Abstract
Over the years, probiotics have been extensively studied within the medical, pharmaceutical, and food fields, as it has been revealed that these microorganisms can provide health benefits from their consumption. Bacterial probiotics comprise species derived from lactic acid bacteria (LAB) (genus Lactobacillus, Leuconostoc, and Streptococcus), the genus Bifidobacterium, and strains of Bacillus and Escherichia coli, among others. The consumption of probiotic products is increasing due to the current situation derived from the pandemic caused by COVID-19. Foods with bacterial probiotics and postbiotics are premised on being healthier than those not incorporated with them. This review aims to present a bibliographic compilation related to the incorporation of bacterial probiotics in food and to demonstrate through in vitro and in vivo studies or clinical trials the health benefits obtained with their metabolites and the consumption of foods with bacterial probiotics/postbiotics. The health benefits that have been reported include effects on the digestive tract, metabolism, antioxidant, anti-inflammatory, anticancer, and psychobiotic properties, among others. Therefore, developing food products with bacterial probiotics and postbiotics is a great opportunity for research in food science, medicine, and nutrition, as well as in the food industry.
1. Introduction
Probiotics are defined as microorganisms that, when consumed in adequate doses or concentrations, can benefit the consumer’s health [1]. Zucko et al. [2] noted that the benefits of probiotics range from the relief of gastrointestinal disorders to help the treatment of allergies, obesity, depression, bacterial vaginosis, and the improvement of the gastrointestinal tract, among many others. Currently, a wide variety of microorganisms are considered bacterial probiotics, of which a considerable portion belongs to the group of lactic acid bacteria (LAB), mainly of the genus Lactobacillus, Leuconostoc, and Streptococcus [3,4]. In addition, other non-LAB microorganisms are also considered bacterial probiotics, including some species of bifidobacteria, Bacillus, and Escherichia coli, among others [4]. Figure 1 shows selected species that could be considered probiotics; however, the strain level should also be checked for specific health benefits.
Due to the recent COVID-19 pandemic, there has been concern about improving health wellbeing and avoiding the spread of the virus. Hence, society began to consume foods that could raise the immune system. Since foods with probiotics can contribute to health (including the immune system), there has been an increase in demand for these products. As a result, the global probiotics market in 2022 was 68.56 billion USD. Still, this value is estimated to increase to 133.92 billion USD by 2030, due to the high consumption of probiotic products triggered by the pandemic [5].
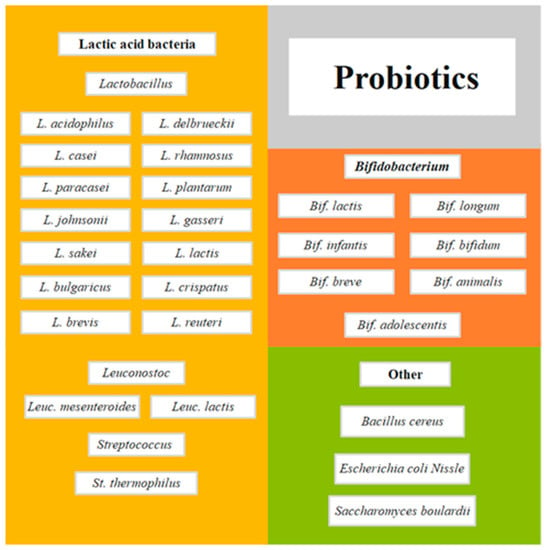
Figure 1.
Selected species of microorganisms as probiotics adapted from Moradi et al. [6].
Furthermore, other factors such as increasing trends in consuming functional foods, new marketing strategies by social networks, and innovative food distribution channels such as online shopping can increase the demand for these products. Therefore, the food and pharmaceutical industries need research to offer humankind better products.
Probiotics are present in different forms for intake, either in drugs such as capsules, tablets, oral suspensions, and powders, or through nourishment. To obtain the benefits of probiotics, it is essential to consider the availability and accessibility of its products. Foods with probiotics are excellent means of offering them, as they can be coupled with the daily diet of society. In manufacturing food products, these can be intentionally incorporated with bacterial probiotics to improve their bioactivity, helping the treatment of specific pathologies (like functional foods) or consumer wellness. Foods related to probiotics include fermented products with probiotics, foods with encapsulated probiotics, and foods with bacterial postbiotics (inactivated bacteria with their metabolites derived from fermentation). Research is constantly advancing to understand and verify the beneficial effects of probiotics on the human body. The benefits that can be provided to the metabolites produced by bacterial probiotic fermentation and foods related to bacterial probiotics have been investigated. Therefore, this work presents a bibliographic compilation of the health benefits of food products incorporated with bacterial probiotics, being either fermented, microencapsulated, or inactivated, as well as the metabolites that are synthesized during fermentation. Studies in vitro, in vivo, and clinical trials show their consumption effects.
2. Food and Probiotic Bacteria
There is a great diversity of foods related to probiotics; for this reason, in this study, these foods are classified into three groups based on what is reported in the literature: (1) fermented foods with probiotics, (2) foods with encapsulated probiotics, and (3) postbiotic foods (new trend). Although this classification is presented in Figure 2, these food groups are detailed further below.
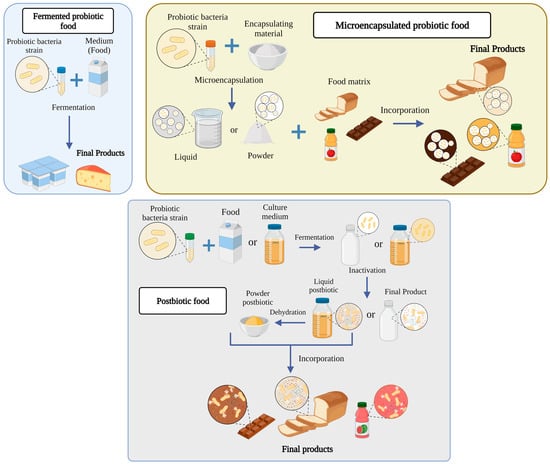
Figure 2.
Types of food with probiotic bacteria.
2.1. Fermented Foods
Fermented foods have been mainly related to probiotic bacteria. Therefore, these fermented products greatly interest the industry and scientific communities. This is because they comprise specific desirable properties, such as an extended shelf-life (owing to the acidification and reduction of the pH), an improvement of organoleptic properties (texture and production of aromas), increased nutritional value, and provide benefits to the consumer [7]. According to Ilango and Antony [8], fermented foods and beverages can be classified in different ways, either (1) by the microorganisms that ferment, (2) by the type of food or beverage, (3) the production method, (4) geographical localization or the producing and consuming communities of these foods. In the first type of fermented foods and beverages, there is a wide variety of fermentative species, of which the most used are the LAB. For example, in yogurt elaboration, lactic cultures include Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. Another fermented food consumed worldwide is cheese, which, in some cases, is added with Lactococcus lactis and Leuconostoc mesenteroides. Other non-LAB bacteria have also been used to produce fermented legumes (Bacillus subtilis ver. natto) and cheese maturation (Propionibacterium freudenreichii) [9]. The second type of foods and beverages can be classified into dairy (yogurt, cheeses, sour cream, buttermilk, kefir, etc.), meat (salami, pepperoni, Serrano ham, etc.), vegetables and tubers (pickles, kimchi, sauerkraut, etc.), fruits (alcoholic beverages such as wine, fruit liqueurs, apple cider, some kinds of vinegar such as apples, grapes, etc.), cereals (beer, bread, and sake), legumes (natto, tempeh, miso, etc.), and in a mixture of foods, such as kimchi, that is sometimes fermented with vegetables and fish [9,10,11].
The third category of fermented foods and beverages based on the production method consists of modifying the food’s intrinsic and extrinsic factors to obtain the best growth conditions for the microorganisms. Among the inherent factors of the physicochemical composition of the food are the regulation of pH, salt concentration, and the addition of sugars and/or additives. In comparison, extrinsic factors modify environmental conditions, such as temperature and atmospheric composition. Fidan et al. [10] noted that intrinsic and extrinsic conditions are vital to obtaining a desired fermented product. For example, if a LAB is required to have antimicrobial properties, it must remain alive at 4 °C for a set amount of time [10]. Oh et al. [12] studied several growth conditions for their optimization, including temperature (15–55 °C), pH (2.5–8.0), and salt concentration (0–10% w/v) for Lactobacillus plantarum PMO 08. Their results showed that the optimal growth temperature was 30 °C, and conditions such as pH and salt concentration could be from 3.5–8.0 for pH and 0–8.0% NaCl. Finally, the fourth type of fermented foods and beverages was assigned by geographical location, or the producing and consuming communities of these foods is mainly based on the culture of each region depending on their traditions and customs, which influence their way of feeding.
In the present work, foods fermented with bacterial probiotics that include microorganisms of the LAB group and bacteria not belonging to this group, such as the genus Bifidobacterium and Bacillus, are reviewed. It is important to delimit the food models that are related to probiotics. Some fermented foods can benefit the consumer, but these foods or drinks do not contain probiotics. A microorganism must meet specific requirements to be considered a probiotic. Pineiro and Stanton [13] outlined several requirements. First, the microorganism with probiotic potential must be named according to the basis on which it can be understood anywhere in the world, such as the International Code of Nomenclature. In addition, it has been recommended that identification be carried out by official methods, such as PFGE DNA/DNA hybridization and 16S RNA sequencing. Subsequently, the microorganism must confer health benefits, which must be determined by in vivo, in vitro, and human tests. Finally, the probiotic must be analyzed for use in industry, evaluating shelf-life conditions and manufacturing practices, including probiotics clearly and concisely in the food labeling expressing the minimum dose contained and the health claims. In terms of regulation, a regulatory framework must be established, which includes strain, efficiency, security, labeling, possible fraud, and appropriate claims. If it is a product fermented with a probiotic, the specific health benefits of the product must be established [13]. Hence, those fermented foods or beverages that do not meet these requirements cannot be considered probiotic products, but they can be considered products with potential probiotic effects. Based on this idea, the term next-generation probiotics (NGPs) has emerged, which are candidate microorganisms to be new probiotics, since they have shown beneficial effects on human health. NGPs are species of the genus Clostridium, Bacteroides, Faecalibacterium, Parabacteroides, and Eggerthellaceae [14].
2.2. Microencapsulated Probiotic Food
Generally, foods containing microencapsulated probiotics do not need fermentation to provide their benefits, as the incorporation of probiotics is commonly carried out in non-dairy food matrices or those foods that conventionally do not provide benefits derived from probiotics [15]. Frakolaki et al. [13] determined that foods with encapsulated probiotics are known as “carrier probiotics” since they mainly transport the probiotics to the desired site, such as the gastrointestinal tract. However, the microencapsulation of probiotics is challenging, since their viability during ingesting must be guaranteed. This implies that the probiotic strains must remain stable and metabolically active during the product’s shelf-life. Furthermore, the encapsulation and the probiotic must be sufficiently resistant to the conditions of the gastrointestinal passage. The stomach is the most critical point because it is the most acidic organ of the human body, with a pH ranging from 1 to 2.5 [16,17].
For the microencapsulation of probiotics, it is essential to consider the food matrix, the probiotic strain, the encapsulation technique, and the encapsulating material [16]. In the food matrix, there are studies where encapsulated probiotics in foods, such as dairy [18,19,20,21,22], meat [23,24], non-dairy beverages [25,26,27], desserts [28,29,30], and bakery products [31,32,33,34] have been investigated. Tripathi and Giri [35] noted that lactobacilli are more resistant to various processes and storage conditions than bifidobacteria, which should be considered when choosing a probiotic strain. Lactobacilli show more resistance to low pH and a better adaptation to milk and other foods. For instance, Lactobacillus casei and Lactobacillus acidophilus have been shown to have resistance in gastric environments (simulation of gastric juice at pH 3.0 at a temperature of 37 °C), unlike L. delbruekii ssp. bulgaricus, which is not resistant [36]. Encapsulation techniques include extrusion, emulsification, coacervation, spray drying, freeze drying, fluidized bed drying, electrospinning, electro spraying, hybridization, and impact aerosol technology [16]. When choosing from these techniques, several factors must be considered, including the economic profitability of the technique (considering the available budget and costs of the equipment), the quality of the final product, and the operating conditions (low temperatures, suitable pH, low oxygen levels, and sterile environment) [37,38].
For the encapsulating agents, a wide variety of materials or biopolymers have been used, such as alginate, chitosan, inulin, starch, maltodextrins, pectins, carboxymethylcellulose, arabic gum, carob gum, xanthan gum, carrageenan, agar, polyols (sorbitol, mannitol, and xylitol), fatty acids, gelatin, zein, gluten, soy protein isolate, milk proteins, β-lactoglobulin, casein, whey, and cellulose acetate phthalate (CAP), among others [16,39,40]. The choice of encapsulating material involves various considerations, since it must be safe for the consumer (recognized as GRAS), resistant to the operating conditions and digestion of the human organism, and subsequently release microorganisms into the intestinal tract. It is proposed to encapsulate the probiotics with these coatings to raise the bioactivity of food. For example, Eratte et al. [41] studied a complex co-encapsulation of probiotic coacervates of tuna oil, whey protein isolates, and arabic gum. The encapsulated probiotic was L. casei 431. Their results demonstrated the addition of tuna oil in the dehydrated encapsulates improved the viability of the probiotic more than in the dry encapsulates without oil.
2.3. Postbiotic Food
The term postbiotic emerged from various investigations suggesting that probiotics’ viability is not necessary to confer health benefits [42,43,44,45]. Multiple researchers have provided several definitions or terminologies to describe postbiotics. Tsilingiri and Rescigno [46] defined postbiotics as “any factor ending the metabolic activity of a probiotic or any released molecule capable of conferring beneficial effects on the host directly or indirectly”. They have also been defined as compounds produced by microorganisms, released from food components or microbial constituents (including non-viable cells) that promote health and wellbeing when administered in adequate amounts [47]. Based on these definitions and that of other authors, such as Aguilar-Toalá et al. [42], Johnson et al. [48], and Wegh et al. [49], who proposed their own definitions, three important features regarding postbiotics can be determined. The first is that they are chemical compounds released or excreted by microorganisms, the second is that they have a bioactive effect on the host and microbiota, and the third is that the microorganisms that produced these substances are non-viable or have been inactivated. Today, there is no official definition of postbiotics, since it is still being studied which compounds and parts of non-living probiotics may belong to this definition. The most current terminology was given by the consensus panel of the International Scientific Association of Probiotics and Prebiotics (ISAPP), which defined the postbiotic as a “preparation of inanimate microorganisms and/or their components that confer a benefit to the health of the host” [50]. However, Aguilar-Toalá et al. [51] disagreed with a reply in which they argued that this concept is confusing due to the word “inanimate”, since this term is generally not used in the field of microbiology. They suggested changing it to “non-viable or inactivated” due to its extensive use in the scientific community.
Postbiotics have technological advantages over probiotics incorporated into foods, since they are more stable, and their storage, handling, and transportation can be more economically feasible [52]. In addition, Homayouni-Rad et al. [53] noted that postbiotics have better absorption through metabolism and a high signaling capacity to various organs and tissues, and can trigger several biological responses that benefit the consumer.
For the formulation of foods with postbiotics, the bacterial probiotic should be considered; the growth medium, either in the feed or a selective culture medium (such as MRS broth); growth conditions such as temperature, pH, substrates, and water activity, among others. In addition, the inactivation technique should be selected, either through conventional (pasteurization or sterilization) or emergent technologies (ultrasound, UV, high pressures, or irradiation), as well as the separation process consisting of centrifugation and filtration that may be optional. This is in addition to the concentration method, such as spray drying or freeze drying, and the incorporation of postbiotics into the food matrix, either as liquid or solid [6].
3. Health Effects of Metabolites from Probiotic Bacteria
The growth of bacterial probiotics is involved in fermentation, which metabolizes the nutrients of the medium and produces a wide variety of metabolic compounds. Metabolites from bacterial probiotics have been studied as they may be the main cause of positive effects on consumer health [42]. As mentioned above, most bacterial probiotics belong to the LAB group, which primarily synthesizes lactic acid from carbohydrate fermentation [54]. Bacterial probiotics excrete secondary metabolites such as organic acids, short-chain fatty acids, enzymes, peptides, teichoic acids, peptidoglycans, exopolysaccharides, vitamins, plasmalogens, neurotransmitters, biosurfactants, amino acids, and flavonoid-derived compounds such as desaminotyrosine, equol daidzein, noratirriol, terpenoids, and phenolic compounds, among others [45,55].
Metabolites from bacterial probiotics have been studied as they may be the main cause of positive effects on consumer health [42]. Figure 3 shows a diagram of the health effects that have been studied concerning bacterial probiotic metabolites. Various components have been investigated, including short-chain fatty acids (SCFAs), bacteriocins, enzymes, teichoic acids, exopolysaccharides (EPS), vitamins, plasmalogens, and biosurfactants.
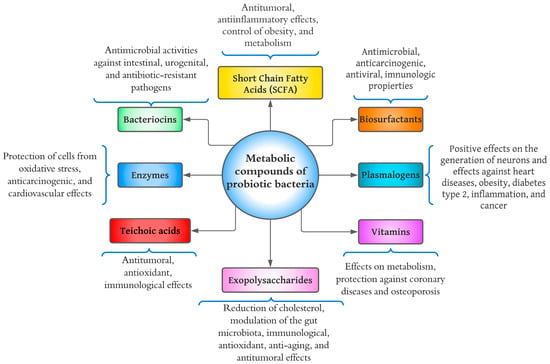
Figure 3.
Diagram of health benefits of metabolic compounds of probiotic bacteria.
3.1. Short-Chain Fatty Acids
SCFAs are the main metabolites produced by the fermentation of intestinal bacteria; these compounds are produced from the fermentation of non-digestible carbohydrates (prebiotics) by the intestinal microbiota [44]. The SCFAs primarily produced are acetate, propionate, and butyrate. The metabolic pathways of acetate are very diverse and depend on the type of bacteria, while the synthesis of propionate and butyrate depends on a specific substrate [56]. Gill et al. [57] determined that homo-fermentative bacteria synthesize SCFAs from carbohydrate fermentation to produce pyruvate via glycolytic, while heterofermentative bacteria, such as bifidobacterial, do so through the phosphoketolase pathway.
The bacterial probiotics that have been studied are mainly of the genus Lactobacillus and Bifidobacterium. For example, one study investigated the production of SCFA from Bif. longum SP 07/3, Bif. bifidum MF 20/5, L. gasseri PA, 16/8, and L. rhamnosus GG [58]. In the results, the highest production of acetate and propionate was with Bif. Bifidum, with a concentration of approximately 125 mM and 130 μM, respectively, while L. rhamnosus GG presented the lowest production of SCFA, with 55 mM of acetate and 100 μM of propionate. SCFAs activate G-specific protein-coupled receptors (GPRs), where GPR41 (known as free fatty acid receptor 3) and GPR43 (free fatty acid receptor 2) have been related to a wide health effect of antitumor and anti-inflammatory benefits on the colon, protection against the development of immune disorders, obesity control, glucose homeostasis control, appetite regulation, and cardiovascular effects [45,57,59]. Gill et al. [57] suggested the consumption of SCFA through the diet as a postbiotic effect, since it has been reported that vinegar and kombucha are the foods with the highest levels of SCFA, with 1015.82 mg of acetate per tablespoon (15 mL) and 1222.95 mg of acetate in a portion of 330 mL, respectively.
In comparison, some cheeses, such as Swiss cheese and blue vein cheese, are high in propionate (109.14 mg) and butyrate (136.54 mg) in a serving of 25 g, respectively [60]. A minimum dose of consumption of SCFAs has not been determined for functional health effects. However, an intervention study tested a beverage with apple cider vinegar containing 25.8 mM of acetate, 0.05 mM of propionate, and 0.04 mM of butyrate. The beverage helped reduce blood pressure, showing potential cardiovascular effects [60]. However, the doses of these compounds and how they can be supplied through food remain to be investigated more thoroughly.
3.2. Plasmalogens
According to Řezanka et al. [61], plasmalogens are lipid compounds with important functions in organisms such as bacteria, protozoa, invertebrates, and mammals. The largest classes of plasmalogens are glycerophosphoryl ethanolamine and glycerophosphorylcholine with a vinyl ether in the position of glycerol sn-1 instead of a fatty acid. At the position of glycerol sn-2, plasmalogens are enriched with a polyunsaturated fatty acid [62]. Regarding bacterial probiotics, only the production of these compounds with Bif. animalis subsp. lactis has been investigated. This research was conducted by Oberg et al. [63], in which plasmalogens were recognized as endogenous antioxidants induced by hydrogen peroxide (H2O2). The effects caused by these compounds are positively involved in neuro-regeneration and the alleviation of heart disease; it has positive effects against type 2 diabetes, obesity, inflammation, and cancer [62].
Regarding neuro-regeneration, plasmalogens are the main components of neuronal tissue, contributing between 50% and 80% of the total glycerophosphoryl ethanolamines of the gray and white matter of the brain [64]. As proof of this claim, Hossain et al. [65] observed that a daily intake of plasmalogens could improve cognition in Alzheimer’s patients. However, research into these metabolites is still very early, and more research is needed concerning their production in bacterial probiotics and their potential health benefits.
3.3. Enzymes
Enzymes excreted by microorganisms have a variety of biochemical, physiological, and regulatory functions. As shown in Figure 3, the health benefits of proteases are the protection of cells from oxidative stress and effects against heart disease and cancer [44]. Microorganisms of the genus Bacillus are probably the most important bacterial source of proteases, as they are capable of producing high amounts of neutral and alkaline proteolytic enzymes with remarkable properties, such as high stability at extreme temperatures, pH, organic solvents, detergents, and oxidizing compounds [66]. The proteases (subtilisin and glutamyl endopeptidase) of B. pumilus have been shown to degrade polymeric extracellular components and significantly eradicate the biofilm generated by Serratia marcescens, known as an opportunistic pathogen responsible for several hospital-acquired infections [67]. Although enzymes have been widely studied in science, more research is still needed regarding their effects in the human body.
3.4. Bacteriocins
Bacteriocins are polypeptides and proteins that form pores in bacterial membranes and inhibit cell wall synthesis [45]. Currently, there are five classes of bacteriocins, as follows:
- Class I: Small proteolytic and heat-resistant peptides substantially modified by transcriptionally specific enzymes. Examples: lantibiotics (nisin), sactipeptide, and loop peptides [68].
- Class II: Divided into four subtypes that are (1) pediocin-like, (2) two peptides, (3) circular, and (4) linear, not pediocin-like. They comprise small peptides resistant to temperature and pH [68].
- Class III: Large thermolabile peptides (>30 kDa) with complex activity and structure. This group includes helveticin, acidophylline, and lactacins (A and B) [69].
- Class IV: It consists of complex proteins conjugated with lipids or carbohydrates. Examples include lactocin S (glycoprotein) and mesenterocin (lipoprotein) [68,69].
- Class V: Peptides with circular structures without post-translational modifications, including enterokine AS-48 and gasericin A [69].
Nisin is one of the most studied and used bacteriocins in food science and industry. This bacteriocin is produced by Lactococcus. lactis subsp. lactis and is approved by the FDA as a Generally Recognized as Safe Additive (“GRAS”) [70]. The antimicrobial spectrum of nisin is broad and can inhibit the growth of Staphylococcus aureus, Cutibacterium acnes, Mycobacterium smegmatis, and strains of Bacillus, Clostridium, Enterococcus, Mycobacterium, and Streptococcus [71]. Furthermore, bacteriocins from Enterococcus faecalis and L. casei have been shown to be effective against urogenital, intestinal, and antibiotic-resistant pathogens [72,73].
3.5. Exopolysaccharides (EPSs)
EPSs are biopolymers produced by microorganisms during their growth. They vary according to the degree of branching from linear to highly branched molecules [74]. EPSs are divided into homopolysaccharides and heteropolysaccharides. Homopolysaccharides are composed of monosaccharides identical in their structure; for example, starch and cellulose. In comparison, heteropolysaccharides consist of different monosaccharides such as xanthan gum, pectin, and galactomannans, among others [75]. The probiotics that have been studied reagrding EPSs belong to the genus Lactobacillus. In a study by Xu et al. [76], they presented that EPSs produced by L. buchneri TCP016 (doses of 200–800 mg) were able to reduce liver damage by regulating the intestinal microbiota, since EPSs reduced the growth of Helicobacteraceae, Lachnospiraceae, and Enterobacteriaceae, but increased the proliferation of Lactobacillus, Rikenellaceae, Bacteroidaceae, and Prevotellaceae. In another study, Wang et al. [77] studied the EPS from L. fermentum S1 by applying doses of 1–4 mg/mL; these EPS showed potent antioxidant activities and prevented the formation of biofilms of E. coli and S. aureus. In addition, EPS from L. casei SB27 showed an increase in the concentration of these compounds (600 μg/mL), and antitumor effects in vitro were increased [78]. EPSs have demonstrated various health effects such as antioxidants, cholesterol-lowering effects, immunity, anti-aging, modulation of the intestinal microbiota, and antitumor [76,77,78,79].
3.6. Teichoic Acids
According to Van der Es et al. [80], teichoic acids are composed of anionic glycopolymers (ribitol) with repeated units of polyols attached to phodiester. There are two types of teichoic acids: Lipoteichoic Acids (LTAs), which are anchored to the membrane by a glycolipid, and Wall Teichoic Acids (WTAs), which are covalently bound to peptidoglycan [81]. Teichoic acids have important roles in bacteria, such as determining cell shape, regulating cell division, and providing vital metabolic aspects for cell physiology, and may confer pathogenesis and antibiotic resistance of Gram-positive bacteria [45]. The most studied teichoic acids in science are LTAs, due to their immune, antitumor, and antioxidant functional properties [82]. For example, several studies have shown that LTAs from L. delbrueckii, L. sakei, L. rhamnosus GG, and L. plantarum K8 have potential anti-inflammatory effects [83,84,85]. Wang et al. [86] studied the LTAs of L. paracasei D3–5 using a dose of 200 mg /kg per day. Their results showed that the compounds benefited the physical, cognitive, and anti-inflammatory functions in mice.
3.7. Vitamins
Vitamins are essential in the diet in small amounts to facilitate various body biological processes. LeBlanc et al. [87] noted that these compounds are important in regulating biochemical reactions in the cell, since some participate as precursors of intracellular coenzymes. Currently, thirteen vitamins are known as essential to human health, and these are classified as fat-soluble (vitamins A, D, E, and K) and water-soluble (vitamin C and vitamins of the B complex) [87]. The human body cannot synthesize most vitamins; therefore, consuming them externally through food is an excellent alternative. Bacterial probiotics can synthesize a large amount of vitamins, such as vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B9 (folate), vitamin B12 (cobalamin), and vitamin K [44,45]. As can be seen, the B complex vitamins are the most produced by bacterial probiotics; production is important, since the B vitamins act in synergy in the body’s homeostasis by participating in metabolic processes, such as energy generation and the synthesis of red blood cells [87]. Deptula et al. [88] noted that vitamin B12 produced through microbial fermentation is preferable to chemical synthesis. Nataraj et al. [45] argued that using microorganisms in vitamin production is more economically feasible than fortifying with chemically synthesized pseudo-vitamins. Bacterial probiotics such as L. brevis, L. fermentum, L. reuteri, and L. salivarius have complete genes (ribA, ribB, ribG, and ribH) for riboflavin synthesis [89]. Bardosono et al. [90] conducted a clinical trial consisting of Bif. animalis subsp. lactis HNO19 supplementation in pregnant women. Their results showed that probiotic supplementation increased the concentration of vitamin B6 in the blood and vitamin B12 in the second and third trimesters, respectively. Therefore, consuming probiotics or foods with probiotics is vital to staying healthy, as vitamins can also be synthesized in the digestive tract.
3.8. Biosurfactants
Biosurfactants represent a wide diversity of polymers synthesized during the early and late logarithmic stationary phases of a microorganism’s growth cycle [91]. Biosurfactants consist of glycolipids, lipopeptides, phospholipids, neutral lipids, protein-polysaccharide complexes, and free fatty acids [45]. The species of Lactobacillus are the most studied concerning biosurfactants. The biosurfactants (methylpentadecanoic acid and eicosanoic acid) of L. jensenii P6A and L. gasseri P6 have shown antimicrobial properties against several pathogens, such as E. coli, Candida albicans, Staphylococcus saprophyticus, Enterobacter aerogenes, and Klebsiella pneumonia [92]. Merghni et al. [93] showed that the biosurfactants of L. casei ATCC 393 have antioxidant and antimicrobial properties against S. aureus (present in the oral cavity), suggesting that biosurfactants could have a significant role in the prevention of oral diseases. Fracchia et al. [94] noted that biosurfactants exhibit a wide variety of properties such as emulsion stabilization, anti-adhesion capacity, anti-biofilm, anticancer, antiviral, immunological, and antimicrobial.
4. Health Benefits of Food Added with Probiotic Bacteria
This section presents the most recent studies regarding the health benefits and consumption of foods with probiotics (fermented or microencapsulated) and some postbiotics. Table 1 shows the scientific studies (in vitro and in vivo) that have been reported concerning the health benefits that can be obtained by consuming foods with probiotics and postbiotics, as well as the probiotics that have been studied and the main obtained results.

Table 1.
Selected studies on the health benefits of consuming food with probiotic bacteria and postbiotics.
4.1. Benefits on Gut and Gastrointestinal Tract
The intestinal tract is a very important organ for the digestive and immune systems; a weak or unhealthy intestinal tract affects the metabolism, immunity, and mental condition [138]. The gut microbiota is a consortium of a wide variety of microorganisms that provide energy and nutrition, and protect the integrity of the gut against pathogenic bacteria [139]. Therefore, regulation of the gut microbiota is vital to prevent gastrointestinal distress such as constipation, diarrhea, and intestinal ulcers. Probiotics are mainly related to the intestinal tract, as their consumption improves the immune system by regulating the intestinal flora and favors the production of metabolites [140]. Gao et al. [138] presented a bibliographic compilation showing how the metabolites produced by probiotics can regulate the intestinal microbiota. EPS, SCFA, and bacteriocins are the main compounds that regulate the intestinal microbiota. For example, SCFAs (acetic, propionic, and butyric acid) regulate the pH of the intestine and stimulate the mucous production of epithelial cells, which can prevent the adhesion and proliferation of pathogenic bacteria [57,140]. Daliri et al. [141] proposed patterns of probiotics’ mechanisms of action in the intestinal micro-ecosystem: modulation of endogenous colonies, protection barrier of the intestinal tract, homeostasis maintenance, regulation of the immune system, and influence on the vagal afferents.
In foods with probiotics, their consumption has been evaluated to understand their effect on the intestinal tract and microbiota. For instance, Liu et al. [95] studied yogurt consumption with Lc. lactis, L. plantarum, and L. casei compared with commercial yogurt (L. bulgaricus and St. thermophilus) in male rods (n = 144) with constipation. Their results showed that yogurt consumption changed the microbiota, giving higher percentages for the phylum Bacteroidetes and lower percentages for the phylum Firmicutes. In addition, consuming both yogurt samples significantly reduced mice defecation by relieving loperamide-induced constipation. Likewise, in rodent models, it has been shown in several studies that milk consumption fermented with L. plantarum B7, L. rhamnosus S1K3, and L. paracasei ST11 can reduce the population of pathogenic bacteria, such as Salmonella enterica serovar Typhimurium [97,98,99]. In clinical trials, Kato-Katoaka et al. [125] studied the effects of fermented milk with L. casei Shirota on abdominal dysfunction in 47 healthy medical students during eight weeks of intervention. In their results, it was observed that the beverage with L. casei Shirota was able to reduce stress-induced abdominal dysfunction by regulating the microbiota, since the group that consumed the fermented beverage significantly reduced the percentage of Bacteroidaceae species compared to the placebo group. In a systematic review by Donovan and Rao [142], several studies were identified that showed that yogurt consumption had a significant effect on infectious diarrhea in healthy and malnourished children. The consumption of milk fermented with L. paracasei and licorice (Glycyrrhiza glabra) demonstrated effects against Helicobacter pylori infection (causing gastric ulcer) in a clinical study by Yoon et al. [143]. The results showed that patients who consumed the product reduced the histological inflammation produced by H. pylori for eight weeks of intervention. In addition, postbiotic food, such as fermented milk with heat-inactivated L. gasseri CP2305, has been reported to regulate bowel function in people with tendencies to constipate (n = 39) [103]. The consumption of foods with probiotics improves the intestinal wellbeing of people. However, we still need to understand more about probiotics’ competence, mechanisms of action, and metabolites during their intestinal tract passage.
4.2. Antioxidant Properties
The study of antioxidant properties has mainly focused on reducing the effects derived from oxidative stress. Oxidative stress is the intracellular state in which oxygen radical levels are elevated, so this increase in radicals can damage lipids, proteins, and DNA [144]. These compounds are commonly referred to as reactive oxygen species (ROS), comprising superoxide anion radicals, hydroxyl radicals, and hydrogen peroxide [145]. ROS at high intracellular levels are detrimental to different biological processes involving stem cell exhaustion, tumorigenesis, autoimmunity (immune response against self-antigens), and accelerated senescence [144].
Naturally, living organisms have various defenses against oxidative damage, such as enzymatic (superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR)), non-enzymatic (glutathione, thioredoxin, vitamin C, and vitamin E) and repair systems of the organism [146]. Despite possessing these defenses, it is not enough to avoid the effects of oxidative stress. Hence, one way to combat it is through diet, which involves the consumption of foods with antioxidant compounds. Several studies (Table 1) have shown that consuming foods with probiotics induces antioxidant effects on the human body. For instance, Shori et al. [105] studied various cashew-plant-based milk yogurt samples with L. rhamnosus ATCC 53103, L. casei ATCC 393, and L. plantarum ATCC 14917. The antioxidant activities by 2,2-diphenyl-1-picrylhydrazyl (DDPH) assay, capacity chelating ferrous ions (FIC), and potential ferric reducing antioxidant (FRAP) were evaluated. Milk-based cashew samples from L. rhamnosus ATCC 53103, L. casei ATCC 393, and L. plantarum ATCC 14917 showed higher values in antioxidant activity tests than the control yogurt made with cow’s milk and commercial cultures (St. thermophilus St1342 and L. delbrueckii ssp. lactis ATCC 7830). The sample with L. rhamnosus ATCC 53103 had the highest antioxidant capacity in the DPPH, FIC, and FRAP tests. However, it is worth mentioning that the sample with L. plantarum ATCC 14917 had a considerable increase in antioxidant effect in the FIC and FRAP tests at 21 days of storage. In another example, Duru et al. [106] developed a fermented oat product with L. acidophilus DSM 13241 and St. thermophilus DSM 15957; they formulated three samples that varied in the amount of isoflavones 0.32, 0.16 mg/mL, and without addition for A, B, and C, respectively. The results showed that antioxidant activity (DPPH assay) increased with the addition of isoflavones, but this was reduced according to longer storage time (up to 28 days). Duru et al. [106] mentioned that adding isoflavones to fermented oats improved the microbial count of bacterial probiotics but did not improve their viability during product storage. Antioxidant properties have also been evaluated in animal models, such as mice. One piece of evidence is the study by Yoon et al. [102], in which a probiotic yogurt was prepared with L. acidophilus, Bif. lactis, St. thermophilus, and L. delbrueckii subsp. bulgaricus fermented at low temperatures (22 °C for 27 h) and conventionally fermented (37 °C for 12 h). Evaluations of antioxidant activities (DPPH assay, 2,20-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) cation assay, and FRAP assay) were applied to mice induced with colitis. The results showed that slow fermentation of probiotic yogurt obtained a higher antioxidant response than rapid fermentation. Yoon et al. [102] argued the reason for this difference is that probiotics had a better absorption of the substrate, given its solubility and diffusivity.
Furthermore, antioxidant properties have been analyzed in foods with encapsulated bacterial probiotics. One study evaluated the antioxidant activities (DPPH assay, 2,20-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) cation assay, oxygen radical absorbance capacity (ORAC) assay, and photochemiluminescence (PCL) assay) of two snack bars made with chickpeas and green lentils with L. plantarum microencapsulated (specifically in the chocolate coating) [107]. The results showed that the snack bar made with chickpeas presented greater antioxidant activity than the bar made with green lentils. It was also mentioned that the total content of polyphenols (TPC) influenced the values of antioxidant activities (ORAC, DPPH, and ABTS). Postbiotic foods have also been reported to exhibit antioxidant activities. For example, Gholamhosseinpour and Hashemi [108] evaluated the antioxidant activity of fermented milk with ultrasound-inactivated L. plantarum AF1 to stop fermentation and to not affect thermolabile compounds. In their results, fermentation was observed to increase antioxidant activity (ABTS assay and DPPH assay), and ultrasound significantly improved this activity, especially the 15-min treatment. They argued that this may be caused by cell lysis of bacteria (release of intracellular compounds), the modification of bacterial extracellular metabolites, and hydrolyzed constituents of milk [108]. Certain compounds derived from probiotic fermentation have been considered antioxidant agents, which include organic acids (SCFA), lactate, 3-phenyllactate, indole-3-lactate, β-hydroxybutyrate, and γ-aminobutyrate, among others [109].
As has been observed, in vitro or in vivo tests show that foods with bacterial probiotics can provide antioxidant activities. However, the best way to verify these activities is through clinical studies focused on oxidative stress. For example, the daily consumption of 200 mL of soy milk with L. plantarum A7 has been evaluated in patients with diabetic kidney disease [110]. The results showed that consumption of probiotic soy milk had a slight effect on the oxidative stress of patients, as oxidized glutathione concentration (ROS) levels were reduced from intake of the probiotic product compared to soy milk without the added probiotic.
Unfortunately, sometimes no improvements or effects in consumption are observed. For example, Bahmani et al. [111] studied the effects of the consumption of symbiotic bread, which contained L. sporogenes and inulin (7 g of inulin per 100 g sample). The results showed that consumption did not have an effect or increased the antioxidant capacity of the plasma. Therefore, the authors argued that a more extended study time was necessary, since the time was short (8 weeks) and to apply more antioxidant activity tests, such as glutathione peroxidase and superoxide dismutase [111]. Another study evaluated the effect against oxidative stress from the supply of probiotic yogurt with L. acidophilus LA5 and Bif. lactis BB12 in pregnant women over nine weeks [112]. The results showed that the consumption of probiotic yogurt did not have significant differences in terms of oxidative stress parameters (total antioxidant activity (TAC), plasma glutathione (GSH), erythrocyte glutathione peroxidase erythrocyte glutathione peroxidase (GR), and serum 8-oxo-7,8-dihydroguanine serum 8-oxo-7,8-dihydroguanine (8-oxo-G)) compared to the control yogurt. Similarly, the authors had the same comments regarding the intervention time, that it was very short, and also referred to the small budget of the clinical study. Therefore, research still needs to be carried out on antioxidant activities, which require more clinical studies evaluating the consumption of foods with probiotics.
4.3. Anti-Inflammatory Properties
According to Plaza-Díaz et al. [147], the consumption of probiotics may provide anti-inflammatory effects that help relieve chronic intestinal diseases, inflammatory bowel diseases (IBDs), necrotizing enterocolitis (NEC), and malabsorption; being the syndromes with the most clinical manifestations. The term IBD describes four pathologies as ulcerative colitis (UC), Crohn’s disease (CD), pouchitis, and microscopic colitis. Table 1 shows clinical studies of probiotic foods with anti-inflammatory effects; there are few because most have been studied with dairy foods, such as yogurt. For example, Mazani et al. [113] conducted a clinical study involving the effect of consuming a probiotic yogurt with Lactobacillus spp. in young women (n = 27) after exercise. Results showed that consumption after exercise of probiotic yogurt for two weeks significantly decreased (p < 0.05) serum tumor necrosis factor-alpha (TNF-α) and biochemical factors matrix metalloproteinase 2 (MMP2), matrix metalloproteinase 9 (MMP9), and malondialdehyde (MDA). In contrast, the control group showed no effect on biochemical factors or anti-inflammatory tests. Another clinical study evaluated the effect of consuming sheep’s milk yogurt with probiotic cultures (St. thermophilus and L. bulgaricus) and commercial yogurt prepared with cow’s milk [114]. The study consisted of thirty participants and lasted approximately five weeks; three yogurt samples were evaluated: whole sheep’s milk, semi-skimmed sheep’s milk, and cow’s milk. Overall, the results showed that there were no significant differences between the samples in terms of inflammatory serum tests (IL-8, TNF-α, and IL-10), but there were decreases in proinflammatory expressions of tumor necrosis factor-alpha (TNF-α) and cytokine IL-8, and increased expression of the anti-inflammatory cytokine IL-10 between the beginning and end of the intervention. Redondo et al. [114] argued that the size of the sample or participants, as well as the time of study, can be considerable influences, so it is recommended to have a large number of participants, at least 50, and a major period of consumption, in addition to the fact that clinical trials are complex because each participant is naturally different. It is not easy to control the diet of each participant.
The study by Lee et al. [115] evaluated the effect of daily consumption (for 12 weeks) of yogurt containing L. paracasei (L. casei 431®) and Bif. lactis (BB-12®) and postbiotic L. plantarum nF1 (inactivated with heat treatment) in 200 individuals over 60 years old. The results showed that the consumption (n = 100) of probiotic and postbiotic yogurt increased cytokine (IL-12) activity, which is linked to inflammatory bowel diseases. Other probiotic foods have only been studied with Kimchi [109] and Inula britannica (East Asian medicinal herb) [116], but studies of these foods have only used in vitro tests to analyze the anti-inflammatory properties. For example, Kim et al. [109] used the gas chromatography technique to investigate metabolites generated during kimchi fermentation with L. plantarum PMO 08. Their results identified around 78 metabolites, some of which have been closely related to anti-inflammatory activities, such as lactate, indole-3-lactate, β-hydroxybutyrate, γ-aminobutyrate, and glycerol. Based on this molecular identification, they presented that kimchi fermented with L. plantarum PMO 08 has anti-inflammatory activities, since in vitro tests showed a significant reduction in levels of proinflammatory cytokine expressions (IL-6 y TNF-α). Despite the breakthrough and the studies reported in the literature regarding anti-inflammatory properties, the mechanisms of action are still unclear and not fully understood, as it has been reported that the activity during immunomodulation is highly specific to the microbial strain and depends on the health status of the host [148].
4.4. Anti-Cancer Properties
Cancer is a disease of high importance in medicine, as it is the second cause of mortality worldwide [149]. The World Health Organization (WHO) [150] reported around 18.1 million new cases in 2018, and this number is expected to rise to 29.4 million by 2040. Colorectal, prostate, lung, stomach, liver, and breast cancers are the highest incidences of this fatal disease [151]. Cancer is a disease caused by DNA repair deficiency or mutations produced during DNA replication, and exposure to the environment, habits of daily life, and genetic inheritance of individuals are the main risk factors [152,153]. Cancer is an uncontrolled cell division usually detected in the late stages of its progression. Once cancer cells spread, they eventually need multiple stages for tumor development to become malignant [154].
Today, cancer treatment is based on the supply of multiple drugs and chemotherapy, which damage not only cancer cells but also healthy cells, causing a certain resistance that can affect future treatments of the patient [155]. Hence, consuming foods with probiotics has been suggested for treating and preventing cancer, as several studies have shown anticancer properties (Table 1). Fatahi et al. [117] evaluated the anticancer activity of kefir (considered a probiotic food), in which different concentrations of kefir extracts interacted with a U87 glioblastoma cell line. Furthermore, anticancer activity was evaluated with the cytotoxic effect on cancer cells using an MTT test. The results found by Fatahi et al. [117] showed that a higher concentration (20 mg/mL) of kefir and fermentation time (48 h) managed to inhibit most malignant cells (83%). They argued that this inhibition might be caused by the interaction of metabolites produced during fermentation and cancer cells that can interfere with regulating proliferation, cell distinction, apoptosis, metastasis, and angiogenesis.
Another example is the evaluation of the anticancer activity of milk fermented with L. casei ATCC 393, which was carried out by Abdel-Hamid et al. [118]. For anticancer activity, it exposed the aqueous extract peptide and its filtrate (<2 kDa) with cell lines of carcinoma MCF-7 and Caco-2 obtained as a response to the anti-proliferative activity using MTT assay. The results showed that peptides with lower molecular weight (<2 kDa) obtained higher values of anti-proliferative activity than the unfiltered aqueous peptide extract in both cell lines, increasing the anticancer properties of cow’s milk fermented with L. casei ATCC 393 [118]. Similarly, it has been shown that peptides from the fermentation of Lc. lactis KX881782 and L. acidophilus DSM9126 in camel’s milk have more cellular inhibition of Caco-2, MCF-7, and HELA than in the fermentation of cow’s milk [119]. It has been argued that peptides from probiotic fermentation, due to their hydrophobicity, amphipathic property, secondary membrane structure, net charge, and oligomerization capacity, are the most important contributors to anticancer activities [156].
In clinical studies, a probiotic drink with L. casei Shirota incorporated with soy isoflavone in healthy people (n = 309) has been evaluated to prevent breast cancer [121]. The results were obtained through clinical questionnaires, which showed a biological interaction between people who consumed the probiotic drink and soy isoflavones. It was observed that the consumption of this product could prevent breast cancer, but it was impossible to identify the amount or daily dose necessary to prevent this disease. Furthermore, Toi et al. [121] suggested that lifestyle and diet must be modified for cancer prevention, including consuming their probiotic drink with L. casei Shirota and soy isoflavone. Similarly, Pala et al. [122] studied the protective effect against colorectal cancer (CRC) through the consumption of commercial yogurt (St. thermophilus and L. delbrueckii subsp. bulgaricus) in a population of 45,241 participants over 12 years of intervention. Again, clinical questionnaires were used to obtain results. During that period, 289 volunteers were diagnosed with CRC. In the results, it was observed that yogurt consumption was inversely associated with the risk of contracting CRC, and the protective effect of yogurt was evident throughout the population.
Furthermore, it was observed that the protective effect was stronger in men than in women. Clinical studies on anticancer properties have been very few and have been based on questionnaires. The results do not show biological or biochemical evidence that could verify whether there certainly is an anticancer effect in human models. More clinical studies are needed to show that the consumption of probiotic foods helps treat and prevent cancer.
4.5. Psychobiotic Effects
Long-term consequences that affect today’s society can be associated as a result of the pandemic generated by the COVID-19 virus. Due to quarantine-induced isolation, the sudden stoppage of the daily lives of millions of people led to increases in loneliness, anxiety, depression, insomnia, the harmful use of alcohol and drugs, and self-harm or suicidal behavior [157,158,159]. For this reason, possible treatments to alleviate mental disorders caused by the pandemic have been investigated, and consuming probiotics or foods could be a viable solution. Recent research has argued that gut microbiota affects gut regulation and influences mental health through the gut-brain axis [124,125]. Although the term “psychobiotics” originated in 2013 [160], it has gained more potency in recent years due to the current situation. Psychobiotics refers to the psychological potential of molecules produced by probiotic bacteria and refers to certain probiotics that include species such as St. thermophilus, Bif. animalis, Bif. bifidum, Bif. longum, L. bulgaricus, Lc. lactis, L. acidophilus, L. plantarum, L. reuteri, L paracasei, L. helveticus, L. rhamnosus, B. coagulans, and Clostridium butyricum, among others. [161]. These bacterial probiotics must be able to produce neuroactive substances, such as γ-aminobutyric acid (GABA) and serotonin [161].
Even the mechanism of action of psychobiotics is not fully understood. Still, it is proposed that the intestinal microbiota communicates with the brain, and vice versa, through the central, autonomic, and enteric nervous systems, as well as the hypothalamic-pituitary-adrenal (HPA) axis [162]. Communication between the gut and the brain must be efficient. Hence, several pathways are concentrated in the nervous system and involve cytokines released by mucosal immune cells and hormones produced by endocrine cells or through the vagus nerve. In addition, the synthesis of neurotransmitters, neurochemicals, and metabolites is important for proper signaling in the brain concerning body stress [161]. Such is the case of serotonin, of which Yano et al. [163] noted that more than 90% of this compound in the body is produced in the intestine and the gut-brain axis, which is vital for the regulation of activation receptors located in enterocytes, enteric neurons, and immune system cells. The main mechanism suggests that when the production of neurotransmitters in the intestine is increased, it causes a decrease in tryptophan in the blood plasma, which generates an increase in the transmitter molecules of the brain and can improve neuropsychiatric parameters such as sleep, appetite, mood, and cognition [164]. Another mechanism proposes that metabolites, such as SCFAs, as they are involved in decreasing pro-inflammatory cytokines, may positively affect the treatment of stress and depression [161,165]. Butyrate is the SCFA that has been the most studied, since bacterial probiotics such as Bif. breve M2CF22M7, Bif. infantis E41 and C. butyricum have demonstrated antidepressant properties in rodents [166,167].
Concerning foods with probiotics, there have been several clinical studies that have evaluated the psychobiotic effects, as shown in Table 1. For instance, Chung et al. [123] studied the psychobiotic effect of fermented milk tablets with L. helveticus IDCC3801 in healthy elderly people aged 60 to 75 years old for 12 weeks. The results showed that fermented milk tablets improved patients’ cognitive functions compared to the placebo group. Similarly, it has been shown that the intake of fermented milk with L. helveticus CM4 with lactononadecapeptide in 61 healthy middle-aged people (50–70 years old) for eight weeks [124]. In the results of the cognitive questionnaires, cognitive metabolism, attention, and memory were demonstrated compared to the placebo group. Concerning serotonin production, Kato-Katoaka et al. [125] investigated the physiological effects and physical stress of consuming fermented milk with L. casei Shirota in 47 medical students undergoing academic examinations for eight weeks. It was possible to demonstrate that the probiotic food consumption significantly increased the levels of fecal serotonin compared to the placebo group. In addition, consumption significantly reduced the stress of students. Other fermented products with probiotics (mainly of the genus Lactobacillus and Bifidobacterium), such as fermented algae (Laminaria japonica) [126], fermented soy [127], kefir [168], and yogurt [128,129], showed psychobiotic effects (Table 1), such as antidepressant, anti-stress, enhanced emotions in women, cognitive enhancement and intelligence, and effects against Alzheimer’s syndrome and anxiety.
Furthermore, dairy foods have been studied with postbiotics, such as the study by Nishida et al. [130], which evaluated the psychobiotic effects of postbiotics from L. gasseri CP2305, developing a fermented milk beverage. The study focused on healthy students (21 men and 11 women) and the intervention time was five weeks with doses of 190 g per day. To promote a state of stress, the study was conducted when students were taking a cadaveric dissection course. It showed that dairy beverages with inactivated LAB improved sleep quality in men more than in women. Furthermore, sleep time and latency increased and decreased in men, respectively. Science has made remarkable findings regarding the psychobiotic effects of consuming foods with probiotics and postbiotics. However, several researchers have concluded that the mechanisms of action and the relationship between the microbiota and the brain still need further investigation.
4.6. Against Heart Diseases and Obesity Reduction
Heart disease is a major concern for the global health system, as one in three people have high cholesterol levels in developed economies [169]. We can also add it to the increase in obesity worldwide owing to a diet high in fatty foods, such as junk foods. Obesity is an extreme state of fat accumulation in the body, closely related to heart and kidney illnesses [169]. Table 1 presents the studies that have been conducted on heart disease and consuming foods with bacterial probiotics. In a study conducted using rats, it was observed that fermented milk incorporated with L. fermentum MTCC significantly reduced levels of low-density lipoprotein cholesterol, serum total cholesterol, liver lipids, coronary artery risk index, and TNF-α and IL-6 mRNA expression of the liver [131]. Beltran-Barrientos et al. [132] conducted a clinical study evaluating the effect of blood pressure reduction on consuming fermented milk with Lc. lactis NRRL B-50571 in 18 patients with a tendency to hypertension with an intervention time of 5 weeks. The results revealed that daily consumption of the probiotic product reduced patients’ systolic and diastolic blood pressure and decreased serum levels of triglycerides, total cholesterol, and low-density lipoproteins. However, the consumption of yogurt with L. acidophilus La5 and Bif. lactis Bb12 has no observed effects against heart disease; the authors argued that this lack of effects was due to the short treatment time (10 weeks) [133]. Regarding anti-obesity effects, it has been suggested that a diet based on probiotic interventions is more effective than drug delivery [169]. Razmpoosh et al. [134] evaluated the effects of the yogurt condensed with L. acidophilus La5 and Bif. lactis Bb12 consumption in 70 overweight or obese women over an intervention time of 8 weeks. The results showed that the intervention group had a greater reduction in triglyceride and cholesterol levels than the placebo group. In addition, fat percentage, body mass index, and waist circumference were significantly more reduced in the intervention group than in the placebo. Similarly, the anti-obesity effects of milk fermented with L. casei Shirota have been reported; its consumption can decrease glycol-albumins and low-density lipoproteins and reduce adipose tissue [135].
4.7. Antiviral Properties
With the recent pandemic caused by COVID-19, research into antiviral properties has gained a lot of attention as the efforts of science and many researchers, as well as vaccines, were obtained in a very short time. Despite the existence of vaccines and drug tests, some people show limited efficiency, so the consumption of probiotics is a viable option to increase defenses in people with a compromised immune system [170]. Muhialdin et al. [171] showed the antiviral activity of probiotics isolated from fermented foods from various studies, most of which had antiviral properties against influenza viruses and enteroviruses; in vitro and in vivo studies were conducted in animal models. As shown in Table 1, few studies have been conducted mainly focusing on the antiviral properties of foods with probiotics or postbiotics. Kim et al. [136] demonstrated the antiviral effects of yogurt with thermally inactivated L. plantarum nF1 (postbiotic) in mice. Their results showed that postbiotic yogurt raised the cytokine Natural Killers (NK) expression, which is closely related to the modulation of the immune system and the primary defense against viral infections.
Similarly, it has been reported that L. delbrueckii subsp. bulgaricus OLL1073R-1 in fermented milk may provide antiviral effects against influenza A [137]. It has been noted that metabolites like proteinase inhibitors and bacteriocins are the main causes of the antiviral activity of probiotics [172]. It is necessary to conduct clinical studies that evaluate this property, as they could help fight viruses such as COVID-19, or be used for the prevention of stationary viruses (Influenza) or new viruses that appear.
5. Probiotics and Postbiotics in the Food Industry
As previously mentioned, probiotics can be presented in various forms for consumption. Therefore, the food industry has a great opportunity to develop products with very high bioactive potential as functional foods, since probiotics and postbiotics can be added to the formulation or preparation of the product. In probiotics, there is a great diversity of products that contain them, most of which are dairy. However, as Reque and Brandelli [173] noted, there are sectors of the population that demand non-dairy products due to either health issues (lactose or milk protein intolerance) or lifestyles (vegetarians or vegans). With the help of microencapsulation of probiotics, products can be designed for people seeking alternatives to dairy products. There are food alternatives with microencapsulated probiotics, such as fruit-based drinks, meat products, desserts, chocolates, and bakery products [16]. While, for lifestyles, more research is required for those that only consume vegetables. Specifically, it is necessary to identify which probiotics are suitable, since their origin must be from vegetable sources. In addition, probiotics help cover the nutritional needs of vegetarians/vegans, such as the production of complex B vitamins or improving the biodigestibility of proteins in the food [174]. In addition, various antinutritional compounds of vegetables are delayed by probiotic growth.
In the same way, postbiotics are an excellent alternative since they are more stable and do not need strict storage, handling, and transport conditions, such as a cold chain. Unfortunately, there have been few studies in which postbiotics are incorporated into foods, but foods such as yogurt and whey-based sports drinks contain inactivated LAB bacteria. A few examples include L. bulgaricus, St. thermophilus, L. acidophilus, L. gasseri CP2305, and L. casei 01, which have already been carried out [103,130,175,176].
A very interesting point regarding probiotics and postbiotics is that they not only provide benefits to the health of the consumer, but they can also participate in food safety because potential applications of postbiotics in food safety (mainly LAB) have been discovered. These include the bio-preservation of dairy, meat, vegetable, and bakery foods, and the production of antimicrobial compounds such as organic acids and/or bacteriocins. This is in addition to its use in the development of bioactive and edible packaging; the prevention and control of biofilms in food machinery; and the reduction and degradation of contaminants, such as biogenic amines, pesticides, and mycotoxins [177]. More research on postbiotics is needed to be able to introduce them into the food industry, since their interaction with food components could produce defects in sensory properties, such as taste, odor, or appearance. Similarly, in products with microencapsulated probiotics, there may be defects that could occur in the shelf-life of the food due to fermentations after processing. Techniques are needed that keep the probiotics partially inactivated or latent. This research is critical to support future regulations and applications with either postbiotics or microencapsulated probiotics incorporated into foods.
6. Conclusions and Future Perspectives
Bacterial probiotics can be incorporated into foods in various ways, whether they are involved in the product’s fermentation, microencapsulated for better bioactivity delivery, or inactivated for better stability in the food product. The fermentation of bacterial probiotics generates a great diversity of metabolites, which have been investigated as demonstrating benefits to human health. Nonetheless, most studies concerning metabolites have been in vitro and in vivo (mostly in rodents), which cannot completely assure that the same benefits would occur in the human organism, so it is therefore necessary to investigate in humans. On the other hand, a large amount of scientific evidence has been presented regarding the consumption of foods with bacterial probiotics. These foods provide health benefits such as the regulation of the microbiota, help relieve gastrointestinal disorders and heart diseases, and have antioxidant, anticancer, anti-inflammatory, psychobiotic, anti-obesity, and antiviral capacities.
Although clinical studies have been the primary sources of valuable information, there are still many loose ends regarding the mechanisms of action of bacterial probiotics and the reasons for alleviating so many ailments. Therefore, in the future, more research involving clinical studies is needed, since foods related to bacterial probiotics could help reduce the consumption of drugs and prevent certain diseases in conjunction with a healthy lifestyle and diet. Furthermore, more research is needed regarding microencapsulated and postbiotic foods, since most have been used in the application of fermented foods, especially in dairy products. However, as previously mentioned, foods with microencapsulated and postbiotic probiotics can be added to non-dairy foods. Therefore, this is a significant opportunity for the food industry and research within nutrition, medicine, and food science.
Author Contributions
Conceptualization, V.E.V.-S., R.H.H.-F., M.T.J.-M., E.M.-L. and A.L.-M.; visualization, V.E.V.-S.; validation, V.E.V.-S., R.H.H.-F., M.T.J.-M., E.M.-L. and A.L.-M.; investigation, V.E.V.-S. and R.H.H.-F.; resources, M.T.J.-M. and A.L.-M.; writing—original draft preparation, V.E.V.-S.; writing—review and editing, V.E.V.-S., R.H.H.-F., M.T.J.-M., E.M.-L. and A.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors Vera-Santander and Hernández-Figueroa acknowledge the financial support for their Ph.D. studies in Food Science from National Council for Science and Technology (CONACyT) and Universidad de las Américas Puebla (UDLAP).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations; World Health Organization. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. In Proceedings of the Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Córdoba, Argentina, 1–4 October 2001; pp. 1–34. [Google Scholar]
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic—Friend or Foe? Curr. Opin. Food Sci. 2020, 32, 45–49. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Wood, B.J.B. Introduction to the LAB. In Lactic Acid Bacteria; Holzapfel, W.H., Wood, B.J.B., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 1–12. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Precedence Research. Probiotics Market (By Ingredient: Bacteria and Yeast; By Application: Food & Beverage, Dietary Supplements, and Animal Feed; By End Use: Human, Animal; By Distribution Channel: Hypermarkets/Supermarkets, Pharmacies/Drugstores, Specialty Stores, Online Stores, Others)—Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2022–2030. Available online: https://www.precedenceresearch.com/probiotics-market (accessed on 27 October 2022).
- Moradi, M.; Molaei, R.; Guimarães, J.T. A Review on Preparation and Chemical Analysis of Postbiotics from Lactic Acid Bacteria. Enzyme Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Recent Insights in the Impact of Emerging Technologies on Lactic Acid Bacteria: A Review. Food Res. Int. 2020, 137, 109544. [Google Scholar] [CrossRef]
- Ilango, S.; Antony, U. Probiotic Microorganisms from Non-Dairy Traditional Fermented Foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Fidan, H.; Esatbeyoglu, T.; Simat, V.; Trif, M.; Tabanelli, G.; Kostka, T.; Montanari, C.; Ibrahim, S.A.; Özogul, F. Recent Developments of Lactic Acid Bacteria and Their Metabolites on Foodborne Pathogens and Spoilage Bacteria: Facts and Gaps. Food Biosci. 2022, 47, 101741. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, T.S.; Moon, H.W.; Lee, S.Y.; Lee, S.Y.; Ji, G.E.; Hwang, K.T. Lactobacillus plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages. Molecules 2020, 25, 5056. [Google Scholar] [CrossRef]
- Pineiro, M.; Stanton, C. Probiotic Bacteria: Legislative Framework—Requirements to Evidence Basis. J. Nutr. 2007, 137, 850S–853S. [Google Scholar] [CrossRef]
- Lin, T.-L.; Shu, C.-C.; Lai, W.-F.; Tzeng, C.-M.; Lai, H.-C.; Lu, C.-C. Investiture of next Generation Probiotics on Amelioration of Diseases—Strains Do Matter. Med. Microecol. 2019, 1–2, 100002. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-Dairy Probiotic Products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A Review of the Microencapsulation Techniques for the Incorporation of Probiotic Bacteria in Functional Foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotics for Gastrointestinal Delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Blaiotta, G.; Murru, N.; Di Cerbo, A.; Succi, M.; Coppola, R.; Aponte, M. Commercially Standardized Process for Probiotic “Italico” Cheese Production. LWT—Food Sci. Technol. 2017, 79, 601–608. [Google Scholar] [CrossRef]
- Frakolaki, G.; Kekes, T.; Lympaki, F.; Giannou, V.; Tzia, C. Use of Encapsulated Bifidobacterium animalis subsp. lactis through Extrusion or Emulsification for the Production of Probiotic Yogurt. J. Food Process Eng. 2022, 45, e13792. [Google Scholar] [CrossRef]
- Khorshidi, M.; Heshmati, A.; Taheri, M.; Karami, M.; Mahjub, R. Effect of Whey Protein- and Xanthan-Based Coating on the Viability of Microencapsulated Lactobacillus acidophilus and Physiochemical, Textural, and Sensorial Properties of Yogurt. Food Sci. Nutr. 2021, 9, 3942–3953. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-Encapsulation of Probiotics with Prebiotics and Their Application in Functional/Synbiotic Dairy Products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef]
- Ribeiro, M.C.E.; Chaves, K.S.; Gebara, C.; Infante, F.N.S.; Grosso, C.R.F.; Gigante, M.L. Effect of Microencapsulation of Lactobacillus acidophilus LA-5 on Physicochemical, Sensory and Microbiological Characteristics of Stirred Probiotic Yoghurt. Food Res. Int. 2014, 66, 424–431. [Google Scholar] [CrossRef]
- Kearney, L.; Upton, M.; McLoughlin, A. Meat Fermentations with Immobilized Lactic Acid Bacteria. Appl. Microbiol. Biotechnol. 1990, 33, 648–651. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.L.; Lara-Labastida, R.; Rodriguez-Huezo, E.; Totosaus, A. Effect of Spray Drying Encapsulation of Thermotolerant Lactic Acid Bacteria on Meat Batters Properties. Food Bioprocess Technol. 2013, 6, 1505–1515. [Google Scholar] [CrossRef]
- Chaikham, P. Stability of Probiotics Encapsulated with Thai Herbal Extracts in Fruit Juices and Yoghurt during Refrigerated Storage. Food Biosci. 2015, 12, 61–66. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of Chitosan-Alginate Encapsulation with Inulin on Survival of Lactobacillus rhamnosus GG during Apple Juice Storage and under Simulated Gastrointestinal Conditions. LWT—Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Praepanitchai, O.-A.; Noomhorm, A.; Anal, A.K. Survival and Behavior of Encapsulated Probiotics (Lactobacillus plantarum) in Calcium-Alginate-Soy Protein Isolate-Based Hydrogel Beads in Different Processing Conditions (PH and Temperature) and in Pasteurized Mango Juice. BioMed Res. Int. 2019, 2019, 9768152. [Google Scholar] [CrossRef]
- Champagne, C.P.; Raymond, Y.; Guertin, N.; Bélanger, G. Effects of Storage Conditions, Microencapsulation and Inclusion in Chocolate Particles on the Stability of Probiotic Bacteria in Ice Cream. Int. Dairy J. 2015, 47, 109–117. [Google Scholar] [CrossRef]
- Gul, O. Microencapsulation of Lactobacillus casei Shirota by Spray Drying Using Different Combinations of Wall Materials and Application for Probiotic Dairy Dessert. J. Food Process. Preserv. 2017, 41, e13198. [Google Scholar] [CrossRef]
- Malmo, C.; La Storia, A.; Mauriello, G. Microencapsulation of Lactobacillus Reuteri DSM 17938 Cells Coated in Alginate Beads with Chitosan by Spray Drying to Use as a Probiotic Cell in a Chocolate Soufflé. Food Bioprocess. Technol. 2013, 6, 795–805. [Google Scholar] [CrossRef]
- Ghasemi, L.; Nouri, L.; Mohammadi Nafchi, A.; Al-Hassan, A.A. The Effects of Encapsulated Probiotic Bacteria on the Physicochemical Properties, Staling, and Viability of Probiotic Bacteria in Gluten-Free Bread. J. Food Process. Preserv. 2022, 46, e16359. [Google Scholar] [CrossRef]
- Hadidi, M.; Majidiyan, N.; Jelyani, A.Z.; Moreno, A.; Hadian, Z.; Mousavi Khanegah, A. Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage. Foods 2021, 10, 2215. [Google Scholar] [CrossRef] [PubMed]
- Penhasi, A.; Reuveni, A.; Baluashvili, I. Microencapsulation May Preserve the Viability of Probiotic Bacteria During a Baking Process and Digestion: A Case Study with Bifidobacterium animalis subsp. lactis in Bread. Curr. Microbiol. 2021, 78, 576–589. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.D.; Boom, R.M.; Schutyser, M.A.I. Survival of Encapsulated Lactobacillus plantarum during Isothermal Heating and Bread Baking. LWT 2018, 93, 396–404. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H. New Perspectives on Probiotics in Health and Disease. Food Sci. Hum. Wellness 2015, 4, 56–65. [Google Scholar] [CrossRef]
- Corona-Hernandez, R.I.; Álvarez-Parrilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; de la Rosa, L.A.; Wall-Medrano, A. Structural Stability and Viability of Microencapsulated Probiotic Bacteria: A Review: Encapsulation of Probiotic Bacteria. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of Bacteria: A Review of Different Technologies and Their Impact on the Probiotic Effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Petrovic, T.; Nedovic, V.; Dimitrijevic-Brankovic, S.; Bugarski, B.; Lacroix, C. Protection of Probiotic Microorganisms by Microencapsulation. Chem. Ind. Chem. Eng. Q. 2007, 13, 169–174. [Google Scholar] [CrossRef]
- Solanki, H.K.; Pawar, D.D.; Shah, D.A.; Prajapati, V.D.; Jani, G.K.; Mulla, A.M.; Thakar, P.M. Development of Microencapsulation Delivery System for Long-Term Preservation of Probiotics as Biotherapeutics Agent. BioMed Res. Int. 2013, 2013, 620719. [Google Scholar] [CrossRef]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-Encapsulation and Characterisation of Omega-3 Fatty Acids and Probiotic Bacteria in Whey Protein Isolate–Gum Arabic Complex Coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Hernández-Granados, M.J.; Franco-Robles, E. Postbiotics in Human Health: Possible New Functional Ingredients? Food Res. Int. 2020, 137, 109660. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What Else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef]
- Collado, M.C.; Vinderola, G.; Salminen, S. Postbiotics: Facts and Open Questions. A Position Paper on the Need for a Consensus Definition. Benef. Microbes 2019, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Kogut, M.H.; Genovese, K.; He, H.; Kazemi, S.; Arsenault, R.J. Administration of a Postbiotic Causes Immunomodulatory Responses in Broiler Gut and Reduces Disease Pathogenesis Following Challenge. Microorganisms 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.-M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When Simplification Fails to Clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Dunand, E.; Burns, P.; Binetti, A.; Bergamini, C.; Peralta, G.H.; Forzani, L.; Reinheimer, J.; Vinderola, G. Postbiotics Produced at Laboratory and Industrial Level as Potential Functional Food Ingredients with the Capacity to Protect Mice against Salmonella Infection. J. Appl. Microbiol. 2019, 127, 219–229. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Aghebati Maleki, L.; Samadi Kafil, H.; Abbasi, A. Postbiotics: A Novel Strategy in Food Allergy Treatment. Crit. Rev. Food Sci. Nutr. 2021, 61, 492–499. [Google Scholar] [CrossRef]
- Stiles, M.E. Biopreservation by Lactic Acid Bacteria. Antonie Van Leeuwenhoek 1996, 70, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Erginkaya, Z.; Konuray-Altun, G. Potential Biotherapeutic Properties of Lactic Acid Bacteria in Foods. Food Biosci. 2022, 46, 101544. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.A.; Bogatyrev, A.; Zelm, M.C.; Gibson, P.R.; Muir, J.G. Delivery of Acetate to the Peripheral Blood after Consumption of Foods High in Short-Chain Fatty Acids. Mol. Nutr. Food Res. 2021, 65, 2000953. [Google Scholar] [CrossRef]
- Řezanka, T.; Křesinová, Z.; Kolouchová, I.; Sigler, K. Lipidomic Analysis of Bacterial Plasmalogens. Folia Microbiol. 2012, 57, 463–472. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the Neglected Regulatory and Scavenging Lipid Species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef]
- Oberg, T.S.; Ward, R.E.; Steele, J.L.; Broadbent, J.R. Identification of Plasmalogens in the Cytoplasmic Membrane of Bifidobacterium animalis subsp. lactis. Appl. Environ. Microbiol. 2012, 78, 880–884. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W. Plasmalogen Deficiency in Early Alzheimer’s Disease Subjects and in Animal Models: Molecular Characterization Using Electrospray Ionization Mass Spectrometry: Plasmalogen Deficiency in Alzheimer’s Disease. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral Ingestion of Plasmalogens Can Attenuate the LPS-Induced Memory Loss and Microglial Activation. Biochem. Biophys. Res. Commun. 2018, 496, 1033–1039. [Google Scholar] [CrossRef]
- Contesini, F.J.; de Melo, R.R.; Sato, H.H. An Overview of Bacillus Proteases: From Production to Application. Crit. Rev. Biotechnol. 2018, 38, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, O.; Mardanova, A.; Evtugyn, V.; Bogomolnaya, L.; Sharipova, M. Effects of Bacillus Serine Proteases on the Bacterial Biofilms. BioMed Res. Int. 2017, 2017, 8525912. [Google Scholar] [CrossRef]
- Hols, P.; Ledesma-García, L.; Gabant, P.; Mignolet, J. Mobilization of Microbiota Commensals and Their Bacteriocins for Therapeutics. Trends Microbiol. 2019, 27, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Preciado, G.M.; EscalanteMinakata, P.; Castro, J.A.O.; Junquera, V.I.; Chávez, J.A.M.; González, C.N.A.; Herrera, R.R. Bacteriocinas: Características y aplicación en alimentos. Investig. Cienc. 2013, 8, 63–69. [Google Scholar]
- Makhal, S.; Kanawjia, S.K.; Giri, A. Effect of MicroGARD on Keeping Quality of Direct Acidified Cottage Cheese. J. Food Sci. Technol. 2015, 52, 936–943. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical Applications of Nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- Abanoz, H.S.; Kunduhoglu, B. Antimicrobial Activity of a Bacteriocin Produced by Enterococcus Faecalis KT11 against Some Pathogens and Antibiotic-Resistant Bacteria. Korean J. Food Sci. Anim. Resour. 2018, 38, 1064–1079. [Google Scholar] [CrossRef]
- Hasan, F.B.; Reza, M.; Masud, H.A.A.; Uddin, M.K.; Uddin, M.S. Preliminary Characterization and Inhibitory Activity of Bacteriocin like Substances from Lactobacillus casei against Multi-Drug Resistant Bacteria. Bangladesh J. Microbiol. 2019, 36, 1–6. [Google Scholar] [CrossRef]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from Lactic Acid Bacteria: Perspectives and Challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Aruhan; Xiu, L.; Sheng, S.; Liang, Y.; Zhang, H.; Liu, Y.; Tong, H.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus buchneri TCP016 Attenuate LPS- and d-GalN-Induced Liver Injury by Modulating the Gut Microbiota. J. Agric. Food Chem. 2019, 67, 11627–11637. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, Partial Characterization and Biological Activity of Exopolysaccharides Produced from Lactobacillus Fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Wang, S.; Yi, H.; Han, X.; Fan, R.; Zhang, Y. Physicochemical Characterization and Antitumour Activity of Exopolysaccharides Produced by Lactobacillus casei SB27 from Yak Milk. Carbohydr. Polym. 2017, 171, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X.; Yan, B. Antioxidant and Immunomodulatory Activity of Selenium Exopolysaccharide Produced by Lactococcus lactis subsp. lactis. Food Chem. 2013, 138, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Van der Es, D.; Hogendorf, W.F.J.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Teichoic Acids: Synthesis and Applications. Chem. Soc. Rev. 2017, 46, 1464–1482. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.; Tytgat, H.L.P.; Verhoeven, T.L.A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M.; De Keersmaecker, S.C.J.; et al. Functional Analysis of Lactobacillus rhamnosus GG Pili in Relation to Adhesion and Immunomodulatory Interactions with Intestinal Epithelial Cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef]
- Kim, K.W.; Kang, S.-S.; Woo, S.-J.; Park, O.-J.; Ahn, K.B.; Song, K.-D.; Lee, H.-K.; Yun, C.-H.; Han, S.H. Lipoteichoic Acid of Probiotic Lactobacillus plantarum Attenuates Poly I:C-Induced IL-8 Production in Porcine Intestinal Epithelial Cells. Front. Microbiol. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Claes, I.J.; Segers, M.E.; Verhoeven, T.L.; Dusselier, M.; Sels, B.F.; De Keersmaecker, S.C.; Vanderleyden, J.; Lebeer, S. Lipoteichoic Acid Is an Important Microbe-Associated Molecular Pattern of Lactobacillus rhamnosus GG. Microb. Cell Factories 2012, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.E.; Kim, H.; Chung, D.K. Lipoteichoic Acid Isolated from Lactobacillus plantarum Maintains Inflammatory Homeostasis through Regulation of Th1- and Th2-Induced Cytokines. J. Microbiol. Biotechnol. 2019, 29, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic Acid from the Cell Wall of a Heat Killed Lactobacillus paracasei D3-5 Ameliorates Aging-Related Leaky Gut, Inflammation and Improves Physical and Cognitive Functions: From C. elegans to Mice. GeroScience 2020, 42, 333–352. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Laiño, J.E.; del Valle, M.J.; de Giori, G.S.; Sesma, F.; Taranto, M.P. B-Group Vitamins Production by Probiotic Lactic Acid Bacteria. In Biotechnology of Lactic Acid Bacteria; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 279–296. [Google Scholar] [CrossRef]
- Deptula, P.; Chamlagain, B.; Edelmann, M.; Sangsuwan, P.; Nyman, T.A.; Savijoki, K.; Piironen, V.; Varmanen, P. Food-Like Growth Conditions Support Production of Active Vitamin B12 by Propionibacterium freudenreichii 2067 without DMBI, the Lower Ligand Base, or Cobalt Supplementation. Front. Microbiol. 2017, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Lule, V.; Kumar, N.; Mandal, S.; Anand, S.; Kumari, V.; Tomar, S. Riboflavin Producing Probiotic Lactobacilli as a Biotechnological Strategy to Obtain Riboflavin-Enriched Fermented Foods. J. Pure Appl. Microbiol. 2016, 10, 161–166. [Google Scholar]
- Bardosono, S.; Wibowo, N.; Sutanto, L.B.; Irwinda, R.; Cannan, R.; Rowan, A.; Dekker, J. Plasma Folate, Vitamin B6 and B12 in Their Relationship to the Presence of Probiotic Strain Bifidobacterium animalis subsp. lactis HNO19 (DR10TM) among Indonesian Pregnant Women in Their Third Semester. World Nutr. J. 2019, 2, 56. [Google Scholar] [CrossRef]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli Species: Properties, Challenges and Potential Biomedical Applications: Biosurfactant/s from Lactobacilli Species. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef]
- Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and Physicochemical Properties of Biosurfactants Produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb. Cell Factories 2017, 16, 155. [Google Scholar] [CrossRef]
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Ben Amor, A.; Mastouri, M. Antioxidant and Antiproliferative Potential of Biosurfactants Isolated from Lactobacillus casei and Their Anti-Biofilm Effect in Oral Staphylococcus aureus Strains. Microb. Pathog. 2017, 104, 84–89. [Google Scholar] [CrossRef]
- Fracchia, L.; Banat, J.J.; Cavallo, M.; Ceresa, C.; Banat, I.M. Potential Therapeutic Applications of Microbial Surface-Active Compounds. AIMS Bioeng. 2015, 2, 144–162. [Google Scholar] [CrossRef]
- Liu, C.-J.; Tang, X.-D.; Yu, J.; Zhang, H.-Y.; Li, X.-R. Gut Microbiota Alterations from Different Lactobacillus Probiotic-Fermented Yoghurt Treatments in Slow-Transit Constipation. J. Funct. Foods 2017, 38, 110–118. [Google Scholar] [CrossRef]
- Acurcio, L.B.; Bastos, R.W.; de Sandes, S.H.C.; de Guimarães, A.C.C.; Alves, C.G.; dos Reis, D.C.; Wuyts, S.; Nunes, Á.C.; Cassali, G.D.; Lebeer, S.; et al. Protective Effects of Milk Fermented by Lactobacillus plantarum B7 from Brazilian Artisanal Cheese on a Salmonella enterica Serovar Typhimurium Infection in BALB/c Mice. J. Funct. Foods 2017, 33, 436–445. [Google Scholar] [CrossRef]
- Acurcio, L.B.; Wuyts, S.; de Cicco Sandes, S.H.; Sant’anna, F.M.; Pedroso, S.H.S.P.; Bastos, R.W.; dos Reis, D.C.; Vieira, A.F.; Cassali, G.D.; Lebeer, S.; et al. Milk Fermented by Lactobacillus paracasei NCC 2461 (ST11) Modulates the Immune Response and Microbiota to Exert Its Protective Effects against Salmonella typhimurium Infection in Mice. Probiotics Antimicrob. Proteins 2020, 12, 1398–1408. [Google Scholar] [CrossRef]
- Kemgang, T.S.; Kapila, S.; Shanmugam, V.P.; Reddi, S.; Kapila, R. Fermented Milk with Probiotic Lactobacillus rhamnosus S1K3 (MTCC5957) Protects Mice from Salmonella by Enhancing Immune and Nonimmune Protection Mechanisms at Intestinal Mucosal Level. J. Nutr. Biochem. 2016, 30, 62–73. [Google Scholar] [CrossRef]
- Guerin-Danan, C.; Chabanet, C.; Pedone, C.; Popot, F.; Vaissade, P.; Bouley, C.; Szylit, O.; Andrieux, C. Milk Fermented with Yogurt Cultures and Lactobacillus casei Compared with Yogurt and Gelled Milk: Influence on Intestinal Microflora in Healthy Infants. Am. J. Clin. Nutr. 1998, 67, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pashapour, N.; Iou, S.G. Evaluation of Yogurt Effect on Acute Diarrhea in 6-24-Month-Old Hospitalized Infants. Turk. J. Pediatr. 2006, 4, 115–118. [Google Scholar]
- Boudraa, G.; Touhami, M.; Pochart, P.; Soltana, R.; Mary, J.-Y.; Desjeux, J.-F. Effect of Feeding Yogurt Versus Milk in Children with Persistent Diarrhea. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 509–512. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Ahn, S.-I.; Jhoo, J.-W.; Kim, G.-Y. Antioxidant Activity of Yogurt Fermented at Low Temperature and Its Anti-Inflammatory Effect on DSS-Induced Colitis in Mice. Food Sci. Anim. Resour. 2019, 39, 162–176. [Google Scholar] [CrossRef]
- Sawada, D.; Sugawara, T.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of Continuous Ingestion of a Beverage Prepared with Lactobacillus gasseri CP2305 Inactivated by Heat Treatment on the Regulation of Intestinal Function. Food Res. Int. 2016, 79, 33–39. [Google Scholar] [CrossRef]
- Sireswar, S.; Dey, G.; Sreesoundarya, T.K.; Sarkar, D. Design of Probiotic-Fortified Food Matrices Influence Their Antipathogenic Potential. Food Biosci. 2017, 20, 28–35. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of Probiotics and Antioxidant Activity of Cashew Milk-Based Yogurt Fermented with Selected Strains of Probiotic Lactobacillus spp. LWT 2022, 153, 112482. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; Belousova, A.V. Production and Assessment of Novel Probiotic Fermented Oat Flour Enriched with Isoflavones. LWT 2019, 111, 9–15. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Arnold, M.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Schmidt, M.; Gramza-Michałowska, A. Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus plantarum. Foods 2022, 11, 309. [Google Scholar] [CrossRef]
- Gholamhosseinpour, A.; Hashemi, S.M.B. Ultrasound Pretreatment of Fermented Milk Containing Probiotic Lactobacillus plantarum AF1: Carbohydrate Metabolism and Antioxidant Activity. J. Food Process Eng. 2019, 42, e12930. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.-B.; Park, J.H.; Kim, K.H. Metabolite Profile Changes and Increased Antioxidative and Antiinflammatory Activities of Mixed Vegetables after Fermentation by Lactobacillus plantarum. PLoS ONE 2019, 14, e0217180. [Google Scholar] [CrossRef] [PubMed]
- Miraghajani, M.; Zaghian, N.; Mirlohi, M.; Feizi, A.; Ghiasvand, R. The Impact of Probiotic Soy Milk Consumption on Oxidative Stress Among Type 2 Diabetic Kidney Disease Patients: A Randomized Controlled Clinical Trial. J. Ren. Nutr. 2017, 27, 317–324. [Google Scholar] [CrossRef]
- Bahmani, F.; Tajadadi-Ebrahimi, M.; Kolahdooz, F.; Mazouchi, M.; Hadaegh, H.; Jamal, A.-S.; Mazroii, N.; Asemi, S.; Asemi, Z. The Consumption of Synbiotic Bread Containing Lactobacillus Sporogenes and Inulin Affects Nitric Oxide and Malondialdehyde in Patients with Type 2 Diabetes Mellitus: Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2016, 35, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Jazayeri, S.; Najafi, M.; Samimi, M.; Mofid, V.; Shidfar, F.; Shakeri, H.; Esmaillzadeh, A. Effect of Daily Consumption of Probiotic Yogurt on Oxidative Stress in Pregnant Women: A Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2012, 60, 62–68. [Google Scholar] [CrossRef]
- Mazani, M.; Nemati, A.; Baghi, A.; Amani, M.; Haedari, K.; Alipanah-Mogadam, R. The Effect of Probiotic Yoghurt Consumption on Oxidative Stress and Inflammatory Factors in Young Females after Exhaustive Exercise. J. Pak. Med. Assoc. 2018, 68, 1748–1754. [Google Scholar]
- Redondo, N.; García-González, N.; Diaz-Prieto, L.E.; Olmedilla-Alonso, B.; Martín-Diana, A.B.; Asensio-Vegas, C.; Nova, E. Effects of Ewe’s Milk Yogurt (Whole and Semi-Skimmed) and Cow’s Milk Yogurt on Inflammation Markers and Gut Microbiota of Subjects with Borderline-High Plasma Cholesterol Levels: A Crossover Study. Eur. J. Nutr. 2019, 58, 1113–1124. [Google Scholar] [CrossRef]
- Lee, A.; Lee, Y.J.; Yoo, H.J.; Kim, M.; Chang, Y.; Lee, D.S.; Lee, J.H. Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity. Nutrients 2017, 9, 558. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Bae, W.-Y.; Yu, H.-S.; Chang, K.-H.; Hong, Y.-H.; Lee, N.-K.; Paik, H.-D. Inula Britannica Fermented with Probiotic Weissella cibaria D30 Exhibited Anti-Inflammatory Effect and Increased Viability in RAW 264.7 Cells. Food Sci. Biotechnol. 2020, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, A.; Soleimani, N.; Afrough, P. Anticancer Activity of Kefir on Glioblastoma Cancer Cell as a New Treatment. Int. J. Food Sci. 2021, 2021, 8180742. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Romeih, E.; Gamba, R.R.; Nagai, E.; Suzuki, T.; Koyanagi, T.; Enomoto, T. The Biological Activity of Fermented Milk Produced by Lactobacillus casei ATCC 393 during Cold Storage. Int. Dairy J. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In Vitro Investigation of Anticancer, Antihypertensive, Antidiabetic, and Antioxidant Activities of Camel Milk Fermented with Camel Milk Probiotic: A Comparative Study with Fermented Bovine Milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Gholamhosseinpour, A. Effect of Ultrasonication Treatment and Fermentation by Probiotic Lactobacillus plantarum Strains on Goat Milk Bioactivities. Int. J. Food Sci. Technol. 2020, 55, 2642–2649. [Google Scholar] [CrossRef]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-Control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt Consumption and Risk of Colorectal Cancer in the Italian European Prospective Investigation into Cancer and Nutrition Cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Kim, D.S.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W. Fermented Milk of Lactobacillus Helveticus IDCC3801 Improves Cognitive Functioning during Cognitive Fatigue Tests in Healthy Older Adults. J. Funct. Foods 2014, 10, 465–474. [Google Scholar] [CrossRef]
- Ohsawa, K.; Nakamura, F.; Uchida, N.; Mizuno, S.; Yokogoshi, H. Lactobacillus Helveticus-Fermented Milk Containing Lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) Improves Cognitive Function in Healthy Middle-Aged Adults: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. J. Food Sci. Nutr. 2018, 69, 369–376. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented Milk Containing Lactobacillus Casei Strain Shirota Prevents the Onset of Physical Symptoms in Medical Students under Academic Examination Stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.N.S.; Ryu, J.; Kim, Y.; Jeon, B.H. The Effects of Fermented Laminaria japonica on Short-Term Working Memory and Physical Fitness in the Elderly. Evid. Based Complement. Alternat. Med. 2018, 2018, e8109621. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The Effects of Probiotics on Mental Health and Hypothalamic-Pituitary-Adrenal Axis: A Randomized, Double-Blind, Placebo-Controlled Trial in Petrochemical Workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef]
- Nishihira, J.; Kagami-Katsuyama, H.; Tanaka, A.; Nishimura, M.; Kobayashi, T.; Kawasaki, Y. Elevation of Natural Killer Cell Activity and Alleviation of Mental Stress by the Consumption of Yogurt Containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a Double-Blind, Placebo-Controlled Clinical Trial. J. Funct. Foods 2014, 11, 261–268. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kawai, T.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Para-psychobiotic Lactobacillus gasseri CP 2305 Ameliorates Stress-related Symptoms and Sleep Quality. J. Appl. Microbiol. 2017, 123, 1561–1570. [Google Scholar] [CrossRef]
- Yadav, R.; Khan, S.H.; Mada, S.B.; Meena, S.; Kapila, R.; Kapila, S. Consumption of Probiotic Lactobacillus fermentum MTCC: 5898-Fermented Milk Attenuates Dyslipidemia, Oxidative Stress, and Inflammation in Male Rats Fed on Cholesterol-Enriched Diet. Probiotics Antimicrob. Proteins 2019, 11, 509–518. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Torres-Inguanzo, E.H.; Astiazarán-García, H.; Esparza-Romero, J.; Vallejo-Cordoba, B. Randomized Double-Blind Controlled Clinical Trial of the Blood Pressure–Lowering Effect of Fermented Milk with Lactococcus Lactis: A Pilot Study12. J. Dairy Sci. 2018, 101, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, B.; Naderi, N.; Janani, L.; Mofid, V.; Hajahmadi, M.; Dehnad, A.; Shidfar, F. Comparison of Probiotic Yogurt and Ordinary Yogurt Consumption on Serum Pentraxin3, NT-ProBNP, OxLDL, and ApoB100 in Patients with Chronic Heart Failure: A Randomized, Triple-Blind, Controlled Trial. Food Funct. 2020, 11, 10000–10010. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Zare, S.; Fallahzadeh, H.; Safi, S.; Nadjarzadeh, A. Effect of a Low Energy Diet, Containing a High Protein, Probiotic Condensed Yogurt, on Biochemical and Anthropometric Measurements among Women with Overweight/Obesity: A Randomised Controlled Trial. Clin. Nutr. ESPEN 2020, 35, 194–200. [Google Scholar] [CrossRef]
- Naito, E.; Yoshida, Y.; Kunihiro, S.; Makino, K.; Kasahara, K.; Kounoshi, Y.; Aida, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Effect of Lactobacillus casei Strain Shirota-Fermented Milk on Metabolic Abnormalities in Obese Prediabetic Japanese Men: A Randomised, Double-Blind, Placebo-Controlled Trial. Biosci. Microbiota Food Health 2018, 37, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chung, W.-C.; Chun, S.; Han, J.H.; Song, M.J.; Lee, K.-W. Enhancing the Natural Killer Cell Activity and Anti-Influenza Effect of Heat-Treated Lactobacillus plantarum NF1-Fortified Yogurt in Mice. J. Dairy Sci. 2018, 101, 10675–10684. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Sawabuchi, T.; Kimoto, T.; Sakai, S.; Kido, H. Lactobacillus delbrueckii ssp. Bulgaricus OLL1073R-1 Feeding Enhances Humoral Immune Responses, Which Are Suppressed by the Antiviral Neuraminidase Inhibitor Oseltamivir in Influenza A Virus–Infected Mice. J. Dairy Sci. 2019, 102, 9559–9569. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Modulation of Gut Health Using Probiotics: The Role of Probiotic Effector Molecules. J. Future Foods 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, Gut Microbiota, and Their Influence on Host Health and Disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Ofosu, F.K.; Xiuqin, C.; Chelliah, R.; Oh, D.-H. Probiotic Effector Compounds: Current Knowledge and Future Perspectives. Front. Microbiol. 2021, 12, 655705. [Google Scholar] [CrossRef]
- Donovan, S.M.; Rao, G. Health Benefits of Yogurt among Infants and Toddlers Aged 4 to 24 Months: A Systematic Review. Nutr. Rev. 2019, 77, 478–486. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Cha, J.M.; Hong, S.S.; Kim, H.K.; Kwak, M.S.; Jeon, J.W.; Shin, H.P. Fermented Milk Containing Lactobacillus paracasei and Glycyrrhiza Glabra Has a Beneficial Effect in Patients with Helicobacter pylori Infection: A Randomized, Double-Blind, Placebo-Controlled Study. Medicine 2019, 98, e16601. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.M.; Kurrey, N.K.; Halami, P.M. In Vitro Anti-Inflammatory Activity among Probiotic Lactobacillus Species Isolated from Fermented Foods. J. Funct. Foods 2018, 47, 19–27. [Google Scholar] [CrossRef]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for Cancer Alternative Prevention and Treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012: Globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer Is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Shaheen, M.; Allen, C.; Nickoloff, J.A.; Hromas, R. Synthetic Lethality: Exploiting the Addiction of Cancer to DNA Repair. Blood 2011, 117, 6074–6082. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Raguz, S.; Yagüe, E. Resistance to Chemotherapy: New Treatments and Novel Insights into an Old Problem. Br. J. Cancer 2008, 99, 387–391. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Identification of Anticancer Peptides from Bovine Milk Proteins and Their Potential Roles in Management of Cancer: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 123–138. [Google Scholar] [CrossRef]
- World Health Organization. Mental Health and COVID-19. Available online: https://www.who.int/europe/emergencies/situations/covid-19/mental-health-and-covid-19 (accessed on 12 November 2022).
- Dey, G.; Mookherjee, S. Probiotics-Targeting New Milestones from Gut Health to Mental Health. FEMS Microbiol. Lett. 2021, 368, fnab096. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, G. Gut–Brain Axis and Mood Disorder. Front. Psychiatry 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Zielińska, D.; Karbowiak, M.; Brzezicka, A. The Role of Psychobiotics to Ensure Mental Health during the COVID-19 Pandemic—A Current State of Knowledge. Int. J. Environ. Res. Public. Health 2022, 19, 11022. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Weiner, H.L. Microbiota Signaling Pathways That Influence Neurologic Disease. Neurotherapeutics 2018, 15, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Kali, A. Psychobiotics: An Emerging Probiotic in Psychiatric Practice. Biomed. J. 2016, 39, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, X.-R.; Peng, L.; Ge, J.-F. Crosstalk between the Microbiota-Gut-Brain Axis and Depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the Role of 5-Hydroxytryptophan Synthesis Regulation Alleviates the Symptom of Depression and Related Microbiota Dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Hu, X.; Yang, C.; Xu, H.; Yao, Y.; Liu, J. Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior in Mice via the Gut-Brain Axis. J. Agric. Food Chem. 2018, 66, 8415–8421. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra e Oliveira, T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell Longev. 2020, 2020, e2638703. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Zhang, H. Trends in Probiotic(s)-Fermented Milks and Their in Vivo Functionality: A Review. Trends Food Sci. Technol. 2021, 110, 55–65. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Kim, J.I.; Park, S.; Yoo, K.; Kim, I.-H.; Joo, W.; Ryu, B.H.; Park, M.S.; Lee, I.; Park, M.-S. Effects of Lactobacillus plantarum and Leuconostoc mesenteroides Probiotics on Human Seasonal and Avian Influenza Viruses. J. Microbiol. Biotechnol. 2018, 28, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Muhialdin, B.J.; Zawawi, N.; Abdull Razis, A.F.; Bakar, J.; Zarei, M. Antiviral Activity of Fermented Foods and Their Probiotics Bacteria towards Respiratory and Alimentary Tracts Viruses. Food Control 2021, 127, 108140. [Google Scholar] [CrossRef]
- Todorov, S.D.; Tagg, J.R.; Ivanova, I.V. Could Probiotics and Postbiotics Function as “Silver Bullet” in the Post-COVID-19 Era? Probiotics Antimicrob. Proteins 2021, 13, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Reque, P.M.; Brandelli, A. Encapsulation of Probiotics and Nutraceuticals: Applications in Functional Food Industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Kwak, H.-S. (Ed.) Nano- and Microencapsulation for Foods; Wiley Blackwell: Chichester, UK, 2014. [Google Scholar]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and Postbiotics: Concepts and Potential Applications in Dairy Products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Zeng, J.; Jiang, J.; Zhu, W.; Chu, Y. Heat-Killed Yogurt-Containing Lactic Acid Bacteria Prevent Cytokine-Induced Barrier Disruption in Human Intestinal Caco-2 Cells. Ann. Microbiol. 2016, 66, 171–178. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics Produced by Lactic Acid Bacteria: The next Frontier in Food Safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).