In Vitro Antibacterial, Antioxidant, Anticholinesterase, and Antidiabetic Activities and Chemical Composition of Salvia balansae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening
2.2. Mineral Content
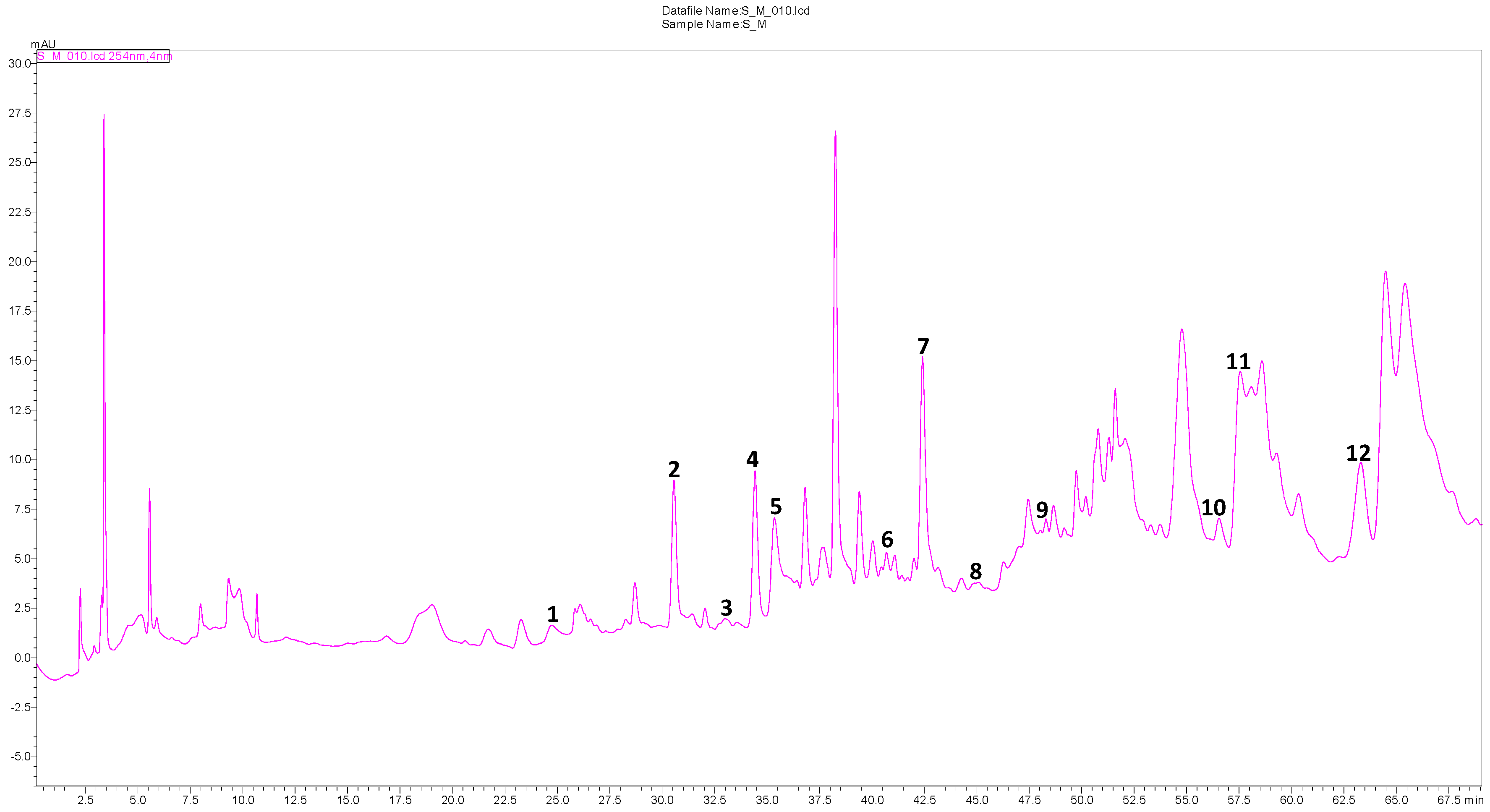
2.3. Quantitative Analysis of Phenolic Compounds by HPLC–DAD
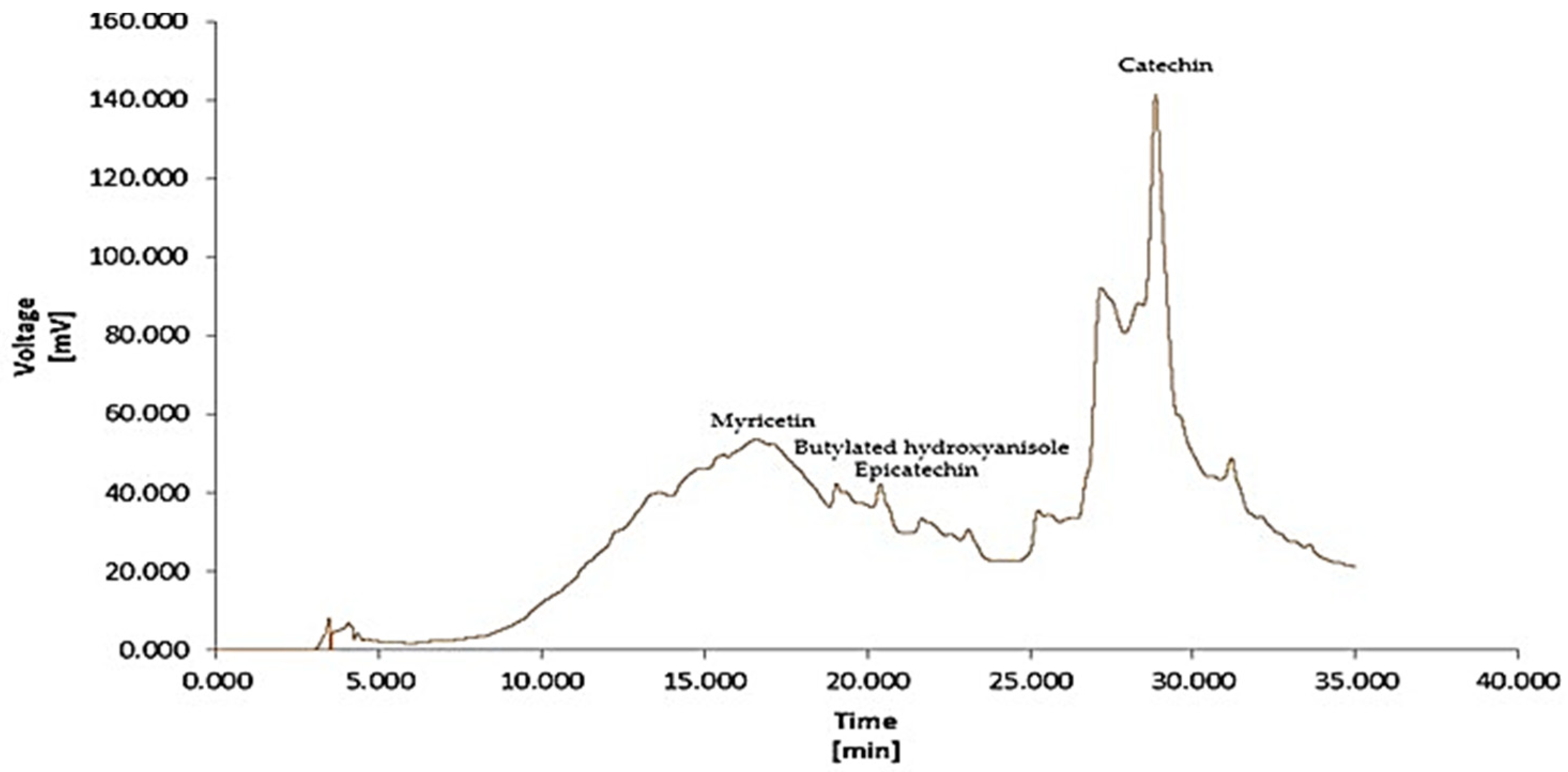
2.4. HPLC-MS Analysis
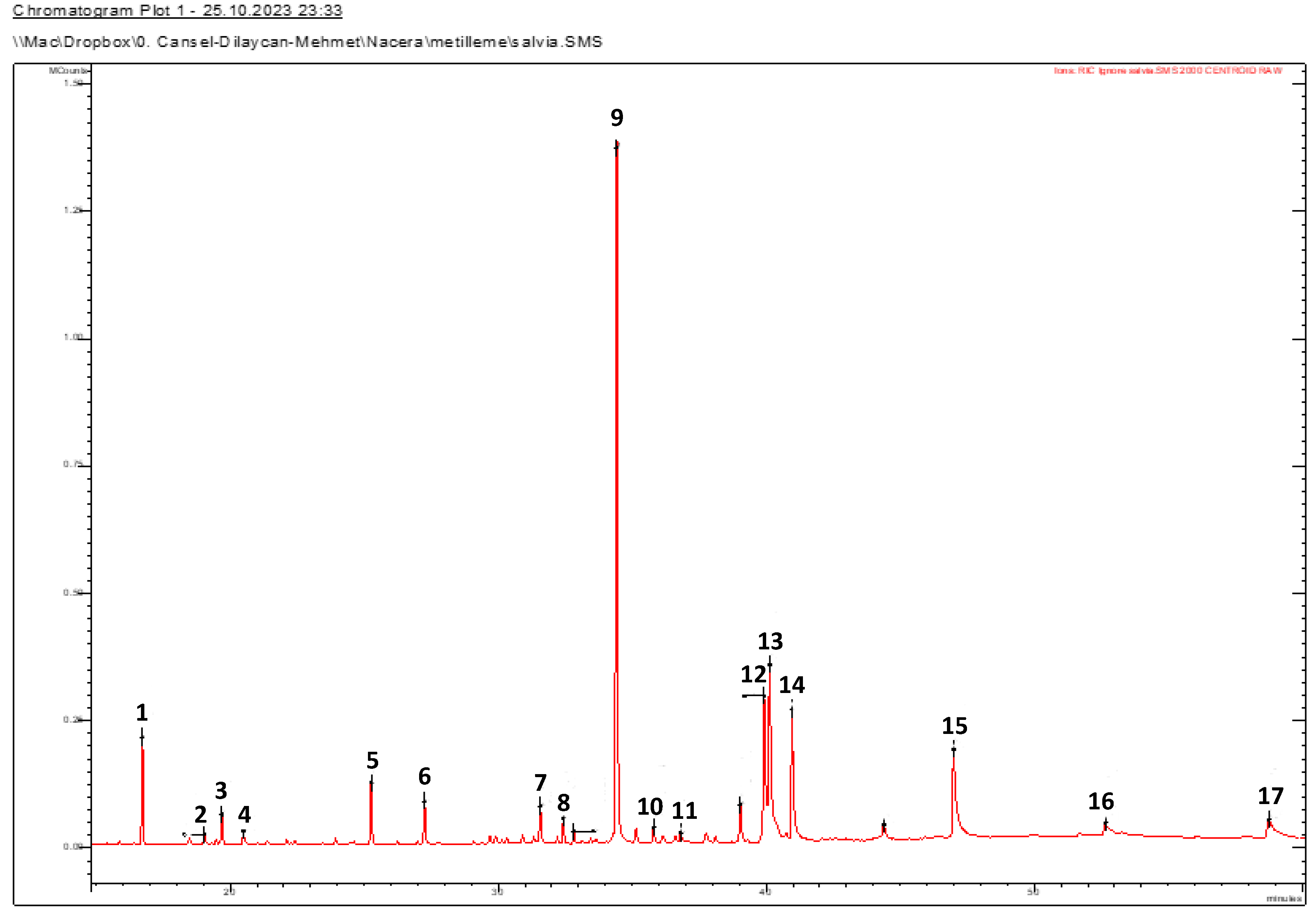
2.5. GC-MS of Fatty Acid Composition
2.6. Biological Activities
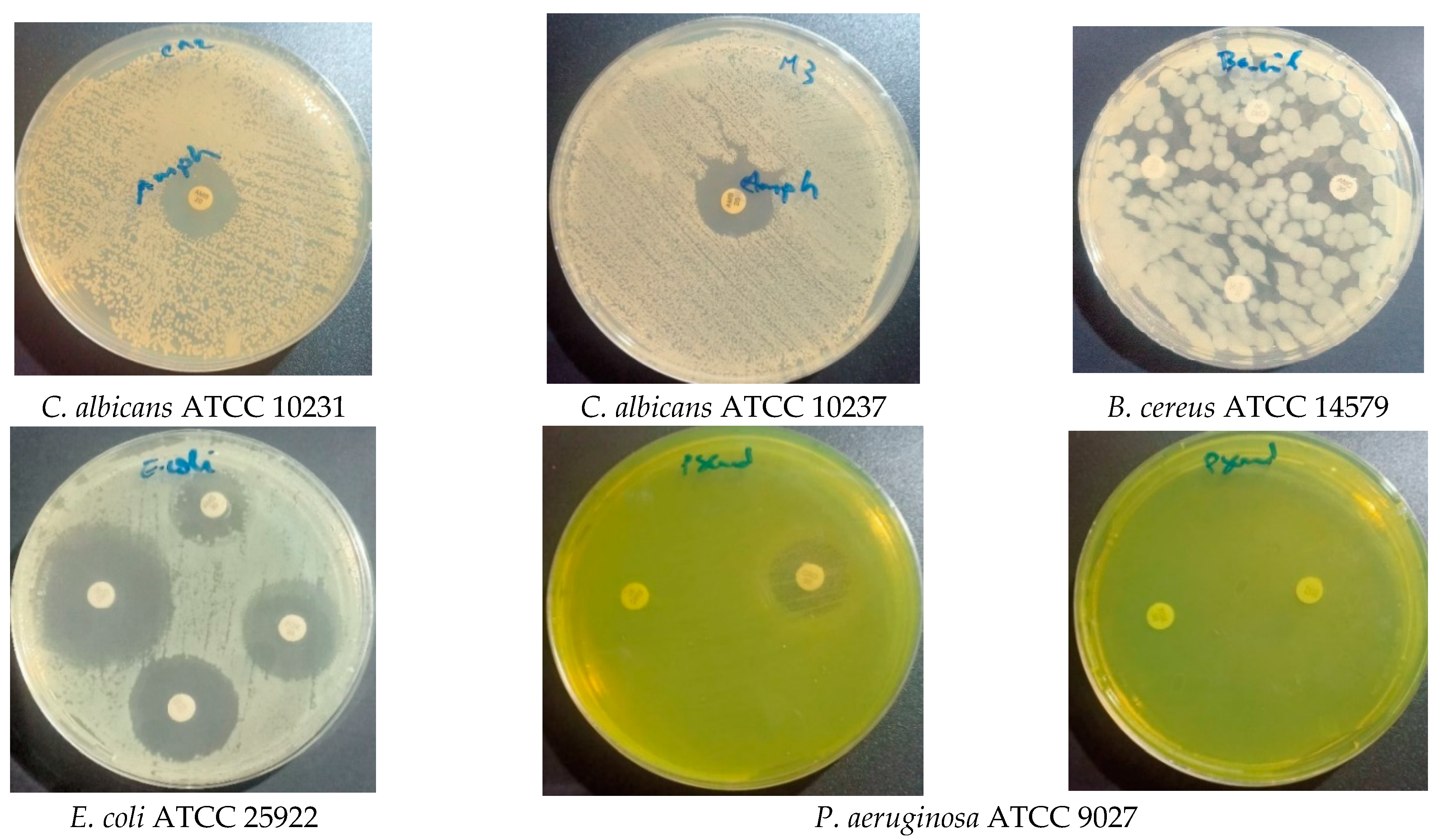
2.6.1. Antimicrobial Activity
2.6.2. Antioxidant Properties
2.6.3. Enzyme Inhibition Activity
3. Materials and Methods
3.1. Chemicals and Spectral Measurements
3.2. Plant Material
3.3. Phytochemical Screening
3.3.1. Test for Tannins
3.3.2. Test for Flavonoids
3.3.3. Test for Phenolic Compounds
3.3.4. Quinones
3.3.5. Anthraquinones
3.3.6. Terpenoids
3.3.7. Saponins
3.3.8. Reducing Compounds
3.4. Preparation of Crude Extracts
3.5. High-Performance Liquid Chromatography Analyses (HPLC-DAD)
3.6. HPLC-MS Analysis
3.7. Fatty Acids Analysis by GC–MS
3.8. Mineral Analyses
3.8.1. Sample Preparation
3.8.2. ICP-MS Analysis
3.9. Biological Activities
3.9.1. Antimicrobial Activities
Determination of Antimicrobial Activities
Determination of Minimum Inhibitory Concentration (MIC)
Minimal Bactericidal Concentration (MBC) and Minimal Fungicidal Concentration (MFC)
3.9.2. Antioxidant Activity
Free radical Scavenging Activity DPPH Assay
ABTS Cation Radical Decolorization Assay
Cupric Reducing Antioxidant Capacity (CUPRAC)
3.9.3. Enzyme inhibition Activity
Anticholinesterase Activity
α-Amylase/α-Glucosidase Inhibitory Activities
- Determination of α-amylase inhibitory activity
- 2
- Determination of α-glucosidase inhibitory activity
3.10. Statistical Examinations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aquaron, M. Plantes qui Nourrissent, Plantes qui Guérissent, Dans L’espace Alpin. Les Actes du Colloque d’Anthropologie Alpine. 2003. Available online: https://www.hominides.com/html/references/colloque_anthropologie_alpine.php (accessed on 25 January 2023).
- Hassan, F.; Ali, E.F.; Mahfouz, S. Comparison between different fertilization sources, irrigation frequency and their combinations on the growth and yield of the coriander plant. Aust. J. Appl. Basic Sci. 2012, 6, 600–615. [Google Scholar]
- Hassan, F.; Al-Yasi, H.; Ali, E.F.; Alamer, K.; Hessini, K.; Attia, H.; El-Shazly, S. Mitigation of salt stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 2021, 101, 157–165. [Google Scholar] [CrossRef]
- Chermat, S.; Gharzouli, R. Ethnobotanical study of medicinal flora in the North East of Algeria-An empirical knowledge in Djebel Zdimm (Setif). J. Mater. Sci. Eng. 2015, 5, 50–59. [Google Scholar] [CrossRef]
- Agisho, H.; Osie, M.; Lambore, T. Traditional medicinal plants utilization, management and threats in Hadiya Zone, Ethiopia. J. Med. Plants 2014, 2, 94–108. [Google Scholar]
- Enyew, A.; Asfaw, Z.; Kelbessa, E.; Nagappan, R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr. Res. J. Biol. Sci. 2014, 6, 154–167. [Google Scholar] [CrossRef]
- Miara, M.D. Importance de la Taxonomie et de la Chorologie Dans la Recherche Ethnobotanique: Analyse Critique de Quelques études Réalisées en Algérie». 16e Séminaire Annuel d’Ethnobotanique du Domaine Européen. Forcalquier, France. 2016. Available online: https://www.researchgate.net/publication/320556534_IMPORTANCE_DE_LA_TAXONOMIE_ET_DE_LA_CHOROLOGIE_DANS_LA_RECHERCHE_ETHNOBOTANIQUE_ANALYSE_CRITIQUE_DE_QUELQUES_ETUDES_REALISEES_EN_ALGERIE (accessed on 20 May 2023).
- Hamlyn, P. The Marshall Cavendish Encyclopedia of Gardening. Garrod Lofthouse Int. 1969, 19, 2034. [Google Scholar]
- Médail, F.; Quézel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Véla, E.; Benhouhou, S. Evaluation d’un nouveau point chaud de biodiversité végétale dans le Bassin méditerranéen (Afrique du Nord). Compt. Rend. Biol. 1997, 330, 589–605. [Google Scholar] [CrossRef]
- Dobignard, A.; Chatelain, C. Index Synonymique et Bibliographique de la Flore d’Afrique du Nord; Conservatoire et Jardin Botaniques de la Ville de Genève: Genève, Switzerland, 2010–2013; pp. 1–5. [Google Scholar]
- Quézel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; CNRS, Ed.; Centre National de la Recherche Scientifique (CNRS): Paris, France, 1962–1963; Volume 2, p. 1170. [Google Scholar]
- Jora. Décret exécutif no 12-03 du 10 Safar 1433 correspondant au 4 janvier 2012 fixant la liste des espèces végétales non cultivées protégées. J. Off. République Algérienne 2012, 51, 12–39. [Google Scholar]
- Walter, K.S.; Gillett, H.J. 1997 IUCN Red List of Threatened Plants; IUCN, Ed.; SSC: Gland, Switzerland, 1998. [Google Scholar]
- Ortiz-Mendoza, N.; Aguirre-Hernández, E.; Fragoso-Martínez, I.; González-Trujano, M.E.; Basurto-Peña, F.A.; Martínez-Gordillo, M.J. A Review on the Ethnopharmacology and Phytochemistry of the Neotropical Sages (Salvia Subgenus Calosphace; Lamiaceae) Emphasizing Mexican Species. Front. Pharmacol. 2022, 13, 867892. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, P.; Lasser, A.; Grandjran, A.; Auberty, R.; Fourey, A. Le climat de l’Algérie. Alger 1946, 4, 219. [Google Scholar]
- Codd, L.E.W. Lamiaceae: Flora of Southern Africa; 28 Botanical Research Institute: Pretoria, Southern Africa, 1985. [Google Scholar]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, G.; Choi, F.F.K.; Ding, L.-S.; Han, Q.B.; Song, J.Z.; Qiao, C.F.; Zhao, Q.S.; Xu, H.X. Qualitative and quantitative analysis of diterpenoids in Salvia, species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 4847–4858. [Google Scholar] [CrossRef]
- Zhang, B.B.; Nie, S.Q.; Liang, J.Y.; Wu, F.H.; Feng, F. Researches on the Chemical Constituents and Bioactivity of Salvia spp. Strait Pharm. J. 2014, 26, 1–5. [Google Scholar]
- Topcu, G. Bioactive Triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef]
- Uysal, I.; Oğuzhan, K.; Falah Saleh, M.; Ömer, L.; Mehmet, D.; Ali Erdem, Ş.; Emre, S.; Filiz Özbas, G.; Mustafa, S. Pharmacological and Nutritional Properties: Genus Salvia. Adv. Pharm. Pharm. 2023, 11, 140–155. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Wang, S.Q. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J. Mol. Cell. Card. 2006, 41, 138–148. [Google Scholar] [CrossRef]
- Li, Y.S.; Yan, L.; Yong, Y.Q. Effect of tanshinone IIA on the transforming growth factor β1 Smads signal pathway in rats with hypertensive myocardial hypertrophy. Chin. J. Integr. Tradit. West. Med. 2010, 30, 499–503. [Google Scholar]
- Mekki, S.; Belhocine, M.; Bouzouina, M.; Chaouad, B.; Mostari, A. Therapeutic effects of Salvia balansae on metabolic disorders and testicular dysfunction mediated by a high-fat diet in Wistar rats. Medit. J. Nutr. Metab. 2023, 16, 21–39. [Google Scholar] [CrossRef]
- Mahdjoub, M.M.; Benzitoune, N.; Maiz, Y.; Aouadi, N.E.H.; Bouhenna, M.M.; Kadri, N. HPLC-DAD screening and antioxidant activity of phenolic compounds of Salvia balansae de Noé leaves extract. J. Res. Pharm. 2023, 27, 1076–1085. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Beto, J.A. The role of calcium in human aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Tel-Çayan, G.; Öztürk, M.; Duru, M.E.; Yabanli, M.; Türkoğlu, A. Content of Minerals and Trace Elements Determined by ICP-MS in Eleven Mushroom Species from Anatolia, Turkey. Chiang Mai J. Sci. 2017, 44, 939–945. [Google Scholar]
- Pham, P.C.; Pham, P.M.; Pham, S.V.; Miller, J.M.; Pham, P.T. Hypomagnesemia in patients with type 2 diabetes. Clin. J. Am. Soc. Nephr. CJASN 2007, 2, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endoc. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.; Bawazeer, N.; Joy, S.S. Variation in macro and trace elements in progression of type 2 diabetes. Sci. World J. 2014, 2014, 461591. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Pande, D.; Karki, K.; Kumar, A.; Khanna, R.S.; Khanna, H.D. Trace elements and antioxidant enzymes associated with oxidative stress in the pre- eclamptic/eclamptic mothers during fetal circulation. Clin. Nutr. 2012, 31, 946–950. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Canada, A.T.; Sacco, C. Trace Elements and Public Health. Ann. Rev. Public Health 1985, 6, 131–146. [Google Scholar] [CrossRef]
- Malenčić, D.R.; Jevrešan, Ž.S.; Popović, M.T. Mineral composition of selected Salvia species growing wild in the Vojvodina province. Mat. Srp. Proc. Nat. Sci. 2003, 105, 25–33. [Google Scholar] [CrossRef]
- Hejaz, H.A.; Sabbobeh, R.; Halayqa, N.; Jahajha, A.; Abu-Lafi, S. Minerals Analysis of Salvia palaestina Leaves: Quantification and Detection. J. Plan. Sci. 2022, 17, 53–61. [Google Scholar] [CrossRef]
- Tapas, A.R.; Sakarkar, D.; Kakde, R. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Topuz, A.; Sahin-Nadeem, H.; Ozdemir, K.S.; Cam, I.B.; Tontul, I.; Gokturk, R.S.; Ay, S.T. A comparative study on phenolic composition, antioxidant activity and essential oil content of wild and cultivated sage (Salvia fruticosa Miller) as influenced by storage. Ind. Crop. Prod. 2012, 39, 170–176. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Lopez-Avila, V.; Milanes, J.; Beckert, W.F. Single-laboratory evaluation of method 8060 for the determination of phthalates in environmental samples. J. Assoc. Off. Anal. Chem. 1991, 74, 793–808. [Google Scholar]
- Turchini, G.M.; Giani, I.; Caprino, F.; Moretti, V.M.; Valfre, F. Discrimination of origin of farmed trout by means of biometrical parameters, fillet composition and flavor volatile compounds. Ital. J. Anim. Sci. 2004, 3, 123–140. [Google Scholar] [CrossRef]
- Senatore, F.; Apostolides Arnold, N.; Bruno, M. Volatile components of Centaurea eryngioides Lam. and Centaurea liberica Trev. var. hermonis Boiss. Lam., two Asteraceae growing wild in Lebanon. Nat. Prod. Res. 2005, 19, 749–754. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Lopez-Hernandez, J.; Paseiro-Losada, P.; Simal-Lozano, J. Study of the effect of light on fatty acids of potato crisps using a gas chromatographic method. Anal. Chim. Acta 2004, 524, 191–200. [Google Scholar] [CrossRef]
- Liang, Y. Retention Data; NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Rout, P.K.; Misra, R.; Sahoo, S.; Sree, A.; Rao, Y.R. Extraction of kewda (Pandanus fascicularis Lam.) flowers with hexane: Composition of concrete, absolute and wax. Flavour Fragr. J. 2005, 20, 442–444. [Google Scholar] [CrossRef]
- Kowalski, R. Analysis of lipophylic fraction from leaves, inflorescences and rhizomes of Siphium perfoliatum L. Acta Soc. Bot. Pol. 2005, 74, 5–10. [Google Scholar] [CrossRef]
- Robinson, A.L.; Adams, D.O.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Influence of geographic origine on the sensory characteristics and wine composition of Vitus viniferas cv. Cabernet Sauvignon wines from Australia (Supplemental data). Am. J. Enol. Vitic. 2012, 64, 467–476. [Google Scholar] [CrossRef]
- Rout, P.K.; Rao, Y.R.; Sree, A.; Naik, S.N. Composition of essential oil, concrete, absolute, wax and headspace volatiles of Murrarya paniculata (Linn.) Jack flowers. Flavour Fragr. J. 2007, 22, 352–357. [Google Scholar] [CrossRef]
- da Camara, C.A.G.; Shepherd, S.L.K.; Joaquim, D.R.G. Analise quimnica da cultura de tecidos do hibrido Clusia paralicola X Clusia weddelliana. Rev. Bras. Farmacogn. 2002, 12, 26–28. [Google Scholar] [CrossRef]
- Remberger, M.; Hynning, P.-A.; Neilson, A.H. Gas chromatographic analysis and gas chromatographic-mass spectrometric identification of compounds in the cyclohexane-extractable fraction from contaminated sediment samples. J. Chromatogr. 1990, 508, 159–178. [Google Scholar] [CrossRef]
- Fuentes, M.J.; Font, R.; Gomez-Rico, M.F.; Martin-Gullon, I. Pyrolysis and combustion of waste lubricant oil from diesel cars: Decomposition and pollutants. J. Anal. Appl. Pyrolysis 2007, 79, 215–226. [Google Scholar] [CrossRef]
- Tepe, B.; Donmez, E.; Unlu, M.; Candan, F.; Daferera, D.; Vardar-Unlu, G. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret Aucher ex Benth.) and Salvia multicaulis (Vahl). J. Food Chem. 2004, 84, 519–525. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Ozkan, G.; Sagdic, O.; Gokturk, R.S.; Unal, O.; Albayrak, S. Study on chemical composition and biological activities of essential oil and extract from Salvia pisidica. LWT Food Sci. Technol. 2010, 43, 186–190. [Google Scholar] [CrossRef]
- Dulger, G.; Dulger, B. Evaluation of Antimicrobial Activities of Salvia verbenaca. Nat. Eng. Sci. 2023, 8, 61–71. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Ceylan, O.; Benabdallah, A.; Tepe, B. Stachys germanica subsp. heldreichii (Boiss.) Hayek: Phytochemical analysis, antioxidant and enzyme inhibitory activities. S. Afr. J. Bot. 2021, 143, 291–300. [Google Scholar] [CrossRef]
- Benabdallah, A.; Betina, S.; Bouchentouf, S.; Boumendjel, M.; Bechkri, S.; Bensouici, C.; Nicoli, F.; Vergine, M.; Carmine, N.; De Bellis, L. Chemical profiling, antioxidant, enzyme inhibitory and in silico modeling of Rosmarinus officinalis L. and Artemisia herba alba Asso. essential oils from Algeria. S. Afr. J. Bot. 2022, 147, 501–510. [Google Scholar] [CrossRef]
- Rungsimakan, S. Phytochemical and biological activity studies on Salvia viridis L. Ph.D. Thesis, University of Bath, Bath, UK, 2011. [Google Scholar]
- Mervić, M.; Bival Štefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative antioxidant, anti-acetylcholinesterase and anti-α-glucosidase activities of Mediterranean Salvia species. Plants 2022, 11, 625. [Google Scholar] [CrossRef]
- Yilmaz, G.; Eruygur, N.; Bona, G.E.; Bona, M.; Akdeniz, M.; Yilmaz, M.A.; Ertas, A. Phytochemical analysis, antioxidant, and enzyme inhibition activity of five Salvia taxa from Turkey. S. Afr. J. Bot. 2023, 152, 212–221. [Google Scholar] [CrossRef]
- Nickavar, B.; Abolhasani, L. Bioactivity-Guided Separation of an α-Amylase Inhibitor Flavonoid from Salvia virgata. Iran. J. Pharm. Res. 2013, 12, 57–61. [Google Scholar]
- Bahadori, M.B.; Salehi, P.; Sonboli, A. Comparative study of the essential oil composition of Salvia urmiensis and its enzyme inhibitory activities linked to diabetes mellitus and Alzheimer’s disease. Int. J. Food. 2017, 20, 2974–2981. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Rathi, D.N.G. Bioactive Components of Salvia and Their Potential Antidiabetic Properties: A Review. Molecules 2021, 26, 3042. [Google Scholar] [CrossRef]
- Raafat, K.; Habib, J. Phytochemical Compositions and Antidiabetic Potentials of Salvia sclarea L. Essential Oils. J. Oleo Sci. 2018, 67, 1015–1025. [Google Scholar] [CrossRef]
- Trease, E.; Evans, W.C. Pharmacognosie, Billiaire Tindall. London 3th Ed. 1987, 61-62. In Karumi, Y.; Onyeyili, P.A.; Ogugduaja, V.O. Identification des principles actifs de l’extrait de feuilles de M. balsamia (Baume de la pomme). J. Med. Sci. 2004, 4, 179–182. [Google Scholar]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 2nd ed.; Tec & Doc Lavoisier: Paris, France, 1993; p. 915. [Google Scholar]
- Harborne, J.B. Phytochemical Methods—A Guide to Modern Techniques of Plant Analysis, 5th ed.; Chapman and Hall Ltd.: London, UK, 1984; pp. 21–72. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biot. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Ertas, A.; Firat, M.; Yener, I.; Akdeniz, M.; Yigitkan, S.; Bakir, D.; Cakir, C.; Abdullah Yilmaz, M.; Ozturk, M.; Kolak, U. Phytochemical Fingerprints and Bioactivities of Ripe Disseminules (Fruit-Seeds) of Seventeen Gundelia (Kenger-Kereng Dikeni) Species from Anatolia with Chemometric Approach. Chem. Biod. 2021, 18, e2100207. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Tel, G.; Ozturk, M.; Duru, M.E.; Turkoglu, A. Antioxidant and anticholinesterase activities of five wild mushroom species with total bioactive contents. Pharm. Biol. 2015, 53, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-L.; Ong, C.-N. Comparative analysis of tea catechins and theaflavins by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2000, 881, 439–447. [Google Scholar] [CrossRef]
- Crupi, P.; Bleve, G.; Tufariello, M.; Corbo, F.; Clodoveo, M.L.; Tarricone, L. Comprehensive identification and quantification of chlorogenic acids in sweet cherry by tandem mass spectrometry techniques. J. Food Compos. Anal. 2018, 73, 103–111. [Google Scholar] [CrossRef]
- Tel, G.; Öztürk, M.; Duru, M.E.; Doğan, B.; Harmandar, M. Fatty Acid Composition, Antioxidant, Anticholinesterase and Tyrosinase Inhibitory Activities of Four Serratula Species from Anatolia. Rec. Nat. Prod. 2013, 7, 86–95. [Google Scholar]
- Gülsen, T.; Öztürk Aydoğmuş, F.; Duru, M.E. The Cooking Effect on Two Edible Mushrooms in Anatolia: Fatty Acid Composition, Total Bioactive Compounds, Antioxidant and Anticholinesterase Activities. Rec. Nat. Prod. 2014, 8, 189–194. [Google Scholar]
- Duru, M.E.; Kivrak, Ş.; Mercan-Doğan, N.; Türkoglu, A.; Özler, M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food. Chem. Toxic. 2011, 49, 1353–1360. [Google Scholar] [CrossRef]
- Tokul-Olmez, O.; Kaplaner, E.; Ozturk, M.; Ullah, Z.; Duru, M.E. Fatty acid profile of four Ganoderma species collected from various host trees with chemometric approach. Biochem. Syst. Ecol. 2018, 78, 91–97. [Google Scholar] [CrossRef]
- Cicero, N.; Gervasi, T.; Durazzo, A.; Lucarini, M.; Macrì, A.; Nava, V.; Giarratana, F.; Tardugno, R.; Vadalà, R.; Santini, A. Mineral and microbiological analysis of spices and aromatic herbs. Foods 2022, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Tel-Cayan, G.; Ullah, Z.; Ozturk, M.; Yabanli, M.; Aydin, F.; Duru, M.E. Heavy metals, trace and major elements in 16 wild mushroom species determined by ICP-MS. At. Spectrosc. 2018, 39, 29–37. [Google Scholar] [CrossRef]
- Nicoletti, M.; Maggi, F.; Papa, F.; Vittori, S.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Ralaibia, E.; et al. In vitro biological activities of the essential oil from the ‘resurrection plant’ Myrothamnus moschatus (Baillon) Niedenzu endemic to Madagascar. Nat. Prod. Res. 2012, 26, 2291–2300. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute CLSI. Performance Standards for Antimicrobial Disk Susceptibility Test; Approved Standard. In CLSI Document M02-A10, 10th ed.; Clinical and Laboratory Standards Institute CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Duraffourd, C.; D’Hervicourt, L.; Lapraz, J.C. Cahiers de phytothérapie clinique. In 1. Examens de Laboratoires Galénique. Eléments Thérapeutiques Synergiques, 2nd ed.; Masson: Paris, France, 1990. [Google Scholar]
- Okusa, P.; Penge, O.; Devleeschouwer, M.; Duez, P. Direct and Indirect Antimicrobial Effects and Antioxidant Activity of Cordia Gilletii De Wild (Boraginaceae). J. Ethnopharmacol. 2007, 112, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Dry, L.; Johnson, M.; Michalak, E.M.; Carson, C.F.; Riley, T.V. Susceptibility of oral bacteria to Melaleuca alternifolia (tea tree) oil in vitro. Oral. Microb. Immunol. 2003, 18, 389–392. [Google Scholar] [CrossRef]
- Levison, M.E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 2004, 18, 451–465. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable Free Radical. Nature 1958, 181, 1119–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gu, K.; Mustafa, O.; Celik, S.E. Mechanism of Antioxidant Capacity Assays and the CUPRAC (Cupric Ion Reducing Antioxidant Capacity) Assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Öztürk, M. Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem. 2012, 134, 48–54. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Topal, M. Secondary metabolites of ethanol extracts of Pinus sylvestris cones from Eastern Anatolia and their antioxidant, cholinesterase and α-glucosidase activities. Rec. Nat. Prod. 2020, 14, 129–138. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Chun, W.J.; Kim, T.Y.; Sun, J.; Yu, C.Y.; Kim, M.J. Rhus verniciflua Stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010, 120, 539–543. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Tran, H.D.; Ahmad, A.; Khanh, T.D.; Dat, T.D. Contribution of momilactones A and B to diabetes inhibitory potential of rice bran: Evidence from in vitro assays. Saudi Pharm. J. 2019, 27, 643–649. [Google Scholar] [CrossRef]



| Plant | Results | |
|---|---|---|
| Compounds | ||
| Phenolic compounds | + | |
| Flavonoids | + | |
| Tannins (Gallic) | + | |
| Quinones | ++ | |
| Anthraquinones | ++ | |
| Terpenoids | + | |
| Saponins | +++ | |
| Reducing compounds | +++ | |
| Plants | S. balansae | Certified and Experimental Values of Studied Metals in NIST-CRM 1203 Drinking Water (mg/kg). a | |||
|---|---|---|---|---|---|
| Mineral Contents | |||||
| Certified Value (mg/kg) | Experimental Value ± S.D. (mg/kg) b | Recovery Value (%) | |||
| Phosphorus (%) | 0.24 ± 0.00 * | - | - | - | |
| Potassium (%) | 2.38 ± 0.07 * | - | - | - | |
| Calcium (%) | 1.52 ± 0.15 | 99.78 ± 0.50 | 100.42 ± 0.95 | 100.64 | |
| Magnesium (%) | 0.45 ± 0.03 | 99.77 ± 0.50 | 100.68 ± 1.02 | 100.23 | |
| Iron (mg L−1) | 1160.36 ± 23.20 * | 200.3 ± 1.0 | 199.89 ± 2.05 | 99.94 | |
| Copper (mg L−1) | 19.53 ± 0.97 | 2000 ± 10 | 202.9 ± 0.12 | 101.45 | |
| Manganese (mg L−1) | 66.22 ± 2.64 * | 50.17 ± 0.25 | 50.02 ± 0.75 | 99.67 | |
| Zinc (mg L−1) | 67.43 ± 2.02 * | 1000 ± 5 | 1003.1 ± 7.8 | 102.59 | |
| Boron (mg L−1) | 30.16 ± 0.60 * | - | - | - | |
| Peak N° | Compounds | RT (min) | DAD Spectra Values | μg/g Extract |
|---|---|---|---|---|
| 1 | Protocatechuic acid | 24.625 | 259, 294 | 50 |
| 2 | p-Hydroxybenzoic acid | 30.867 | 208, 255 | 60 |
| 3 | 6,7-Dihydroxycoumarin | 33.435 | 208, 257, 298, 345 | tr |
| 4 | Vanillic acid | 34.758 | 208, 260, 292 | 180 |
| 5 | Caffeic acid | 35.280 | 215, 324 | tr |
| 6 | p-Coumaric acid | 40.874 | 208, 309 | tr |
| 7 | Ferulic acid | 42.564 | 216, 233, 324 | 450 |
| 8 | Coumarin | 45.178 | 278, 309 | tr |
| 9 | trans-2-Hydroxycinnamic acid | 48.243 | 212, 276, 325 | tr |
| 10 | trans-Cinnamic acid | 56.203 | 204, 275 | tr |
| 11 | Luteolin | 57.872 | 254, 350 | 480 |
| 12 | Kaempferol | 62.485 | 255, 363 | 130 |
| N | RT (min) | Area (mV.s) | Height (mV) | Area (%) | Height (%) | W05 (min) | Compound Name |
|---|---|---|---|---|---|---|---|
| 1 | 3.460 | 85.937 | 7.920 | 0.7 | 5.1 | 0.14 | Sinapinic acid |
| 2 | 3.603 | 10.603 | 1.968 | 0.1 | 1.3 | 0.09 | Nicotinamide |
| 3 | 4.613 | 9.040 | 0.603 | 0.1 | 0.4 | 0.12 | Ascorbic acid |
| 4 | 16.590 | 2686.466 | 15.792 | 21.7 | 10.1 | 3.24 | Myricetin |
| 5 | 19.060 | 131.010 | 5.637 | 1.1 | 3.6 | 0.48 | Butylated hydroxyanisole |
| 6 | 20.403 | 156.513 | 7.668 | 1.3 | 4.9 | 0.25 | Epicatechin |
| 7 | 21.667 | 111.398 | 3.643 | 0.9 | 2.3 | 0.52 | genistein |
| 8 | 23.097 | 85.366 | 4.143 | 0.7 | 2.7 | 0.27 | Kaempferol |
| 9 | 28.863 | 8965.095 | 101.406 | 72.5 | 65.1 | 0.63 | Catechin |
| 10 | 31.190 | 124.939 | 6.918 | 1.0 | 4.4 | 0.24 | ND |
| Total | 12366.366 | 155.697 | 100.0 | 100.0 |
| Peak N° | RT | RI a | RI b | Compound Name | Concentration (%) | Literature |
|---|---|---|---|---|---|---|
| 1 | 16.723 | 1431 | 1452.5 | Dimethyl phthalate | 4.80 | Lopez-Avila et al. [44] |
| 2 | 19.026 | 1443 | 1473 | Buthylatedhydroxytoluen | 0.55 | Turchini et al. [45] |
| 3 | 19.69 | 1499 | 1486 | Dihydroactinidiolide | 1.51 | Senatore et al. [46] |
| 4 | 20.489 | 1540 | 1540.9 | Nonanedioic acid (Azaleic acid) | 0.77 | Sanches-Silvia et al. [47] |
| 5 | 25.262 | 1689 | 1689 | Isobutyl methyl phthalate | 3.11 | Liang [48] |
| 6 | 27.251 | 1725 | 1723 | Myristic acid (C14:0) | 2.24 | Rout et al. [49] |
| 7 | 31.568 | 1842 | 1847 | Phytone | 1.92 | Kowalski [50] |
| 8 | 32.429 | 1863 | 1864 | Diisobutyl phthalate | 1.33 | Lopez-Avila et al. [44] |
| 9 | 34.418 | 1938 | 1926 | Palmitic acid (C16:0) | 36.72 | Robinson et al. [51] |
| 10 | 35.143 | 1943 | 1949 | Isophytol | 0.86 | Rout et al. [52] |
| 11 | 36.815 | 1995 | 1996 | 15-Methyl-hexadecanoic acid | 0.49 | da Camara et al. [53] |
| 12 | 39.923 | 2090 | 2096 | Lineloic acid (C18:2) | 9.39 | Rout et al. [52] |
| 13 | 40.126 | 2095 | 2106 | Oleic acid (C18:1) | 12.62 | Rout et al. [49] |
| 14 | 40.963 | 2128 | 2128 | Stearic acid (C18:0) | 8.97 | Rout et al. [49] |
| 15 | 46.993 | 2324 | 2321.8 | Eicosanoic acid (C20:0) | 8.37 | Remberger et al. [54] |
| 16 | 52.657 | 2531 | 2531 | Docosanoic acid (C22:0) | 0.78 | Kowalski [50] |
| 17 | 58.747 | 2600 | 2600 | n-Hexacosane | 0.97 | Fuentes [55] |
| Strains | S. aureus (MRSA) ATCC 34300 | S. aureus ATCC 6538 | P. aeruginosa ATCC 9027 | E. coli ATCC 8739 | E. coli ATCC 25922 | B. cereus ATCC 14579 | C. albicans (CA2) ATCC 10231 | C. albicans (M3) ATCC 10237 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract | |||||||||||
| S. balansae | Diameter of inhibition zone (mm) * | 50 (mg/mL) | 13 ± 0.66 | 10 ± 1.11 | 10 ± 0 | 13.33 ± 0.44 | 10.66 ± 0.44 | 10.66 ± 0.44 | 10.66 ± 0.44 | 12.33 ± 0.44 | |
| 100 (mg/mL) | 14 ± 0 | 11 ± 0 | 13 ± 0 | 14 ± 0 | 11 ± 0 | 11 ± 0 | 12 ± 0 | 15 ± 0 | |||
| 200 (mg/mL) | 16 ± 0 | 11 ± 0 | 14 ± 0 | 17 ± 0 | 12 ± 0 | 11 ± 0 | 15 ± 0 | 18 ± 0 | |||
| MIC (μg/mL) | 2000 | <2000 | 1000 | 2000 | 2000 | <2000 | <2000 | 2000 | |||
| MBC (μg/mL) | 2000 | 2000 | 2000 | 2000 | 2000 | 2000 | - | - | |||
| MFC (μg/mL) | - | - | - | - | - | - | 2000 | 2000 | |||
| MBC/MIC ratio | 1 | Nd | 2 | 1 | 1 | Nd | - | - | |||
| MFC/MIC ratio | - | - | - | - | - | - | Nd | 1 | |||
| Strains | S. aureus (MRSA) ATCC 34300 | S. aureus ATCC 6538 | P. aeruginosa ATCC 9027 | E. coli ATCC 8739 | E. coli ATCC 25922 | B. cereus ATCC 14579 | C. albicans (CA2) ATCC 10231 | C. albicans (M3) ATCC 10237 | |
|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | |||||||||
| inhibition zone (mm) | Amoxicillin (AMC) 30 µg | 40 | 22 | 00 | 34 | 20 | 15 | - | - |
| Cefazolin (CZ) 30 µg | 00 | 23 | 00 | 34 | 25 | 00 | - | - | |
| Ceftriaxon (CRO) 30 µg | 00 | 15 | 18 | 44 | 36 | 00 | - | - | |
| Cefoxitin (FOX) 30 µg | 22 | 12 | 00 | 40 | 25 | 00 | - | - | |
| Amph (AMB) 20 µg | - | - | - | - | - | - | 22 | 20 |
| Extracts and Compounds | Antioxidant Activity | ||
|---|---|---|---|
| DPPH Assay a | ABTS Assay a | CUPRAC Assay a | |
| IC50 (µg/mL) | IC50 (µg/mL) | A0.5(µg/mL) | |
| Methanol extract | 242.7 ± 7.44 | 124.1 ± 9.70 | 222.9 ± 6.05 |
| Petroleum ether extract | NA | NT | NT |
| BHA b | 3.44 ± 0.09 | 1.88 ± 0.06 | 5.62 ± 0.08 |
| Extracts | Anticholinesterase Activity | Antidiabetic Activity | ||
|---|---|---|---|---|
| AChE Assay a | BChE Assay a | α-Amylase Inhibitory Assay a | α-Glucosidase Inhibitory Assay a | |
| IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | |
| Methanol extract | >200 | >200 | >400 | >400 |
| Petroleum ether extract | >200 | 82.33 ± 2.13 | >400 | >400 |
| Galantamine b | 4.31 ± 0.03 | 45.29 ± 0.06 | - | - |
| Acarbose b | - | - | Nd | Nd |
| No | Compounds | RT a (min) | Calibration Equation | R2 b | Linear Range (μg/mL) | λmax, nm | LOD c (μg/mL) | LOQ c (μg/mL) | Recovery (%) | RSD d within Day (n = 7) | RSD between Days (n = 7) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Protocatechuic acid | 24.625 | y = 76181x − 88801 | 0.9995 | 3.13–100 | 254 | 3.42 | 10.35 | 102.35 ± 4.21 | 3.19 | 1.22 |
| 2 | 4-Hydroxybenzoic acid | 30.867 | y = 111102x + 21691 | 0.9993 | 1.56–50.0 | 254 | 1.58 | 4.79 | 100.82 ± 3.89 | 4.00 | 2.41 |
| 3 | 6,7-Dihydroxycoumarin | 33.435 | y = 34377x − 32740 | 0.9940 | 5.00–50 | 254 | 3.98 | 12.07 | 104.11 ± 5.06 | 4.94 | 3.72 |
| 4 | Vanillic acid | 34.758 | y = 74653x − 9634.1 | 0.9998 | 1.56–100 | 254 | 1.56 | 4.68 | 103.58 ± 4.43 | 5.06 | 3.88 |
| 5 | Caffeic acid | 35.280 | y = 67972x − 32965 | 0.9880 | 3.00–30.0 | 254 | 4.54 | 13.75 | 102.67 ± 4.92 | 4.01 | 5.87 |
| 6 | p-Coumaric acid | 40.874 | y = 18300x + 6153.3 | 0.9998 | 6.25–400 | 254 | 5.46 | 16.56 | 101.60 ± 2.36 | 3.14 | 0.44 |
| 7 | Ferulic acid | 42.564 | y = 35737x + 12977 | 0.9999 | 2.34–300 | 254 | 3.96 | 11.99 | 100.99 ± 3.54 | 3.20 | 0.51 |
| 8 | Coumarin | 45.178 | y = 36021x − 23215 | 0.9999 | 3.13–100 | 254 | 2.21 | 6.69 | 101.74 ± 4.83 | 3.59 | 1.08 |
| 9 | trans-2-Hydroxycinnamic acid | 48.243 | y = 53843x + 124308 | 0.9996 | 3.13–400 | 254 | 3.09 | 9.27 | 99.75 ± 3.75 | 2.85 | 0.75 |
| 10 | trans-Cinnamic acid | 56.203 | y = 87505 + 4540.2 | 0.9999 | 1.25–50.0 | 254 | 0.58 | 1.74 | 100.85 ± 1.58 | 5.78 | 5.66 |
| 11 | Luteolin | 57.872 | y = 9895.8x + 159212 | 0.9950 | 4.84–620 | 254 | 2.75 | 8.34 | 100.00 ± 4.91 | 2.88 | 2.39 |
| 12 | Kaempferol | 62.485 | y = 68024x + 7902.3 | 0.9999 | 1.56–100 | 254 | 1.01 | 3.06 | 98.57 ± 3.84 | 1.87 | 5.04 |
| Instrument | Agilent™ 7700x ICP-MS |
| RF power | 1600 W |
| RF match | 2.10 V |
| Sampling depth | 10.0 nm |
| Nebulizer gas | 0.57 L/min |
| S/C temperature | 2 °C |
| Nebulizer type | MicroMist |
| Spray chamber | Scott-type double-pass |
| Ar flow rate | Plasma: 15 L/min; Auxiliary: 0.9 L/min; Nebulizer: 1.0–1.1 L/min |
| Solution uptake rate | 1.8 mL/min |
| VacuumInterface | 4 torr, quadrupole: 2 105 torr |
| Data acquisition | Peak hopping; Replicate time 200 ms; Dwell time 200 ms; Sweeps/reading 3; Readings/replicate 3; Number of replicates 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, A.; Souhila, T.; Nacéra, B.; Amina, B.; Alghonaim, M.I.; Öztürk, M.; Alsalamah, S.A.; Miara, M.D.; Boufahja, F.; Bendif, H. In Vitro Antibacterial, Antioxidant, Anticholinesterase, and Antidiabetic Activities and Chemical Composition of Salvia balansae. Molecules 2023, 28, 7801. https://doi.org/10.3390/molecules28237801
Mokhtar A, Souhila T, Nacéra B, Amina B, Alghonaim MI, Öztürk M, Alsalamah SA, Miara MD, Boufahja F, Bendif H. In Vitro Antibacterial, Antioxidant, Anticholinesterase, and Antidiabetic Activities and Chemical Composition of Salvia balansae. Molecules. 2023; 28(23):7801. https://doi.org/10.3390/molecules28237801
Chicago/Turabian StyleMokhtar, Amırat, Tabak Souhila, Bouriah Nacéra, Benabdallah Amina, Mohammed I. Alghonaim, Mehmet Öztürk, Sulaiman A. Alsalamah, Mohamed Djamel Miara, Fehmi Boufahja, and Hamdi Bendif. 2023. "In Vitro Antibacterial, Antioxidant, Anticholinesterase, and Antidiabetic Activities and Chemical Composition of Salvia balansae" Molecules 28, no. 23: 7801. https://doi.org/10.3390/molecules28237801
APA StyleMokhtar, A., Souhila, T., Nacéra, B., Amina, B., Alghonaim, M. I., Öztürk, M., Alsalamah, S. A., Miara, M. D., Boufahja, F., & Bendif, H. (2023). In Vitro Antibacterial, Antioxidant, Anticholinesterase, and Antidiabetic Activities and Chemical Composition of Salvia balansae. Molecules, 28(23), 7801. https://doi.org/10.3390/molecules28237801







