Forward or Backward: Lessons Learned from Small Molecule Drugs Approved by FDA from 2012 to 2022
Abstract
1. Introduction
2. Multi-Angle Analysis of Small Molecule Drugs Approved by the FDA from 2012 to 2022
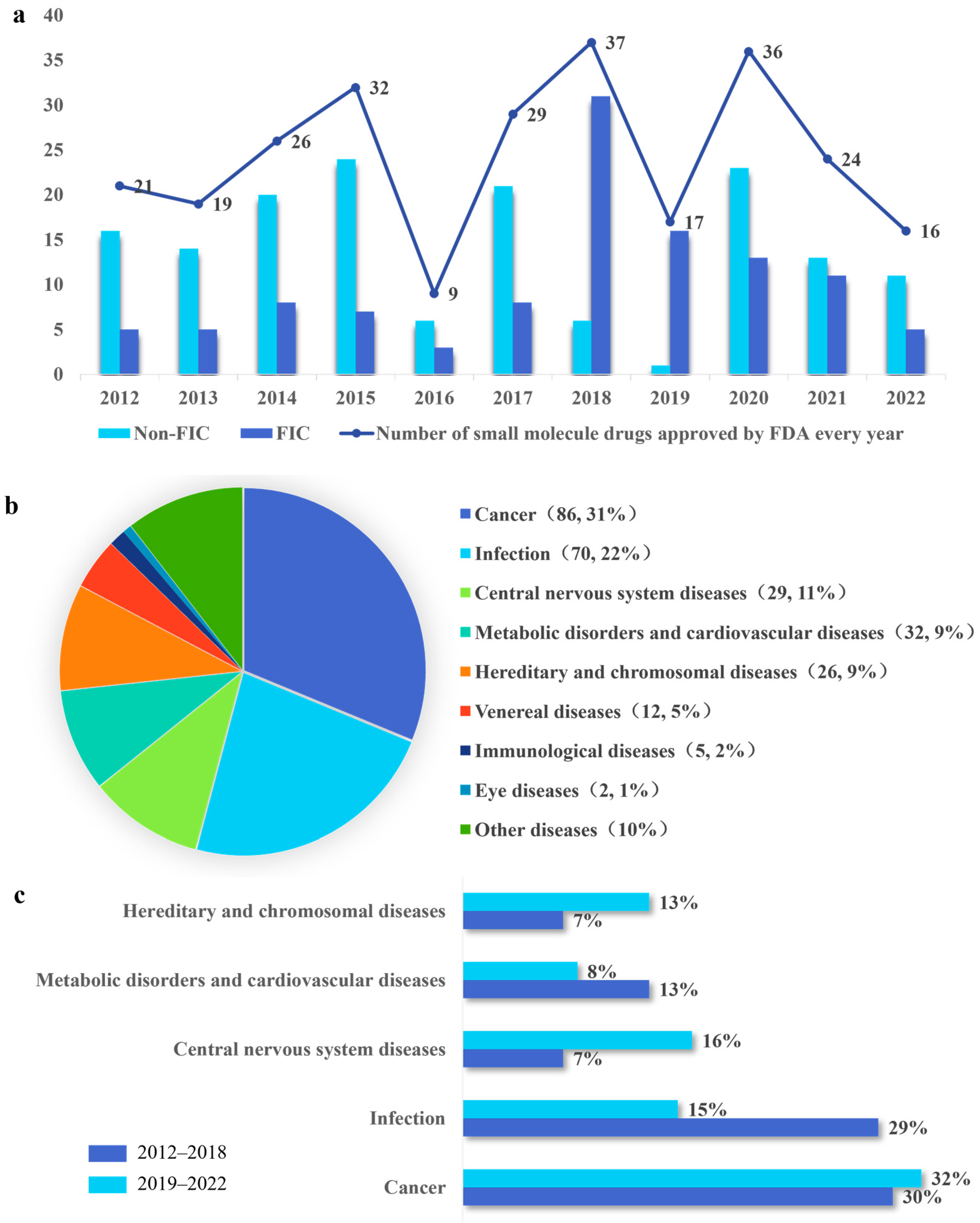
2.1. Analysis of Overall Trends in FDA Approval of NMEs
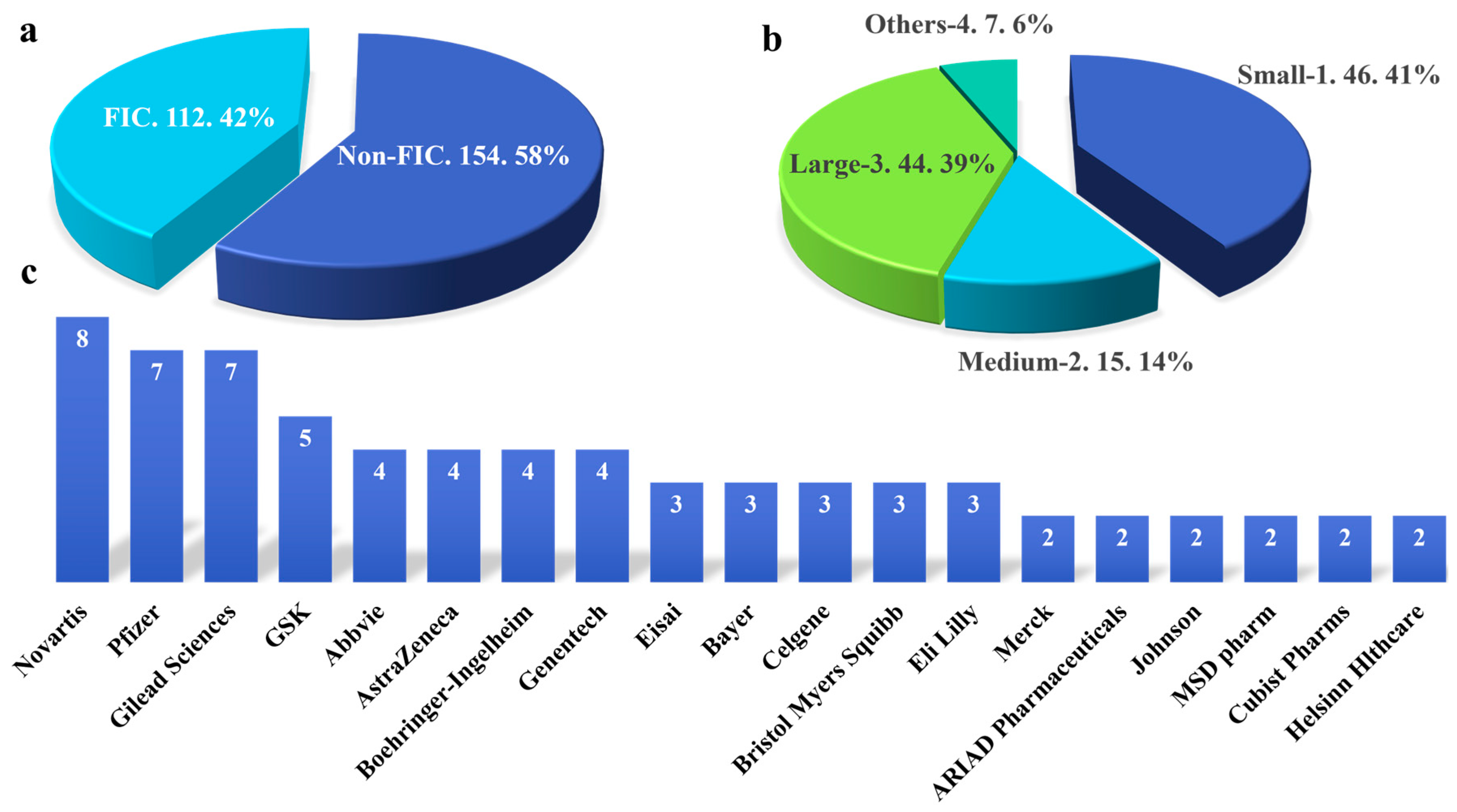
2.2. The Relationship between Pharmaceutical Enterprises or Academic Institutions and the Development of FIC Drugs
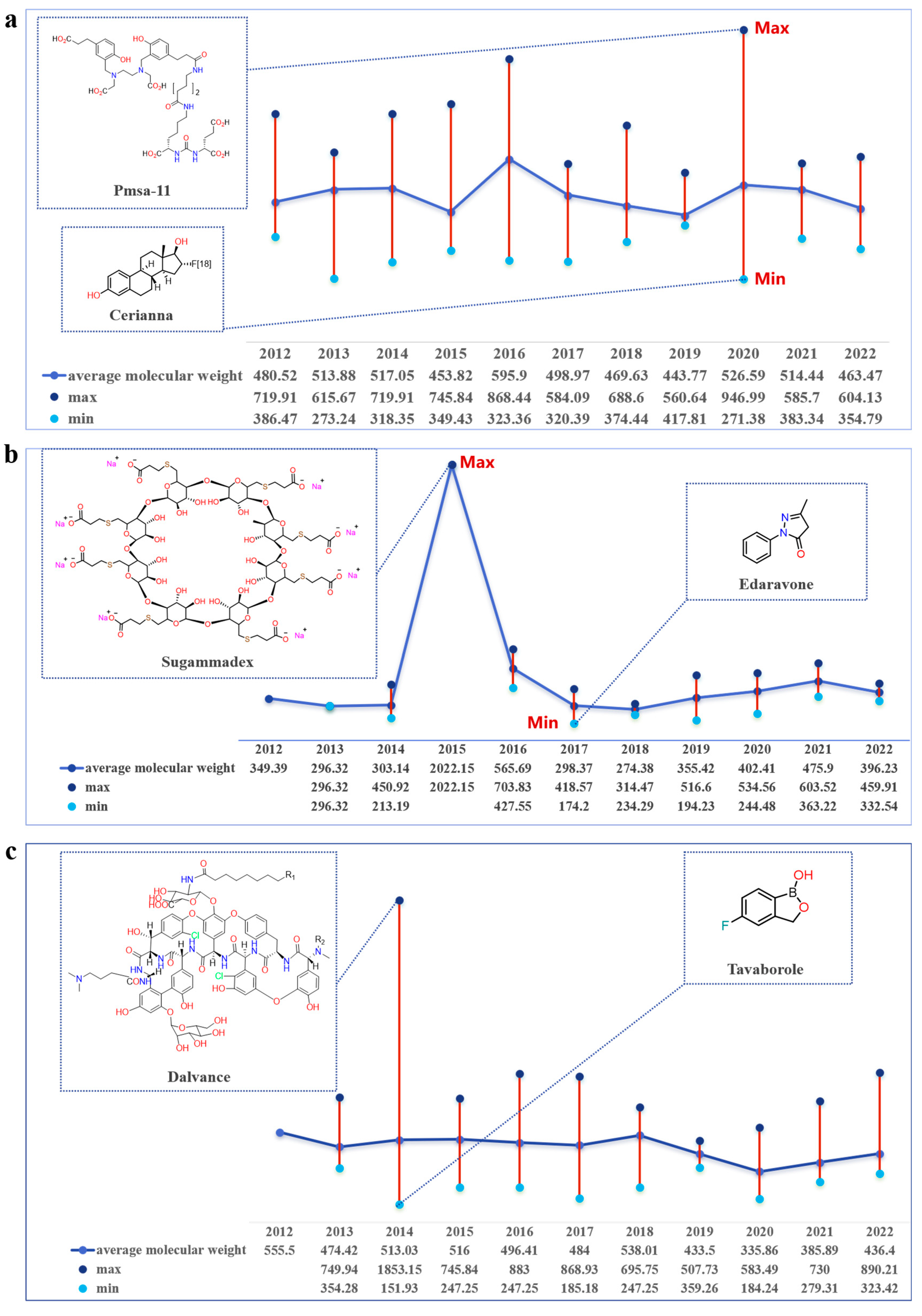
2.3. Trends in Molecular Weight Variation of FIC Small-Molecule Drugs
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Dhillon, S.; Keam, S.J. Umbralisib: First Approval. Drugs 2021, 81, 857–866. [Google Scholar] [CrossRef]
- Savage, D.G.; Antman, K.H. Imatinib mesylate—A new oral targeted therapy. N. Engl. J. Med. 2002, 346, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Chang, M.T.; Jonsson, P.; Drilon, A. Fusions in solid tumours: Diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 2017, 14, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Gasch, C.; Ffrench, B.; O’Leary, J.J.; Gallagher, M.F. Catching moving targets: Cancer stem cell hierarchies, therapy-resistance & considerations for clinical intervention. Mol. Cancer 2017, 16, 43. [Google Scholar] [CrossRef]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019, 234, 116781. [Google Scholar] [CrossRef]
- Mele, L.; Del Vecchio, V.; Liccardo, D.; Prisco, C.; Schwerdtfeger, M.; Robinson, N.; Desiderio, V.; Tirino, V.; Papaccio, G.; La Noce, M. The role of autophagy in resistance to targeted therapies. Cancer Treat. Rev. 2020, 88, 102043. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Hussain, S.; Singh, A.; Nazir, S.U.; Tulsyan, S.; Khan, A.; Kumar, R.; Bashir, N.; Tanwar, P.; Mehrotra, R. Cancer drug resistance: A fleet to conquer. J. Cell Biochem. 2019, 120, 14213–14225. [Google Scholar] [CrossRef]
- Boumahdi, S.; de Sauvage, F.J. The great escape: Tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 2020, 19, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Yuan, W.E.; Su, J.; Liu, Y.; Chen, J. Recent advances in small molecule based cancer immunotherapy. Eur. J. Med. Chem. 2018, 157, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Offringa, R.; Kötzner, L.; Huck, B.; Urbahns, K. The expanding role for small molecules in immuno-oncology. Nat. Rev. Drug Discov. 2022, 21, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, A.; Tournier, C. Immuno-oncology. Essays Biochem. 2023, 67, 903. [Google Scholar] [CrossRef]
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Gammon, K. Neurodegenerative disease: Brain windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef]
- Wobst, H.J.; Mack, K.L.; Brown, D.G.; Brandon, N.J.; Shorter, J. The clinical trial landscape in amyotrophic lateral sclerosis-Past, present, and future. Med. Res. Rev. 2020, 40, 1352–1384. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; Moufawad El Achkar, C.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef]
- O’Donnell, P.; Rosen, L.; Alexander, R.; Murthy, V.; Davies, C.H.; Ratti, E. Strategies to Address Challenges in Neuroscience Drug Discovery and Development. Int. J. Neuropsychopharmacol. 2019, 22, 445–448. [Google Scholar] [CrossRef]
- Wang, S. Historical Review: Opiate Addiction and Opioid Receptors. Cell Transplant. 2019, 28, 233–238. [Google Scholar] [CrossRef]
- Doughty, B.; Morgenson, D.; Brooks, T. Lofexidine: A Newly FDA-Approved, Nonopioid Treatment for Opioid Withdrawal. Ann. Pharmacother. 2019, 53, 746–753. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves the First Non-Opioid Treatment for Management of Opioid Withdrawal Symptoms in Adults. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-non-opioid-treatment-management-opioid-withdrawal-symptoms-adults (accessed on 11 October 2023).
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Ahmed, A.B.F.; Bhattacharjee, S.; Slama, P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public. Health 2020, 17, 9411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Coronavirus Treatment Acceleration Program (CTAP). Available online: https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap (accessed on 12 May 2023).
- FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 22 October 2020).
- Bathurst, I.; Hentschel, C. Medicines for Malaria Venture: Sustaining antimalarial drug development. Trends Parasitol. 2006, 22, 301–307. [Google Scholar] [CrossRef]
- Frampton, J.E. Tafenoquine: First Global Approval. Drugs 2018, 78, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Wobst, H.J. A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A.T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef]
- Van Camp, G. Cardiovascular disease prevention. Acta Clin. Belg. 2014, 69, 407–411. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Sclavo, M. Cardiovascular risk factors and prevention in women: Similarities and differences. Ital. Heart J. Suppl. 2001, 2, 125–141. [Google Scholar]
- Ruffolo, R.R. Why has R&D productivity declined in the pharmaceutical industry? Expert. Opin. Drug Discov. 2006, 1, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons learned from the fate of AstraZeneca’s drug pipeline: A five-dimensional framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Pammolli, F.; Magazzini, L.; Riccaboni, M. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 2011, 10, 428–438. [Google Scholar] [CrossRef]
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Approval and Regulation of Pharmaceuticals, 1983–2018. JAMA 2020, 323, 164–176. [Google Scholar] [CrossRef]
- The Drug Development Process. Available online: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process (accessed on 4 January 2018).
- Mullard, A. 2016 FDA drug approvals. Nat. Rev. Drug Discov. 2017, 16, 73–76. [Google Scholar] [CrossRef]
- Yu, W.; MacKerell, A.D., Jr. Computer-Aided Drug Design Methods. Methods Mol. Biol. 2017, 1520, 85–106. [Google Scholar] [CrossRef]
- Blundell, T.L.; Wright, P.E. Structural biology—Painting the mechanistic landscape of biomolecules. Curr. Res. Struct. Biol. 2022, 4, iv. [Google Scholar] [CrossRef]
- CzarnikEditor, A.W. Journal of Combinatorial Chemistry: Our Next Millennium. J. Comb. Chem. 2000, 2, 1. [Google Scholar] [CrossRef][Green Version]
- Makurvet, F.D. Biologics vs. small molecules: Drug costs and patient access. Med. Drug Discov. 2021, 9, 100075. [Google Scholar] [CrossRef]
- Garvey, M. Non-Mammalian Eukaryotic Expression Systems Yeast and Fungi in the Production of Biologics. J. Fungi 2022, 8, 1179. [Google Scholar] [CrossRef]
- Takebe, T.; Imai, R.; Ono, S. The Current Status of Drug Discovery and Development as Originated in United States Academia: The Influence of Industrial and Academic Collaboration on Drug Discovery and Development. Clin. Transl. Sci. 2018, 11, 597–606. [Google Scholar] [CrossRef]
- Mullard, A. 2012 FDA drug approvals. Nat. Rev. Drug Discov. 2013, 12, 87–90. [Google Scholar] [CrossRef]
- Mullard, A. 2013 FDA drug approvals. Nat. Rev. Drug Discov. 2014, 13, 85–89. [Google Scholar] [CrossRef]
- Mullard, A. 2014 FDA drug approvals. Nat. Rev. Drug Discov. 2015, 14, 77–81. [Google Scholar] [CrossRef]
- Novel Drug Approvals for 2015. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015 (accessed on 13 October 2023).
- Novel Drugs Summary 2016. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drugs-summary-2016 (accessed on 13 October 2023).
- Novel Drug Approvals for 2017. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017 (accessed on 13 October 2023).
- Noval Drug Approvals for 2018. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2018 (accessed on 13 October 2023).
- Noval Drug Approvals for 2019. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed on 13 October 2023).
- Novel Drug Approvals for 2020. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 (accessed on 15 October 2023).
- Novel Drug Approvals for 2021. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 (accessed on 23 October 2023).
- Novel Drug Approvals for 2022. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022 (accessed on 26 October 2023).
- Bedair, A.; Mansour, F.R. Insights into the FDA 2018 New Drug Approvals. Curr Drug Discov Technol 2021, 18, 293–306. [Google Scholar] [CrossRef]
- Lanthier, M.; Miller, K.L.; Nardinelli, C.; Woodcock, J. An improved approach to measuring drug innovation finds steady rates of first-in-class pharmaceuticals, 1987–2011. Health Aff. 2013, 32, 1433–1439. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Ribeiro, A.; Rodrigues, L.O.; Harada, G.; Nobre, M.R.C. U.S. Food and Drug Administration anticancer drug approval trends from 2016 to 2018 for lung, colorectal, breast, and prostate cancer. Int. J. Technol. Assess Health Care 2020, 36, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, R. Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin. Pharmaceutics 2023, 15, 1794. [Google Scholar] [CrossRef] [PubMed]
- Bennani, Y.L. Drug discovery in the next decade: Innovation needed ASAP. Drug Discov. Today 2011, 16, 779–792. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Hogan, G.; Tangney, M. The Who, What, and Why of Drug Discovery and Development. Trends Pharmacol. Sci. 2018, 39, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Ullman, F.; Boutellier, R. Drug discovery: Are productivity metrics inhibiting motivation and creativity? Drug Discov. Today 2008, 13, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Nwaka, S.; Ridley, R.G. Virtual drug discovery and development for neglected diseases through public-private partnerships. Nat. Rev. Drug Discov. 2003, 2, 919–928. [Google Scholar] [CrossRef]
- Kiriiri, G.K.; Njogu, P.M.; Mwangi, A.N. Exploring different approaches to improve the success of drug discovery and development projects: A review. Future J. Pharm. Sci. 2020, 6, 27. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef]
- Vicenti, I.; Zazzi, M.; Saladini, F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert. Opin. Ther. Pat. 2021, 31, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, A.E. Small molecule-RNA targeting: Starting with the fundamentals. Chem. Commun. 2020, 56, 14744–14756. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Fluoroestradiol F18. In Drugs and Lactation Database (LactMed®); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2006. [Google Scholar]
- Hawkins, J.; Khanna, S.; Argalious, M. Sugammadex for Reversal of Neuromuscular Blockade: Uses and Limitations. Curr. Pharm. Des. 2019, 25, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Karalapillai, D.; Kaufman, M.; Weinberg, L. Sugammadex. Crit. Care Resusc. 2013, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.T.; Sprung, J.; Jankowski, C.J. Sugammadex: A novel agent for the reversal of neuromuscular blockade. Pharmacotherapy 2007, 27, 1181–1188. [Google Scholar] [CrossRef]
- Kheterpal, S.; Vaughn, M.T.; Dubovoy, T.Z.; Shah, N.J.; Bash, L.D.; Colquhoun, D.A.; Shanks, A.M.; Mathis, M.R.; Soto, R.G.; Bardia, A.; et al. Sugammadex versus Neostigmine for Reversal of Neuromuscular Blockade and Postoperative Pulmonary Complications (STRONGER): A Multicenter Matched Cohort Analysis. Anesthesiology 2020, 132, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Lee, J.; Moreland, N.; Sadoughi, A.; Borna, R.; Salehi, A.; Jahr, J.S. Sugammadex: Clinical Pharmacokinetics and Pharmacodynamics. Curr. Anesthesiol. Rep. 2018, 8, 168–177. [Google Scholar] [CrossRef]
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef]
- Barberán, J.; de la Cuerda, A.; Barberán, L.C. Dalbavancin. Rev. Esp. Quimioter. 2021, 34 (Suppl. 1), 26–28. [Google Scholar] [CrossRef]
- Belley, A.; Arhin, F.F.; Sarmiento, I.; Deng, H.; Rose, W.; Moeck, G. Pharmacodynamics of a simulated single 1,200-milligram dose of oritavancin in an in vitro pharmacokinetic/pharmacodynamic model of methicillin-resistant staphylococcus aureus infection. Antimicrob. Agents Chemother. 2013, 57, 205–211. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Murdan, S. Drug delivery to the nail following topical application. Int. J. Pharm. 2002, 236, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Coronado, D.; Merchant, T.; Chanda, S.; Zane, L.T. In Vitro Nail Penetration and Antifungal Activity of Tavaborole, a Boron-Based Pharmaceutical. J. Drugs Dermatol. 2015, 14, 609–614. [Google Scholar] [PubMed]
- Wienkers, L.C.; Heath, T.G. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov. 2005, 4, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Lu, A.Y. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol. Rev. 1997, 49, 403–449. [Google Scholar] [PubMed]
- Lin, J.H. Species similarities and differences in pharmacokinetics. Drug Metab. Dispos. 1995, 23, 1008–1021. [Google Scholar]
- Tang, C.; Prueksaritanont, T. Use of in vivo animal models to assess pharmacokinetic drug-drug interactions. Pharm. Res. 2010, 27, 1772–1787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, M.; Sun, S.; You, Q.; Wang, L. Forward or Backward: Lessons Learned from Small Molecule Drugs Approved by FDA from 2012 to 2022. Molecules 2023, 28, 7941. https://doi.org/10.3390/molecules28247941
Gu M, Sun S, You Q, Wang L. Forward or Backward: Lessons Learned from Small Molecule Drugs Approved by FDA from 2012 to 2022. Molecules. 2023; 28(24):7941. https://doi.org/10.3390/molecules28247941
Chicago/Turabian StyleGu, Mingxiao, Sudan Sun, Qidong You, and Lei Wang. 2023. "Forward or Backward: Lessons Learned from Small Molecule Drugs Approved by FDA from 2012 to 2022" Molecules 28, no. 24: 7941. https://doi.org/10.3390/molecules28247941
APA StyleGu, M., Sun, S., You, Q., & Wang, L. (2023). Forward or Backward: Lessons Learned from Small Molecule Drugs Approved by FDA from 2012 to 2022. Molecules, 28(24), 7941. https://doi.org/10.3390/molecules28247941







