Abstract
An asymmetric aza-BODIPY analogue bearing quinoxaline moiety was synthesized via a titanium tetrachloride-mediated Schiff-base-forming reaction of 6,7-dimethyl-1,4-dihydroquinoxaline-2,3-dione and benzo[d]thiazol-2-amine. This novel aza-BODIPY analogue forms a complementary hydrogen-bonded dimer due to the quinoxaline moiety in the crystal structure. It also shows intense absorption and fluorescence, with fluorescence quantum yields close to unity. The electrochemical measurements and the DFT calculations revealed the presence of the low-lying HOMO, which benefits their potential applications as an electron-transporting material.
1. Introduction
Boron dipyrromethenes (BODIPYs) and their meso-nitrogen-substituted analogues called aza-BODIPYs have received considerable research attention owing to their fascinating optical properties and potential applications, such as bioimaging [1,2,3,4,5,6,7], chemosensors [8,9,10,11], laser dyes [12], sensitizers for photodynamic [13,14,15] and photothermal therapies [16,17], and optoelectronic materials for light-emitting diodes [18,19,20] and organic photovoltaics [10,21]. Despite their excellent chemical and photophysical properties, the fluorescent emission of most BODIPY derivatives is virtually quenched in the solid state due to the small Stokes shifts and strong intermolecular π-π stacking interactions enhanced by the symmetric, planar structure [22,23,24,25,26]. To address these drawbacks, considerable research efforts have been devoted to developing BODIPY analogues with asymmetric structures, for which large Stokes shifts can be expected, owing to the structural changes between the ground and excited states. In addition, asymmetric BODIPY analogues tend to take head-to-tail stacking patterns in the solid state to minimize dipolar interactions [5,27,28,29,30,31,32,33,34,35,36,37,38,39]. However, compared with the conventional symmetric BODIPYs, there is still a great challenge for the synthesis of asymmetric BODIPYs.
Recently, we have reported a Schiff-base-forming reaction of azaarylamines and diketopyrrolopyrrole, in which the lactam moieties of diketopyrrolopyrrole were successfully converted to aza-BODIPY structures in the presence of titanium tetrachloride (TiCl4) and triethylamine (NEt3) [40,41]. A series of asymmetric aza-BODIPYs were also synthesized from monolactams based on the TiCl4-based method. The asymmetric compounds exhibited moderately high fluorescence quantum yields of 0.13–0.27 (Chart 1, 1a,b) [39]. Lu et al. revealed that 1,8-naphthalenimide, isoindole-1,3-dione, and their derivatives can also be converted to asymmetric aza-BODIPYs in simple ethanol reflux with azaarylamines, followed by boron complexation (Chart 1, 3a,b) [29]. The aza-BODIPYs thus synthesized, which have a ketone moiety, showed intense fluorescence in solution and moderate solid-state emission (ΦF = 0.005–0.22) due to the asymmetric structures. Jiao et al. reported the synthesis of similar asymmetric aza-BODIPYs from isoindole-1,3-dione based on the TiCl4-mediated method (Chart 1, 2a–c) [28]. In contrast to conventional symmetric BODIPYs, these asymmetric aza-BODIPYs exhibited large Stoke shifts and high photostability. The above-mentioned studies indicate that the Schiff-base-forming reaction is a versatile method of synthesizing asymmetric aza-BODIPYs with unique optical and electronic properties.
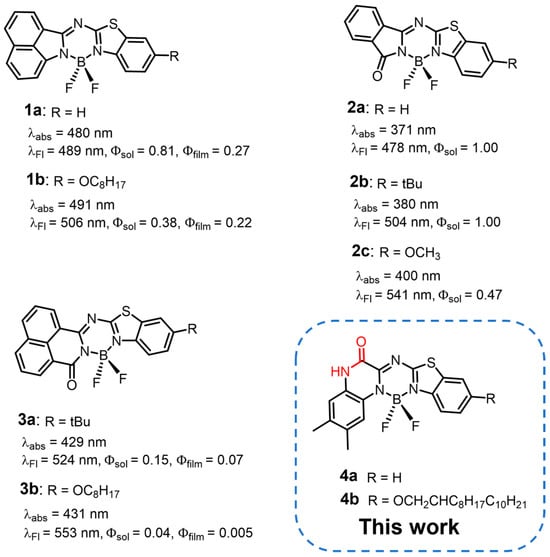
Chart 1.
Structures of asymmetric aza-BODIPYs synthesized via a Schiff-base-forming reaction.
To broaden the scope of the TiCl4-mediated Schiff-base-forming reaction for the synthesis of asymmetric aza-BODIPYs, herein, we investigated the synthesis of novel asymmetric aza-BODIPYs (4a and 4b) bearing a quinoxaline moiety. The target compounds were synthesized from 6,7-dimethyl-1,4-dihydroquinoxaline-2,3-dione and 2-aminobenzothiazole. These asymmetric aza-BODIPYs showed intense absorption and fluorescence in solution. In the crystal structure of 4a, a complementary hydrogen-bonded dimer and the J-type arrangement of the dimer were observed in the packing diagram.
2. Results and Discussion
2.1. Synthesis and Characterization
The Schiff-base-forming reaction of 6,7-dimethyl-1,4-dihydroquinoxaline-2,3-dione and benzo[d]thiazol-2-amine in the presence of TiCl4 and NEt3 and subsequent boron complexation provided the target asymmetric aza-BODIPYs, 4a and 4b (Scheme 1). The aza-BODIPY 4a was obtained in a higher yield of 42% than 4b (27%) despite its lower solubility than 4b. We characterized 4a and 4b by NMR spectroscopy (Figures S1–S4).
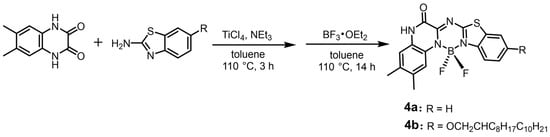
Scheme 1.
Synthesis of 4a and 4b.
The structure of 4a was unambiguously elucidated by X-ray crystallography. In a unit cell, an acetonitrile molecule was also found as a solvent molecule. The single crystal of 4a was obtained via the slow diffusion of acetonitrile into a chloroform solution of 4a (Figure 1). The aza-BODIPY 4a features a highly coplanar structure in the crystal structure. The C9–O1 bond length of 4a (1.228(3) Å) is a typical C=O bond length (ca. 1.22 Å). The longer bond distance of B1–N3 (1.577(4) Å) than that of B1–N1 (1.543(4) Å) implies the asymmetric structure of 4a. Similar trends have been reported for the B-N bonds in other asymmetric amido/imino BF2 complexes [28,29,31,42,43]. In the unit cell, two molecules were stacked with each other to form π-π stackings (Figure S5). The amide unit of the quinoxaline moiety formed a hydrogen bond with the neighboring molecule with N–H⋯O distance of 2.85 Å (Figure 2). The hydrogen bonds mean that the molecules are nearly parallel to each other. In the molecular packing diagram, hydrogen-bonded dimers are slip-stacked in a J-type arrangement. The interplanar distances between the mean planes of the next neighbors are 3.16 and 3.38 Å (Figure 2). The above results indicate that hydrogen bonding is critical for influencing molecular packing and determining the crystal structure.
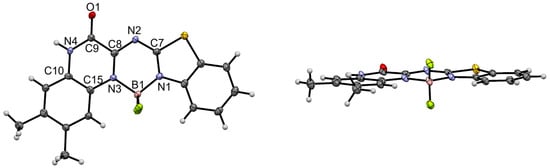
Figure 1.
X-ray crystal structure of 4a (top view: left; side view: right). The thermal ellipsoids were scaled at 50% probability.
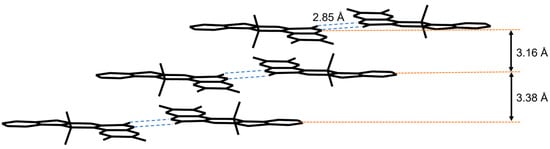
Figure 2.
Molecular packing diagram of 4a. Hydrogen bonds are shown as blue dashed lines. The interplanar distances between the mean planes of molecules excluding the two fluorine atoms are shown.
To give a deep insight into the hydrogen-bonding interactions in solution, temperature-dependent chemical shifts of the 1H NMR spectra of 4a were investigated in 1,1,2,2-tetrachloroethane-d2. As shown in Figure 3, the downfield shift of the NH proton signal from 9.39 to 11.23 ppm, together with significant broadening, was observed when the temperature decreased from 40 °C to –40 °C due to the formation of intermolecular hydrogen bonds at low temperatures.
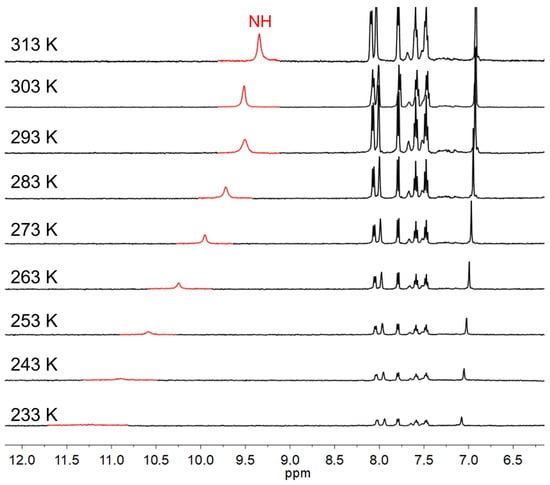
Figure 3.
Variable-temperature 1H NMR spectra of 4a in 1,1,2,2-tetrachloroethane-d2. The NH proton signals are highlighted in red.
2.2. Photophysical Properties
The photophysical properties of 4a and 4b were measured in chloroform solution and film (Figure 4). The details are summarized in Table 1. The aza-BODIPY 4a exhibits absorption with distinctive vibronic structures comprising the less intense 0-0 band at 449 nm and the 0-1 vibronic band at 423 nm as a main band in the visible region. A similar absorption spectral profile with the intense 0-1 vibronic band at 460 nm is also observed for 4b. The molar absorption coefficients are 1.24 × 104 M−1cm−1 and 1.97 × 104 M−1cm−1 for 4a and 4b in tetrahydrofuran solution, respectively (Figure S8). Despite the aza-BODIPY-like structure, the absorption spectra of 4a and 4b exhibit blueshifts from those of the conventional BODIPY 5 with maximum wavelength (λmax) at 500 nm and aza-BODIPY 6 with λmax at 713 nm [44,45] (Chart 2). As detailed in the following section, the DFT calculations revealed that the blueshifts can be ascribed to the different frontier molecular orbital (MO) distribution patterns from those of regular BODIPYs.
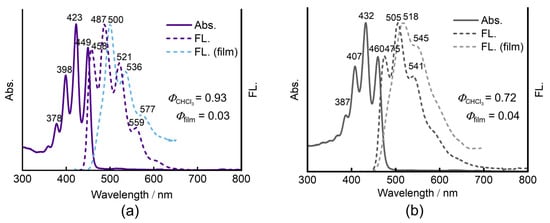
Figure 4.
UV/vis absorption (solid line) and fluorescence (dashed line) spectra of (a) 4a and (b) 4b in CHCl3.

Table 1.
Summary of optical and electrochemical properties of 4a and 4b.
Chart 2.
Structures of BODIPY 5 and aza-BODIPY 6.
The aza-BODIPYs 4a and 4b display intense fluorescence at 458 and 475 nm with small Stokes shifts of 438 and 687 cm−1, respectively, as a mirror image to the absorption spectra. The ΦF values are almost unity for 4a (0.93) and 0.72 for 4b. The slightly smaller ΦF value of 4b is mainly due to the structural flexibility arising from the long alkoxy chain, which enhances the nonradiative decay processes.
In contrast to the reported asymmetric aza-BODIPYs such as 1a and 1b, which exhibit moderate quantum yields in the film state (0.27 for 1a and 0.22 for 1b), the fluorescence of 4a and 4b is virtually quenched in the film state, exhibiting ΦF values as law as ca. 0.03–0.04. In the film state, the 0-0 emission band disappeared due to self-absorption. The significant overlap of the absorption and emission due to the small Stokes shifts causes the self-absorption quenching in the solid state.
The impact of pH values on the stability of 4a was also examined (Figure S9). The absorption and fluorescence spectral profiles of 4a in acetonitrile do not change in the range from pH = 2 to pH = 11. At a higher pH than pH = 12, both absorption and fluorescence spectra showed redshifts, and the fluorescence spectra became broad and structureless. These spectral changes are probably due to the deprotonation of the amide unit of the quinoxaline moiety. The observed high stability of 4a over the wide range of pH benefits its use under physiological conditions.
2.3. Electrochemical Properties
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed in dichloromethane containing 0.1 M tetrabutylammonium perchlorate (TBAP) as a supporting electrolyte (Figure 5). Table 1 summarizes the redox potentials and experimental LUMO and HOMO energy levels estimated from the onset of the first reduction waves and the optical band gaps and LUMO values, respectively. The estimated LUMO (−3.66 eV for 4a and −3.63 eV for 4b) and HOMO (−6.29 eV for 4a and −6.16 eV for 4b) values are significantly lower than those of the widely used electron-transporting material, Alq3 (aluminum tris(8-hydroxyquinolinnate)) (−3.0 eV for LUMO and −5.7 eV for HOMO), implying their potential applications for such a purpose in organic light-emitting diodes.
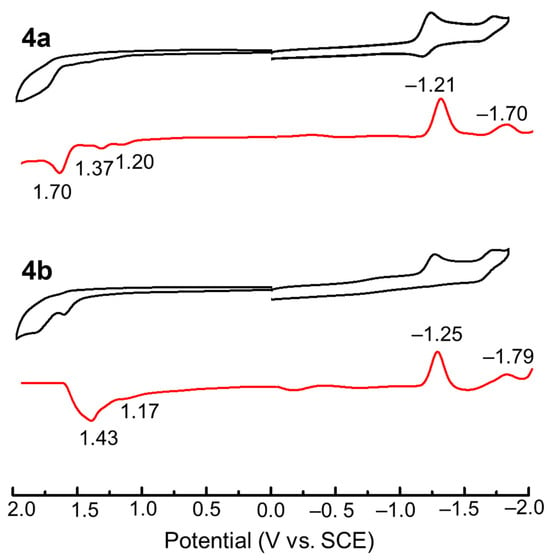
Figure 5.
CV (black) and DPV (red) of 4a (top) and 4b (bottom) (0.5 mM) in CH2Cl2 containing 0.1 M TBAP as a supporting electrolyte (scan rate: 100 mV−1 for CV; pulse amplitude: 0.05 V, pulse width: 0.2 s for DPV).
2.4. Theoretical Calculations
Density functional theory (DFT) and time-dependent (TD)DFT calculations on a model structure of 4 at the B3LYP/6-31G(d) level were conducted to give a detailed insight into the electronic structures (Figure 6 and Figure 7). The TDDFT calculations assign the main absorption band as a HOMO–LUMO transition (Table 2). The TDDFT calculations revealed the wavelength of the S1 excited state at 400 nm with the oscillator strength of 0.46. The S1 state mainly comprises the HOMO-to-LUMO transition. Although the transition energy is slightly overestimated, the TDDFT calculation reproduced the observed absorption spectrum. Because of the absence of an s-indacene-like conjugated system in the structure of 4, the HOMO and LUMO distribution patterns of 4 are different from those of regular aza-BODIPYs, exhibiting larger MO coefficients of the HOMO on the meso-nitrogen atom compared with the LUMO. Therefore, the HOMO is more stabilized than the LUMO by the electron-deficient nitrogen atom at the meso-position. The observed blueshifts of the absorption spectrum of 4 can be explained by the resultant wide HOMO–LUMO gap.
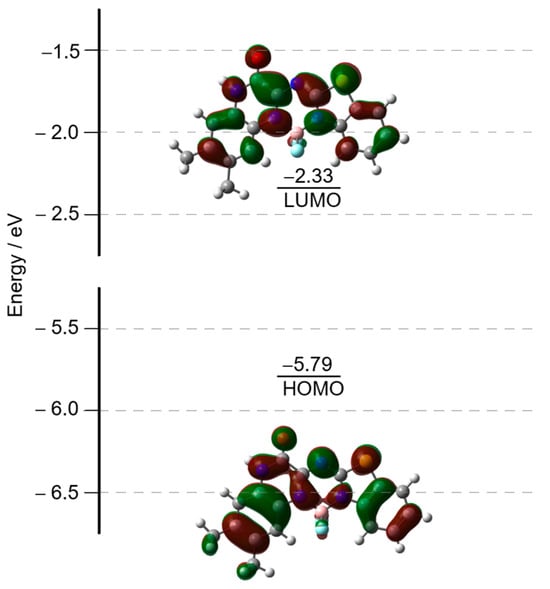
Figure 6.
Frontier molecular orbitals of 4 (B3LYP/6-31G(d)).
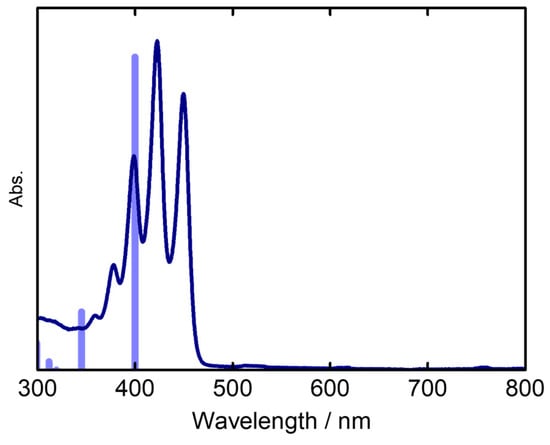
Figure 7.
Theoretical absorption bands (blue bars) at the B3LYP/6-31G(d) level and the observed absorption spectrum of 4a in CHCl3 (blue line).

Table 2.
Summary of the TDDFT calculation of 4a at the B3LYP/6-31G(d) level.
3. Materials and Methods
3.1. Instrumentation and Measurements
Electronic absorption spectra were recorded on a JASCO V-770 spectrophotometer. Fluorescence spectra were recorded on an SPEX Fluorolog-3-NIR spectrometer (HORIBA) with an NIR-PMT R5509 photomultiplier tube (Hamamatsu). Absolute fluorescence quantum yields were measured using a Hamamatsu Photonics C9920-03G calibrated integrating sphere system with self-absorption correction. The thin films for solid fluorescent measurement were fabricated via spin-coating a solution of 4a and 4b in CHCl3, respectively. 1H NMR spectra were recorded on a JEOL JNM-ECX500 spectrometer (operating at 495 MHz for 1H) using a residual solvent as an internal reference for 1H (δ = 7.26 ppm for CDCl3). CV and DPV measurements were conducted in a CH2Cl2 solution containing 0.1 M TBAP and 0.1 M samples with a scan rate of 0.1 V s−1 under a nitrogen atmosphere. A glassy carbon electrode and a platinum wire were used as the working and counter electrodes, respectively. A saturated calomel electrode (SCE) was used as a reference electrode. The voltammogram display follows the IUPAC convention. Preparative separations were performed using silica gel column chromatography (KANTO Silica Gel 60 N, spherical, neutral, 40–50 mm). All reagents and solvents used for reactions were of commercial reagent grade and were used without further purification unless noted otherwise. All solvents used in optical measurements were of commercial spectroscopic grade.
3.2. Crystallographic Data Collection and Structure Refinement
Suitable crystals of 4a for X-ray analysis were obtained from the vapor diffusion of acetonitrile into a chloroform solution of 4a. Data collection was carried out at −173 °C on a Rigaku Saturn724 diffractometer with MoKα radiation. The structure was solved via a direct method (SHELXT) and refined using a full-matrix least-squares technique (SHELXL). CCDC 2299504 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 1 November 2023), or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-1223-336033.
3.3. Computational Methods
The Gaussian 16, Revision A.03 [46] software package was used to carry out DFT and TDDFT calculations at the B3LYP/6-31G(d) level of theory. Structural optimizations were performed on model compounds.
3.4. Synthesis
4a: 6,7-Dimethyl-1,4-dihydroquinoxaline-2,3-dione (48 mg, 0.25 mmol) and benzo[d]thiazole-2-amine (169 mg, 1.13 mmol) were added to dry toluene (6 mL), and the mixture was refluxed at 110 °C. Then, titanium tetrachloride (TiCl4) (0.15 mL, 1.38 mmol) and triethylamine (NEt3) (0.5 mL, 3.63 mmol) were added to the reaction mixture. After the imine formation was confirmed by TLC analysis in ca. 3 h, borontrifluoride etherate (BF3·OEt2) (0.48 mL, 3.98 mmol) was added. After refluxing for 14 h, the reaction mixture was added to water and extracted with CHCl3. The combined organic extracts were dried over sodium sulfate and concentrated in vacuo to provide yellow solids. The crude compound was chromatographed on a silica gel column using ethyl acetate/hexane = 1:3 to 1:1 (v/v), then ethyl acetate) to afford 4a as yellow solids (48 mg, 54%).
1H NMR (495 MHz, CDCl3, 298 K): δ [ppm] = 10.12 (br, 1H), 8.13 (d, J = 9.0 Hz, 1H), 8.07 (s, 1H), 7.78 (d, J = 7.5 Hz, 1H), 7.60 (dt, J1 = 9.0 Hz, J2 = 1.0 Hz, 1H), 7.47 (dt, J1 = 9.0 Hz, J2 = 1.0 Hz, 1H), 7.02 (s, 1H), 2.40 (s, 3H), 2.35 (s, 3H).
4b: 4b was synthesized and purified in a similar manner to 4a, using 6,7-dimethyl-1,4-dihydroquinoxaline-2,3-dione (48 mg, 0.25 mmol), 6-((2-octyldodecyl)oxy)benzo[d]thiazol-2-amine (503 mg, 1.13 mmol), TiCl4 (0.15 mL, 1.38 mmol), NEt3 (0.5 mL, 3.63 mmol), and BF3·OEt2 (0.48 mL, 3.98 mmol). The crude mixture was chromatographed on silica gel column (ethyl acetate/hexane = 1:5 (v/v)) to afford 4b as yellow solids (27 mg, 16%).
1H NMR (495 MHz, CDCl3, 298 K): δ [ppm] = 11.33 (br, 1H), 8.05(s, 1H), 8.00 (d, J = 9.5 Hz, 1H), 7.22 (d, J = 2.0 Hz, 1H), 7.17–7.15 (dd, J1 = 9.5 Hz, J2 = 2.0 Hz, 2H), 7.14 (s, 1H), 3.90 (d, J = 5.5 Hz, 1H), 2.39 (s, 3H), 2.35 (s, 3H), 1.82 (m, 1H), 1.48–1.28 (m, 32H), 0.89–0.86 (m, 6H).
4. Conclusions
In summary, we successfully converted 6,7-dimethyl-1,4-dihydroquinoxaline-2,3-dione as a bislactam precursor to the asymmetric aza-BODIPYs by the Schiff-base-forming reaction. The novel aza-BODIPYs exhibit intense fluorescence in solution, whereas the fluorescence emission is nearly quenched in the film state due to the self-absorption. The crystal structure of 4a and its molecular packing diagram reveal that 4a forms a complementary hydrogen-bonded dimer with the next neighbor, and the dimer is further assembled into J-aggregates. The low-lying HOMO based on the electrochemical measurements indicates the potential applications of 4 as an electron-transporting material. An investigation along these lines is being intensively carried out in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28247940/s1, Figures S1–S4. 1H NMR and 1H-1H COSY spectra of 4a and 4b in CDCl3. Figure S5: (a) Unit cell of the X-ray crystal structure and (b) hydrogen bonding dimer of 4a in the crystal structure. Hydrogen bonds are shown as dashed lines. Table S1: Crystallographic data of 4a. Figure S6: X-ray crystal structure of 4a with labels. Table S2: Bond length for 4a. Table S3: Bond angels for 4a. Figure S7: UV/vis absorption spectra of 4a in various solvents. Figure S8: UV/vis absorption spectra of 4a (a) and 4b (b) in THF at different concentrations. Figure S9: UV/vis absorption and fluorescence spectra of 4a at various pH values in CH3CN solution.
Author Contributions
Investigation, Visualization, Data curation, Formal analysis, Writing—original draft, R.F.; Methodology, Z.C. Supervision, Methodology, Y.W.; Resources, Supervision, Writing—review and editing, J.P.; Funding acquisition, Project administration, Resources, Supervision, Writing—review and editing, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Grants-in-Aids from JSPS (No. JP22H02064), Naohiko Fukuoka Memorial Foundation, China Postdoctoral Science Foundation (No. 2023M731405), and the Project Startup Foundation for Distinguished Scholars of Jiangsu University (No. 5501310024 (R.F.)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
Author Ru Feng was employed by the company Jiangsu Chunlan Clean Energy Academy Co., Ltd.; Author Yue Wang was employed by the company Jiangsu Agrochem Laboratory Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.E.; Thompson, A. Advances in the Chemistry of Dipyrrins and Their Complexes. Chem. Rev. 2007, 107, 1831–1861. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural Modification Strategies for the Rational Design of Red/NIR Region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, J. Far-Red and near Infrared BODIPY Dyes: Synthesis and Applications for Fluorescent PH Probes and Bio-Imaging. Org. Biomol. Chem. 2014, 12, 3774. [Google Scholar] [CrossRef]
- Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent Materials: Locking π-Conjugated and Heterocyclic Ligands with Boron(III). Angew. Chem. Int. Ed. 2014, 53, 2290–2310. [Google Scholar] [CrossRef]
- Jean-Gérard, L.; Vasseur, W.; Scherninski, F.; Andrioletti, B. Recent Advances in the Synthesis of [a]-Benzo-Fused BODIPY Fluorophores. Chem. Commun. 2018, 54, 12914–12929. [Google Scholar] [CrossRef]
- Shukla, V.K.; Chakraborty, G.; Ray, A.K.; Nagaiyan, S. Red and NIR Emitting Ring-Fused BODIPY/Aza-BODIPY Dyes. Dye. Pigment. 2023, 215, 11124. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Bessette, A.; Hanan, G.S. Design, Synthesis and Photophysical Studies of Dipyrromethene-Based Materials: Insights into Their Applications in Organic Photovoltaic Devices. Chem. Soc. Rev. 2014, 43, 3342–3405. [Google Scholar] [CrossRef]
- Sonkaya, Ö.; Soylukan, C.; Pamuk Algi, M.; Algi, F. Aza-BODIPY-Based Fluorescent and Colorimetric Sensors and Probes. Curr. Org. Synth. 2023, 20, 20–60. [Google Scholar] [PubMed]
- Zhang, D.; Martín, V.; García-Moreno, I.; Costela, A.; Pérez-Ojeda, M.E.; Xiao, Y. Development of Excellent Long-Wavelength BODIPY Laser Dyes with a Strategy That Combines Extending π-Conjugation and Tuning ICT Effect. Phys. Chem. Chem. Phys. 2011, 13, 13026. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of Small Molecule Aza-BODIPY: From Rational Structural Design to in vivo Investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-BODIPY-Based Nanomedicines in Cancer Phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925. [Google Scholar] [CrossRef]
- Mao, Z.; Kim, J.H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J.S. Engineering of BODIPY-Based Theranostics for Cancer Therapy. Coord. Chem. Rev. 2023, 476, 214908. [Google Scholar] [CrossRef]
- Bonardi, L.; Kanaan, H.; Camerel, F.; Jolinat, P.; Retailleau, P.; Ziessel, R. Fine-Tuning of Yellow or Red Photo- and Electroluminescence of Functional Difluoro-Boradiazaindacene Films. Adv. Funct. Mater. 2008, 18, 401–413. [Google Scholar] [CrossRef]
- Squeo, B.M.; Pasini, M. BODIPY Platform: A Tunable Tool for Green to NIR OLEDs. Supramol. Chem. 2020, 32, 56–70. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent Advances of BODIPY Based Derivatives for Optoelectronic Applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-Based Molecules, a Platform for Photonic and Solar Cells. Molecules 2020, 26, 153. [Google Scholar] [CrossRef]
- Ozdemir, T.; Atilgan, S.; Kutuk, I.; Yildirim, L.T.; Tulek, A.; Bayindir, M.; Akkaya, E.U. Solid-State Emissive BODIPY Dyes with Bulky Substituents as Spacers. Org. Lett. 2009, 11, 2105–2107. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.; Gai, L.; Li, Z.; Deng, Y.; Xiao, X.; Lai, G.; Shen, Z. Tuning the Solid-State Luminescence of BODIPY Derivatives with Bulky Arylsilyl Groups: Synthesis and Spectroscopic Properties. Chem. Eur. J. 2012, 18, 7852–7861. [Google Scholar] [CrossRef]
- Bochkov, A.Y.; Akchurin, I.O.; Dyachenko, O.A.; Traven, V.F. NIR-Fluorescent Coumarin-Fused BODIPY Dyes with Large Stokes Shifts. Chem. Commun. 2013, 49, 11653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wen, Y.; Xiao, Y.; Yu, G.; Liu, Y.; Qian, X. Bulky 4-Tritylphenylethynyl Substituted Boradiazaindacene: Pure Red Emission, Relatively Large Stokes Shift and Inhibition of Self-Quenching. Chem. Commun. 2008, 4777–4779. [Google Scholar] [CrossRef] [PubMed]
- Hepp, A.; Ulrich, G.; Schmechel, R.; Von Seggern, H.; Ziessel, R. Highly Efficient Energy Transfer to a Novel Organic Dye in OLED Devices. Synth. Met. 2004, 146, 11–15. [Google Scholar] [CrossRef]
- Bukowska, P.; Piechowska, J.; Loska, R. Azine-Imidazole Aza-BODIPY Analogues with Large Stokes Shift. Dye. Pigment. 2017, 137, 312–321. [Google Scholar] [CrossRef]
- Gao, N.; Cheng, C.; Yu, C.; Hao, E.; Wang, S.; Wang, J.; Wei, Y.; Mu, X.; Jiao, L. Facile Synthesis of Highly Fluorescent BF2 Complexes Bearing Isoindolin-1-One Ligand. Dalton Trans. 2014, 43, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, H.; Zhou, Z.; Shimizu, S.; Li, Z.; Kobayashi, N.; Shen, Z. Asymmetric Core-Expanded Aza-BODIPY Analogues: Facile Synthesis and Optical Properties. Chem. Commun. 2015, 51, 1713–1716. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, H.; Wang, S.; Li, Z.; Shen, Z. Asymmetric Boron-Complexes Containing Keto-Isoindolinyl and Pyridyl Groups: Solvatochromic Fluorescence, Efficient Solid-State Emission and DFT Calculations. J. Mater. Chem. C 2015, 3, 12281–12289. [Google Scholar] [CrossRef]
- Liu, H.; Lu, H.; Xu, J.; Liu, Z.; Li, Z.; Mack, J.; Shen, Z. Boron-Pyridyl-Imino-Isoindoline Dyes: Facile Synthesis and Photophysical Properties. Chem. Commun. 2014, 50, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tong, S.; Wang, Q.Q.; Ao, Y.F.; Wang, D.X.; Zhu, J. Thiazole Boron Difluoride Dyes with Large Stokes Shift, Solid State Emission and Room-Temperature Phosphorescence. Chem. Eur. J. 2022, 28, e202202507. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, L.; Yang, M.; Zhang, H.; Lan, J.; Zhou, F.; Chen, X.; Wu, D.; You, J. Pd-Catalyzed Direct C-H Functionalization/Annulation of BODIPYs with Alkynes to Access Unsymmetrical Benzo[b]-Fused BODIPYs: Discovery of Lysosome-Targeted Turn-On Fluorescent Probes. J. Org. Chem. 2018, 83, 9538–9546. [Google Scholar] [CrossRef]
- Maeda, C.; Nomoto, S.; Takaishi, K.; Ema, T. Aggregation-Induced Circularly Polarized Luminescence from Boron Complexes with a Carbazolyl Schiff Base. Chem. Eur. J. 2020, 26, 13016–13021. [Google Scholar] [CrossRef]
- Yu, C.; Yu, C.; Yu, C.; Fang, X.; Wu, Q.; Jiao, L.; Sun, L.; Li, Z.; So, P.K.; Wong, W.Y.; et al. A Family of BODIPY-like Highly Fluorescent and Unsymmetrical Bis(BF2) Pyrrolyl-Acylhydrazone Chromophores: BOAPY. Org. Lett. 2020, 22, 4588–4592. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Zhou, X.; Wu, Y.; Ma, C.; Liu, W.; Zhang, C. Asymmetric Anthracene-Fused BODIPY Dye with Large Stokes Shift: Synthesis, Photophysical Properties and Bioimaging. Dye. Pigment. 2016, 126, 232–238. [Google Scholar] [CrossRef]
- Ying, Z.; Yi, X.; Shaoming, C.; Xuhong, Q. Isomeric Boron-Fluorine Complexes with Donor-Acceptor Architecture: Strong Solid/Liquid Fluorescence and Large Stokes Shift. Org. Lett. 2008, 10, 633–663. [Google Scholar]
- Zhou, Y.; Xiao, Y.; Li, D.; Fu, M.; Qian, X. Novel Fluorescent Fluorine-Boron Complexes: Synthesis, Crystal Structure, Photoluminescence, and Electrochemistry Properties. J. Org. Chem. 2008, 73, 1571–1574. [Google Scholar] [CrossRef]
- Shimizu, S.; Murayama, A.; Haruyama, T.; Iino, T.; Mori, S.; Furuta, H.; Kobayashi, N. Benzo[c,d]Indole-Containing Aza-BODIPY Dyes: Asymmetrization-Induced Solid-State Emission and Aggregation-Induced Emission Enhancement as New Properties of a Well-Known Chromophore. Chem. Eur. J. 2015, 21, 12996–13003. [Google Scholar] [CrossRef]
- Shimizu, S.; Iino, T.; Araki, Y.; Kobayashi, N. Pyrrolopyrrole Aza-BODIPY Analogues: A Facile Synthesis and Intense Fluorescence. Chem. Commun. 2013, 49, 1621–1623. [Google Scholar] [CrossRef]
- Shimizu, S. Aza-BODIPY Synthesis towards Vis/NIR Functional Chromophores Based on a Schiff Base Forming Reaction Protocol Using Lactams and Heteroaromatic Amines. Chem. Commun. 2019, 55, 8722–8743. [Google Scholar] [CrossRef] [PubMed]
- Araneda, J.F.; Piers, W.E.; Heyne, B.; Parvez, M.; McDonald, R. High Stokes Shift Anilido-Pyridine Boron Difluoride Dyes. Angew. Chem. Int. Ed. 2011, 50, 12214–12217. [Google Scholar] [CrossRef] [PubMed]
- Nawn, G.; Oakley, S.R.; Majewski, M.B.; McDonald, R.; Patrick, B.O.; Hicks, R.G. Redox-Active, near-Infrared Dyes Based on ‘Nindigo’ (Indigo-N,N′-Diarylimine) Boron Chelate Complexes. Chem. Sci. 2013, 4, 612–662. [Google Scholar] [CrossRef]
- Lu, H.; Xue, Z.; Mack, J.; Shen, Z.; You, X.; Kobayashi, N. Specific Cu2+-Induced J-Aggregation and Hg2+-Induced Fluorescence Enhancement Based on BODIPY. Chem. Commun. 2010, 46, 356. [Google Scholar] [CrossRef]
- Lu, H.; Shimizu, S.; Mack, J.; Shen, Z.; Kobayashi, N. Synthesis and Spectroscopic Properties of Fused-Ring-Expanded Aza-Boradiazaindacenes. Chem Asian J. 2011, 6, 1026–1037. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).