The Preventive Effect of Specific Collagen Peptides against Dexamethasone-Induced Muscle Atrophy in Mice
Abstract
1. Introduction
2. Results
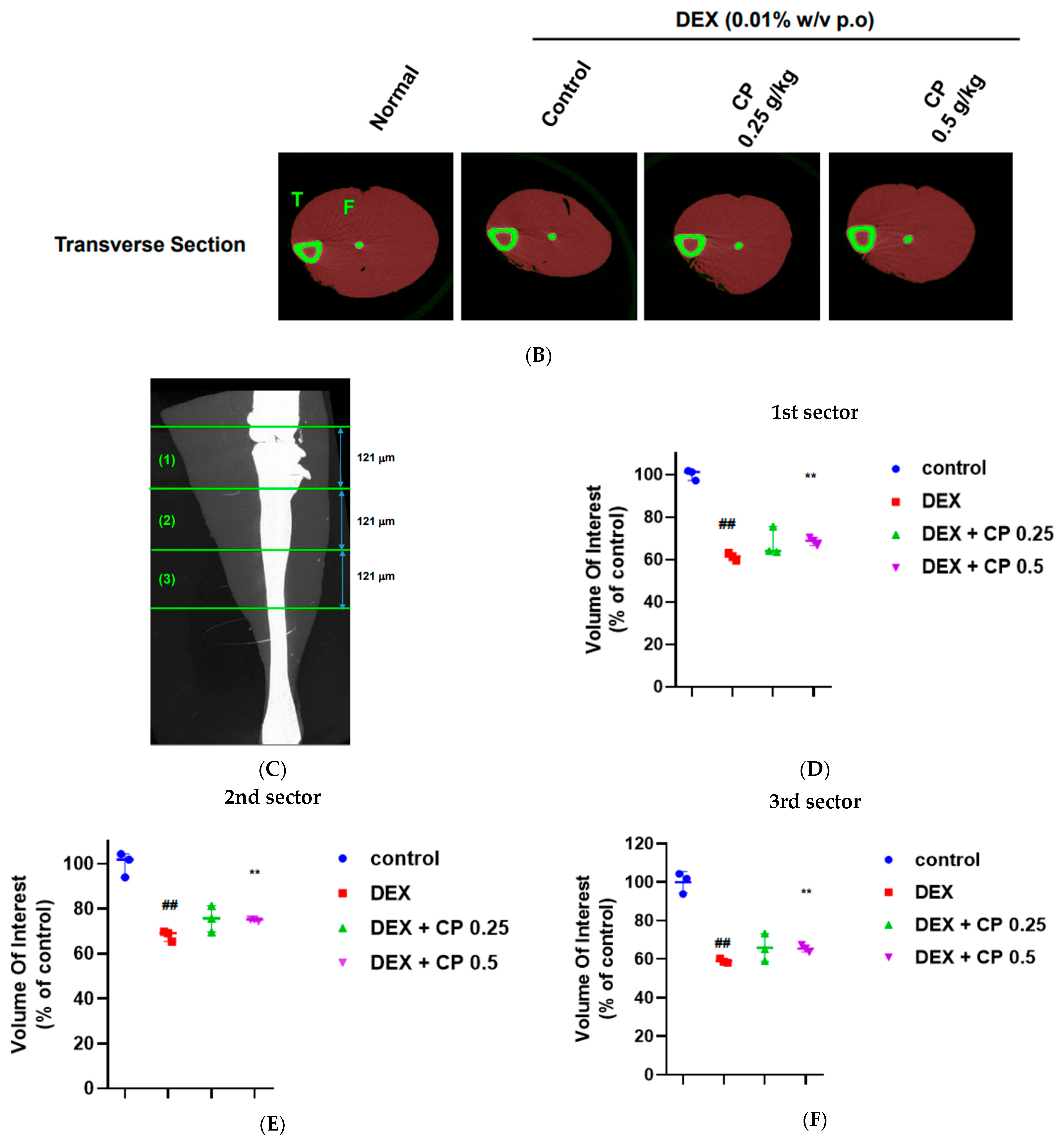
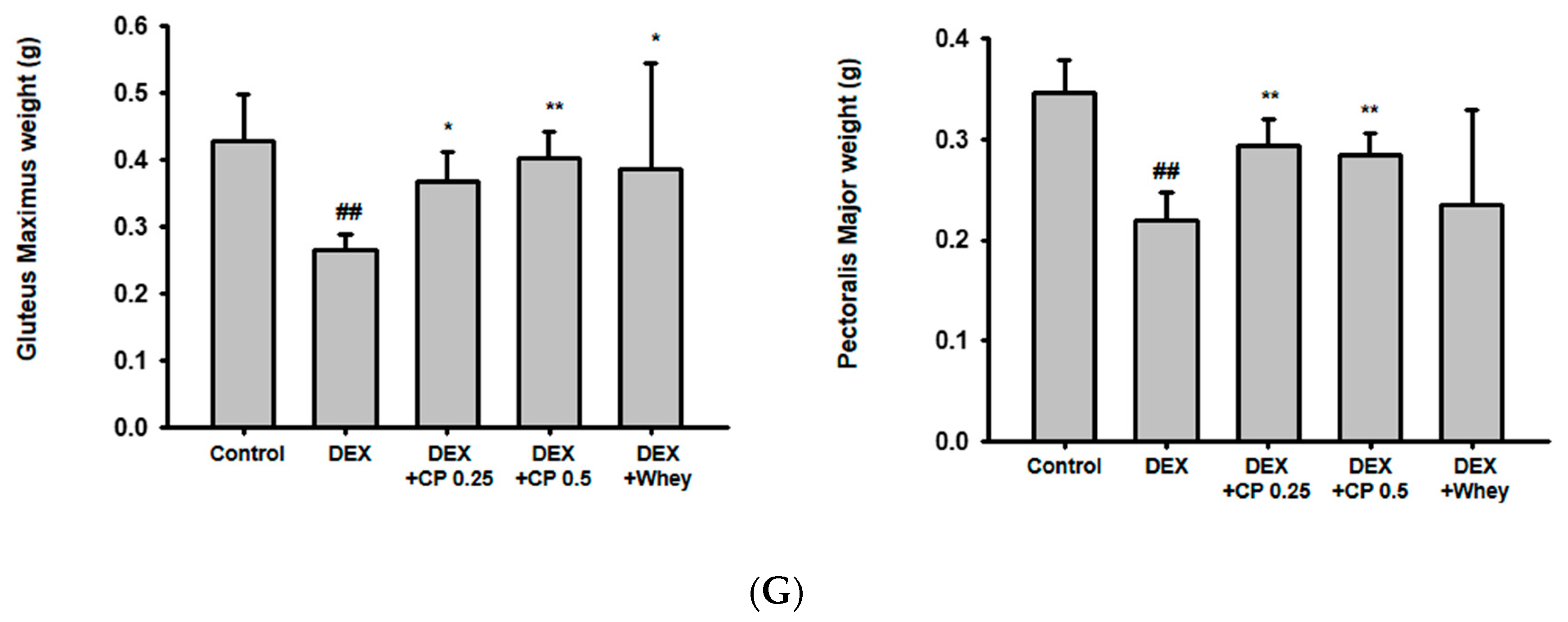
2.1. CP Administration Beneficially Affects Muscle Volume and Weight in Mice with DEX-Induced Muscle Wasting
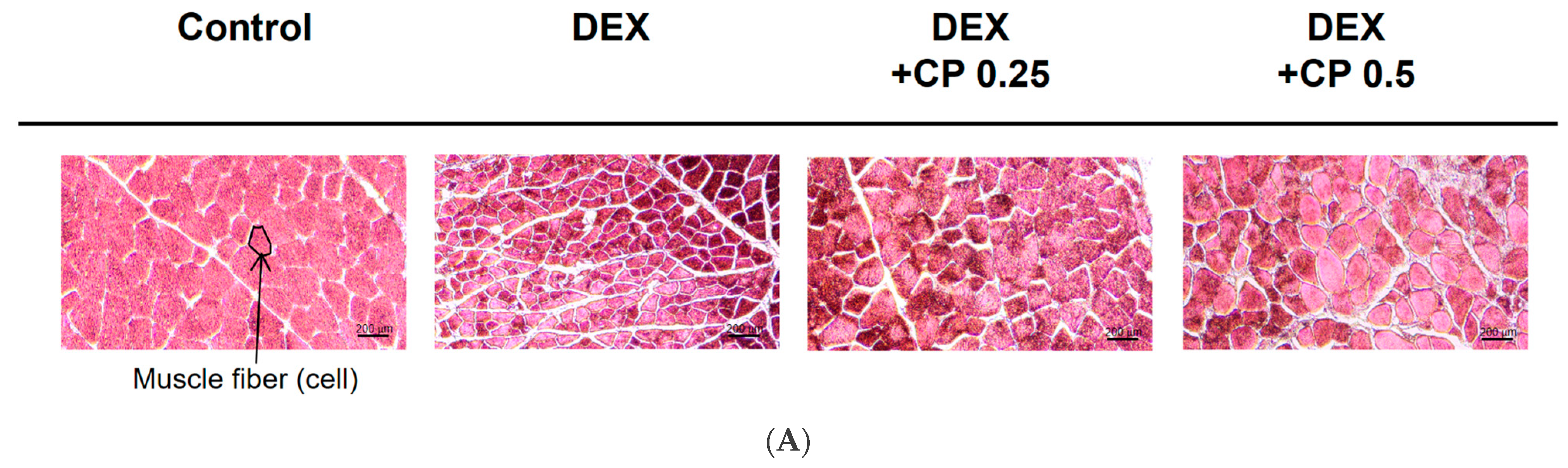
2.2. CP Intake Increases Fiber CSA in Mice with DEX-Induced Muscle Atrophy
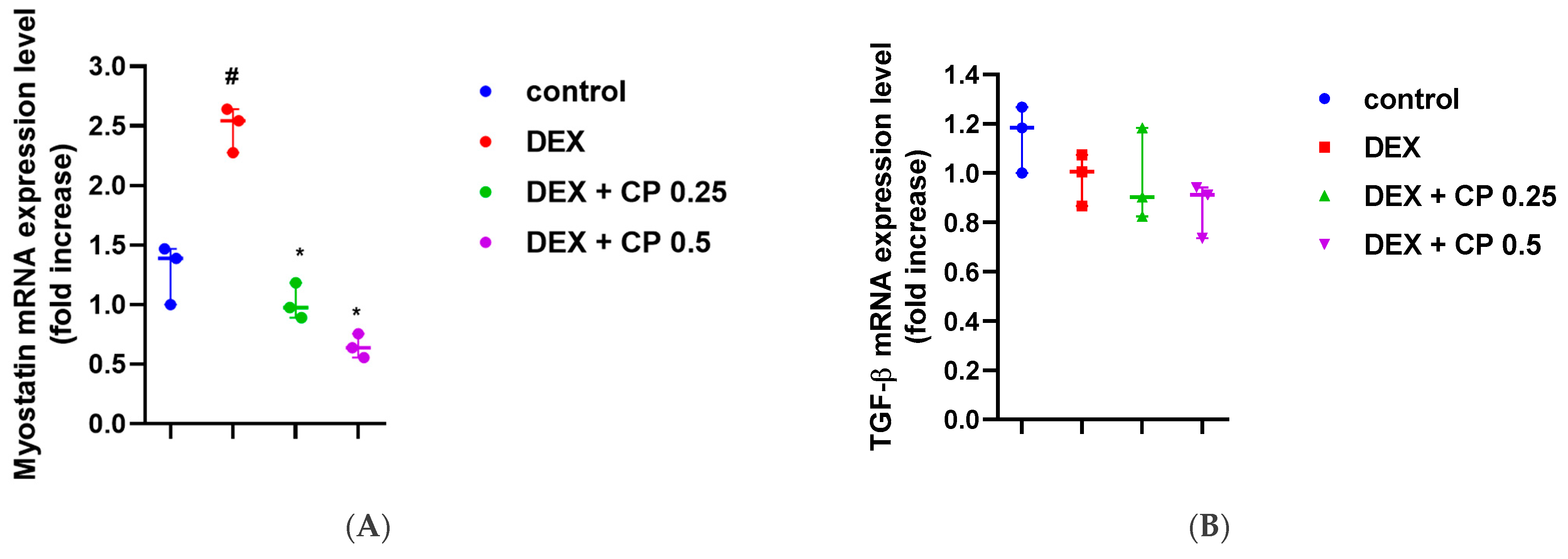
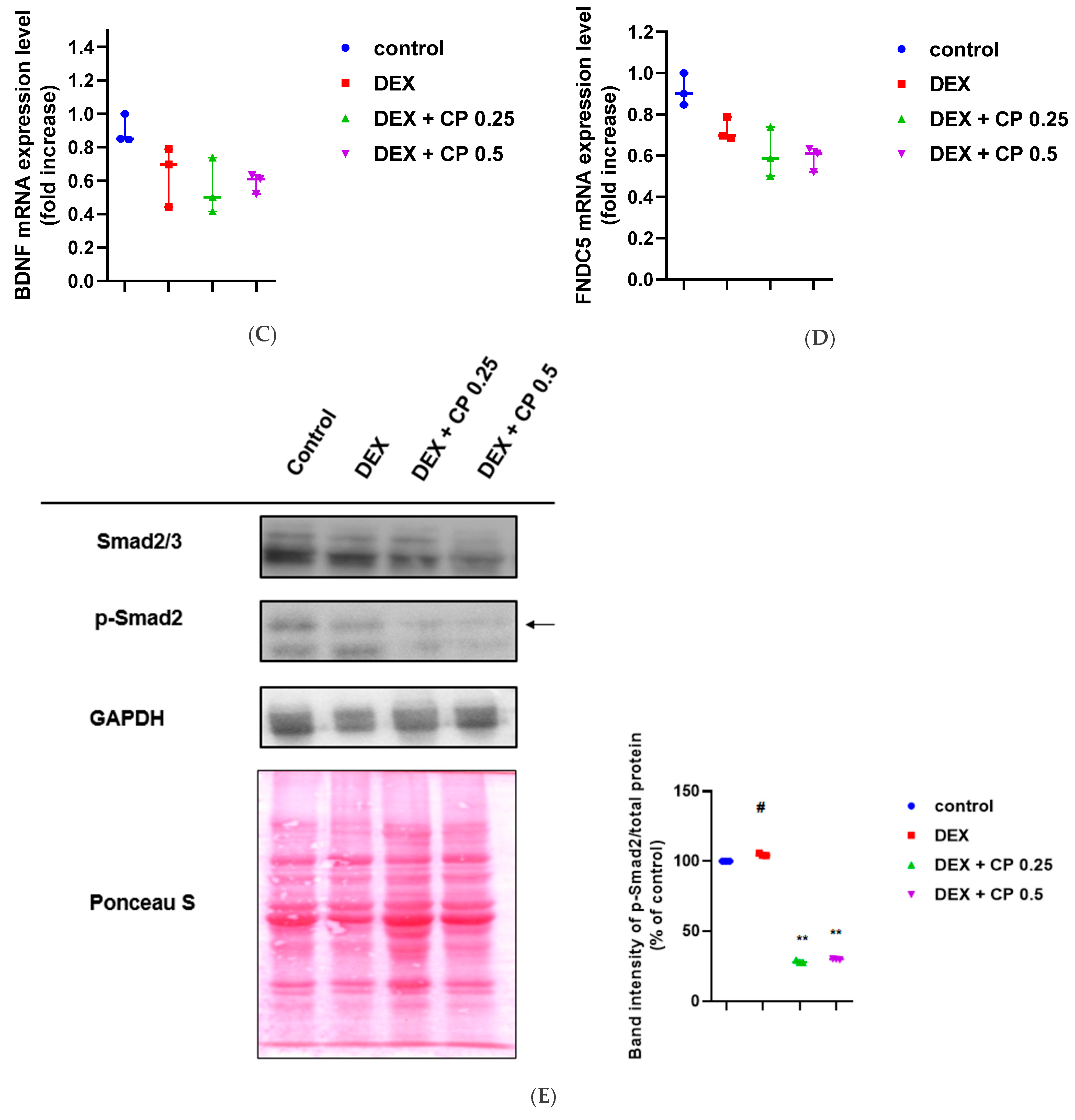
2.3. CP Suppresses the Myostatin/Smad Pathways Activated by DEX
2.4. CP Did Not Affect the Expression of Atrogin-1, MuRF 1, and Myogenin, Genes Related to the Catabolic Pathway
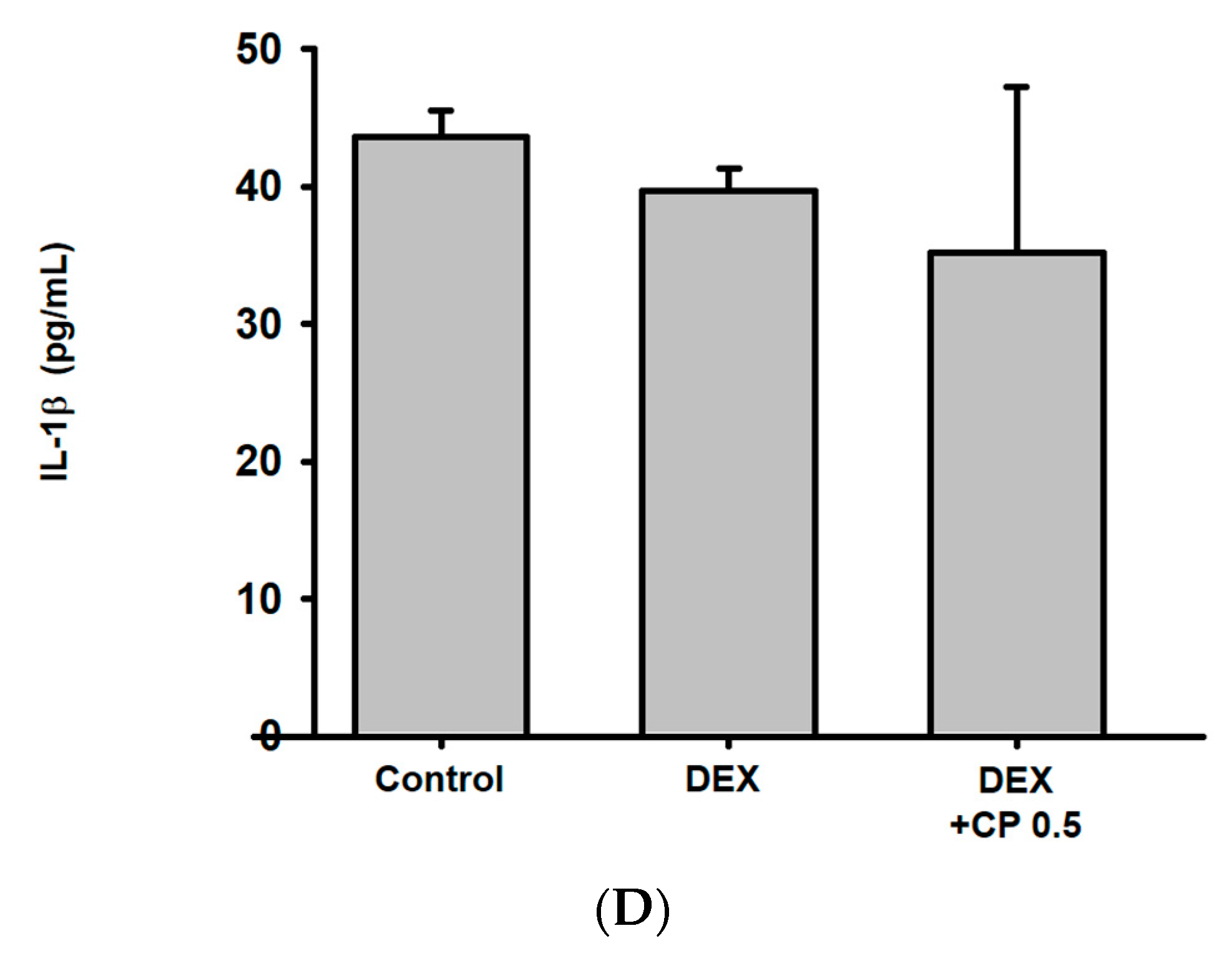
2.5. CP Decreased Serum Albumin Levels and Increased TNF-a Gene Expressions
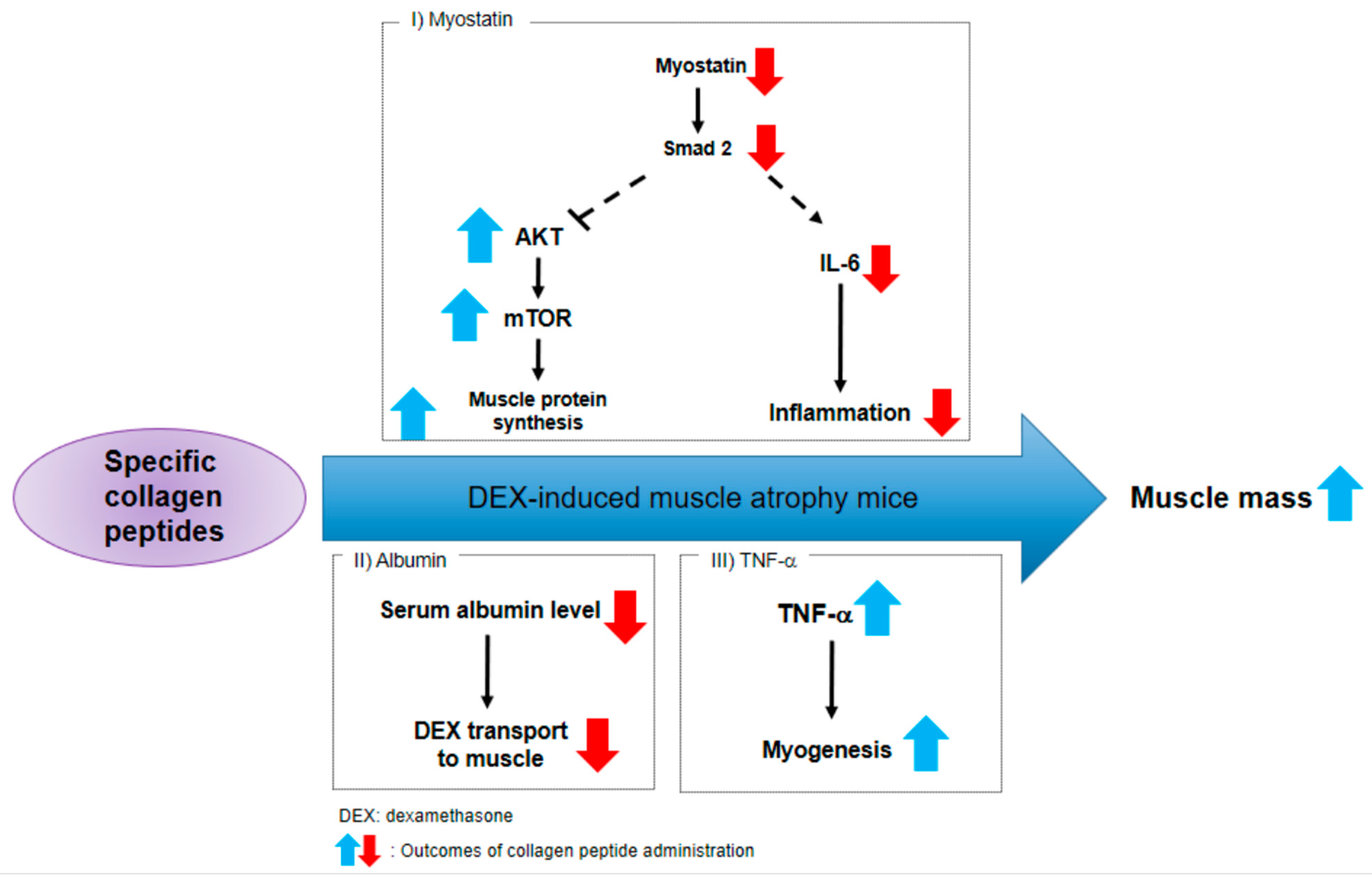
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animal Experiment
4.3. Micro-Computed Tomography (microCT)
4.4. Muscle Histology
4.5. RNA Extraction and cDNA Synthesis
4.6. Real-Time PCR
4.7. Immunoblotting
4.8. mTOR ELISA
4.9. Evans Blue Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brioche, T.; Pagano, A.F.; Py, G.; Chopard, A. Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention. Mol. Asp. Med. 2016, 50, 56–87. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Van Loon, L.J. Skeletal muscle atrophy during short-term disuse: Implications for age-related sarcopenia. Ageing Res. Rev. 2013, 12, 898–906. [Google Scholar] [CrossRef]
- Morley, J.E.; Thomas, D.R.; Wilson, M.-M.G. Cachexia: Pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006, 83, 735–743. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Gorgey, A.S. Skeletal muscle mitochondrial health and spinal cord injury. World J. Orthop. 2016, 7, 628. [Google Scholar] [CrossRef]
- Yao, S.; Shi, F.; Mu, N.; Li, X.; Ma, G.; Wang, Y.; Sun, X.; Liu, X.; Su, L. Angio-associated migratory cell protein (AAMP) interacts with cell division cycle 42 (CDC42) and enhances migration and invasion in human non-small cell lung cancer cells. Cancer Lett. 2021, 502, 1–8. [Google Scholar] [CrossRef]
- Nagumo, Y.; Kandori, S.; Tanuma, K.; Nitta, S.; Chihara, I.; Shiga, M.; Hoshi, A.; Negoro, H.; Kojima, T.; Mathis, B.J.; et al. PLD1 promotes tumor invasion by regulation of MMP-13 expression via NF-kappaB signaling in bladder cancer. Cancer Lett. 2021, 511, 15–25. [Google Scholar] [CrossRef]
- Oh, H.J.; Jin, H.; Nah, S.Y.; Lee, B.Y. Gintonin-enriched fraction improves sarcopenia by maintaining immune homeostasis in 20- to 24-month-old C57BL/6J mice. J. Ginseng Res. 2021, 45, 744–753. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, D.; Gai, L.; Wu, X.; Shi, Y.; He, Y.; Liu, C.; Zhang, C.; Zhou, G.; Yuan, C. Saponins from Panax japonicus ameliorate age-related renal fibrosis by inhibition of inflammation mediated by NF-kappaB and TGF-beta1/Smad signaling and suppression of oxidative stress via activation of Nrf2-ARE signaling. J. Ginseng Res. 2021, 45, 408–419. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hubner, C.; Riebel, T.; Komen, W.; Braun, T.; Tobin, J.F.; Lee, S.J. Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- Pirruccello-Straub, M.; Jackson, J.; Wawersik, S.; Webster, M.T.; Salta, L.; Long, K.; McConaughy, W.; Capili, A.; Boston, C.; Carven, G.J.; et al. Blocking extracellular activation of myostatin as a strategy for treating muscle wasting. Sci. Rep. 2018, 8, 2292. [Google Scholar] [CrossRef]
- Zhong, F.J.; Sun, B.; Cao, M.M.; Xu, C.; Li, Y.M.; Yang, L.Y. STMN2 mediates nuclear translocation of Smad2/3 and enhances TGFbeta signaling by destabilizing microtubules to promote epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2021, 506, 128–141. [Google Scholar] [CrossRef]
- Saneyasu, T.; Honda, K.; Kamisoyama, H. Myostatin Increases Smad2 Phosphorylation and Atrogin-1 Expression in Chick Embryonic Myotubes. J. Poult. Sci. 2019, 56, 224–230. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, L.; Tergaonkar, V. sORF-Encoded MicroPeptides: New players in inflammation, metabolism, and precision medicine. Cancer Lett. 2021, 500, 263–270. [Google Scholar] [CrossRef]
- Roberts, M.D.; Bayless, D.S.; Company, J.M.; Jenkins, N.T.; Padilla, J.; Childs, T.E.; Martin, J.S.; Dalbo, V.J.; Booth, F.W.; Rector, R.S.; et al. Elevated skeletal muscle irisin precursor FNDC5 mRNA in obese OLETF rats. Metabolism 2013, 62, 1052–1056. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.-K. The novel myokine irisin: Clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef]
- Chang, J.S.; Kong, I.D. Irisin prevents dexamethasone-induced atrophy in C2C12 myotubes. Pflug. Arch. 2020, 472, 495–502. [Google Scholar] [CrossRef]
- Clow, C.; Jasmin, B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sport. Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.S.; Krause Neto, W.; Calefi, A.S.; Georgetti, M.; Guerreiro, L.; Zocoler, C.A.S.; Gama, E.F. Significant Acute Response of Brain-Derived Neurotrophic Factor Following a Session of Extreme Conditioning Program Is Correlated With Volume of Specific Exercise Training in Trained Men. Front. Physiol. 2018, 9, 823. [Google Scholar] [CrossRef]
- Omura, T.; Sano, M.; Omura, K.; Hasegawa, T.; Doi, M.; Sawada, T.; Nagano, A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Peripher. Nerv. Syst. 2005, 10, 293–300. [Google Scholar] [CrossRef]
- Delezie, J.; Weihrauch, M.; Maier, G.; Tejero, R.; Ham, D.J.; Gill, J.F.; Karrer-Cardel, B.; Ruegg, M.A.; Tabares, L.; Handschin, C. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 16111–16120. [Google Scholar] [CrossRef]
- Song, Z.; Moore, D.R.; Hodson, N.; Ward, C.; Dent, J.R.; O’Leary, M.F.; Shaw, A.M.; Hamilton, D.L.; Sarkar, S.; Gangloff, Y.G.; et al. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci. Rep. 2017, 7, 5028. [Google Scholar] [CrossRef]
- Goodman, C.A. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev. Physiol. Biochem. Pharmacol. 2014, 166, 43–95. [Google Scholar]
- Kimball, S.R. Regulation of global and specific mRNA translation by amino acids. J. Nutr. 2002, 132, 883–886. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Bell, A.L.; Nayananjalie, W.A.; Escobar, J.; Hanigan, M.D. Essential amino acids regulate both initiation and elongation of mRNA translation independent of insulin in MAC-T cells and bovine mammary tissue slices. J. Nutr. 2011, 141, 1209–1215. [Google Scholar] [CrossRef]
- Pi, R.; Yang, Y.; Hu, X.; Li, H.; Shi, H.; Liu, Y.; Wang, X.; Tong, A.; Lu, T.; Wei, Y.; et al. Dual mTORC1/2 inhibitor AZD2014 diminishes myeloid-derived suppressor cells accumulation in ovarian cancer and delays tumor growth. Cancer Lett. 2021, 523, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Shu, J.; Chen, X.; Pan, H.; Chen, G.; Bi, Y.; Cui, D.; Li, X.; Liu, D.; Wang, L.; et al. DEPTOR inhibits lung tumorigenesis by inactivating the EGFR-mTOR signals. Cancer Lett. 2021, 519, 263–276. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, P.C.; Wang, X.; Goldman, D. Myogenin regulates denervation-dependent muscle atrophy in mouse soleus muscle. J. Cell Biochem. 2011, 112, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.K.; Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Park, C.H.; Yoon, C.; Kim, N.D.; Kim, M.K.; Chung, H.Y. beta-Hydroxy beta-methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS ONE 2014, 9, e102947. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Yang, X.; Wang, R.X.; Jiao, H.C.; Zhao, J.P.; Song, Z.G.; Lin, H. Leucine alleviates dexamethasone-induced suppression of muscle protein synthesis via synergy involvement of mTOR and AMPK pathways. Biosci. Rep. 2016, 36, e00346. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Herningtyas, E.H.; Ikeshita, N.; Shibahara, H.; Sugiyama, Y.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y.; et al. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve 2010, 41, 819–827. [Google Scholar] [CrossRef]
- Yoshida, T.; Kakizawa, S.; Totsuka, Y.; Sugimoto, M.; Miura, S.; Kumagai, H. Effect of endurance training and branched-chain amino acids on the signaling for muscle protein synthesis in CKD model rats fed a low-protein diet. Am. J. Physiol. Ren. Physiol. 2017, 313, F805–F814. [Google Scholar] [CrossRef]
- Sadri, S.; Sharifi, G.; Jalali Dehkordi, K. Nano branched-chain amino acids enhance the effect of uphill (concentric) and downhill (eccentric) treadmill exercise on muscle gene expression of Akt and mTOR on aged rats. Sport Sci. Health 2022, 18, 481–490. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; Konig, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Jendricke, P.; Centner, C.; Zdzieblik, D.; Gollhofer, A.; Konig, D. Specific Collagen Peptides in Combination with Resistance Training Improve Body Composition and Regional Muscle Strength in Premenopausal Women: A Randomized Controlled Trial. Nutrients 2019, 11, 892. [Google Scholar] [CrossRef]
- Kirmse, M.; Oertzen-Hagemann, V.; de Marees, M.; Bloch, W.; Platen, P. Prolonged Collagen Peptide Supplementation and Resistance Exercise Training Affects Body Composition in Recreationally Active Men. Nutrients 2019, 11, 1154. [Google Scholar] [CrossRef]
- Centner, C.; Jerger, S.; Mallard, A.; Herrmann, A.; Varfolomeeva, E.; Gollhofer, S.; Oesser, S.; Sticht, C.; Gretz, N.; Aagaard, P.; et al. Supplementation of Specific Collagen Peptides Following High-Load Resistance Exercise Upregulates Gene Expression in Pathways Involved in Skeletal Muscle Signal Transduction. Front. Physiol. 2022, 13, 838004. [Google Scholar] [CrossRef]
- El Shafey, N.; Guesnon, M.; Simon, F.; Deprez, E.; Cosette, J.; Stockholm, D.; Scherman, D.; Bigey, P.; Kichler, A. Inhibition of the myostatin/Smad signaling pathway by short decorin-derived peptides. Exp. Cell Res. 2016, 341, 187–195. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Gong, Q.; Yin, J.; Wang, M.; He, L.; Lei, F.; Luo, Y.; Yang, S.; Feng, Y.; Li, J.; Du, L. Comprehensive study of dexamethasone on albumin biogenesis during normal and pathological renal conditions. Pharm. Biol. 2020, 58, 1252–1262. [Google Scholar] [CrossRef]
- Shabalin, I.G.; Czub, M.P.; Majorek, K.A.; Brzezinski, D.; Grabowski, M.; Cooper, D.R.; Panasiuk, M.; Chruszcz, M.; Minor, W. Molecular determinants of vascular transport of dexamethasone in COVID-19 therapy. IUCrJ 2020, 7, 1048–1058. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Ong, J.M.; Garvey, W.T.; Henry, R.R.; Kern, P.A. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J. Clin. Investig. 1996, 97, 1111–1116. [Google Scholar] [CrossRef]
- Torrente, Y.; El Fahime, E.; Caron, N.J.; Del Bo, R.; Belicchi, M.; Pisati, F.; Tremblay, J.P.; Bresolin, N. Tumor necrosis factor-alpha (TNF-alpha) stimulates chemotactic response in mouse myogenic cells. Cell Transplant. 2003, 12, 91–100. [Google Scholar] [CrossRef]
- Busillo, J.M.; Azzam, K.M.; Cidlowski, J.A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef]
- Frank, M.G.; Hershman, S.A.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology 2014, 40, 191–200. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Otis, J.S.; Ashikhmin, Y.I.; Brown, L.A.; Guidot, D.M. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Res. Ther. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.; Chi, M.; Yang, Q.; Guo, C. Drugs for the Treatment of Muscle Atrophy. In Background and Management of Muscular Atrophy; IntechOpen: London, UK, 2021. [Google Scholar]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]
- Golan, T.; Geva, R.; Richards, D.; Madhusudan, S.; Lin, B.K.; Wang, H.T.; Walgren, R.A.; Stemmer, S.M. LY2495655, an antimyostatin antibody, in pancreatic cancer: A randomized, phase 2 trial. J. Cachexia Sarcopenia Muscle 2018, 9, 871–879. [Google Scholar] [CrossRef]
- Egerman, M.A.; Cadena, S.M.; Gilbert, J.A.; Meyer, A.; Nelson, H.N.; Swalley, S.E.; Mallozzi, C.; Jacobi, C.; Jennings, L.L.; Clay, I.; et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015, 22, 164–174. [Google Scholar] [CrossRef]
- Souza, T.A.; Chen, X.; Guo, Y.; Sava, P.; Zhang, J.; Hill, J.J.; Yaworsky, P.J.; Qiu, Y. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol. Endocrinol. 2008, 22, 2689–2702. [Google Scholar] [CrossRef]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, B.D.; Eldridge, J.A. Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and brain. Endocrinology 2015, 156, 3885–3888. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jang, I.-Y.; Jung, H.-W.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Lee, E.; Kim, B.-J.J.E.G. Serum irisin level is independent of sarcopenia and related muscle parameters in older adults. Exp. Gerontol. 2022, 162, 111744. [Google Scholar] [CrossRef] [PubMed]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Myostatin signaling regulates Akt activity via the regulation of miR-486 expression. Int. J. Biochem. Cell Biol. 2014, 47, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A.; McNally, R.M.; Hoffmann, F.M.; Hornberger, T.A. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol. Endocrinol. 2013, 27, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, N.; Baker, S.J. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. 2009, 4, 127–150. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Lu, L.; Jiang, P.; Sun, H.; Wang, H.J.P.O. Inhibition of miR-29 by TGF-beta-Smad3 signaling through dual mechanisms promotes transdifferentiation of mouse myoblasts into myofibroblasts. PLoS ONE 2012, 7, e33766. [Google Scholar] [CrossRef]
- Patil, R.H.; Naveen Kumar, M.; Kiran Kumar, K.M.; Nagesh, R.; Kavya, K.; Babu, R.L.; Ramesh, G.T.; Chidananda Sharma, S. Dexamethasone inhibits inflammatory response via down regulation of AP-1 transcription factor in human lung epithelial cells. Gene 2018, 645, 85–94. [Google Scholar] [CrossRef]
- Atsaves, V.; Zhang, R.; Ruder, D.; Pan, Y.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. Constitutive control of AKT1 gene expression by JUNB/CJUN in ALK+ anaplastic large-cell lymphoma: A novel crosstalk mechanism. Leukemia 2015, 29, 2162–2172. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef]
- Caldow, M.K.; Ham, D.J.; Trieu, J.; Chung, J.D.; Lynch, G.S.; Koopman, R. Glycine Protects Muscle Cells From Wasting in vitro via mTORC1 Signaling. Front. Nutr. 2019, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, B. Collagen supplementation: Therapy for skin disorders: A review. World J. Pharm. Res. 2020, 9, 2504–2518. [Google Scholar]
- Osawa, Y.; Mizushige, T.; Jinno, S.; Sugihara, F.; Inoue, N.; Tanaka, H.; Kabuyama, Y. Absorption and metabolism of orally administered collagen hydrolysates evaluated by the vascularly perfused rat intestine and liver in situ. Biomed. Res. 2018, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food. Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, S.B.; Jung, J.H.; Piao, Z.; Chung, H.J. Orally Available Collagen Tripeptide: Enzymatic Stability, Intestinal Permeability, and Absorption of Gly-Pro-Hyp and Pro-Hyp. J. Agric. Food Chem. 2016, 64, 7127–7133. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Kimira, Y.; Osawa, Y.; Shimizu, J.; Kataoka-Matsushita, A.; Mano, H. Collagen-derived dipeptide prolyl hydroxyproline directly binds to Foxg1 to change its conformation and inhibit the interaction with Runx2. Biosci. Biotechnol. Biochem. 2019, 83, 2027–2033. [Google Scholar] [CrossRef]
- Otsuka, Y.; Egawa, K.; Kanzaki, N.; Izumo, T.; Rogi, T.; Shibata, H. Quercetin glycosides prevent dexamethasone-induced muscle atrophy in mice. Biochem. Biophys. Rep. 2019, 18, 100618. [Google Scholar] [CrossRef]
- Nicastro, H.; Gualano, B.; de Moraes, W.M.; de Salles Painelli, V.; da Luz, C.R.; dos Santos Costa, A.; de Salvi Guimaraes, F.; Medeiros, A.; Brum, P.C.; Lancha, A.H., Jr. Effects of creatine supplementation on muscle wasting and glucose homeostasis in rats treated with dexamethasone. Amino Acids 2012, 42, 1695–1701. [Google Scholar] [CrossRef]
- de Salvi Guimaraes, F.; de Moraes, W.M.; Bozi, L.H.; Souza, P.R.; Antonio, E.L.; Bocalini, D.S.; Tucci, P.J.; Ribeiro, D.A.; Brum, P.C.; Medeiros, A. Dexamethasone-induced cardiac deterioration is associated with both calcium handling abnormalities and calcineurin signaling pathway activation. Mol. Cell Biochem. 2017, 424, 87–98. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Biol. 2015, 13, e1002128. [Google Scholar] [CrossRef]





| Gene | Direction | Primer sequence (5′→3′) |
|---|---|---|
| BDNF | F | TACCTGGATGCCGCAAACAT |
| R | TGCTTCAGTTGGCCTTTGGA | |
| FNDC5 | F | CACGCGAGGCTGAAAAGATG |
| R | GAGCTATAACACCTGCCCACA | |
| Myostatin | F | TCACGCTACCACGGAAACAA |
| R | AGGAGTCTTGACGGGTCTGA | |
| Myogenin | F | AGGAGATCATTTGCTCGCGG |
| R | GTTGGGCATGGTTTCGTCTG | |
| TGF-β | F | AACAATTCCTGGCGTTACCTT |
| R | CTGCCGTACAACTCCAGTGA | |
| IL-6 | F | AGCCAGAGTCCTTCAGAGAGAT |
| R | AGGAGAGCATTGGAAATTGGGG | |
| MuRF1 | F | GAGGGGCTACCTTCCTCTCA |
| R | AGAGGAACGCTGCCTTTCAA | |
| Atrogin-1 | F | TTCAGCAGCCTGAACTACGA |
| R | AGTATCCATGGCGCTCCTTC | |
| GAPDH | F | GGTTGTCTCCTGCGACTTCA |
| R | CATTGAGAGCAATGCCAGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Park, S.H.; Kim, D.S.; Choi, W.; Jang, J.; Rahmawati, L.; Jang, W.Y.; Lim, H.K.; Hwang, J.Y.; Gu, G.R.; et al. The Preventive Effect of Specific Collagen Peptides against Dexamethasone-Induced Muscle Atrophy in Mice. Molecules 2023, 28, 1950. https://doi.org/10.3390/molecules28041950
Oh J, Park SH, Kim DS, Choi W, Jang J, Rahmawati L, Jang WY, Lim HK, Hwang JY, Gu GR, et al. The Preventive Effect of Specific Collagen Peptides against Dexamethasone-Induced Muscle Atrophy in Mice. Molecules. 2023; 28(4):1950. https://doi.org/10.3390/molecules28041950
Chicago/Turabian StyleOh, Jieun, Sang Hee Park, Dong Seon Kim, Wooram Choi, Jiwon Jang, Laily Rahmawati, Won Young Jang, Hyun Kyung Lim, Ji Yeon Hwang, Ga Rin Gu, and et al. 2023. "The Preventive Effect of Specific Collagen Peptides against Dexamethasone-Induced Muscle Atrophy in Mice" Molecules 28, no. 4: 1950. https://doi.org/10.3390/molecules28041950
APA StyleOh, J., Park, S. H., Kim, D. S., Choi, W., Jang, J., Rahmawati, L., Jang, W. Y., Lim, H. K., Hwang, J. Y., Gu, G. R., Geum, J.-H., Choi, S.-Y., Kim, J. H., & Cho, J. Y. (2023). The Preventive Effect of Specific Collagen Peptides against Dexamethasone-Induced Muscle Atrophy in Mice. Molecules, 28(4), 1950. https://doi.org/10.3390/molecules28041950








