Antioxidant Properties of Maqui Berry Extract (Aristotelia chilensis (Mol.) Stuntz) and Its Potential Photoprotective Role on Human Skin Fibroblasts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Antioxidant Activities
2.2. Chemical Composition and Toxic Element Levels
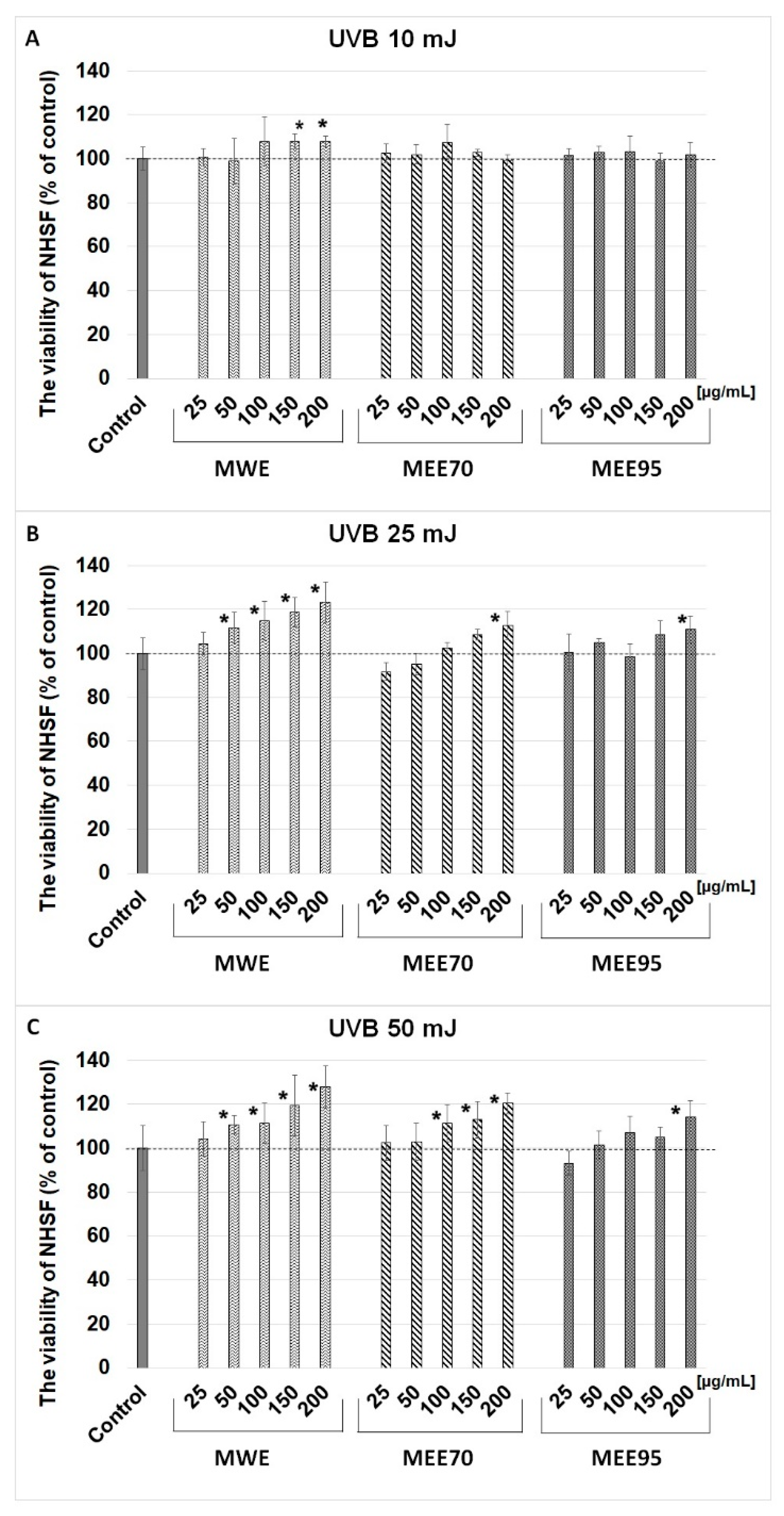
2.3. Cell Viability, DNA Synthesis, and Photoprotective Role
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Material and Extracts Preparation
3.3. Determination of Antioxidant Capacity Using DPPH Assays
3.4. Determination of Total Phenolic Content (TPC) Analysis
3.5. Determination of Toxic Metal Levels—As, Cd, and Pb
3.6. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.7. Cell Culture
Cytotoxicity Assay
3.8. DNA Synthesis Assay
3.9. UVB Irradiation
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schön, C.; Wacker, R.; Micka, A.; Steudle, J.; Lang, S.; Bonnländer, B. Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients 2018, 10, 1720. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of antioxidant compounds and -Glucosidase-Amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Bribiesca-Cruz, I.; Moreno, D.A.; García-Viguera, C.; Gallardo, J.M.; Segura-Uribe, J.J.; Pinto-Almazán, R.; Guerra-Araiza, C. Maqui berry (Aristotelia chilensis) extract improves memory and decreases oxidative stress in male rat brain exposed to ozone. Nutr. Neurosci. 2021, 24, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Osorio, M.J.; Parada, J.; Robert, P. Stability and bioaccessibility of anthocyanins from maqui (Aristotelia chilensis [Mol.] Stuntz) juice microparticles. J. Food Sci. Technol. 2018, 91, 549–556. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M. Evaluation of phenolic profiles and antioxidant capacity of maqui (Aristotelia chilensis) berries and their relationships to drying methods. J. Sci. Food Agric. 2018, 98, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Go’mez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Torres, M.D.; Camilo, C.; Mariotti-Celis, M.S.; Domínguez, H.; Pérez-Correa, J.R. Multi-response optimal hot pressurized liquid recovery of extractable polyphenols from leaves of maqui (Aristotelia chilensis [Mol.] Stuntz). Food Chem. 2021, 357, 129729, Advance online publication. [Google Scholar] [CrossRef]
- Guo, H.; Weng, W.; Zhang, S.; Rinderknecht, H.; Braun, B.; Breinbauer, R.; Gupta, P.; Kumar, A.; Ehnert, S.; Histing, T.; et al. Maqui Berry and Ginseng Extracts Reduce Cigarette Smoke-Induced Cell Injury in a 3D Bone Co-Culture Model. Antioxidants 2022, 11, 2460. [Google Scholar] [CrossRef]
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Zúniga, G.E.; Tapia, A.; Arenas, A.; Contreras, R.A.; Zúñiga-Libano, G. Phytochemistry and biological properties of Aristotelia chilensis a Chilean blackberry: A review. Phytochem. Rev. 2017, 16, 1081–1094. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic efects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Perez, D.D.; Vasquez, L.; Martinez, A.L.F.; Leighton, F. Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Femenias, A.; Martínez-Garza, Ú.; Sanz-Lamora, H.; Castagnini, J.M.; Quifer-Rada, P.; Lamuela-Raventós, R.M.; Marrero, P.F.; Haro, D.; Relat, J. Lyophilized Maqui (Aristotelia chilensis) Berry Induces Browning in the Subcutaneous White Adipose Tissue and Ameliorates the Insulin Resistance in High Fat Diet-Induced Obese Mice. Antioxidants 2019, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Romero-González, J.; Shun Ah-Hen, K.; Lemus-Mondaca, R.; Muñoz-Fariña, O. Total phenolics, anthocyanin profile and antioxidant activity of maqui, Aristotelia chilensis (Mol.) Stuntz, berries extract in freeze-dried polysaccharides microcapsules. Food Chem. 2020, 313, 126115. [Google Scholar] [CrossRef]
- Céspedes, C.; Valdez-Morales, M.; Avila, J.; El-Hafidi, M.; Alarcón, J.; Paredes-López, O. Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae). Food Chem. 2010, 1, 886–895. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Pavon, N.; Dominguez, M.; Alarcon, J.; Balbontin, C.; Kubo, I.; El-Hafidi, M.; Avila, J.G. The Chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), Maqui as mediator in inflammation-associated disorders. Food Chem. Toxicol. 2017, 108, 438–450. [Google Scholar] [CrossRef]
- Agulló, V.; González-Trujano, M.E.; Hernandez-Leon, A.; Estrada-Camarena, E.; Pellicer, F.; García-Viguera, C. Antinociceptive effects of maqui-berry (Aristotelia chilensis (Mol.) Stuntz). Int. J. Food Sci. Nutr. 2021, 72, 947–955. [Google Scholar] [CrossRef]
- Dara, A.; Feizy, J.; Naji-Tabasi, S.; Fooladi, E.; Rafe, A. Intensified extraction of anthocyanins from Berberis vulgaris L. by pulsed electric field, vacuum-cold plasma, and enzymatic pretreatments: Modeling and optimization. Chem. Biol. Technol. Agric. 2023, 10, 93. [Google Scholar] [CrossRef]
- Cadet, J.; Mouret, S.; Ravanat, J.L.; Douki, T. Photoinduced damage to cellular DNA: Direct and photosensitized reactions. Photochem. Photobiol. 2012, 88, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, G.; Meng, X.S.; Fu, H.Y.; Mo, Q.G.; Wang, Y.W. Photoprotection of maqui berry against ultraviolet B-induced photodamage in vitro and in vivo. Food Funct. 2020, 11, 2749–2762. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Kadekaru, T.; Ogawa, K.; Hitoe, S.; Shimoda, H.; Hara, H. Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. J. Funct. Foods 2013, 27, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Menaa, A. Skin photoprotection by polyphenols in animal models and humans. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 64; pp. 831–838. [Google Scholar]
- Gonzalez, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crops Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Sobaszek, P.; Różyło, R.; Dziki, L.; Gawlik-Dziki, U.; Biernacka, B.; Panasiewicz, M. Evaluation of Color, Texture, Sensory and Antioxidant Properties of Gels Composed of Freeze-Dried Maqui Berries and Agave Sugar. Processes 2020, 8, 1294. [Google Scholar] [CrossRef]
- Issis, Q.F.; Antonio, V.G.; Elsa, U.; Valeria, V.; Nicole, C.; Jacqueline, P. Vacuum drying application to maqui (Aristotelia chilensis [Mol] Stuntz) berry: Weibull distribution for process modelling and quality parameters. J. Food Sci. Technol. 2019, 56, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert Canales, P.S. Comparision of the total phonolic content, total anthocyanins content andantioxidant activity of polyphenol-rich fruits grown in Chile. Cienc. Investig. Agrar. 2014, 41, 49–60. [Google Scholar]
- Guerrero, J.; Ciampi, L.; Castilla, A.; Medel, F.; Schalchli, H.; Hormazabal, E.; Bensch, E.; Alberdi, M. Antioxidant capacity, anthocyanins, and total phenols of wild and cultivated berries in Chile. Chil. J. Agric. Res. 2010, 70, 537–544. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Przygoński, K.; Wojtowicz, E. The antioxidant properties of exotic fruit juices from acai, maqui berry and noni berries. Eur. Food Res. Technol. 2018, 244, 1897–1905. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Bastias-Montes, J.M.; Choque-Chávez, M.C.; Alarcón-Enos, J.E.; Quevedo-León, R.A.; Muñoz-Fariña, O.; Vidal-San-Martín, C. Effect of spray drying at 150, 160, and 170 °C on the physical and chemical properties of maqui extract (Aristotelia chilensis (Molina) Stuntz). Chil. J. Agric. Res. 2019, 79, 144–152. [Google Scholar] [CrossRef]
- Muñoz, O.; Ramos, F. Quantitative analysis of phytosterols in Aristotelia chilensis (Maqui) leaves using GC/MS. Int. Food Res. J. 2016, 23, 822–826. [Google Scholar]
- Crisóstomo-Ayala, K.A.; Sabater-Jara, A.B.; Pérez Manriquez, C.; Ferreres, F.; Gil-Izquierdo, Á.; Pedreño, M.Á.; Hernández de la Torre, M.; Sanchez-Olate, M.; Ríos Leal, D.G. Comparative Study of Metabolomic Profile and Antioxidant Content of Adult and In Vitro Leaves of Aristotelia chilensis. Plants 2021, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Wacewicz-Muczyńska, M.; Socha, K.; Soroczyńska, J.; Niczyporuk, M.; Borawska, M.H. Cadmium, lead and mercury in the blood of psoriatic and vitiligo patients and their possible associations with dietary habits. Sci. Total Environ. 2021, 757, 143967. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Kim, Y.J.; Lee, E.H.; Cho, E.B.; Kim, D.H.; Kim, B.O.; Kang, I.K.; Jung, H.Y.; Cho, Y.J. Protective effects of galangin against UVB irradiation-induced photo-aging in CCD-986sk human skin fibroblasts. Appl. Biol. Chem. 2019, 62, 40. [Google Scholar] [CrossRef]
- Bae, J.Y.; Lim, S.S.; Kim, S.J.; Choi, J.S.; Park, J.; Ju, S.M.; Han, S.J.; Kang, I.J.; Kang, Y.H. Bog blueberry anthocyanins alleviate photoaging in ultraviolet-B irradiation-induced human dermal fibroblasts. Mol. Nutr. Food Res. 2009, 53, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Lecci, R.M.; D’Antuono, I.; Cardinali, A.; Garbetta, A.; Linsalata, V.; Logrieco, A.F.; Leone, A. Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes. Molecules 2021, 26, 2153. [Google Scholar] [CrossRef]
- Sanchez Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Sarkis, J.; Michel, I.; Tessaro, I.; Marczak, L. Optimization of phenolics extraction from sesame seed cake. Sep. Purif. Technol. 2014, 122, 506–514. [Google Scholar] [CrossRef]
- Isidorov, V.A. Identification of Biologically and Environmentally Significant Organic Compounds: Mass Spectra and Retention Indices Library of Trimethylsilyl Derivatives, 1st ed.; PWN: Warszawa, Poland, 2015. [Google Scholar]


| Type of Extract | DPPH (mg Tx/L) | DPPH (% of Free Radical Scavenging) | TPC (mg GAE/100 g) |
|---|---|---|---|
| MWE | 283.63 ± 7.29 | 66.04 | 57.75 ± 0.44 |
| MEE70 | 284.60 ± 4.31 | 64.35 | 56.47 ± 0.19 |
| MEE95 | 211.80 ± 6.14 | 46.48 | 56.65 ± 0.69 |
| Group of Compounds | MME (%) |
|---|---|
| Unsaturated fatty acids and esters | 34.77 |
| Saturated fatty acids and esters | 23.18 |
| Fitosterols | 10.90 |
| Monoglycerides | 9.23 |
| Phenols | 4.56 |
| Carbohydrates | 3.17 |
| Phenylpropenoids | 2.65 |
| Pyrans | 1.27 |
| Aromatic Acids | 0.83 |
| Other compounds | 9.44 |
| Sum | 100 |
| Components, TMS Derivative | Rt (min) | LTPRI Exp | LTPRI Lit | Relative Composition (%) |
|---|---|---|---|---|
| N,N-73, 75, 117, 103 | 12.77 | 1032 | - | 0.92 |
| N,N-97, 68, 98, 42 | 16.44 | 1108 | 0.38 | |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 17.74 | 1141 | 1151 | 0.89 |
| Maltol | 20.90 | 1210 | 1208 | 0.39 |
| N,N-120, 174, 91, 65 | 21.42 | 1221 | - | 0.11 |
| 5-Hydroxymethylfurfural | 21.78 | 1230 | 1233 | 3.55 |
| Benzoic Acid | 22.59 | 1248 | 1249 | 0.34 |
| Octanoic acid | 23.53 | 1266 | 1266 | 0.22 |
| Benzeneacetic acid | 24.83 | 1298 | 1302 | 0.37 |
| 2-Methoxy-4-vinylphenol | 25.66 | 1317 | 1317 | 1.11 |
| Nonanoic acid | 28.01 | 1363 | 1355 | 2.50 |
| 1,2,3-Benzenetriol | 28.36 | 1381 | 1386 | 3.10 |
| Phenol, 2-methoxy-4-(1-propenyl)- | 31.27 | 1451 | 1450 | 1.87 |
| Decanoic acid | 31.67 | 1461 | 1460 | 0.13 |
| β-D-Glucopyranose, 1,6-anhydro- | 32.77 | 1488 | 1487 | 2.68 |
| N,N-73, 129, 75, 145 | 33.00 | 1493 | - | 1.64 |
| N,N-73, 75, 117, 129 | 33.35 | 1503 | - | 1.24 |
| N,N-73, 239, 44. 75 | 34.04 | 1520 | - | 0.29 |
| Cinnamic acid | 34.98 | 1550 | 1542 | 0.30 |
| 4-Hydroxybenzoic acid | 38.38 | 1635 | 1635 | 0.13 |
| Ethanone, 1-[4-(methylsulfonyl)phenyl]- | 38.75 | 1645 | 1649 | 0.12 |
| Homovanillic acid | 38.94 | 1650 | 1658 | 0.26 |
| Dodecanoic acid | 39.23 | 1658 | 1655 | 0.22 |
| Levoglucosan | 41.87 | 1710 | 1694 | 0.19 |
| (E)-Coniferyl alcohol | 42.15 | 1740 | 1743 | 0.48 |
| Myristic acid | 44.12 | 1796 | 1794 | 0.22 |
| D-(-)-Tagatofuranose, pentakis(trimethylsilyl) ether (isomer 1) | 44.27 | 1801 | 1801 | 0.30 |
| Protocatechoic acid | 45.47 | 1836 | 1835 | 0.09 |
| Tetradecanoic acid | 46.06 | 1854 | 1850 | 0.46 |
| N, N-73, 310, 44, 254 | 46.61 | 1870 | 0.31 | |
| Hexadecanoic acid, methyl ester | 48.46 | 1928 | 1926 | 0.50 |
| Pentadecanoic acid | 49.24 | 1952 | 1950 | 0.11 |
| Hexadecanoic acid, ethyl ester | 50.63 | 1996 | 1993 | 1.98 |
| 9-Hexadecenoic acid, (Z)- | 51.64 | 2029 | 2027 | 0.29 |
| Palmitic Acid | 52.33 | 2052 | 2050 | 14.92 |
| Linoleic acid, methyl ester | 53.65 | 2096 | 2092 | 0.57 |
| 6-Octadecenoic acid, methyl ester, (Z)- | 53.83 | 2102 | 2105 | 0.69 |
| cis-Vaccenic acid | 55.02 | 2143 | 2139 | 2.51 |
| Linoleic acid ethyl ester | 55.63 | 2164 | 2162 | 4.31 |
| Oleic acid, ethyl ester | 55.81 | 2170 | 2173 | 3.83 |
| Octadecanoic acid, ethyl ester | 56.58 | 2196 | 2195 | 0.28 |
| 9,12-Octadecadienoic acid (Z,Z) | 57.12 | 2216 | 2212 | 7.49 |
| Oleic Acid, (Z) | 57.29 | 2222 | 2218 | 14.17 |
| 13-Octadecenoic acid | 57.46 | 2228 | 2228 | 0.96 |
| Stearic acid | 58.02 | 2248 | 2246 | 1.65 |
| N, N-103, 73, 44, 131 | 66.41 | 2568 | - | 0.81 |
| 1-Monopalmitin | 67.36 | 2607 | 2607 | 0.14 |
| 2-linoleoylglycerol | 70.47 | 2739 | 2739 | 7.33 |
| 1-Monooleoylglycerol | 71.25 | 2772 | 2784 | 1.77 |
| β-Sitosterol | 82.57 | 3214 | 3200 | 5.79 |
| β-Sitosterol | 83.23 | 3334 | 3344 | 5.13 |
| Sum | - | - | - | 100 |
| As | Cd | Pb | ||||
|---|---|---|---|---|---|---|
| Type of Extract | As (µg/kg) | Norm * | Cd (µg/kg) | Norm ** (µg/kg) | Pb (µg/kg) | Norm ** (µg/kg) |
| MWE | 11.83 | - | 433.33 | 1000 | 258.81 | 3000 |
| MEE70 | 3.37 | 14.31 | 77.45 | |||
| MEE95 | 1.58 | 7.89 | 78.38 | |||
| Phase | Temperature (°C) | Pressure (atm) | Time (min) | Power (%) |
|---|---|---|---|---|
| I | 170 | 20 | 10 | 90 |
| II | 190 | 30 | 10 | 90 |
| III | 210 | 40 | 10 | 90 |
| IV | 50 | 40 | 18 | 0 |
| Parameter | Analytical Conditions | ||
|---|---|---|---|
| As | Cd | Pb | |
| Mode | KED | Standard | Standard |
| Mass (amu) | 75 | 110 111 113 114 | 206 207 208 |
| Dwell time per amu (ms) | 50 | 50 | 50 |
| Integration time (ms) | 1000 | 1000 | 1000 |
| Detector calibration mode | Dual | Dual | Dual |
| Replicants | 5 | 5 | 5 |
| Element | Precision (%) | Recovery (%) | Declared Concentration in CRM (µg/kg) |
|---|---|---|---|
| As | 3.3 | 99.0 | 10 |
| Cd | 2.5 | 99.1 | 7 |
| Pb | 2.4 | 99.5 | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacewicz-Muczyńska, M.; Moskwa, J.; Puścion-Jakubik, A.; Naliwajko, S.K.; Niczyporuk, M.; Socha, K. Antioxidant Properties of Maqui Berry Extract (Aristotelia chilensis (Mol.) Stuntz) and Its Potential Photoprotective Role on Human Skin Fibroblasts. Molecules 2023, 28, 7802. https://doi.org/10.3390/molecules28237802
Wacewicz-Muczyńska M, Moskwa J, Puścion-Jakubik A, Naliwajko SK, Niczyporuk M, Socha K. Antioxidant Properties of Maqui Berry Extract (Aristotelia chilensis (Mol.) Stuntz) and Its Potential Photoprotective Role on Human Skin Fibroblasts. Molecules. 2023; 28(23):7802. https://doi.org/10.3390/molecules28237802
Chicago/Turabian StyleWacewicz-Muczyńska, Marta, Justyna Moskwa, Anna Puścion-Jakubik, Sylwia K. Naliwajko, Marek Niczyporuk, and Katarzyna Socha. 2023. "Antioxidant Properties of Maqui Berry Extract (Aristotelia chilensis (Mol.) Stuntz) and Its Potential Photoprotective Role on Human Skin Fibroblasts" Molecules 28, no. 23: 7802. https://doi.org/10.3390/molecules28237802
APA StyleWacewicz-Muczyńska, M., Moskwa, J., Puścion-Jakubik, A., Naliwajko, S. K., Niczyporuk, M., & Socha, K. (2023). Antioxidant Properties of Maqui Berry Extract (Aristotelia chilensis (Mol.) Stuntz) and Its Potential Photoprotective Role on Human Skin Fibroblasts. Molecules, 28(23), 7802. https://doi.org/10.3390/molecules28237802











