esatbeyoglu T, Chai T-T. Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture. Molecules. 2023; 28(3):1233. https://doi.org/10.3390/molecules28031233
Chicago/Turabian StyleQuah, Yixian, Shi-Ruo Tong, Joanna Bojarska, Katrin Giller, Sheri-Ann Tan, Zyta Maria Ziora, Tuba Esatbeyoglu, and Tsun-Thai Chai. 2023. "Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture" Molecules 28, no. 3: 1233. https://doi.org/10.3390/molecules28031233
APA StyleQuah, Y., Tong, S.-R., Bojarska, J., Giller, K., Tan, S.-A., Ziora, Z. M., Esatbeyoglu, T., & Chai, T.-T. (2023). Bioactive Peptide Discovery from Edible Insects for Potential Applications in Human Health and Agriculture. Molecules, 28(3), 1233. https://doi.org/10.3390/molecules28031233









