Ag@AuNP-Functionalized Capillary-Based SERS Sensing Platform for Interference-Free Detection of Glucose in Urine Using SERS Tags with Built-In Nitrile Signal
Abstract
:1. Introduction
2. Results and Discussion
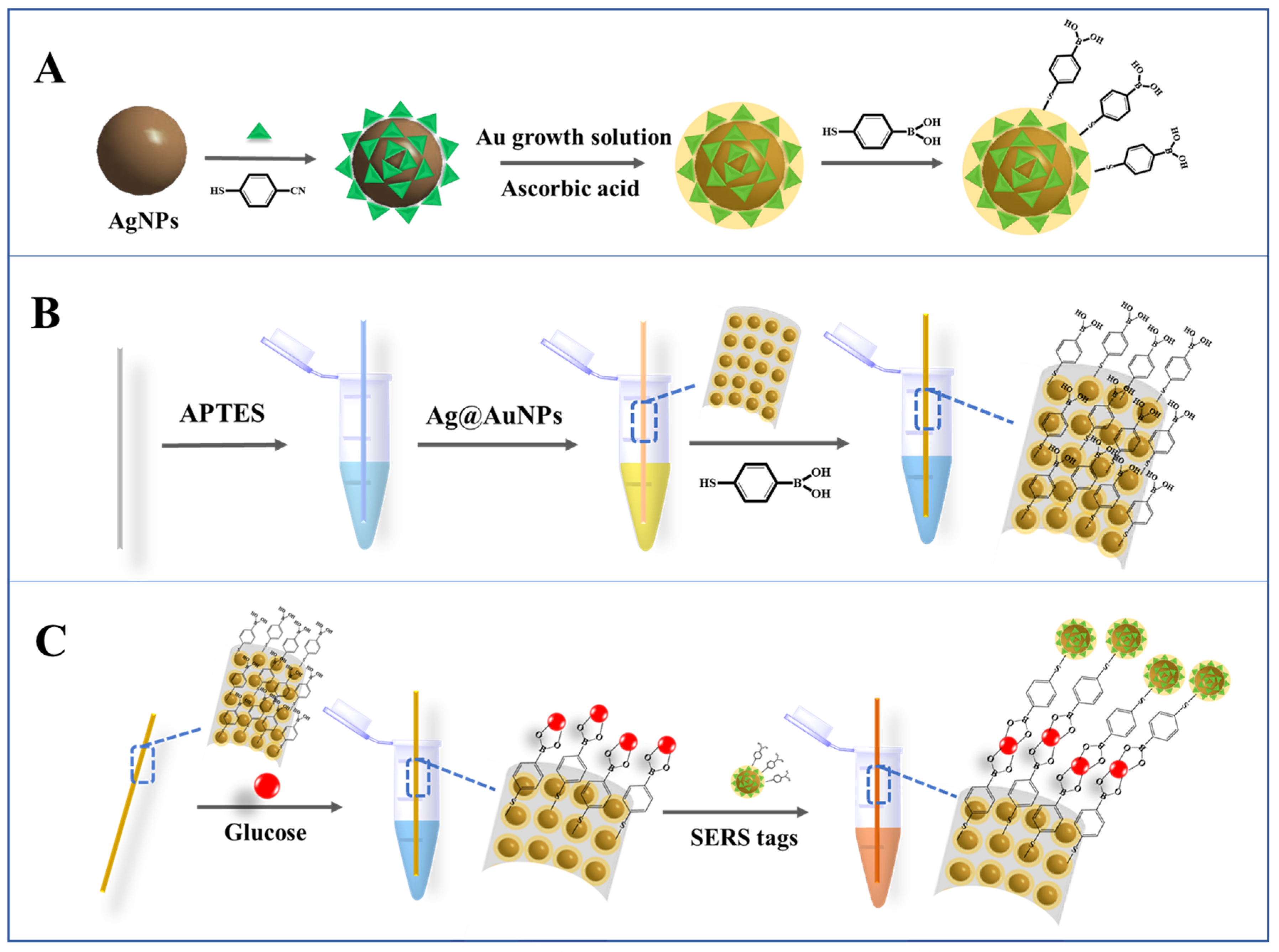
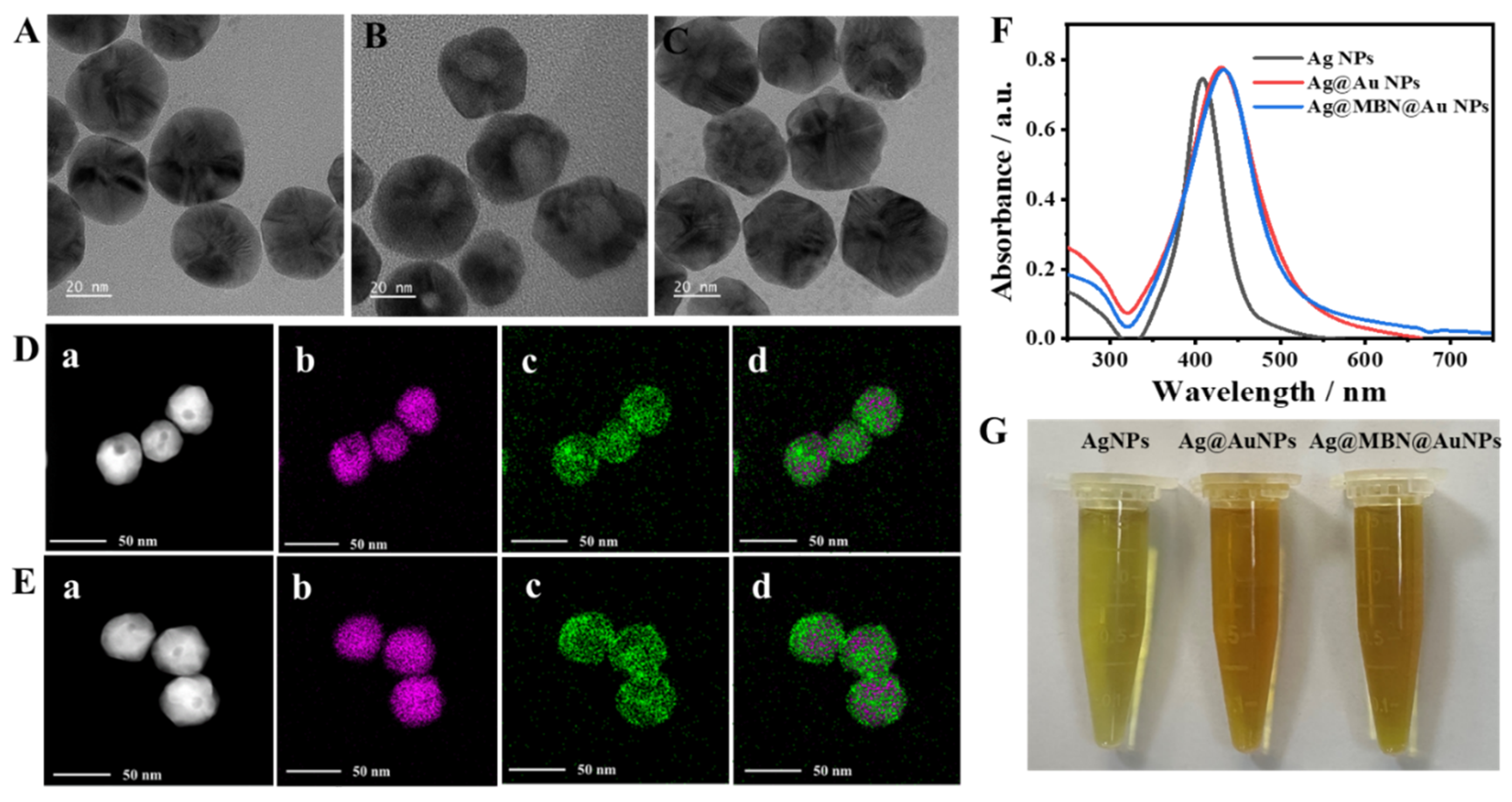
2.1. Characterization of the Ag@AuNPs and Ag@MBN@AuNPs
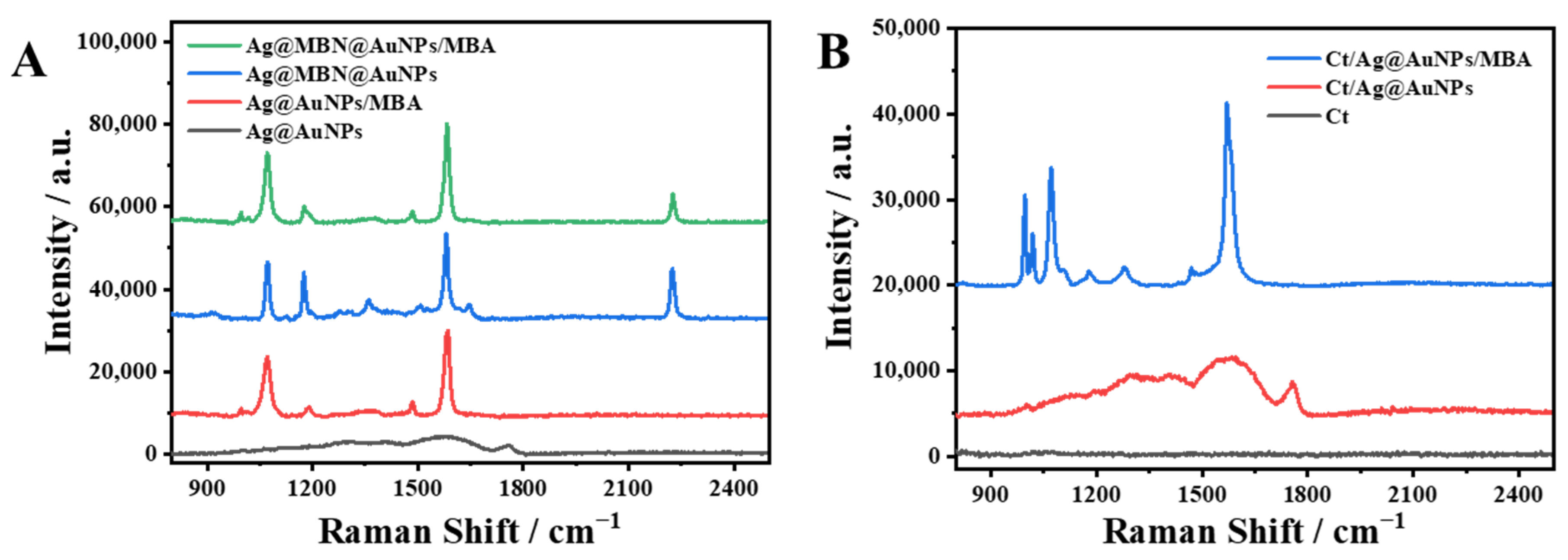
2.2. Establishment of Ag@AuNP-Functionalized Capillary-Based SERS Capture Substrate
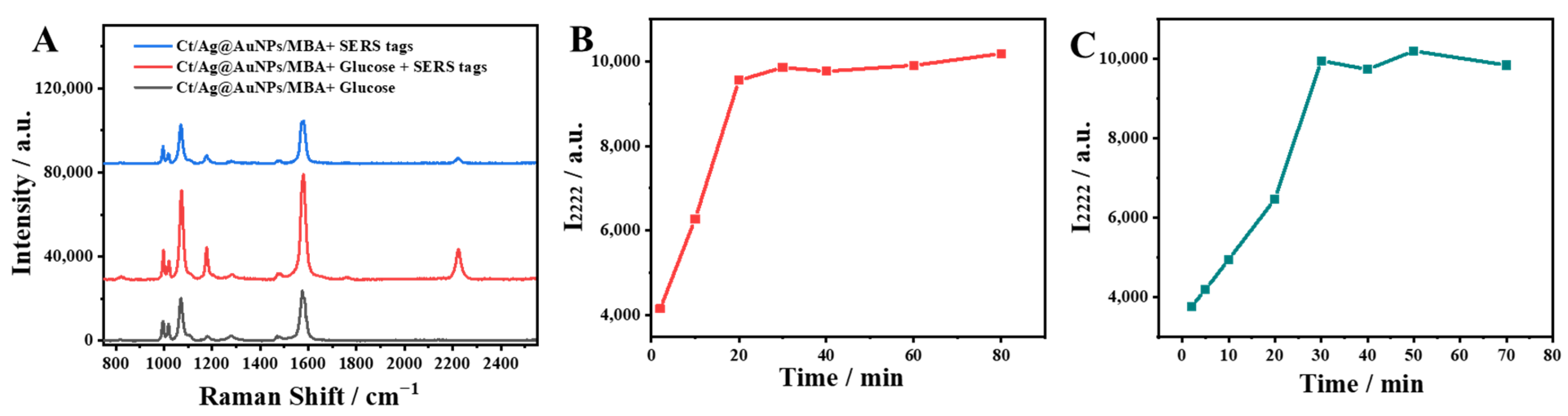
2.3. Feasibility of Glucose Analysis by the Capillary-Based SERS Sensing Platform and Optimization of Main Detection Conditions
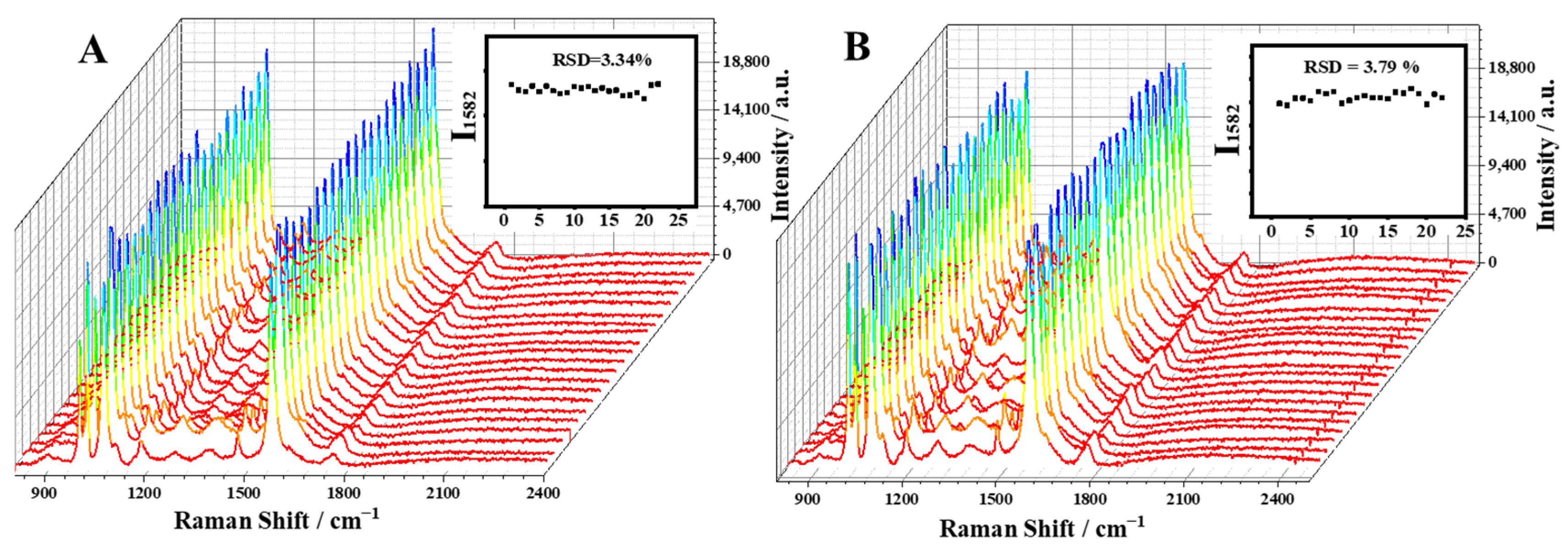
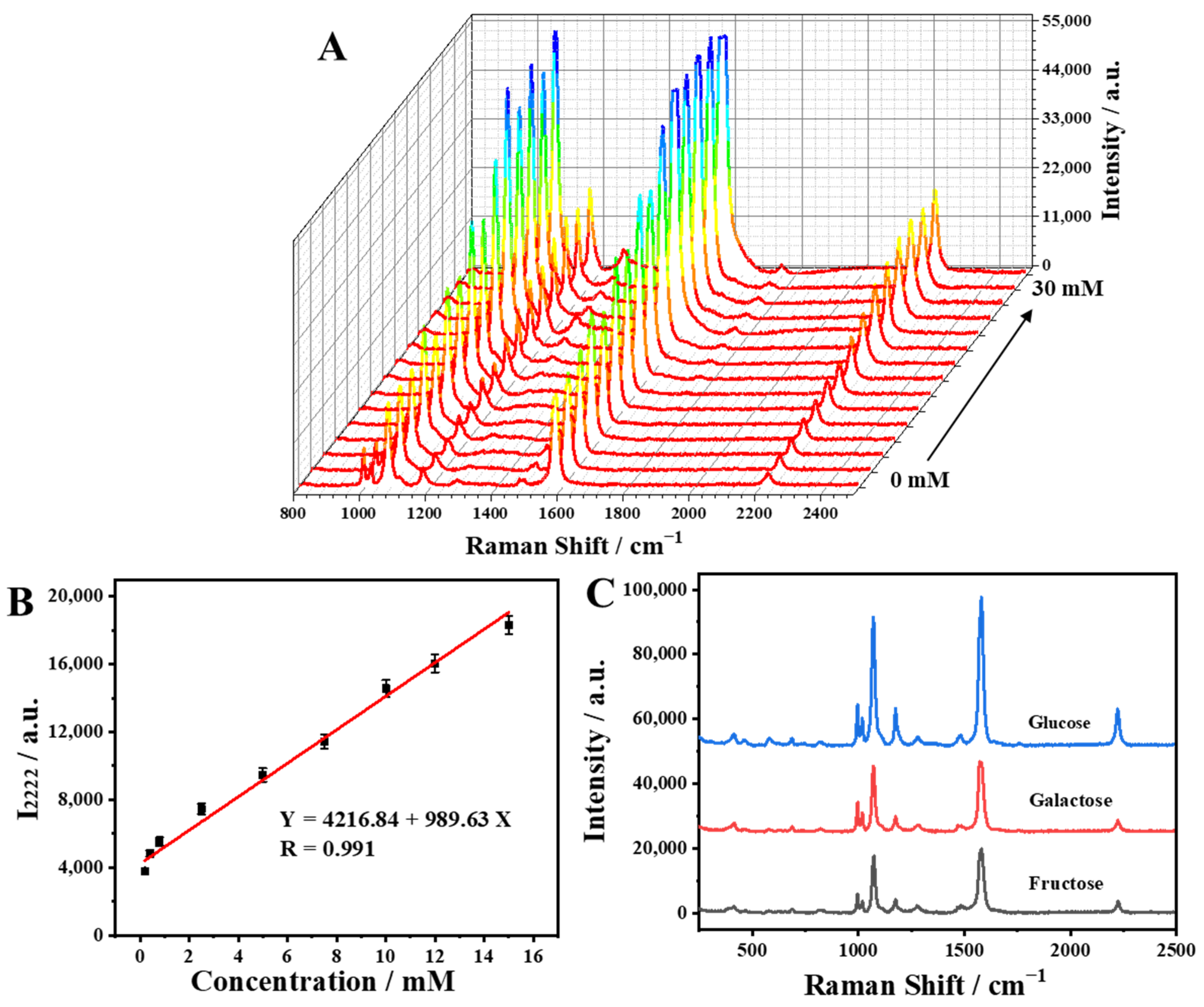
2.4. SERS Determination of Glucose Levels Using the Capillary-Based SERS Sensing Platform
2.5. Practical Analysis of Glucose in Artificial Urine
3. Experimental Section
3.1. Materials and Methods
3.1.1. Chemical Reagents
3.1.2. Characterizations
3.1.3. SERS Detection Parameters
3.2. Preparation of Ag@AuNPs
3.2.1. AgNPs
3.2.2. Ag@AuNPs
3.3. Preparation of SERS Tags (Ag@MBN@AuNPs@MBA)
3.4. Fabrication of Capillary-Based SERS Capture Substrate
3.5. SERS Analysis of Glucose Using the Capillary-Based SERS Sensing Platform
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rayfield, E.J.; Ault, M.J.; Keusch, G.T.; Brothers, M.J.; Nechemias, C.; Smith, H. Infection and diabetes: The case for glucose control. Am. J. Med. 1982, 72, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Ripsin, C.M.; Kang, H.; Urban, R.J. Management of blood glucose in type 2 diabetes mellitus. Am. Fam. Physician 2009, 79, 29–36. [Google Scholar] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: Open cohort study in primary care. BMJ 2016, 352, i1450. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of oxidative stress during diabetes mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Ohigashi, S.; Hikita, S.; Kitamura, N.; Motoyoshi, M.; Tatsuno, I. Acute pulmonary edema caused by hypoglycemia due to insulin overdose. Intern. Med. J. 2004, 43, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Maciver, N.J.; Jacobs, S.R.; Wieman, H.L.; Wofford, J.A.; Coloff, J.L.; Rathmell, J.C. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukocyte Biol. 2008, 84, 949–957. [Google Scholar] [CrossRef]
- Rivas, A.M.; Nugent, K. Hyperglycemia, Insulin, and Insulin Resistance in Sepsis. Am. J. Med. Sci. 2021, 3, 297–302. [Google Scholar] [CrossRef]
- Kong, K.V.; Lam, Z.Y.; Lau, W.K.; Leong, W.K.; Olivo, M. A transition metal carbonyl probe for use in a highly specific and sensitive SERS-based assay for glucose. J. Am. Chem. Soc. 2013, 135, 18028–18031. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Rahman, M.M.; Daniele, S.; Canzonieri, V.; Rizzolio, F. Glucose detection devices and methods based on metal–organic frameworks and related materials. Adv. Funct. Mater. 2021, 31, 2106023. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Martínez-Paredes, G.; Añorga, L.; Grande, H.J. Glucose biosensor based on disposable electrochemical paper-based transducers fully fabricated by screen-printing. Biosens. Bioelectron. 2018, 109, 8–12. [Google Scholar] [CrossRef]
- Karyakin, A.A. Glucose biosensors for clinical and personal use. Electrochem. Commun. 2021, 125, 106973. [Google Scholar] [CrossRef]
- Naderi Asrami, P.; Aberoomand Azar, P.; Saber Tehrani, M.; Mozaffari, S.A. Glucose oxidase/nano-ZnO/thin film deposit FTO as an innovative clinical transducer: A sensitive glucose biosensor. Front. Chem. 2020, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yang, J.; Song, W.; Luo, P.; Zou, J.; Peng, J.; Huang, B.; Luo, Z. Au@ Ag nanoparticle sensor for sensitive and rapid detection of glucose. New J. Chem. 2021, 45, 3059–3066. [Google Scholar] [CrossRef]
- Hou, G.; Zhang, H.; Xie, G.; Xiao, K.; Wen, L.; Li, S.; Tian, Y.; Jiang, L. Ultratrace detection of glucose with enzyme-functionalized single nanochannels. J. Mater. Chem. A 2014, 2, 19131–19135. [Google Scholar] [CrossRef]
- Syshchyk, O.; Skryshevsky, V.A.; Soldatkin, O.O.; Soldatkin, A.P. Enzyme biosensor systems based on porous silicon photoluminescence for detection of glucose, urea and heavy metals. Biosens. Bioelectron. 2015, 66, 89–94. [Google Scholar] [CrossRef]
- Si, Y.; Xu, L.; Deng, T.; Zheng, J.; Li, J. Catalytic hairpin self-assembly-based SERS sensor array for the simultaneous measurement of multiple cancer-associated miRNAs. ACS Sens. 2020, 5, 4009–4016. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. [Google Scholar] [CrossRef]
- Yonzon, C.R.; Haynes, C.L.; Zhang, X.; Walsh, J.T.; Van Duyne, R.P. A glucose biosensor based on Surface-Enhanced Raman Scattering: Improved partition layer, temporal stability, reversibility, and resistance to serum protein interference. Anal. Chem. 2004, 76, 78–85. [Google Scholar] [CrossRef]
- Shafer-Peltier, K.E.; Haynes, C.L.; Glucksberg, M.R.; Van Duyne, R.P. Toward a glucose biosensor based on surface-enhanced Raman scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef]
- Si, Y.; Bai, Y.; Qin, X.; Li, J.; Zhong, W.; Xiao, Z.; Li, J.; Yin, Y. Alkyne–DNA-functionalized alloyed Au/Ag nanospheres for ratiometric surface-enhanced Raman scattering imaging assay of endonuclease activity in live cells. Anal. Chem. 2018, 90, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ren, J.Q.; Zhang, X.G.; Wu, D.Y.; Shen, A.G.; Hu, J.M. Alkyne-modulated surface-enhanced Raman scattering-palette for optical interference-free and multiplex cellular imaging. Anal. Chem. 2016, 88, 6115–6119. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, H.; Dodo, K.; Palonpon, A.; Ando, J.; Fujita, K.; Kawata, S.; Sodeoka, M. Alkyne-tag Raman imaging for visualization of mobile small molecules in live cells. J. Am. Chem. Soc. 2012, 134, 20681–20689. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tian, X.; Hong, S.; Dai, P.; You, Q.; Wang, R.; Feng, L.; Xie, C.; Tian, Z.Q.; Chen, X. A bioorthogonal Raman reporter strategy for SERS detection of glycans on live cells. Angew. Chem. Int. Ed. 2013, 52, 7266–7271. [Google Scholar] [CrossRef]
- Dzhagan, V.; Smirnov, O.; Kovalenko, M.; Mazur, N.; Hreshchuk, O.; Taran, N.; Plokhovska, S.; Pirko, Y.; Yemets, A.; Yukhymchuk, V.; et al. Spectroscopic study of phytosynthesized Ag nanoparticles and their activity as SERS substrate. Chemosensors 2022, 10, 129. [Google Scholar] [CrossRef]
- Zavatski, S.; Popov, A.I.; Chemenev, A.; Dauletbekova, A.; Bandarenka, H. Wet chemical synthesis and characterization of Au coatings on meso- and macroporous Si for molecular analysis by SERS spectroscopy. Crystals 2022, 12, 1656. [Google Scholar] [CrossRef]
- Bandarenka, H.; Burko, A.; Girel, K.; Laputsko, D.; Orel, E.; Mizgailo, A.; Sharopov, U.; Podelinska, A.; Shapel, U.; Pankratov, V.; et al. Improvement of heat dissipation in Ag/Ni substrates for testing Cu-TiO2/TiO2-modified filters using SERS spectroscopy. Crystals 2023, 13, 749. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Gao, M.; Zhang, X. High-sensitive bioorthogonal SERS tag for live cancer cell imaging by self-assembling core-satellites structure gold-silver nanocomposite. Talanta 2017, 172, 176–181. [Google Scholar] [CrossRef]
- Ma, S.; Li, Q.; Yin, Y.; Yang, J.; Liu, D. Interference-free surface-enhanced Raman scattering tags for single-cell molecular imaging with a high signal-to-background ratio. Small 2017, 13, 1603340. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, S.; Liao, Y.; Zhu, X.; Chen, Y.; Chen, L.; Liu, F.; Hu, X.; Tu, H.; Zhang, L.; et al. Isotopic graphene–isolated-Au-nanocrystals with cellular Raman-silent signals for cancer cell pattern recognition. Chem. Sci. 2018, 9, 2842–2849. [Google Scholar] [CrossRef]
- Gandra, N.; Singamaneni, S. Bilayered Raman-intense gold nanostructures with hidden tags (BRIGHTs) for high-resolution bioimaging. Adv. Mater. 2012, 25, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Lin, X.; Jiang, C.; Li, C.; Lin, H.; Huang, J.; Wang, S.; Liu, G.; Yan, X.; Zhong, Q.; et al. Reliable quantitative SERS analysis facilitated by core–shell nanoparticles with embedded internal standards. Angew. Chem. Int. Ed. 2015, 54, 7308–7312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, W.; Sun, K.; Chen, L. Reporter-embedded SERS tags from gold nanorod seeds: Selective immobilization of reporter molecules at the tip of nanorods. ACS Appl. Mater. Interfaces 2016, 8, 28105–28115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, N.; Xing, Y.; Deng, D. Boronic acid-based electrochemical sensors for detection of biomolecules. Int. J. Electrochem. Sci. 2013, 8, 11161–11174. [Google Scholar] [CrossRef]
- Xia, N.; Wu, D.H.; Sun, T.; Wang, Y.L.; Ren, X.Y.; Zhao, F.; Liu, L.; Yi, X.Y. Magnetic bead-based electrochemical and colorimetric methods for the detection of poly(ADP-ribose) polymerase-1 with boronic acid derivatives as the signal probes. Sens. Actuators B Chem. 2021, 327, 128913. [Google Scholar] [CrossRef]
- Xia, N.; Wu, D.H.; Yu, H.Q.; Sun, W.W.; Yi, X.Y.; Liu, L. Magnetic bead-based electrochemical and colorimetric assays of circulating tumor cells with boronic acid derivatives as the recognition elements and signal probes. Talanta 2021, 221, 121640. [Google Scholar] [CrossRef]
- Liu, L.L.; Chang, Y.; Xia, N.; Peng, P.Z.; Zhang, L.P.; Jiang, M.S.; Zhang, J.B.; Liu, L. Simple, sensitive and label–free electrochemical detection of microRNAs based on the in situ formation of silver nanoparticles aggregates for signal amplification. Biosens. Bioelectron. 2017, 94, 235–242. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, C.; Chang, Y.; Ma, H.Y.; Hao, Y.Q. Two sensitive electrochemical strategies for the detection of protein kinase activity based on the 4-mercaptophenylboronic acid-induced in situ assembly of silver nanoparticles. Sens. Actuators B 2017, 248, 178–186. [Google Scholar] [CrossRef]
- Xia, N.; Cheng, C.; Liu, L.; Peng, P.Z.; Liu, C.Y.; Chen, J.X. Electrochemical glycoprotein aptasensors based on the in-situ aggregation of silver nanoparticles induced by 4-mercaptophenylboronic acid. Microchim. Acta 2017, 184, 4393–4400. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.H.; Chang, Y.; Guo, H.; Wang, W.Q. Biosensors with boronic acid-based materials as the recognition elements and signal labels. Biosensors 2023, 13, 785. [Google Scholar] [CrossRef]
- Li, J.; Bai, Z.M.; Mao, Y.J.; Sun, Q.Q.; Ning, X.H.; Zheng, J.B. Disposable sandwich-type electrochemical sensor for selective detection of glucose based on boronate affinity. Electroanalysis 2017, 29, 2307–2315. [Google Scholar] [CrossRef]
- Bi, X.; Du, X.; Jiang, J.; Huang, X. Facile and sensitive glucose sandwich assay using in situ-generated Raman reporters. Anal. Chem. 2015, 87, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, J.; Xu, X.; Fu, G.; Zhang, L.; Sun, P.; Yan, X.; Liu, F.; Wang, C.; Liu, X.; et al. Gold-trisoctahedra-coated capillary-based SERS platform for microsampling and sensitive detection of trace fentanyl. Anal. Chem. 2022, 94, 4850–4858. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, S.; Schwenke, A.M.; Soliveri, G.; Ardizzone, S.; Weber, K.; Cialla-May, D.; Hoeppener, S.; Schubert, U.S.; Popp, J. Trace detection of tetrahydrocannabinol (THC) with a SERS-based capillary platform prepared by the in situ microwave synthesis of AgNPs. Anal. Chim. Acta 2016, 939, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.P.; Paul, P.K. Structures of carbohydrate–boronic acid complexes determined by NMR and molecular modelling in aqueous alkaline media. Org. Biomol. Chem. 2004, 2, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Jiang, L.; Zhang, T.; Lin, M.; Tian, D.B.; Huang, H. Colorimetric detection of glucose using a boronic acid derivative receptor attached to unmodified AuNPs. Chin. Chem. Lett. 2014, 25, 77–79. [Google Scholar] [CrossRef]
- Norrild, J.C.; Eggert, H. Evidence for mono-and bisdentate boronate complexes of glucose in the furanose form. Application of 1JC-C coupling constants as a structural probe. J. Am. Chem. Soc. 1995, 117, 1479–1484. [Google Scholar] [CrossRef]
- Csapó, E.; Oszkó, A.; Varga, E.; Juhász, Á.; Buzás, N.; Korösi, L.; Majzik, A.; Dékány, I. Synthesis and characterization of Ag/Au alloy and core(Ag)–shell(Au) nanoparticles. Colloids Surfaces A 2012, 415, 281–287. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21. [Google Scholar] [CrossRef]
- Liu, H.L.; Yang, L.B.; Liu, J.H. Three-dimensional SERS hots pots for chemical sensing: Towards developing a practical analyzer. TrAC-Trend. Anal. Chem. 2016, 80, 364–372. [Google Scholar] [CrossRef]
- Hansen, J.S.; Christensen, J.B.; Petersen, J.F.; Hoeg-Jensen, T.; Norrild, J.C. Arylboronic acids: A diabetic eye on glucose sensing. Sens. Actuators B 2012, 161, 45–79. [Google Scholar] [CrossRef]
- Eggert, H.; Frederiksen, J.; Morin, C. A New glucose-selective fluorescent bisboronic acid. First report of strong α-furanose complexation in aqueous solution at physiological pH1. J. Org. Chem. 1999, 64, 3846–3852. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.; Zhang, L.; Han, L.; Gao, C.; Yin, Y. Etching-free epitaxial growth of gold on silver nanostructures for high chemical stability and plasmonic activity. Adv. Funct. Mater. 2015, 25, 5435–5443. [Google Scholar] [CrossRef]
- He, X.N.; Wang, Y.N.; Wang, Y.; Xu, Z.R. Accurate quantitative detection of cell surface sialic acids with a background-free SERS probe. Talanta 2020, 209, 120579. [Google Scholar] [CrossRef]
- Muhammad, P.; Tu, X.Y.; Liu, J.; Wang, Y.J.; Liu, Z. Molecularly imprinted plasmonic substrates for specific and ultrasensitive immunoassay of trace glycoproteins in biological samples. ACS Appl. Mater. Interfaces 2017, 9, 12082–12091. [Google Scholar] [CrossRef]
- Peng, R.Y.; Si, Y.M.; Deng, T.; Zheng, J.; Li, J.S.; Yang, R.H.; Tan, W.H. A novel SERS nanoprobe for the ratiometric imaging of hydrogen peroxide in living cells. Chem. Commun. 2016, 52, 8553–8556. [Google Scholar] [CrossRef]
| Sample | Analyte | Added (mM) | Found (mM) | Recovery (%) |
|---|---|---|---|---|
| Artificial urine | Glucose | 1 | 1.0855 ± 0.0421 | 108.55 |
| 2 | 2.0560 ± 0.2489 | 102.80 | ||
| 5 | 4.9086 ± 0.7176 | 98.17 | ||
| 10 | 10.7915 ± 1.4868 | 107.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.; Wang, H.; Yan, Y.; Li, B.; Ni, Z.; Shi, H. Ag@AuNP-Functionalized Capillary-Based SERS Sensing Platform for Interference-Free Detection of Glucose in Urine Using SERS Tags with Built-In Nitrile Signal. Molecules 2023, 28, 7939. https://doi.org/10.3390/molecules28247939
Si Y, Wang H, Yan Y, Li B, Ni Z, Shi H. Ag@AuNP-Functionalized Capillary-Based SERS Sensing Platform for Interference-Free Detection of Glucose in Urine Using SERS Tags with Built-In Nitrile Signal. Molecules. 2023; 28(24):7939. https://doi.org/10.3390/molecules28247939
Chicago/Turabian StyleSi, Yanmei, Hua Wang, Yehao Yan, Bingwen Li, Zeyun Ni, and Hongrui Shi. 2023. "Ag@AuNP-Functionalized Capillary-Based SERS Sensing Platform for Interference-Free Detection of Glucose in Urine Using SERS Tags with Built-In Nitrile Signal" Molecules 28, no. 24: 7939. https://doi.org/10.3390/molecules28247939
APA StyleSi, Y., Wang, H., Yan, Y., Li, B., Ni, Z., & Shi, H. (2023). Ag@AuNP-Functionalized Capillary-Based SERS Sensing Platform for Interference-Free Detection of Glucose in Urine Using SERS Tags with Built-In Nitrile Signal. Molecules, 28(24), 7939. https://doi.org/10.3390/molecules28247939





