Peptide-Drug Conjugates: A New Hope for Cancer Management
Abstract
1. Introduction
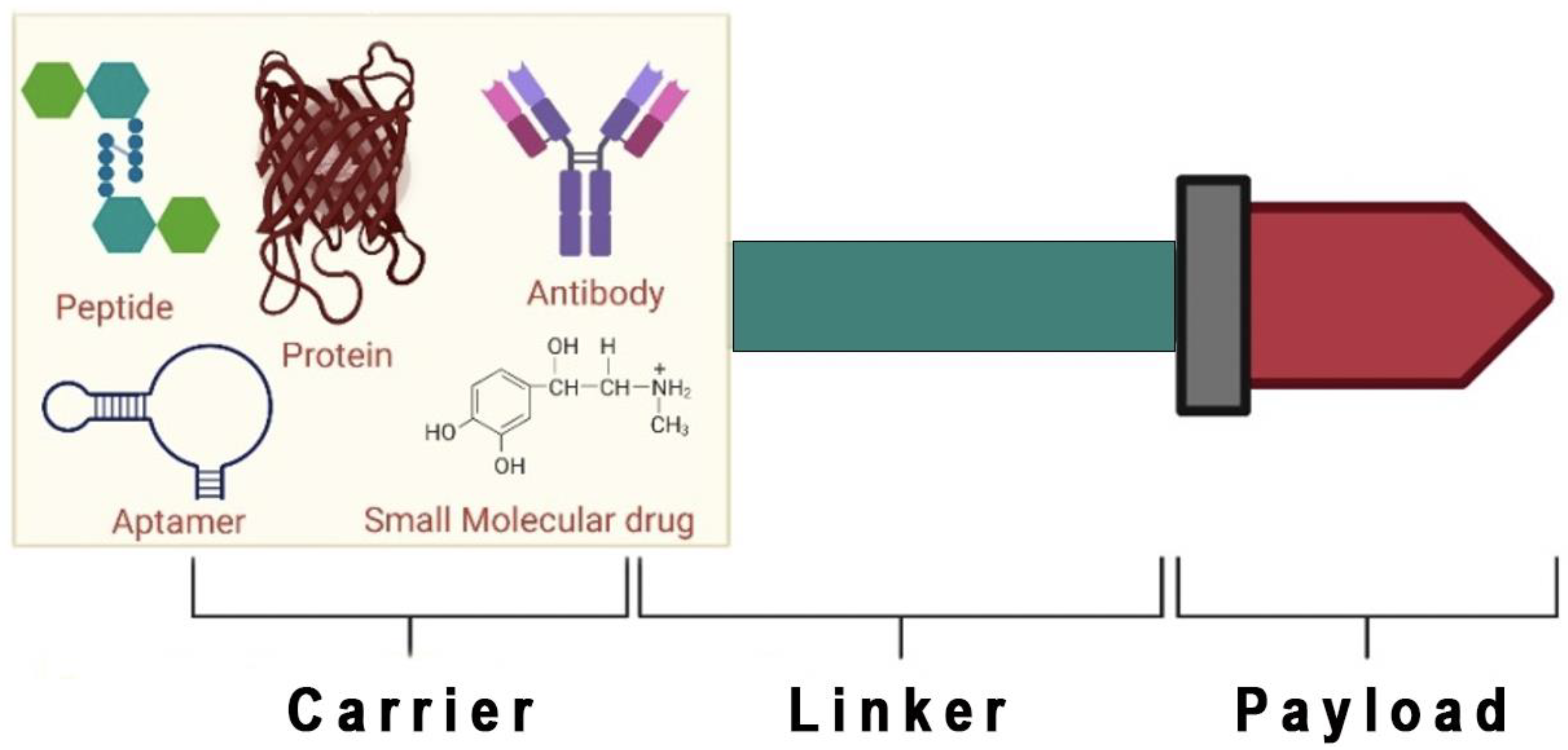
2. Peptide-Drug Conjugates
3. Components of Peptide-Drug Conjugates
3.1. Homing Peptide
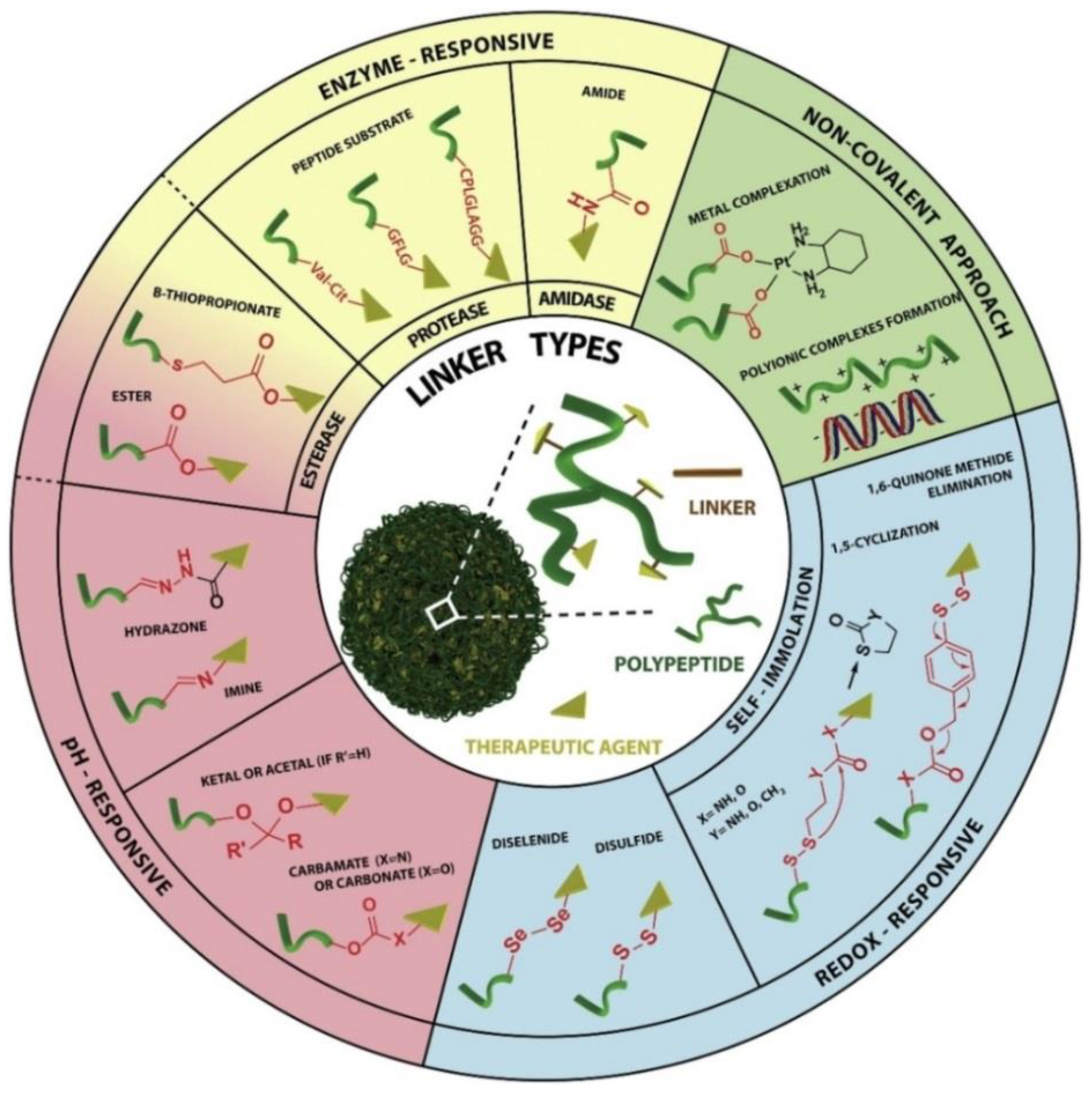
3.2. Linker
3.2.1. pH-Responsive Linkers
3.2.2. Enzyme Responsive Linkers
3.2.3. Redox Responsive Linkers
3.2.4. Non-Covalent Interaction Linkers
3.3. Payloads
3.3.1. Radionuclides
3.3.2. Chemotherapeutic Agents
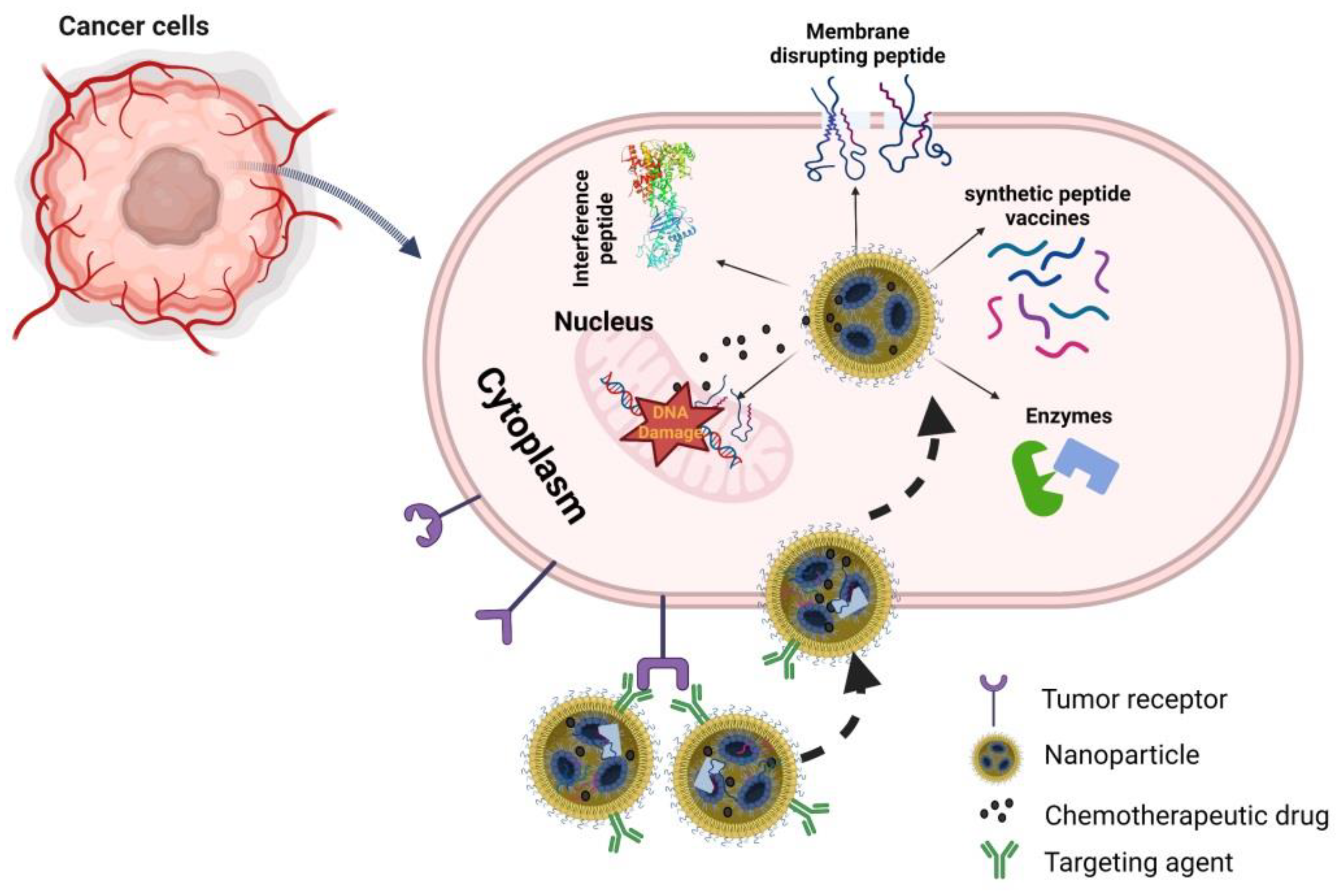
4. Delivery of Peptide–Drug-Conjugates to the Desired Targeted Tissue
5. Applications
5.1. Anti-Cancer Therapy
5.2. Apoptosis
5.3. Tumor Accumulation
5.4. Cancer Immunotherapy
5.5. Nanotechnology
5.6. Bicycle–Toxin Conjugates
5.7. Peptide–Dendrimer Conjugates
5.8. Cell-Penetrating Peptides
6. Human Clinical Trials
7. Challenges for the Delivery of Peptide-Drug Conjugates: The Way Forward
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chavda, V.P.; Ertas, Y.N.; Walhekar, V.; Modh, D.; Doshi, A.; Shah, N.; Anand, K.; Chhabria, M. Advanced Computational Methodologies Used in the Discovery of New Natural Anticancer Compounds. Front. Pharmacol. 2021, 12, 702611. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.X.; Chen, Z.S.; Hou, K. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ. Precis. Oncol. 2019, 3, 7. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.; Park, S. iRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 1906. [Google Scholar] [CrossRef]
- Chavda, V.P. Nanotherapeutics, and Nanobiotechnology. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–13. [Google Scholar]
- Chavda, V.P.; Vihol, D.; Mehta, B.; Shah, D.; Patel, M.; Vora, L.K.; Pereira-Silva, M.; Paiva-Santos, A.C. Phytochemical-loaded liposomes for anti-cancer therapy: An updated review. Nanomedicine 2022, 7, 547. [Google Scholar] [CrossRef]
- Puig-Saus, C.; Rojas, L.A.; Laborda, E.; Figueras, A.; Alba, R.; Fillat, C.; Alemany, R. iRGD tumor-penetrating peptide-modified oncolytic adenovirus shows enhanced tumor transduction, intratumoral dissemination, and antitumor efficacy. Gene Ther. 2014, 21, 767. [Google Scholar] [CrossRef]
- Safari-Alighiarloo, N.; Taghizadeh, M.; Rezaei-Tavirani, M.; Goliaei, B.; Peyvandi, A.A. Protein-protein interaction networks (PPI) and complex diseases. Gastroenterol Hepatol. Bed Bench 2014, 7, 17. [Google Scholar]
- De Las Rivas, J.; Fontanillo, C. Protein–Protein Interactions Essentials: Key Concepts to Building and Analyzing Interactome Networks. PLoS Comput. Biol. 2010, 6, e1000807. [Google Scholar] [CrossRef]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N.S. Protein-protein interaction detection: Methods and analysis. Int. J. Proteom. 2014, 2014, 147648. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, Z.S.; Zarei, M.; Fath, M.K.; Ganji, M.; Farahani, M.S.; Afsharnouri, F.; Pourzardosht, N.; Khalesi, B.; Jahangiri, A.; Rahbar, M.R.; et al. In silico Approaches for the Design and Optimization of Interfering Peptides Against Protein–Protein Interactions. Front. Mol. Biosci. 2021, 8, 669431. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Patel, Z.; Parmar, Y.; Chavda, D. In Silico Protein Design and Virtual Screening. Comput. Bioinform. 2021, 5, 85–99. [Google Scholar]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Bojarska, J.; Feehan, J.; Matsoukas, J.; Wolf, W.M. Smart therapies for pandemics: A potential of short peptides. Front. Pharmacol. 2022, 13, 914467–914475. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Feehan, J.; Kaczmarek, K.; Matsoukas, J.M.; Paredes Lopez, O.; Saviano, M.; Skwarczynski, M.; Smith-Carpenter, J.; et al. New Advances in Short Peptides: Looking Forward. Molecules 2022, 27, 3635. [Google Scholar] [CrossRef]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-based Scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef]
- Bojarska, J.; Wolf, W.M. Ultra-short cyclo-peptides as bio-inspired therapeutics: Proline-based 2,5-diketopiperazines (DKP). Proceedings 2021, 79, 10. [Google Scholar]
- Bojarska, J.; Breza, M.; Remko, M.; Czyz, M.; Gajos-Michniewicz, A.; Zimecki, M.; Kaczmarek, K.; Madura, I.D.; Wojciechowski, J.M.; Wolf, W.M. Structural and Biofunctional Insights into the Cyclo(Pro-Pro-Phe-Phe-) Scaffold from Experimental and In Silico Studies: Melanoma and Beyond. Int. J. Mol. Sci. 2022, 23, 7173. [Google Scholar] [CrossRef]
- Bojarska, J. Advances in Research of Short Peptides. Molecules 2022, 27, 2446. [Google Scholar] [CrossRef] [PubMed]
- Bojarska, J.; New, R.; Borowiecki, P.; Remko, M.; Breza, M.; Madura, I.D.; Fruzinski, A.; Pietrzak, A.; Wolf, W.M. The First Insight Into the Supramolecular System of D,L-α-Difluoromethylornithine: A New Antiviral Perspective. Front. Chem. 2021, 9, 679776. [Google Scholar] [CrossRef] [PubMed]
- Bojarska, J.; Remko, M.; Breza, M.; Madura, I.; Fruzinski, A.; Wolf, W.M. A Proline-Based Tectons and Supramolecular Synthons for Drug Design 2.0: A Case Study of ACEI. Pharmaceuticals 2020, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Bojarska, J.; Remko, M.; Madura, I.D.; Kaczmarek, K.; Zabrocki, J.; Wolf, W.M. Synthesis, Experimental and in Silico Studies of N-Fluorenylmethoxycarbonyl-O-Tert-Butyl-N-Methyltyrosine, Coupled with CSD Data: A Survey of Interactions in the Crystal Structures of Fmoc-Amino Acids. Acta Crystallogr. C 2020, 76, 328–345. [Google Scholar] [CrossRef]
- Bojarska, J.; Remko, M.; Madura, I.D.; Wojciechowski, J.M.; Olczak, A.; Kaczmarek, K.; Zabrocki, J.; Wolf, W.M. Supramolecular Synthon Polymorphism in Modified Amino Acids. Structural, Conformational and Energy Landscapes of N-Benzoyl-20-Hydroxy-3-Methylisovaline. J. Mol. Struct. 2019, 1190, 11–22. [Google Scholar] [CrossRef]
- Bojarska, J.; Remko, M.; Breza, M.; Madura, I.D.; Kaczmarek, K.; Zabrocki, J.; Wolf, W.M. A Supramolecular Approach to Structure-Based Design with A Focus on Synthons Hierarchy in Ornithine-Derived Ligands: Review, Synthesis, Experimental and in Silico Studies. Molecules 2020, 25, 1135. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151. [Google Scholar] [CrossRef]
- Shaji, J.; Patole, V. Protein and peptide drug delivery: Oral approaches. Indian J. Pharm. Sci. 2008, 70, 269–277. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Habault, J.; Poyet, J.L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef]
- Yan, L.; Rosen, N.; Arteaga, C. Targeted cancer therapies. Chin. J. Cancer 2011, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136. [Google Scholar] [CrossRef] [PubMed]
- Tsomaia, N. Peptide therapeutics: Targeting the undruggable space. Eur. J. Med. Chem. 2015, 94, 459. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.G.; Rudolph, J.; Bailey, D. Phenotypic screening in cancer drug discovery—Past, present and future. Nat. Rev. Drug Discov. 2014, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Rodríguez, J.J.; Ochoa-Zarzosa, A.; López-Gómez, R.; López-Meza, J.E. Plant antimicrobial peptides as potential anticancer agents. Biomed. Res. Int. 2015, 2015, 735087. [Google Scholar] [CrossRef]
- Alas, M.; Saghaeidehkordi, A.; Kaur, K. Peptide-Drug Conjugates with Different Linkers for Cancer Therapy. J. Med. Chem. 2021, 64, 216. [Google Scholar] [CrossRef]
- Chavda, V.P.; Ajabiya, J.; Teli, D.; Bojarska, J.; Apostolopoulos, V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules 2022, 27, 4315. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. (177)Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023. [Google Scholar] [CrossRef]
- Deonarain, M.P.; Yahioglu, G.; Stamati, I.; Pomowski, A.; Clarke, J.; Edwards, B.M.; Diez-Posada, S.; Stewart, A.C. Small-format drug conjugates: A viable alternative to ADCs for solid tumors? Antibodies 2018, 7, 16. [Google Scholar] [CrossRef]
- Yang, S.B.; Banik, N.; Han, B.; Lee, D.-N.; Park, J. Peptide-Based Bioconjugates and Therapeutics for Targeted Anticancer Therapy. Pharmaceutics 2022, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110, 112. [Google Scholar] [CrossRef] [PubMed]
- Jong, M.D.; Valkema, R.; Jamar, F.; Kvols, L.K.; Kwekkeboom, D.J.; Breeman, W.A.P.; Bakker, W.H.; Smith, C.; Pauwels, S.; Krenning, E.P. Somatostatin receptor-targeted radionuclide therapy of tumors: Preclinical and clinical findings. Semin. Nucl. Med. 2002, 32, 133. [Google Scholar] [CrossRef] [PubMed]
- Jamous, M.; Haberkorn, U.; Mier, W. Synthesis of Peptide Radiopharmaceuticals for the Therapy and Diagnosis of Tumor Diseases. Molecules 2013, 18, 3379. [Google Scholar] [CrossRef]
- Capper, C.P.; Rae, J.M.; Auchus, R.J. The Metabolism, Analysis, and Targeting of Steroid Hormones in Breast and Prostate Cancer. Horm. Cancer 2016, 7, 149. [Google Scholar] [CrossRef]
- Mateos, M.V.; Blade, J.; Bringhen, S.; Ocio, E.M.; Efebera, Y.; Pour, L.; Gay, F.; Sonneveld, P.; Gullbo, J.; Richardson, P.G. Melflufen: A peptide-drug conjugtae for the treatment of multiple myeloma. J. Clin. Med. 2020, 9, 3120. [Google Scholar] [CrossRef]
- Lindberg, J.; Nilvebrant, J.; Lehmann, F. Progress and future directions with peptide-drug conjugates for targeted cancer therapy. Molecules 2021, 26, 6042. [Google Scholar] [CrossRef]
- Kurzock, R.; Gabrail, N.; Chandhasin, C.; Moulder, S.; Smith, C.; Brenner, A.; Sankhala, K.; Mita, A.; Elian, K.; Bouchard, D.; et al. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumours. Mol. Cancer Ther. 2012, 11, 308. [Google Scholar] [CrossRef]
- Li, F.; Tang, S.C. Targeting metastatic breast cancer with ANG1005, a novel peptide-paclitaxel conjugate that crosses the blood-brain-barrier (BBB). Genes Dis. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Allred, J.B.; Liu, H.; Hobday, T.J.; Rodacker, M.W.; Lyss, A.P.; Fitch, T.R.; Perez, E.A. Phase 2 trial of paclitaxel polyglumex with capecitabine for metastatic breast cancer. Am. J. Clin. Oncol. 2014, 37, 167–171. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 9. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.B.; López, C.Q.; Manczak, T.; Martinez, K.; Simonsen, H.T. Thapsigargin—From Thapsia L. to Mipsagargin. Molecules 2015, 20, 6113. [Google Scholar] [CrossRef] [PubMed]
- Emos, G.; Gorchev, G.; Sehouli, J.; Wimberger, P.; Stahle, A.; Hanker, L.; Hilpert, F.; Sindermann, H.; Grundker, C.; Harter, P. Efficacy and safety of AEZS-108 (INN: Zoptarelin doxorubicin acetate) an LHRH agonist linked to doxorubicin in women with platinum refractory or resistant ovarian cancer expressing LHRH receptors: A multicenter phase II trial of the ago-study group (AGO GYN 5). Gynecol. Oncol. 2014, 133, 427. [Google Scholar]
- BT1718 in Patients with Advanced Solid Tumours. Available online: https://clinicaltrials.gov/ct2/show/NCT03486730 (accessed on 30 August 2022).
- Schoffski, P.; Delord, J.P.; Brain, E.; Robert, J.; Dumez, H.; Gasmi, J.; Trouet, A. First-in-man phase I study assessing the safety and pharmacokinetics of a 1-hour intravenous infusion of the doxorubicin prodrug DTS-201 every 3 weeks in patients with advanced or metastatic solid tumours. Eur. J. Cancer 2017, 86, 240. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Register—Search for DTS-201. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=DTS-201 (accessed on 29 April 2008).
- Pinato, D.J.; Black, J.R.M.; Ramaswami, R.; Tan, T.M.; Adjogatse, D.; Sharma, R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med. Oncol. 2016, 33, 47. [Google Scholar] [CrossRef]
- Forsell-Aronsson, E.; Bernhardt, P.; Nilsson, O.; Tisell, L.E.; Wangberg, B.; Ahlman, H. Biodistribution data from 100 patinets i.v. injected with In-111-DTPA-D-Phe(1)-octreotide. Acta Oncolog. 2004, 43, 436–442. [Google Scholar] [CrossRef]
- Lorusso, D.; Scambia, G.; Amadio, G.; di Legge, A.; Pietragalla, A.; De Vincenzo, R.; Masciullo, V.; Stefano, M.D.; Mangili, G.; Citterio, G.; et al. Phase II study of NGR-hTNF in combination with doxorubicin in relapsed ovarian cancer patients. Br. J. Cancer 2012, 107, 37–42. [Google Scholar] [CrossRef]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Busselberg, D. Paclitaxel`s mechanistic and clinical effects on breast cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef]
- Curtis, K.K.; Sarantopoulos, J.; Northfelt, D.W.; Weiss, G.J.; Barnhart, K.M.; Whisnant, J.K.; Leuschner, C.; Alila, H.; Borad, M.J.; Ramanathan, R.K. Novel LHRH-receptor-targeted cytolytic peptide, EP-100: First-in-human phase I study in patients with advanced LHRH-receptor-expressing solid tumors. Cancer Chemother. Pharm. 2014, 73, 931–941. [Google Scholar] [CrossRef]
- Ley, K.; Rivera-Nieves, J.; Sandborn, W.J.; Shattil, S. Integrin-based therapeutics: Biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Toschi, L.; Finocchiaro, G.; Bartolini, S.; Gioia, V.; Cappuzzo, F. Role of gemcitabine in cancer therapy. Future Oncol. 2005, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Pezhman Shirmardi, S.; Gandomkar, M.; Maragheh, M.G.; Shamsaei, M. Preclinical evaluation of a new bombesin analog for imaging of gastrin-releasing peptide receptors. Cancer Biother. Radiopharm. 2011, 26, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, M.; Rosenthal-Aizman, K.; Saar, K.; Eiriksdottir, E.; Jiang, Y.; Sassian, M.; Ostlund, P.; Hallbrink, M.; Langel, U. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem. Pharmacol. 2006, 71, 416–425. [Google Scholar] [CrossRef]
- Biri-Kovács, B.; Adorján, A.; Szabó, I.; Szeder, B.; Bősze, S.; Mező, G. Structure-Activity Relationship of HER2 Receptor Targeting Peptide and Its Derivatives in Targeted Tumor Therapy. Biomolecules 2020, 10, 183. [Google Scholar] [CrossRef]
- Hernandez Vargas, S.; Kossatz, S.; Voss, J.; Ghosh, S.C.; Tran Cao, H.S.; Simien, J.; Reiner, T.; Dhingra, S.; Fisher, W.E.; Azhdarinia, A. Specific Targeting of Somatostatin Receptor Subtype-2 for Fluorescence-Guided Surgery. Clin. Cancer Res. 2019, 25, 4332. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef]
- Kondo, E.; Iioka, H.; Saito, K. Tumor-homing peptide and its utility for advanced cancer medicine. Cancer Sci. 2021, 112, 2118–2125. [Google Scholar] [CrossRef]
- Fujiwara, K.; Toda, H.; Ikeguchi, M. Dependence of α-helical and β-sheet amino acid propensities on the overall protein fold type. BMC Struct. Biol. 2012, 12, 18. [Google Scholar] [CrossRef]
- Bouayad-Gervais, S.; St-Cyr, D.J.; Courcelles, M.; Bonneil, É.; Gohard, F.H.; Thibault, P.; Earnshaw, C.; Tyers, M. Head-to-tail cyclization of side chain-protected linear peptides to recapitulate genetically-encoded cyclized peptides. Pept. Sci. 2022, 114, e24254. [Google Scholar] [CrossRef]
- Katsara, M.; Tselios, T.; Deraos, S.; Deraos, G.; Matsoukas, M.T.; Lazoura, E.; Matsoukas, J.; Apostolopoulos, V. Round and round we go: Cyclic peptides in disease. Curr. Med. Chem. 2006, 13, 2221–2232. [Google Scholar] [PubMed]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, J. Apostolopoulos. Design of novel cyclic altered peptide ligands of myelin basic protein MBP83-99 that modulate immune responses in SJL/J mice. J. Med. Chem. 2008, 51, 3971–3978. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Chatzantoni, K.; Matsoukas, M.T.; Tselios, T.; Deraos, S.; Katsara, M.; Papathanasopoulos, P.; Vynios, D.; Apostolopoulos, V.; Mouzaki, A.; et al. Cittrulination of linear and cyclic altered peptide ligands from myelin basic protein (MBP87-99) epitope elicits a Th1 polarized responses by T cells isolated from multiple sclerosis patients: Implications in triggering disease. J. Med. Chem. 2008, 51, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, M.T.; Friligou, I.; Matsoukas, J.; Apostolopoulos, V. Design and synthesis of a cyclic double mutant peptide (cyclo(87-99)[A91,A96]MBP87-99) induces altered responses in mice after conjugation to mannan: Implications in the immunotherapy of multiple sclerosis. J. Med. Chem. 2009, 52, 214–218. [Google Scholar] [CrossRef]
- Lourbopoulos, A.; Matsoukas, M.T.; Katsara, M.; Deraos, G.; Giannakopoulos, A.; Lagoudaki, R.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Cyclization of PLP139-151 peptide reduces its encephalitogenic potential in experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2018, 26, 2221–2228. [Google Scholar] [CrossRef]
- Garg, C.; Priyam, A.; Kumar, P.; Kumar Sharma, A.; Gupta, A. In vitro assessment of core-shell micellar nanostructures of amphiphilic cationic polimer-peptide conjugates as efficient gene and drug carriers. J. Pharm. Sci. 2020, 109, 2847–2853. [Google Scholar] [CrossRef]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-based drug-delivery systems in biotechnological applications: Recent advances and perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Chi-Lung Lee, A.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A comprehensive review on current advances in peptide drug development and design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar]
- Sarkar, G.; Curran, G.L.; Sarkaria, J.N.; Lowe, V.J.; Jenksins, R.B. Peptide carrier-mediated non-covalent delivery of unmodified cisplatin, methotrexate and other agents via intravenous route to the brain. PLoS ONE 2014, 9, e979655. [Google Scholar] [CrossRef]
- Shah, J.N.; Guo, G.Q.; Krishnan, A.; Ramesh, M.; Kumar Katari, N.; Shahbaaz, M.; Abdellattif, M.H.; Singh, S.K.; Dua, K. Peptide-based therapeutics: Emerging potential therapeutic agents for COVID-19. Therapie 2022, 77, 319–328. [Google Scholar] [CrossRef]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Harris, L.K.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2022, 2, e1600349. [Google Scholar] [CrossRef] [PubMed]
- Anti-Cancer Peptide Drug Conjugates: An Overwiew (2021). Available online: https://www.biochempeg.com/article/199.html (accessed on 9 July 2021).
- Sharma, A.; Kapoor, P.; Gautam, A.; Chaudhary, K.; Kumar, R.; Chauhan, J.S.; Tyagi, A.; Raghava, G.P.S. Computational approach for designing tumor homing peptides. Sci. Rep. 2013, 3, srep01607. [Google Scholar] [CrossRef] [PubMed]
- Laakonen, P.; Porkka, K.; Hoffman, J.A.; Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 2002, 8, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Singh, H.; Gautam, A.; Chaudhary, K.; Kumar, R.; Raghava, G.P.S. TumorHoPe: A database of tumor homing peptides. PLoS ONE 2012, 7, e35187. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Meka, R.R.; Venkatesha, S.H.; Lees, J.; Teesalu, T.; Moudgil, K.D. A novel CNS-homing peptide for targeting neuroinflammatory lesions in experimental autoimmune encephalomyelitis. Mol. Cell. Probes 2020, 51, 101530. [Google Scholar] [CrossRef]
- Khandia, R.; Sachan, S.; Munjal, A.K.; Tiwari, R.; Dhama, K. Tumor homing peptides: Promising futuristic hope for cancer therapy. In Topics in Anti-Cancer Research; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; Volume 44, pp. 43–86. [Google Scholar]
- Ruoslahti, E. Homing peptides. Encycl. Ref. Cancer 2001, 417–418. [Google Scholar]
- Cho, C.-F.; Farquhar, C.E.; Fadzen, C.M.; Scott, B.; Zhuang, P.; von Spreckelsen, N.; Loas, A.; Hartrampf, N.; Pentelute, B.L.; Lawler, S.E. A Tumor-Homing Peptide Platform Enhances Drug Solubility, Improves Blood–Brain Barrier Permeability and Targets Glioblastoma. Cancers 2022, 14, 2207. [Google Scholar] [CrossRef]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Tselios, T.; Deraos, G.; Lourbopoulos, A.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Altered peptide ligands of myelin basic protein (MBP87-99) conjugated to reduces mannan modulate immune responses in mice. Immunology 2009, 128, 521–533. [Google Scholar] [CrossRef]
- Xing, P.; Michael, M.; Apostolopulos, V.; Prenzoska, J.; Marshall, C.; Bishop, J.; McKenzie, I.F.C. Phase-I study of synthetic MUC1 peptides in breast-cancer. Int. J. Oncol. 1995, 6, 1283–1289. [Google Scholar] [CrossRef]
- Karanikas, V.; Hwang, L.A.; Pearson, J.; Ong, C.S.; Apostolopoulos, V.; Vaughan, H.; Xing, P.X.; Jamieson, G.; Pietersz, G.; Tait, B.; et al. Antibody and T cell responses of patients with adenocarcinoma immunized with manna-MUC1 fusion protein. J. Clin. Investig. 1997, 100, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Stojanovska, L.; McKenzie, I.F.C.; Vassilaros, S. Dendritic cell immunotherapy: Clinical outcomes. Clin. Transl. Immunol. 2014, 3, e21. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Lofthouse, S.A.; Popovski, V.; Chelvanayagam, G.; Sandrim, M.S.; McKenzie, I.F.C. Peptide mimics of a tumor antigen induce functional cytotoxic T cells. Nat. Biotechnol. 1998, 16, 276–280. [Google Scholar] [CrossRef]
- Wilsinso, B.L.; Day, S.; Chapman, R.; Perrier, S.; Apostolopoulos, V.; Payne, R.J. Synthesis and immunological evaluation of self-assebing and self-adjuvanting tricomponent glycopeptide cancer-vaccine candidates. Chem. Eur. J. 2012, 18, 16540–16548. [Google Scholar]
- Morris, M.C.; Depollier, J.; Heitz, F.; Divita, G. A peptide carrier from the delivery of biologiclly active protein sinto mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, H.; Moore, G.J.; Mavromoustakos, T.; Tsiodras, S.; Ligielli, I.; Kelaidonis, K.; Chasapis, C.T.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; et al. Discovery of a new generation of angiotensyn receptor blocking drugs: Receptor mechanisms and in silico binding to enzymes relevant to SARS-CoV-2. Comput. Struct. Biotechnol. J. 2022, 20, 2091–2111. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.M.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; Kelaidonis, K.; Ligielli, I.; Moschovou, K.; Georgiou, N.; Plotas, P.; Chasapis, C.T.; et al. Diminazene Aceturate Reduces Angiotensin II Constriction and Interacts with the Spike Protein of Severe Acute Respiratory Syndrome Coronavirus 2. Biomedicines 2022, 10, 1731. [Google Scholar] [CrossRef]
- Melnyk, T.; Dordevic, S.; Conejos-Sanchez, I.; Vincent, M.J. Therapeutic potential of polypeptide-based conjugates: Rational design and analytical tools that can boost clinical translation. Adv. Drug Deliv. Rev. 2020, 160, 136–169. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Yu, Z.; Sun, M.; Lin, L.; Liu, W.; Han, Q.; Wei, M.; Jin, Y. The Analysis of Key Factors Related to ADCs Structural Design. Front. Pharmacol. 2019, 10, 373. [Google Scholar] [CrossRef]
- Ding, H.; Gangalum, P.R.; Galstyan, A.; Fox, I.; Patil, R.; Hubbard, P.; Murali, R.; Ljubimova, J.Y.; Holler, E. HERG-2 positive breast cancer targeting and treatment by a peptide-conjugated mini nanodrug. Nanomedicine 2017, 13, 631–639. [Google Scholar] [CrossRef]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current multistage drug delivery systems based on the tumor microenviron ment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef] [PubMed]
- Praveen, K.; Das, S.; Dhaware, V.; Pandey, B.; Mondal, B.; Gupta, S.S. pH-responsive ‘supra-amphiphilic’ nanoparticles based on homoarginine polypeptides. ACS Appl. Bio Mater. 2019, 2, 4162–4172. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 1, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Thayumanavan, S. Substituent effects on the pH sensitivity of acetals and ketals and their correlation with encapsulation stability in polymeric nanogels. J. Am. Chem. Soc. 2017, 139, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Kalia, J.; Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: Destruction or repair? Nat. Clin. Pract. Rheumatol. 2008, 4, 128–135. [Google Scholar] [CrossRef]
- de la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef]
- Heeswijk, W.A.R.; Hoes, C.J.T.; Stoffer, T.; Eenink, M.J.D.; Potman, W.; Feijen, J. The synthesis and characterization of polypeptide-adriamycin conjugates and its complexes with adriamycin. Part I. J. Control. Release 1985, 1, 301–315. [Google Scholar] [CrossRef]
- Mahmood, U.; Tung, C.H.; Bogdanov, A.; Weissleder, R. Near-infrared optical imaging of protease activity for tumor detection. Radiology 1999, 213, 866–870. [Google Scholar] [CrossRef]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bogdanov, A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef]
- Tung, C.H.; Mahmood, U.; Bredow, S.; Weissleder, R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000, 60, 4953–4958. [Google Scholar] [PubMed]
- Klahan, B.; Seidi, F.; Crespy, D. Oligo(thioether-ester)s blocks in polyurethanes for slowly releasing active payloads. Macromol. Chem. Phys. 2018, 219, 1800392. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathionemetabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Taresco, V.; Alexander, C.; Singh, N.; Pearce, A.K. Stimuli-responsive prodrug chemistries for drug delivery. Adv. Ther. 2018, 1, 1800030. [Google Scholar] [CrossRef]
- Yue, D.; Cheng, G.; He, Y.; Nie, Y.; Jiang, Q.; Cai, X.; Gu, Z. Influence of reduction-senitive diselenide bonds and isulfide bonds on oligoethylenimine conjugates for gene delivery. J. Mater. Chem. 2014, 2, 7210–7221. [Google Scholar]
- Mochida, Y.; Cabral, H.; Kataoka, K. Polymeric micelles for targeted tumor therapy of Platinum anti-cancer drugs. Expert Opin. Drug Deliv. 2017, 14, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Zagorodko, O.; Arroyo-Crespo, J.J.; Nebot, V.J.; Vincent, M.J. Polypeptide-based conjugates as therapeutics: Opportunities and challenges. Macromol. Biosci. 2017, 17, 1600316. [Google Scholar] [CrossRef]
- Ha, J.H.; Lee, D.Y.; Lee, S.H.; Yun, C.O.; Kim, Y.C. Development of apoptsis-inducing polypeptide via simultaneous mitochondrial via membranę disruption and Ca2+ delivery. Biomaterials 2019, 197, 51–59. [Google Scholar] [CrossRef]
- Chia-Hung, C.; Min-Liang, K.; Jen-Ling, W.; Wei-Chuan, L.; Li-Ching, C.; Leong-Perng, C.; Johnson, L. CCM-AMI, a polyethylene glycol micelle with amifostine, as an acute radiation syndrome protectant in C57BL/6 Mice. Health Phys. 2015, 109, 242–248. [Google Scholar]
- Wang, C.H.; Cheng, C.Y.; Chen, C.H.; Liao, W.C. Chelating complex micelles for delivering cytoprotectant amifostine and its application in radiation protection. J. Pharmacovigil. 2018, 6, 3. [Google Scholar]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 2019, 48, 4361. [Google Scholar] [CrossRef] [PubMed]
- Vrettos, E.I.; Mező, G.; Tzakos, A.G. On the design principles of peptide-drug conjugates for targeted drug delivery to the malignant tumor site. Beilstein J. Org. Chem. 2018, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.J.; Bargh, J.D.; Dannheim, F.M.; Hanby, A.R.; Seki, H.; Counsell, A.J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y.; et al. Site-selective modification strategies in antibody–drug conjugates. Chem. Soc. Rev. 2021, 50, 1305. [Google Scholar] [CrossRef]
- Bargh, J.D.; Walsh, S.J.; Isido-Llobet, A.; Omarjee, S.; Caroll, J.S.; Spring, D.R. Sulfatase-cleavable linkers for antibody-drug conjugates. Chem. Sci. 2020, 11, 2375–2380. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef]
- Majumdar, S.; Siahaan, T.J. Peptide-mediated targeted drug delivery. Med. Res. Rev. 2012, 32, 637. [Google Scholar] [CrossRef]
- Staben, L.R.; Koenig, S.G.; Lehar, S.M.; Vandlen, R.; Zhang, D.; Chuh, J.; Yu, S.F.; Ng, C.; Guo, J.; Liu, Y.; et al. Targeted drug delivery through the traceless release of tertiary and heteroaryl amines from antibody-drug conjugates. Nat. Chem. 2016, 8, 1112. [Google Scholar] [CrossRef]
- Anami, Y.; Yamazaki, C.M.; Xiong, W.; Gui, X.; Zhang, N.; An, Z.; Tsuchikama, K. Glutamic acid–valine–citrulline linkers ensure stability and efficacy of antibody–drug conjugates in mice. Nat. Commun. 2018, 9, 2512. [Google Scholar] [CrossRef]
- Jeffrey, S.C.; Andreyka, J.B.; Bernhardt, S.X.; Kissler, K.M.; Kline, T.; Lenox, J.S.; Moser, R.F.; Nguyen, M.T.; Okeley, N.M.; Stone, I.J.; et al. Development and properties of beta-glucuronide linkers for monoclonal antibody-drug conjugates. Bioconjug. Chem. 2006, 17, 831. [Google Scholar] [CrossRef]
- Su, Z.; Xiao, D.; Xie, F.; Liu, L.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Antibody-drug conjugates: Recent advances in linker chemistry. Acta Pharm. Sin. B 2021, 11, 3889–3907. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Q.; Yang, J.; Wang, H.; Xu, J.; Zheng, J. IRGD as a tumor-penetrating peptide for cancer therapy (Review). Mol. Med. Rep. 2017, 15, 2925–2930. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Hoskin, D.W. Power Coombs MR. Anti-cancer Activities of Natural and Synthetic Peptides. Adv. Exp. Med. Biol. 2019, 1117, 131. [Google Scholar] [PubMed]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems. J. Pharmacol. Exp. Ther. 2019, 370, 570. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wan, K.; Zhou, N.; Wei, G.; Su, Z. Supramolecular peptide nano-assemblies for cancer diagnosis and therapy: From molecular design to material synthesis and function-specific applications. J. Nanobiotechnol. 2021, 19, 253. [Google Scholar] [CrossRef]
- Gokhale, A.S.; Satyanarayanajois, S. Peptides and peptidomimetics as immunomodulators. Immunotherapy 2014, 6, 755. [Google Scholar] [CrossRef]
- Liu, M.; Fang, X.; Yang, Y.; Wang, C. Peptide-Enabled Targeted Delivery Systems for Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 701504. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alrokayan, S.A.; Kumar, S. Targeted anti-cancer therapy: Overexpressed receptors and nanotechnology. Clin. Chim. Acta. 2014, 436, 787. [Google Scholar] [CrossRef]
- Mckertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.T.; Moody, A.; Schorzman, A.N.; Zamboni, W.C. Importance and Considerations of Antibody Engineering in Antibody-Drug Conjugates Development from a Clinical Pharmacologist’s Perspective. Antibodies 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Worm, D.J.; Els-Heindl, S.; Beck-Sickinger, A.G. Targeting of peptide-binding receptors on cancer cells with peptide-drug conjugates. Pept. Sci. 2020, 112, e24171. [Google Scholar] [CrossRef]
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Millian, M.; Griebenow, K. Smart Targeting to Improve Cancer Therapeutics. Drug Des. Devel. Ther. 2019, 13, 3753. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Lubin, B.C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J. Nanobiotechnol. 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Lelle, M.; Frick, S.U.; Steinbrink, K.; Peneva, K. Novel cleavable cel-penetrating peptide-drug conjugates: Synthesis and characteization. J. Pept. Sci. 2014, 20, 323–333. [Google Scholar] [CrossRef]
- Kaloyanova, S.; Lelle, M.; Mullen, K.; Peneva, K. Branched cel-penetrating peptide drug conjugates for overcoming drug resistance. Eur. J. Cancer 2014, 50, S207–S208. [Google Scholar] [CrossRef]
- Hingorani, D.V.; Allevato, M.M.; Camargo, M.F.; Lesperance, J.; Quaraishi, M.A.; Aguilera, J.; Franiak-Pietryga, I.; Scanderberg, D.J.; Wang, Z.; Molinolo, A.A.; et al. Monomethyl auristatin antibody and peptide drug conjugates for trimodal cancer chemo-radio-immunotherapy. Nat. Commun. 2022, 13, 3869. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Stojanovska, L.; Gargosky, S.E. MUC1 (CD227): A multi-tasked molecule. Cell. Mol. Life Sci. 2015, 72, 4475–4500. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; McKenzie, I.F. MUC1 and breast cancer. Curr. Opin. Mol. Ther. 1999, 1, 98–103. [Google Scholar]
- Apostolopoulos, V.; McKenzie, I.F.C. Cellular mucins: Targets for immunotherapy. Crit. Rev. Immunol. 2017, 37, 421–437. [Google Scholar] [CrossRef]
- Swaan, P.W. Recent advances in intestinal macromolecular drug delivery via receptor-mediated transport pathways. Pharmaceut. Res. 1998, 15, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Nelepcu, I.; Hui, D.; Oo, H.Z.; Truong, S.; Zhao, S.; Tahiry, Z.; Esfandnia, S.; Ghaidi, F.; Adomat, H.; et al. Internalization and trafficking of CSPG-bound recombinant VAR2CSA lectins in cancer cells. Sci. Rep. 2022, 12, 3075. [Google Scholar] [CrossRef] [PubMed]
- Diez-Torrubia, A.; Cabrera, S.; de Castro, S.; Garcia-Aparicio, C.; Mulder, G.; De Meester, I.; Camarasa, M.J.; Balzarini, J.; Velazquez, S. Novel water-soluble prodrugs of acyclovir cleavable by the dipeptidyl-peptidase IV (DPP IV/CD26) enzyme. Eur. J. Med. Chem. 2013, 70, 456. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Lei, Y.; Wang, R.; Zhan, M.; Liu, J.; Zhou, Y.A.Y.; Zhan, J.; Yin, F.; Li, Z. Design and evaluation of a novel peptide-drug conjugate covalently targeting SARS-CoV-2 papain-like protease. J. Med. Chem. 2022, 65, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, Z.; Kratky, M.; Vosatka, R.; Szabo, E.; Senoner, Z.; David, S.; Stolarikova, J.; Vinsova, J.; Bosze, S. In vitro biological evaluation of new antimycobacterial salicylanilide-tuftsin conjugates. Eur. J. Med. Chem. 2017, 133, 152. [Google Scholar] [CrossRef]
- Long, Y.; Sun, J.; Song, T.Z.; Liu, T.; Tang, F.; Zhang, X.; Ding, L.; Miao, Y.; Weiliang, Z.; Xiaoyan, P.; et al. CoVac501, a self-adjuvanting peptide vaccine conjugated with TLR7 agonists, against SARS-CoV-2 induces protective immunity. Cell Discov. 2022, 8, 1. [Google Scholar] [CrossRef]
- Ferreira, S.Z.; Carneiro, H.C.; Lara, H.A.; Alves, R.B.; Resende, J.M.; Oliveira, H.M.; Silva, L.M.; Santos, D.A.; Freitas, R.P. Synthesis of a new peptide-coumarin conjugate: A potential agent against cryptococcosis. ACS Med. Chem. Lett. 2015, 6, 271. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Day, S.; Malins, L.R.; Apostolopoulos, V.; Payne, R.I. Self-adjuvanting multicomponent cancer candidates combining Per-glycosylated MUC1 glycopeptides and the toll-like receptor 2 agonist Pam3CysSer. Angew. Chem. Int. Ed. 2011, 50, 1635–1639. [Google Scholar] [CrossRef]
- Shokri, B.; Zarghi, A.; Shahhoseini, S.; Mohammadi, R.; Kobarfard, F. Design, synthesis and biological evaluation of peptide-NSAID conjugates for targeted cancer therapy. Arch. Pharm. 2019, 352, e1800379. [Google Scholar] [CrossRef]
- Schieb, H.; Weidlich, S.; Schlechtingen, G.; Linning, P.; Jennings, G.; Gruner, M.; Wiltfang, J.; Klafki, H.W.; Knolker, H.J. Structural design, solid-phase synthesis and activity of membrane-anchored ß-secretase inhibitors on Aß generation from wild-type and Swedish-mutant APP. Chem. Eur. J. 2010, 16, 14412. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cao, B.; Wu, H.; Cheng, G. Cholesterol-peptide hybrids to form liposome-like vesicles for gene delivery. PLoS ONE 2013, 8, e54460. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus particles for biological imaging and sensing. Analyst 2016, 141, 3526. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B. Highlights of Prescribing Information. Regulatory Education for Industry. 2015. Available online: www.fda.gov (accessed on 3 November 2015).
- Wang, Z.; Liang, C.; Shi, F.; He, T.; Gong, C.; Wang, L.; Yang, Z. Cancer vaccines using supramolecular hydrogels of NSAID-modified peptides as adjuvants abolish tumorigenesis. Nanoscale 2017, 9, 14058–14064. [Google Scholar] [CrossRef]
- Mohammadi, R.; Shokri, B.; Shamshirian, D.; Zarghi, A.; Shahhosseini, S. Synthesis and biological evaluation of RGD conjugated with Ketoprofen/Naproxen and radiolabeled with [99mTc] via N4(GGAG) for αVβ3 integrin-targeted drug delivery. DARU. J. Pharm. Sci. 2020, 28, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, Y.; Won, W.R.; Lim, C.; Kim, J.C.; Kang, K.; Husni, P.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of AE147 Peptide-Conjugated Nanocarriers for Targeting uPAR-Overexpressing Cancer Cells. Int. J. Nanomed. 2021, 16, 5437. [Google Scholar] [CrossRef]
- Szabo, I.; Bosze, S.; Orban, E.; Sipos, E.; Halmos, G.; Kovacs, M.; Mezo, G. Comparative in vitro biological evaluation of daunorubicin containing GnRH-I and GnRH-II conjugates developed for tumor targeting. J. Pept. Sci. 2015, 21, 426. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, J.Y.; Xu, X.D.; Qiu, W.X.; Lei, Q.; Han, K.; Cheng, Y.J.; Zhang, X.Z. Activable cell-penetrating peptide conjugated prodrug for tumor targeted drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 16061. [Google Scholar] [CrossRef]
- Bera, S.; Zhanel, G.G.; Schweizer, F. Synthesis and antibacterial activity of amphiphilic lysine-ligated neomycin B conjugates. Carbohydr. Res. 2011, 346, 560. [Google Scholar] [CrossRef]
- Brezden, A.; Mohamed, M.F.; Nepal, M.; Harwood, J.S.; Kuriakose, J.; Seleem, M.N.; Chmielewski, J. Dual Targeting of Intracellular Pathogenic Bacteria with a Cleavable Conjugate of Kanamycin and an Antibacterial Cell-Penetrating Peptide. J. Am. Chem. Soc. 2016, 138, 10945. [Google Scholar] [CrossRef]
- Horváti, K.; Bacsa, B.; Szabó, N.; Fodor, K.; Balka, G.; Rusvai, M.; Kiss, E.; Mezo, G.; Grolmusz, V.; Vertessy, B.; et al. Antimycobacterial activity of peptide conjugate of pyridopyrimidine derivative against Mycobacterium tuberculosis in a series sssof in vitro and in vivo models. Tuberculosis 2015, 95, S207. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Teixeira, C.; Figueiras, M.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Gomes, P. Novel cinnamic acid/4-aminoquinoline conjugates bearing non-proteinogenic amino acids: Towards the development of potential dual action antimalarials. Eur. J. Med. Chem. 2012, 54, 887. [Google Scholar] [CrossRef] [PubMed]
- Mura, F.; Zuniga-Nunez, D.; Mallet, J.M.; Lavielle, S.; Matton, P.; Barrias, P.; Fuentealba, D.; Aspee, A. A microenvironment-sensitive coumarin-labeled pepotde for the assessment of lipid-peptide interactions. Dye. Pigment. 2020, 176, 108234. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Vale, R.; Zemlak, T.S.; Hoskin, D.W. Generation of a hematologic malignancy-selective membranolytic peptide from the antimicrobial core (RRWQWR) of bovine lactoferricin. Exp. Mol. Pathol. 2013, 95, 192. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Y.; Hua, H.; Li, D.; Tang, C. Marine Antitumor Peptide Dolastatin 10: Biological Activity, Structural Modification and Synthetic Chemistry. Mar. Drugs 2021, 19, 363. [Google Scholar] [CrossRef]
- Um, W.; Park, J.; Ko, H.; Lim, S.; Yoon, H.Y.; Shim, M.K.; Lee, S.; Ko, Y.J.; Kim, M.J.; Park, J.H.; et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials 2019, 224, 119494. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Sha, H.; Zhang, L.; Bian, X.; Han, X.; Liu, B. Tumor-penetrating peptide fused to a pro-apoptotic peptide facilitates effective gastric cancer therapy. Oncol. Rep. 2017, 37, 2063. [Google Scholar] [CrossRef]
- Redko, B.; Tuchinsky, H.; Segal, T.; Tobi, D.; Luboshits, G.; Ashur-Fabian, O.; Pinhasov, A.; Gerlitz, G.; Gellerman, G. Toward the development of a novel non-RG cyclic peptide drug conjugate for treatment of human metastatic melanoma. Oncotarget 2017, 8, 757–768. [Google Scholar] [CrossRef]
- Brunetti, J.; Pillozzi, S.; Falciani, C.; Depau, L.; Tenori, E.; Scali, S.; Lozzi, L.; Pini, A.; Arcangeli, A.; Menichetti, S.; et al. Tumor-selective peptide-carrier delivery of Paclitaxel increases in vivo activity of the drug. Sci. Rep. 2015, 5, 17736. [Google Scholar] [CrossRef]
- Salem, A.F.; Wang, S.; Billet, S.; Chen, J.-F.; Udompholkul, P.; Gambini, L.; Baggio, C.; Tseng, H.-R.; Posadas, E.M.; Bhowmick, N.A.; et al. Reduction of Circulating Cancer Cells and Metastases in Breast-Cancer Models by a Potent EphA2-Agonistic Peptide-drug Conjugate. J. Med. Chem. 2018, 61, 2052. [Google Scholar] [CrossRef]
- Kuol, N.; Yan, X.; Barriga, V.; Karakkat, J.; Vassilaros, S.; Fyssas, I.; Tsimpanis, A.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Pilot Study: Immune Checkpoints Polymorphisms in Greek Primary Breast Cancer Patients. Biomedicines 2022, 10, 1827. [Google Scholar] [CrossRef] [PubMed]
- Barriga, V.; Kuol, K.; Apostolopoulos, V. The complex interaction between the tumor micro-environment and immune checkpoints in breast cancer. Cancers 2019, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. PD-1/PD-L1 in disease. Immunotherapy 2018, 10, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. The mechanisms tumor cells utilize to evade the host`s immune system. Maturitas 2017, 105, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Gu, G.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Song, Q.; Yao, L.; Tu, Y.; et al. Activatable cell penetrating peptide-conjugated nanoparticles with enhanced permeability for site-specific targeting delivery of anticancer drug. Bioconjug. Chem. 2013, 20, 419–430. [Google Scholar] [CrossRef]
- Zhu, W.; Bai, Y.; Zhang, N.; Yan, J.; Chen, J.; He, Z.; Sun, Q.; Pu, Y.; He, B.; Ye, X. A tumor extracellular pH-sensitive PD-L1 binding peptide nanoparticle for chemo-immunotherapy of cancer. J. Mater. Chem. B 2021, 9, 4201. [Google Scholar] [CrossRef]
- Liu, M.; Wang, H.; Liu, L.; Wang, B.; Sun, G. Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy. J. Transl. Med. 2016, 14, 155. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Yang, Y.; Liu, Y.; Xie, H.; Yu, Q.; Tian, L.; Tang, X.; Ren, K.; Li, J.; et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-gamma and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J. Control. Release 2020, 321, 23. [Google Scholar] [CrossRef]
- Park, C.C.; Ali, S.; Janoff, A.S.; Meers, P. Triggerable liposomal fusion by enzyme cleavage of a novel-lipid conjugate. Biochim. Biophys. Acta 1998, 1372, 13–27. [Google Scholar]
- Baines, I.C.; Colas, P. Peptide aptamers as guides for small molecule drug discovery. Drug Discov. Today 2006, 11, 334–341. [Google Scholar] [CrossRef]
- Hassanzadeganroudsari, M.; Soltani, M.; Heydarinasab, A.; Apostolopoulos, V.; Akbarzadehkhiyavi, A.; Nurgali, K. Targeted nano-drug delivery system for glioblastoma therapy: In vitro and in vivo study. J. Drug Deliv. Sci. Technol. 2020, 60, 102039. [Google Scholar] [CrossRef]
- Maraming, P.; Daduang, J.; Yong Kah, J.C. Conjugation with gold nanoparticles improves the stability of the KT2 peptide and maintains its anti-cancer properties. RSC Adv. 2022, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater. Sci. 2019, 7, 461. [Google Scholar] [CrossRef]
- Caldwell, C., Jr.; Johnson, C.E.; Balaji, V.N.; Balaji, G.A.; Hammer, R.D.; Kannan, R. Identification and Validation of a PD-L1 Binding Peptide for Determination of PDL1 Expression in Tumors. Sci. Rep. 2017, 7, 13682. [Google Scholar] [CrossRef]
- Lee, S.; Trinh, T.H.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.-B.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef]
- Zhang, L.; Giraudo, E.; Hoffman, J.A.; Hanahan, D.; Ruoslahti, E. Lymphatic zip codes in premalignant lesions and tumors. Cancer Res. 2006, 66, 5696–5706. [Google Scholar] [CrossRef]
- Ferro-Flores, G.; Ramirez, F.d.M.; Melendez-Alafort, L.; Santos-Cuevas, C.L. Peptides for in vitro target-specific cancer imaging. Med. Chem. 2010, 10, 87–97. [Google Scholar]
- Haubner, R.; Beer, A.; Wang, H.; Chen, X. Positron emission tomography tracers for imaging angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 86–103. [Google Scholar] [CrossRef]
- Kuil, J.; Velders, A.H.; van Leeuwen, F.W.B. Multimodal tumor-targeting peptides functionalized with both a radio- and a fluorescent label. Bioconjug. Chem. 2010, 21, 1709–1719. [Google Scholar] [CrossRef]
- Mudd, G.E.; Brown, A.; Chen, J.; van Rietschoten, K.; Teufel, D.P.; Pavan, S.; Lani, R.; Huxley, P.; Bennett, G.S. Identification and optimization of EphA2-selective bicycles for the delivery of cytotoxic payloads. J. Med. Chem. 2020, 63, 4107. [Google Scholar] [CrossRef] [PubMed]
- Sapra, R.; Verma, R.P.; Maurya, G.P.; Dhawan, S.; Babu, J.; Haridas, V. Designer peptide and protein dendrimers: A cross-sectional analysis. Chem. Rev. 2019, 119, 11391. [Google Scholar] [CrossRef]
- Li, N.; Li, N.; Yi, Q.; Luo, K.; Guo, C.; Pan, D.; Gu, Z. Amphiphilic peptide dendritic copolymer-doxorubicin nanoscale conjugate self-assembled to enzyme-responsive anti-cancer agent. Biomaterials 2014, 35, 9529. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Carvalho, C.R.; Maia, F.R.; Caballero, D.; Kundu, S.C.; Reis, R.L.; Oliveira, J.M. Peptide-Modified Dendrimer Nanoparticles for Targeted Therapy of Colorectal Cancer. Adv. Ther. 2019, 2, 1900132. [Google Scholar] [CrossRef]
- Cooper, B.M.; Iegre, J.; O’Donovan, D.H.; Halvarsson, M.O.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates. Chem. Soc. Rev. 2021, 50, 1480. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.A.; Pouniotis, D.S.; Sheng, K.C.; Apostolopoulos, V.; Pietersz, G.A. A membrane penetrating multiple antigen peptide (MAP) incorporating ovalbumin CD8 epitope induces potent immune responses in mice. Biochim. Biophys. Acta Biomembr. 2010, 12, 2286–2295. [Google Scholar] [CrossRef]
- Sheng, K.C.; Kalkanidis, M.; Pouniotis, D.S.; Esparon, S.; Tang, C.K.; Apostolopoulos, V.; Pietersz, G.A. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur. J. Immunol. 2008, 38, 424–436. [Google Scholar] [CrossRef]
- Pouniotis, D.S.; Apostolopoulos, V.; Pietersz, G.A. Penetration tandemly linked to a CTL peptide induces anti-tumour T-cell responses via a cross-presentation pathway. Immunology 2005, 117, 329–339. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pounotis, D.S.; van Maanen, P.J.; Andriessen, R.W.; Lodding, J.; Xing, P.X.; McKenzie, I.F.C.; Loveland, B.E.; Pietersz, G.A. Delivery of tumor associated antigens to antigen presenting cells using penetratin induces potent immune responses. Vaccine 2006, 12, 3191–3202. [Google Scholar] [CrossRef]
- Brooks, N.A.; Pouniotis, D.S.; Tang, C.K.; Apostolopoulos, V.; Pietersz, G.A. Cell-penetrating peptides: Application in vaccine delivery. Biochim. Biophys. Acta Rev. Cancer 2020, 1805, 25–34. [Google Scholar] [CrossRef]
- Pouniotis, D.S.; Esparon, S.; Apostolopoulos, V.; Pietersz, G.A. Whole protein and defined CD8+ and CD4+ peptides linked to penetratin targets both MHC class I and II antigen presentation pathways. Immunol. Cell Biol. 2011, 89, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Pouniotis, D.; Tang, C.K.; Apostolopoulos, V.; Pietersz, G. Vaccine delivery by penetratin: Mechanism of antigen presentation by dendritic cells. Immunol. Res. 2016, 64, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zhang, L.; Chen, P. Membrane Internalization Mechanisms and Design Strategies of Arginine-Rich Cell-Penetrating Peptides. Int. J. Mol. Sci. 2022, 23, 9038. [Google Scholar] [CrossRef] [PubMed]
- Del Genio, V.; Falanga, A.; Allard-Vannier, E.; Hervé-Aubert, K.; Leone, M.; Bellavita, R.; Uzbekov, R.; Chourpa, I.; Galdiero, S. Design and Validation of Nanofibers Made of Self-Assembled Peptides to Become Multifunctional Stimuli-Sensitive Nanovectors of Anticancer Drug Doxorubicin. Pharmaceutics 2022, 14, 1544. [Google Scholar] [CrossRef]
- Miller, D.S.; Scambia, G.; Bondarenko, I.; Westermann, A.M.; Oaknin, A.; Oza, A.M.; Lisyanskaya, A.S.; Vergote, I.; Wenham, R.M.; Temkin, S.M.; et al. ZoptEC: Phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer (NCT01767155). J. Clin. Oncol. 2018, 36, 5503. [Google Scholar] [CrossRef]
- Engel, J.B.; Tinneberg, H.R.; Rick, F.G.; Berkes, E.; Schally, A.V. Targeting of peptide cytotoxins to LHRH receptors for treatment of cancer. Curr. Drug Targets 2016, 17, 488. [Google Scholar] [CrossRef]
- Langer, C.J.; O’Byrne, K.J.; Socinski, M.A.; Mikhailov, S.M.; Lesniewski-Kmak, K.; Smakal, M.; Ciuleanu, T.E.; Orlov, S.V.; Dediu, M.; Heigener, D.; et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naïve advanced non-small cell lung cancer. J. Thorac. Oncol. 2018, 3, 623. [Google Scholar] [CrossRef]
- Graybill, W.S.; Coleman, R.L. Vintafolide: A novel targeted agent for epithelial ovarian cancer. Future Oncol. 2014, 10, 541. [Google Scholar] [CrossRef]
- Balogh, B.; Ivanczi, M.; Nizami, B.; Beke-Somfai, T.; Mandity, I.M. ConjuPeptDB: A database of peptide-drug conjugates. Nucl. Ac. Res. 2021, 49, 1. [Google Scholar] [CrossRef]
- Therapeutics, Programs. Available online: https://www.bicycletherapeutics.com/programs/#bicycle-conjugates (accessed on 14 May 2020).
- Fang, Y.; Wang, H. Molecular Engineering of Peptide-drug Conjugates for Therapeutics. Pharmaceutics 2022, 14, 212. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Xiang, T.; Liu, H.; Chen, L.; Jiang, B.; Yao, J.; Zhu, H.; Hu, R.; Chen, Z. Multifunctional building elemenst for the construction of peptide drug conjugates. Eng. Regen. 2022, 3, 92–109. [Google Scholar]
- Fu, C.; Yu, L.; Miao, Y.; Liu, X.; Yu, Z.; Wei, M. Peptide-drug conjugates (PDCs). A novel trend of research and development on targeted therapy, hype or hope? Acta Pharm. Sin. B 2022. [Google Scholar] [CrossRef]
| Development Phase | Peptide-Drug-Conjugates | References | ||
|---|---|---|---|---|
| US FDA approved | Lu177 dotatate | Peptide | Somatostatin analogue | [43,44,45,46] |
| Drug | Radio-therapeutic agent | |||
| Linker | Amide (chelating agent Lu177 chelates with meta-chelating agent DOTA) | |||
| Action | Somatostatin receptor-2 mediated delivery of nucleotide | |||
| Indication | Neuroendocrine cancer | |||
| Melflufen (melphalan flufenamide) amino peptide | Peptide | [47,48] | ||
| Drug | ||||
| Linker | Enzymatically cleaved linker | |||
| Action | Targets aminopeptidases. Rapidly releases alkylating agent into cancer cells | |||
| Indication | Multiple myeloma, ovarian cancer, breast cancer, acute myeloid leukemia, hematologic malignancies, and solid tumors | |||
| Clinical development | Paclitaxel with Angiopep-2 | Peptide | Angiopep-2 | [49,50] |
| Drug | Paclitaxel | |||
| Linker | Ester | |||
| Action | LPR-1 mediated brain uptake | |||
| Indication | Brain cancer | |||
| Paclitaxel with Poliglumex | Peptide | Poliglumex | [51,52,53] | |
| Drug | Paclitaxel | |||
| Linker | Ester | |||
| Action | Prolongs the exposure of the tumour to the active form of drug by minimizing the systemic enhancing the permeability of tumor vasculature | |||
| Indication | To treat a wide variety of cancers | |||
| Thapsigargin with Tetrapeptide | Peptide | Tetrapeptide | [52,53] | |
| Drug | Thapsigargin | |||
| Linker | Ester | |||
| Action | Tumor activation (extracellularly) | |||
| Indication | To treat a wide variety of cancers | |||
| Doxorubicin-GnRH | Peptide | GnRH | [54] | |
| Drug | Doxorubicin | |||
| Linker | Ester | |||
| Action | GnRH-mediated delivery to cancer cells | |||
| Indication | Ovarian and endometrial cancer | |||
| Maytansinoid with Bicyclic peptide | Peptide | Bicyclic peptide | [55] | |
| Drug | Maytansinoid | |||
| Linker | Disulfide | |||
| Action | Membrane type-1 matrixmetalloproteinase delivery to toxin material | |||
| Indication | Cancer | |||
| Doxorubicin-Tetrapeptide | Peptide | Tetrapeptide | [56,57] | |
| Drug | Doxorubicin | |||
| Linker | Amide | |||
| Action | Tumor activation (extracellularly) | |||
| Indication | Cancer | |||
| 123I-Glutamate urea lysine | Peptide | Glutamate urea lysine | [58] | |
| Drug | Radiotherapeutic agent (123I) | |||
| Linker | Amide | |||
| Action | Prostate-specific membrane antigen-mediated delivery of nucleotide | |||
| Indication | Specifically used for prostate cancer | |||
| 111In-DTPA-D-Phe-1-octreotide | Peptide | D-phe-1-octreotide | [59] | |
| Drug | 111In-DTPA | |||
| Linker | Amido bond | |||
| Action | Diagnosis of neuroendocrine tumors (somatostatin receptor-positive tumors) using SPECT or planar scintigraphy | |||
| Indication | Diagnostic Radiology | |||
| Aminopeptides N (CD13) peptide-hTumour Necrosis Factor (NGR-TNF) | Peptide | NGR | [60] | |
| Drug | hTNFα | |||
| Linker | Amido bond | |||
| Action | Targets angiogenic tumor blood vessels (vasculature), through the NGR motif | |||
| Indication | Elapsed ovarian cancer | |||
| Paclitaxel-Poliglumex conjugate CT2103 (Opaxio™) | Peptide | Poliglumex | [61] | |
| Drug | Paclitaxel (chemotherapeutic drug) | |||
| Linker | Ester bond | |||
| Action | Paclitaxel therapeutic index is restricted due to poor tumor exposure and high systemic exposure. Paclitaxel poliglumex (PPX) combination increases tumor exposure by taking advantage of tumor tissue’s hyperpermeable vasculature and reduced lymphatic clearance. The release of paclitaxel is dependent, at least in part, on PPX metabolism by the lysosomal protease cathepsin B, which is increased in many tumor types. | |||
| Indication | Metastatic breast cancer | |||
| Paclitaxel linked to brain delivery vector angiopep-2 (ANG1005) | Peptide | Angiopep-2 | [62] | |
| Drug | Paclitaxel | |||
| Linker | Ester bond | |||
| Action | Paclitaxel is blocked from attaining its target in malignant gliomas by the presence of the efflux pump P-glycoprotein (P-gp) at the blood-brain barrier. Angiopep-2 peptide vectors have the potential to improve their efficacy in the treatment of brain tumors. | |||
| Indication | Recurrent malignant glioma | |||
| Natural luteinizing hormone-releasing hormone (LHRH) ligand linked to a cationic membrane disrupting peptide (EP-100) | Peptide | LHRH | [63] | |
| Drug | CLIP71 | |||
| Linker | Amino bond | |||
| Action | EP-100 interacts with the negatively charged membrane upon accumulation on the cell membrane via LHRH receptor targeting, resulting in lysis and cell death. | |||
| Indication | LHRH-receptor-expressing solid tumors | |||
| Preclinical development | Gemcitabine conjugation to an integrin binding knotting peptide-Ecbellium elaterium trypsin inhibitor (EETI)-2.5Z, via Val-Ala-PABC linker (EETI-2.5Z-Val-Ala-PABC-genciabine) | Peptide | Knotting peptide | [64,65] |
| Drug | Gemcitabine | |||
| Linker | Amide, ester, cathepsin-B, carbamate | |||
| Action | DNA replication block assisted by integrin knotting peptide that specifically allows tumor delivery | |||
| Indication | Breast cancer, ovarian cancer, geo-blastoma, and pancreatic cancer | |||
| DOTA (bifunctional chelating ligand; 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) linked to bombesin (BBN) (DOTA-GABA-BBN) | Peptide | Bombesin 7–14, from frog skin | [66] | |
| Drug | DOTA | |||
| Linker | g-aminobutyric acid | |||
| Action | Bombesin is overexpressed on cancer cells. DOTA-GABA-BBN is a specific radioligand for gastrin-releasing peptide-positive tumors. Used in targeted PET imaging | |||
| Indication | Used for diagnostic radiology | |||
| MTX-YTA2 | Peptide | Acetyl-YTAIAWVKAFIRKLRK -amide | [67] | |
| Drug | Methotrexate (MTX) | |||
| Linker | Amino bond | |||
| Action | Cell-penetrating peptide (YTA2) conjugated to MTX as therapeutic for drug-resistant cancer cells | |||
| Indication | Breast cancer | |||
| Homing Peptide | Receptor Site [71,73,73,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] |
|---|---|
| Somatostatin (SST) | Somatostatin receptor |
| Gonadotropin-releasing hormone (GnRH) | Receptor version of hormone-GnRH-R |
| Angiopep-2 | Low-density lipoprotein receptor-related protein (LRP-1) |
| Epidermal growth factor (EGF) | Epidermal growth factor receptor (EGFR): HER1, HER2, HER3, HER4 |
| REG | Integrin αvβ3 receptor |
| iREG | Integrin αvβ3/αvβ5 receptor |
| GE11 | Epidermal growth factor receptor (EGFR): ErbB1 |
| D-Lys6-LHRH | Luteinizing hormone-releasing hormone receptor (LHRH-R) |
| Octreotide | Somatostatin receptor 2/5 (SSTR2/5) |
| Type of Linker | Name | Comment |
|---|---|---|
| pH-responsive | Acetal/Ketal bond | An acidic environment in endosomes |
| Hydrazone bond | ||
| Vinyl ether bond | ||
| Enzyme responsive | Gly-Phe-Leu-Gly | Cathepsin B |
| Phe-Lys | ||
| Val-Citrulline | ||
| Redox responsive | Disulfide bond | Reduction in endosomes by glutathione |
| Non-covalent interaction | Amido bond | The potential of modifying the particle’s surface peptide layer to stabilize the particle for systemic in vivo injection or for targeting opens fascinating possibilities for improving cargo trafficking |
| Carbon chain | ||
| Ether bond |
| Peptide | Drug/Vaccine | Indications | Reference |
|---|---|---|---|
| Somatostatin peptide analogue | 177Lu-dotate | Gastroenteropancreatic neuroendocrine tumors | [168] |
| Di-amino acid | Melflufen | Multiple myeloma | [169] |
| Arginine-glycine-aspartic acid | Naproxen/Ibuprofen | Anti-inflammatory/additives therapy with anti-cancer drugs to provide specific targeted delivery | [170] |
| Asparagine-glycine-arginine | Naproxen/ Ibuprofen | ||
| Arginine-glycine-aspartic acid/Asparagine-glycine-arginine | Ketoprofen | Anti-cancer therapy | [171] |
| AE147 peptide | Docetaxel | Advanced therapy for metastatic tumor | [172] |
| Gonadotropin-releasing hormone I/II | Daunorubicin | Targeted tumor therapy with high binding affinity and provides apoptotic effects | [173] |
| Dimethylmaleic anhydride | Doxorubicin | Targeted tumor therapy | [174] |
| Lysine | Neomycin-B | Antibiotics mainly evaluate their activity against gram-positive bacteria | [175] |
| Disulfide | Kanamycin | Successfully shows the activity in Mycobacterium tuberculosis | [176] |
| Polylactide-co-glycolide | Pyridopyrimidine derivatives | Anti-tubercular activity | [177] |
| Dipeptide | Cinnamic acid | Anti-protozoal agent | [178] |
| Ubiquicidin | Coumarin derivatives | Anti-fungal agent | [179] |
| Self-adjuvanting peptide vaccine conjugated toll-like receptor 7 agonists | CoVac501 | SARS-CoV-2 and other infectious diseases | [162] |
| Name of PDCs | TTP | Payload | Linker | Indication | Development Phase | Clinical Trials Registry |
|---|---|---|---|---|---|---|
| ANG1005 | Angiopep-2 | Paclitaxel | Succinic acid | Leptomeningeal disease from breast cancer | Phase III | NCT02048059 NCT03613181 |
| NGR015, NGR-hTNF | CNGRCG (1,5SS) | Human TNF | Amide | Malignant pleural mesothelioma | Phase III | NCT01098266 NCT03804866 NCT00484276 NCT00483080 |
| [18F]-AlF-NOTA-octreotide | Octreotide | 18F | NOTA | PET or GEP-NETs | Phase II/III | NCT04552847 |
| BT1718 | MT1-MMP binder | DM1 | Disulfide | Solid tumors | Phase II | NCT03486730 |
| [18F]-Fluciclatide | RGD | 18F | PEG | PET imaging | Phase II | NCT00918281 NCT00565721 NCT01176500 NCT01633255 NCT01788280 |
| [18F]-RGD-K5 | Cyclo-(RGDfK) | 18F | NOTA | PET imaging | Phase II | NCT00988936 NCT02490891 NCT03364270 |
| G-202 (mipsagargin) | DγEγEγEγE | Thapsigargin | Amide | Solid tumors | Phase II | NCT02381236 NCT02067156 NCT02607553 NCT01777594 NCT01056029 |
| 68Ga-NODAGA-E[cyclo(RGDyK)] 2 | E[cyclo(RGDyK)]2 | 68Ga | NODAGA | PET imaging | Phase II | NCT03445884 NCT03271281 |
| PEN-221 | fCYwKTCC (2,7 SS) | DM-1 | Disulfide | Neuroendocrine and small cell lung cancers | Phase II | NCT02936323 |
| 90Y-DOTATOC | 3 Tyr-octreotat | 90Y | DOTA | PRRT | Phase II | NCT03273712 |
| GRN1005 | Trevatide | Paclitaxel | Ester | Non-small Cell Lung Cancer (NSCLC) With Brain Metastases | Phase II | NCT01497665 |
| BT5528 | EphA2 binder | MMAE | Amide | EphA2-positive NSCLC | Phase I/II | NCT04180371 |
| 68Ga-NOTA-BBN-RGD | cyclo(RGDyK) and BBN | 68Ga | NOTA | PET/CT and PET imaging | Phase I | NCT02749019 NCT02747290 |
| tTF-NG | GNGRAHA | tTF | Amide | Malignant solid tumors | Phase I | NCT02902237 |
| TH1902 | Docetaxel peptide–drug conjugate | Alexa488 | Cleavable ester linker | Solid Tumor, TNBC-Triple-Negative Breast Cancer, Hormone Receptor-positive Breast Cancer, Epithelial Ovarian Cancer, Endometrial Cancer, Cutaneous Melanoma, Thyroid Cancer, Small-cell Lung Cancer, Prostate Cancer | Phase I | NCT04706962 |
| MB1707 | CXC | Paclitaxel | Ester | Advanced Solid Tumor, Breast Cancer, Non-Small Cell Lung Cancer, Ovary Cancer, Pancreatic Neoplasms | Early Phase I | NCT05465590 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Solanki, H.K.; Davidson, M.; Apostolopoulos, V.; Bojarska, J. Peptide-Drug Conjugates: A New Hope for Cancer Management. Molecules 2022, 27, 7232. https://doi.org/10.3390/molecules27217232
Chavda VP, Solanki HK, Davidson M, Apostolopoulos V, Bojarska J. Peptide-Drug Conjugates: A New Hope for Cancer Management. Molecules. 2022; 27(21):7232. https://doi.org/10.3390/molecules27217232
Chicago/Turabian StyleChavda, Vivek P., Hetvi K. Solanki, Majid Davidson, Vasso Apostolopoulos, and Joanna Bojarska. 2022. "Peptide-Drug Conjugates: A New Hope for Cancer Management" Molecules 27, no. 21: 7232. https://doi.org/10.3390/molecules27217232
APA StyleChavda, V. P., Solanki, H. K., Davidson, M., Apostolopoulos, V., & Bojarska, J. (2022). Peptide-Drug Conjugates: A New Hope for Cancer Management. Molecules, 27(21), 7232. https://doi.org/10.3390/molecules27217232









