Green Synthesis of Copper Oxide Nanoparticles from the Leaves of Aegle marmelos and Their Antimicrobial Activity and Photocatalytic Activities
Abstract
:1. Introduction
2. Results
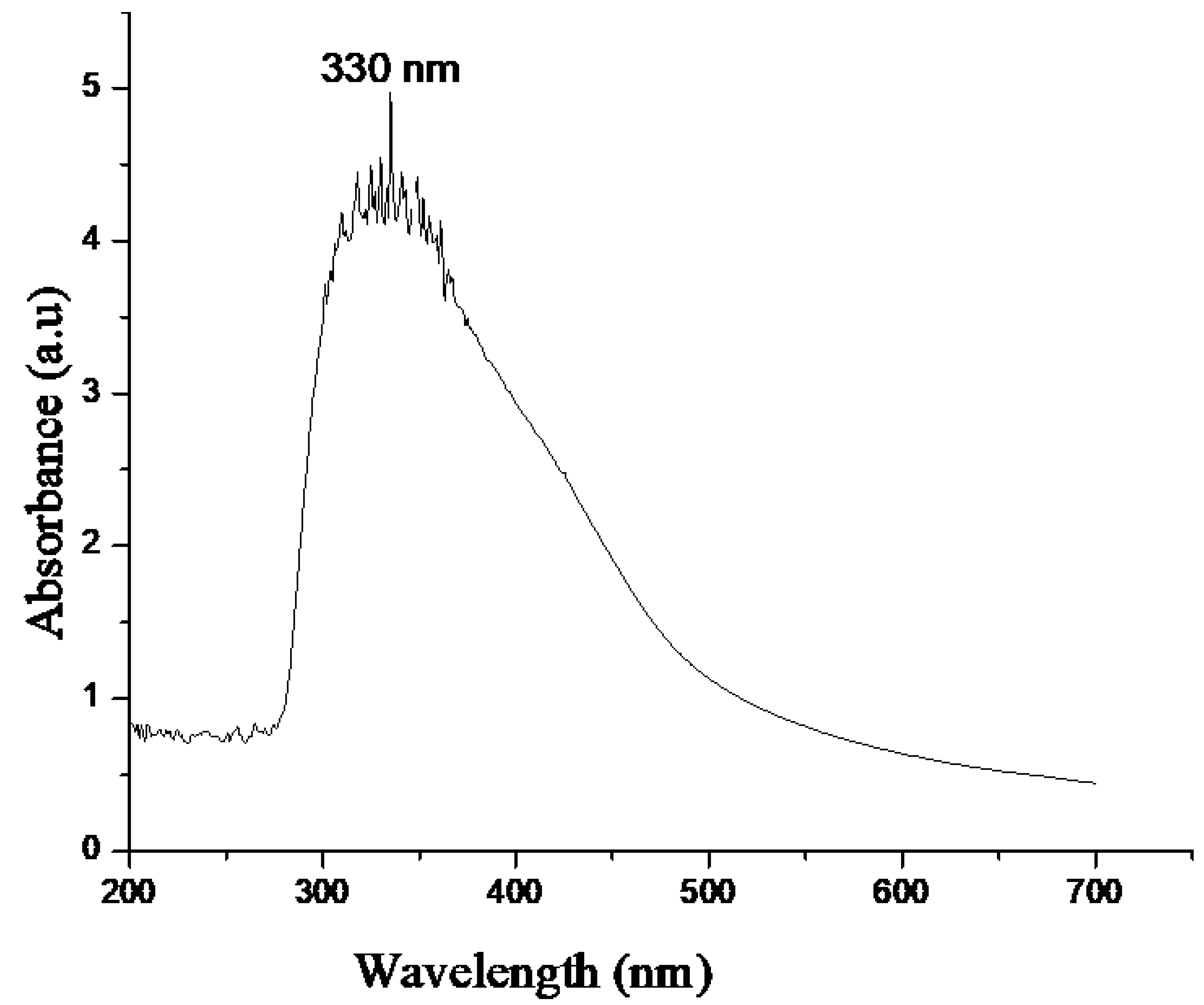
2.1. UV-Vis
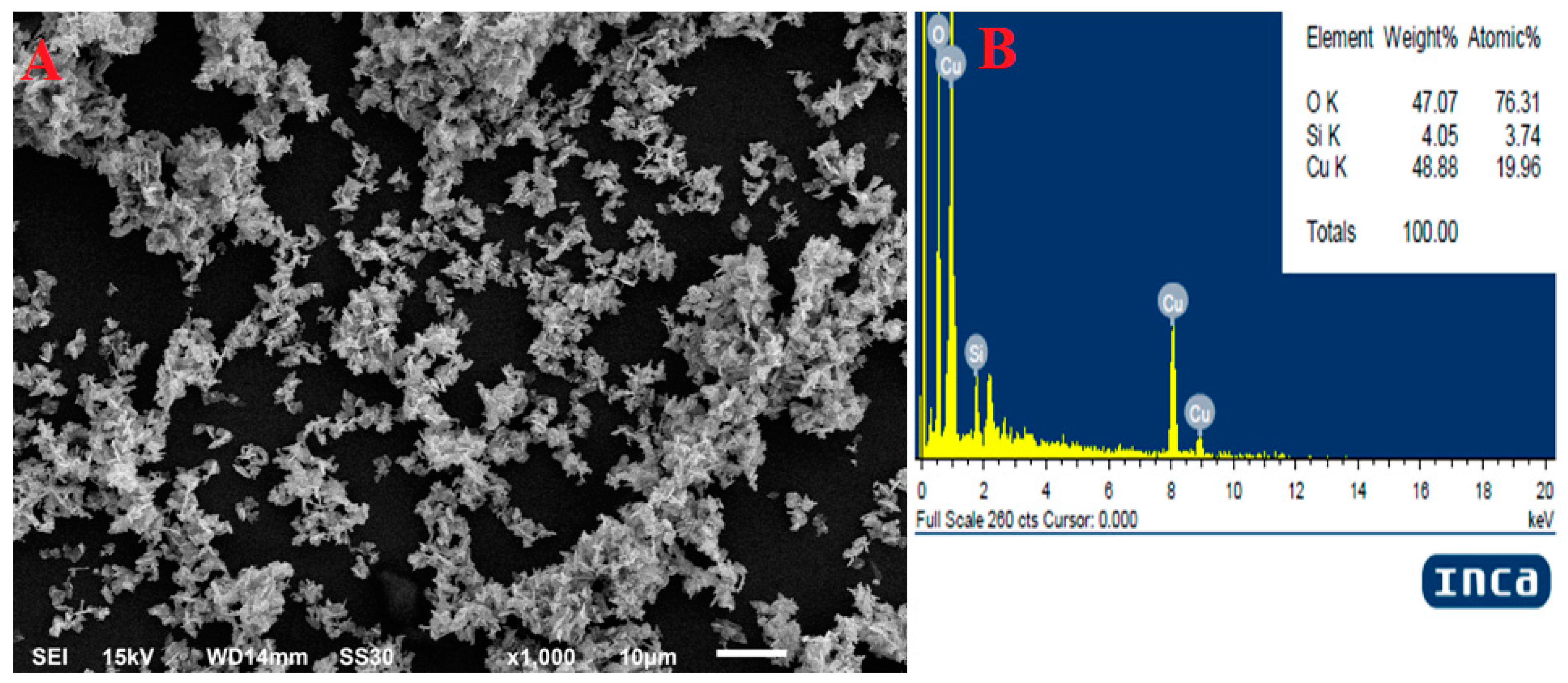
2.2. SEM with EDX
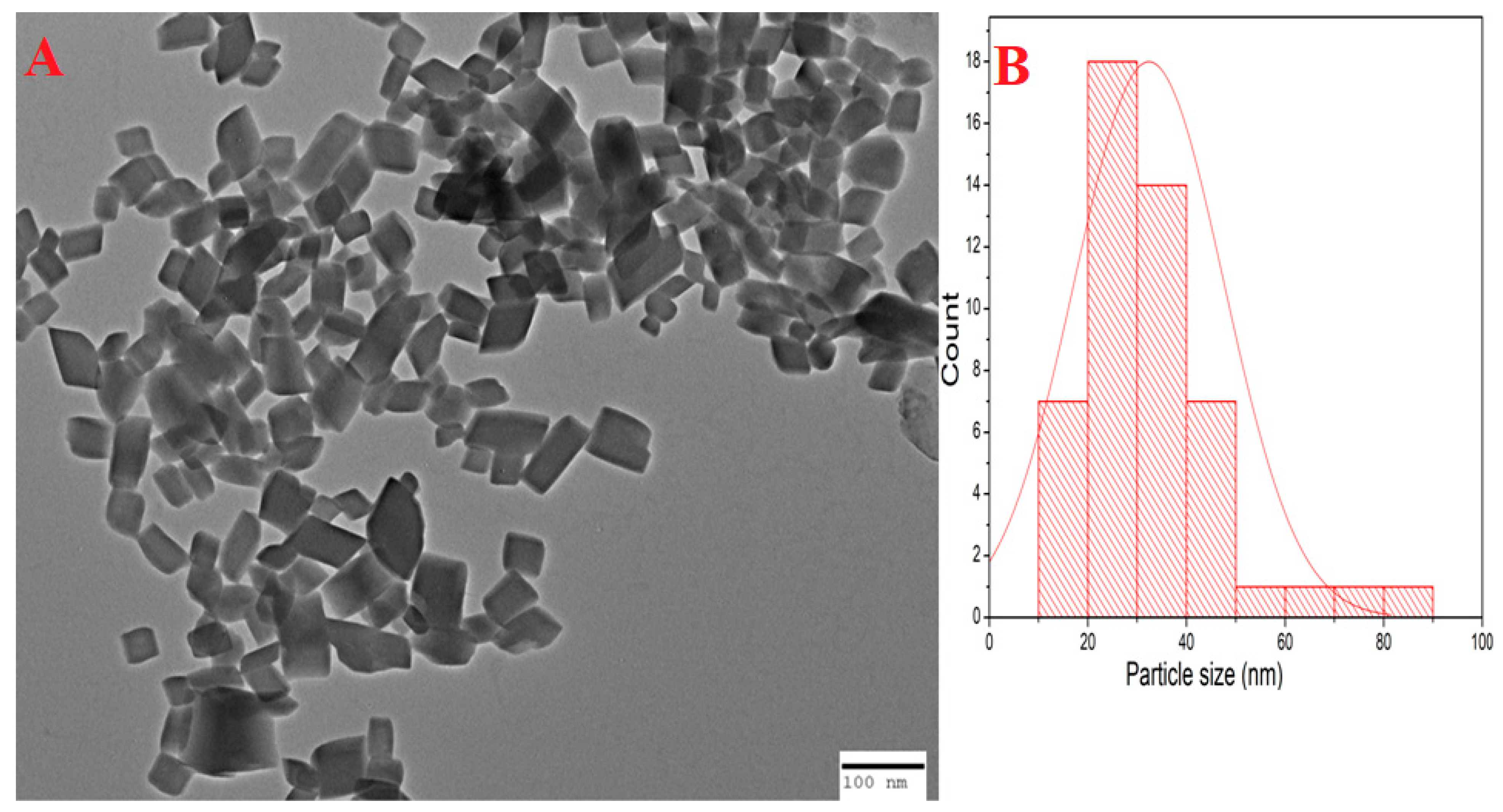
2.3. TEM
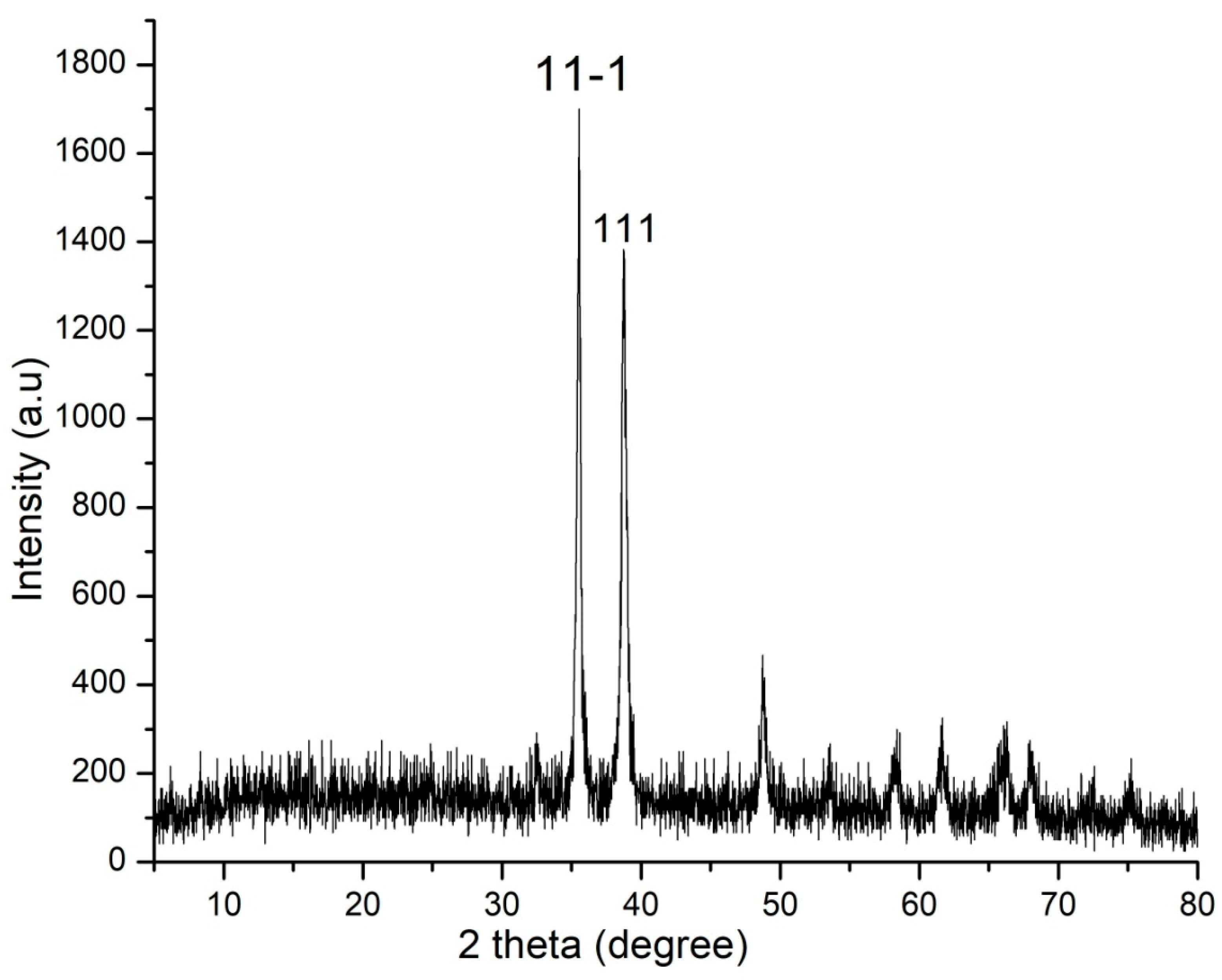
2.4. XRD
2.5. MIC
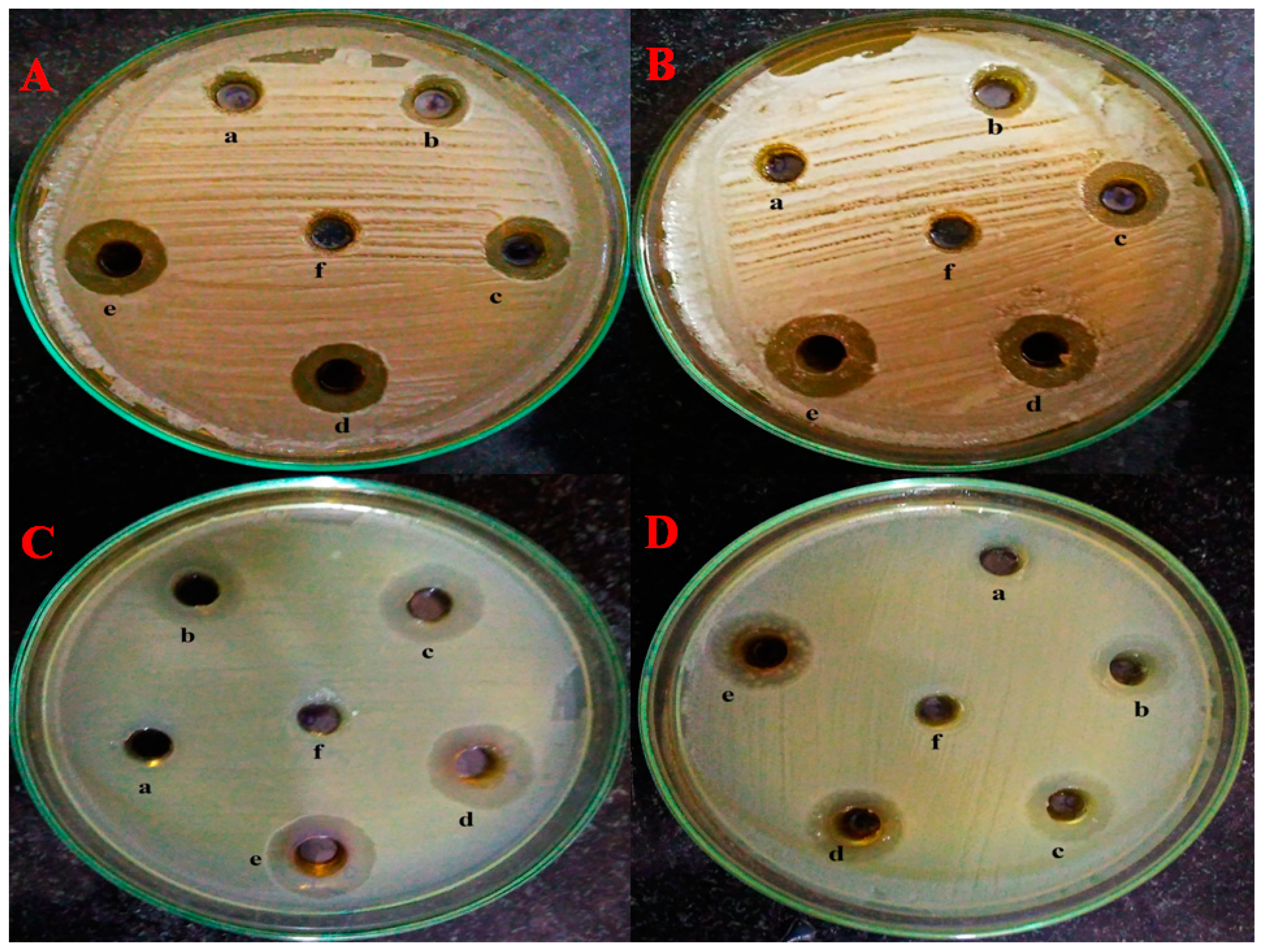
2.6. Well Diffusion Assay
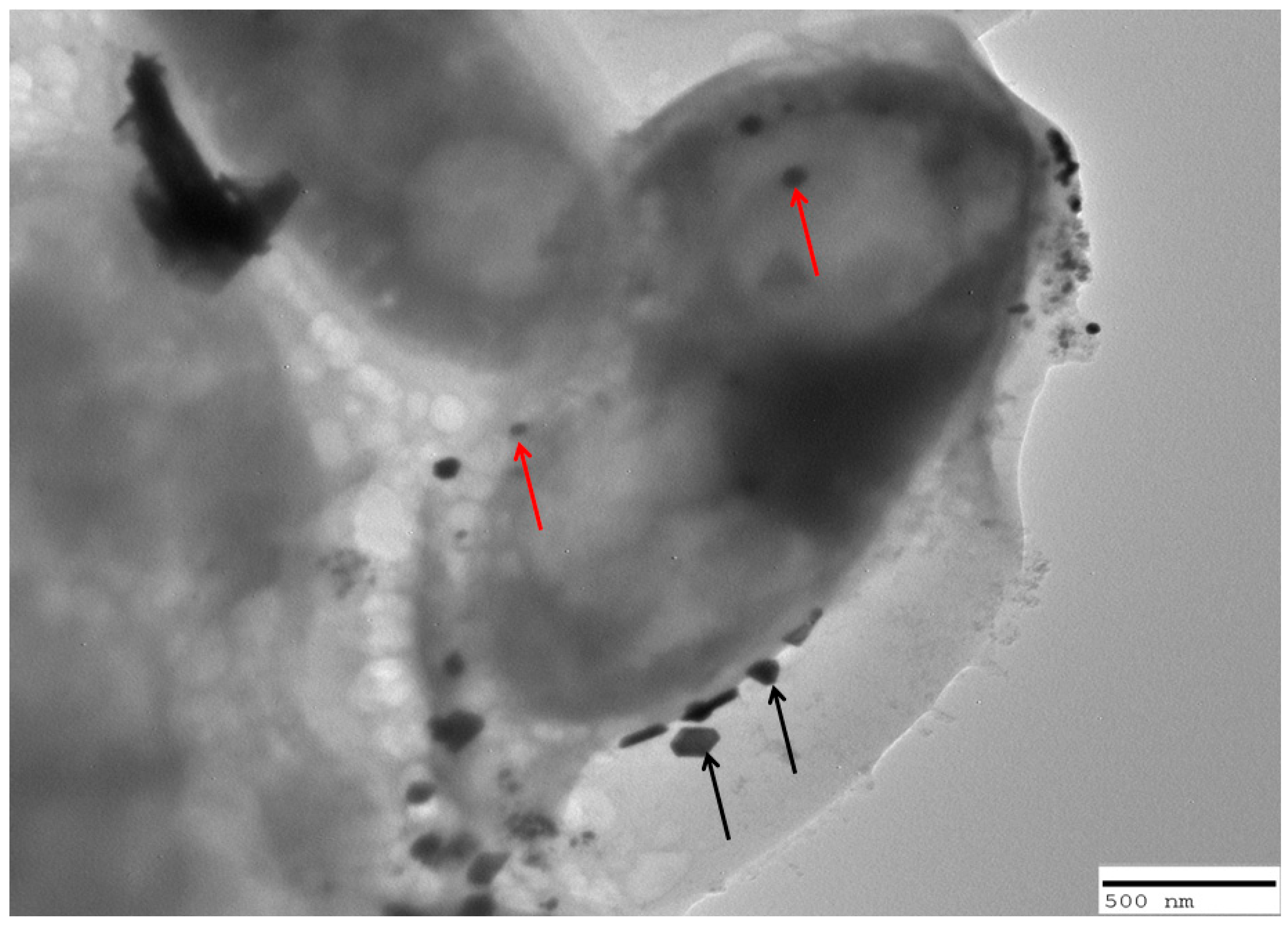
2.7. Interaction of C. albicans Cells with Nanoparticles
2.8. Photocatalytic Degradation of MB and MO
3. Discussion
4. Materials and Methods
4.1. Chemicals and Microorganisms
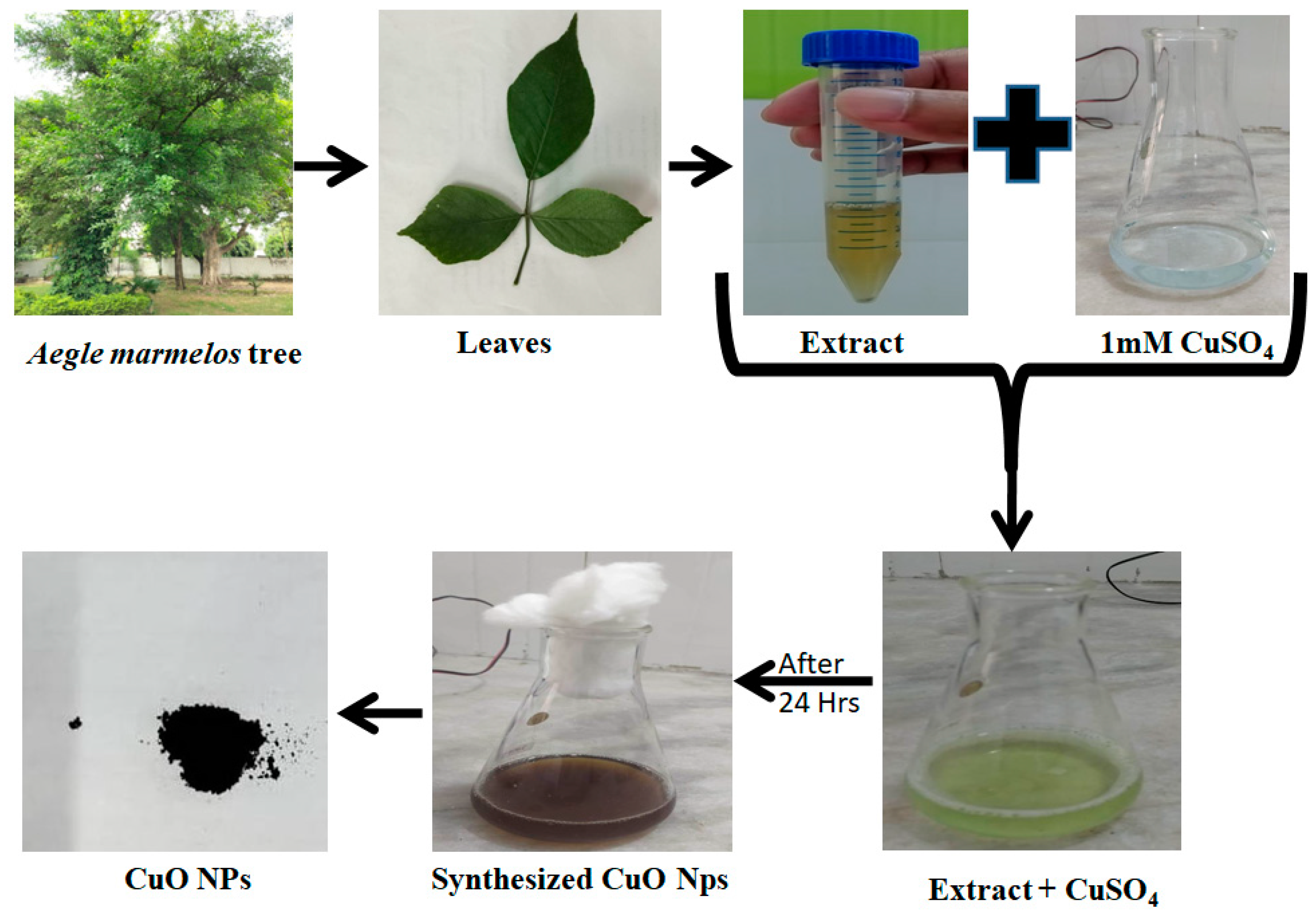
4.2. Preparation of Aegle Marmelos Leaf Extract
4.3. Synthesis of Aegle Marmelos Copper Oxide Nanoparticles (AM-CuO NP’s)
4.4. UV–Vis Spectroscopy
4.5. SEM with EDX
4.6. TEM
4.7. XRD
4.8. Determination of Minimum Inhibitory Concentration
4.9. Well Diffusion Assay
4.10. TEM Analysis of Interaction of Copper Oxide Nanoparticles with Candidal Cells
4.11. Photocatalytic Activity of CuO NPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Chernikova, N.; Hassan, T.; Mandzhieva, S.; Sushkova, S.; Lysenko, V.; Soldatov, M.A.; Burachevskaya, M. Effects of Zinc Oxide Nanoparticles on Physiological and Anatomical Indices in Spring Barley Tissues. Nanomaterials 2021, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Bhavyasree, P.; Xavier, T. Green synthesised copper and copper oxide based nanomaterials using plant extracts and their application in antimicrobial activity: Review. Curr. Res. Green Sustain. Chem. 2021, 5, 100249. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS ONE 2023, 18, e0274679. [Google Scholar] [CrossRef]
- Tuutijärvi, T.; Lu, J.; Sillanpää, M.; Chen, G. As(V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, H.S. Handbook of Nanostructured Materials and Nanotechnology, Five-Volume Set; Academic Press: Cambridge, MA, USA, 1999; ISBN 9780080533643. [Google Scholar]
- Buazar, F.; Sweidi, S.; Badri, M.; Kroushawi, F. Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach. Green Process. Synth. 2019, 8, 691–702. [Google Scholar] [CrossRef]
- Sukumar, S.; Rudrasenan, A.; Nambiar, D.P. Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia bonducella Seed Extract and Their Applications. ACS Omega 2020, 5, 1040–1051. [Google Scholar] [CrossRef]
- Sukhwal, A.; Jain, D.; Joshi, A.; Rawal, P.; Kushwaha, H.S. Biosynthesised silver nanoparticles using aqueous leaf extract of Tagetes patula L. and evaluation of their antifungal activity against phytopathogenic fungi. IET Nanobiotechnology 2017, 11, 531–537. [Google Scholar] [CrossRef]
- Jain, D.; Kour, R.; Bhojiya, A.A.; Meena, R.H.; Singh, A.; Mohanty, S.R.; Rajpurohit, D.; Dev Ameta, K. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci. Rep. 2020, 10, 13865. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Rashidian, G.; Hoseinifar, S.H.; Kamel, S.; Zare, M.; Ghafarifarsani, H.; Algharib, S.A.; Moonmanee, T.; Van Doan, H. Protective effects of Allium hirtifolium extract against foodborne toxicity of Zinc oxide nanoparticles in Common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109345. [Google Scholar] [CrossRef]
- Jia, B.; Mei, Y.; Cheng, L.; Zhou, J.; Zhang, L. Preparation of copper nanoparticles coated cellulose films with antibacterial properties through one-step reduction. ACS Appl. Mater. Interfaces 2012, 4, 2897–2902. [Google Scholar] [CrossRef]
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima, T.; Shivashangari, K.S.; Ravikumar, V. Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Babaei, Z. Protein nanoparticle: A unique system as drug delivery vehicles. Afr. J. Biotechnol. 2008, 7, 4926–4934. [Google Scholar]
- Sobha, K.; Surendranath, K.; Meena, V.; Jwala, T.K.; Swetha, N.; Latha, K.S.M. Emerging trends in nanobiotechnology. Biotechnol. Mol. Biol. Rev. 2010, 5, 01–12. [Google Scholar]
- Jain, D.; Kushwaha, H.S.; Rathore, K.S.; Stephen, B.J.; Daima, H.K.; Jain, R.; Singh, A. Fabrication of iron oxide nanoparticles from ammonia vapor and their importance in plant growth and dye degradation. Part. Sci. Technol. 2021, 40, 97–103. [Google Scholar] [CrossRef]
- Kadri, S.S. Key Takeaways from the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 5 August 2023).
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar]
- Laupland, K.B.; Church, D.L. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin. Microbiol. Rev. 2014, 27, 647–664. [Google Scholar] [CrossRef]
- Palmer, K.L.; Mashburn, L.M.; Singh, P.K.; Whiteley, M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 2005, 187, 5267–5277. [Google Scholar] [CrossRef] [PubMed]
- Viale, P.; Giannella, M.; Lewis, R.; Trecarichi, E.M.; Petrosillo, N.; Tumbarello, M. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev. Anti Infect. Ther. 2013, 11, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Y.; Zhang, W.; Zheng, S.; Zhang, Q.; Bai, C.; Zhang, P. Risk factors for hospital-acquired bloodstream infections caused by extended-spectrum β-lactamase Klebsiella pneumoniae among cancer patients. Ir. J. Med. Sci. 2013, 183, 463–469. [Google Scholar] [CrossRef]
- Biasoli, M. Candidiasis. 2013. Available online: http://www.fbioyf.unr.edu.ar/evirtual/file.php/118/materiales_2013/teoricos_20 (accessed on 28 September 2022).
- McCullough, M.; Ross, B.; Reade, P. Candida albicans: A review of its history, taxonomy, epidemiology, virulence attributes, and methods of strain differentiation. Int. J. Oral Maxillofac. Surg. 1996, 25, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-resistant Candida: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.P.; Sánchez, M.A.; González, C.P.; García, L.A. Antidiarrhoeal activity of different plants used in traditional medicine. Afr J Biotechnol. 2007, 6, 2988–2994. [Google Scholar]
- Mazumder, R.; Bhattacharya, S.; Mazumder, A.; Pattnaik, A.K.; Tiwary, P.M.; Chaudhary, S. Antidiarrhoeal evaluation of Aegle marmelos (Correa) Linn. root extract. Phytother Res. 2006, 20, 82–84. [Google Scholar] [CrossRef]
- Venkatesan, D.; Karunakaran, M.; Kumar, S.S.; Palaniswamy, P.; Ramesh, G. Antimicrobial activity of Aegle marmelos against pathogenic organism compared with control drug. Ethnobot. Leafl. 2009, 13, 968–974. [Google Scholar]
- Meena, A.; Rao, M.; Kandale, A.; Sannd, R.; Kiran, N.U.; Yadav, A. Standardisation of desmodium gangeticum—A tradition ayurvedic plant. Drug Invent. Today. 2010, 2, 182. [Google Scholar]
- Tanwar, S.; Parauha, Y.R.; There, Y.; Dhoble, S.J. Green synthesis-assisted copper nanoparticles using Aegle marmelos leaves extract: Physical, optical, and antimicrobial properties. Luminescence 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Takele, E.; Bogale, R.F.; Shumi, G.; Kenasa, G. Green synthesis, Characerization, and Antibacterial activity of CuO/ZnO Nanocomposite Using Zingiber officinale Rhizome extract. J. Chem. 2023, 3481389–3481399. [Google Scholar]
- Jayakumari, G.; Gokulpriya, C.; Sudhapriya, R.; Sharmila, G.; Muthukumaran, C. Phytofabrication and characterization of monodisperse copper oxide nanoparticles using Albizia lebbeck leaf extract. Appl. Nanosci. 2015, 5, 1017–1021. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Jalal, M.; AlYahya, S.; Asiri, S.M.M.; Khan, H.M. Effect of Biosynthesized ZnO Nanoparticles on Multi-Drug Resistant Pseudomonas Aeruginosa. Antibiotics 2020, 9, 260. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Jalal, M.; Ali, S.G.; Pal, R.; Musarrat, J. Green synthesis of Al2O3 nanoparticles and their bactericidal potential against clinical isolates of multi-drug resistant Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2014, 31, 153–164. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic Gold Nanoparticles as Potent Antibacterial and Antibiofilm Nano-Antibiotics against Pseudomonas aeruginosa. Antibiotics 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Ansari, M.A.; Shukla, A.K.; Ali, S.G.; Khan, H.M.; Pal, R.; Alam, J.; Cameotra, S.S. Green synthesis and antifungal activity of Al2O3NPs against fluconazole-resistant Candida spp isolated from a tertiary care hospital. RSC Adv. 2016, 6, 107577–107590. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Ali, S.G.; Khan, H.M.; Rehman, S. Anticandidal activity of bioinspired ZnO NPs: Effect on growth, cell morphology and key virulence attributes of Candida species. Artif. Cells Nanomed. Biotechnol. 2018, 46, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Veer, B.; Singh, R. Phytochemical Screening and Antioxidant Activities of Aegle marmelos Leaves. Anal. Chem. Lett. 2019, 9, 478–485. [Google Scholar] [CrossRef]
- Shanmugapriya, J.; Reshma, C.A.; Srinidhi, V.; Harithpriya, K.; Ramkumar, K.M.; Umpathy, D.; Gunasekaran, K.; Subashini, R. Green Synthesis of Copper Nanoparticles Using Withania somnifera and Its Antioxidant and Antibacterial Activity. J. Nanomater. 2022, 5, 7967294. [Google Scholar] [CrossRef]
- Peng, F.; Sun, Y.; Lu, Y.; Yu, W.; Ge, M.; Shi, J.; Cong, R.; Ha, J.; Dai, N. Studies on Sensing Properties and Mechanism of CuO Nanoparticles to H2S Gas. Nanomaterials 2020, 10, 774. [Google Scholar] [CrossRef]
- Panacek, A.; Kolar, M.; Vecerova, R.; Prucek, R.; Soukupova, J.; Krystof, V.; Hamal, P.; Zboril, R.; Kvitek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Fozia Khattak, B.; Alotaibi, A.; Qasim, M.; Ahmad, I.; Ullah, R.; Bourhia, M.; Gul, A.; Zahoor, S.; Ahmad, R. Green Synthesis of Copper Oxide Nanoparticles Using Aerva javanica Leaf Extract and Their Characterization and Investigation of In Vitro Antimicrobial Potential and Cytotoxic Activities. Evid.-Based Complement. Altern. Med. 2021, 2021, 5589703–5589802. [Google Scholar] [CrossRef] [PubMed]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Ejobi, F.; Tweyongyere, R.; Lubwama, M.; Kirabira, J.B.; Wampande, E.M. Nanotechnological solutions for controlling transmission and emergence of antimicrobial-resistant bacteria, Future Prospects and challenges: A Systematic Review. J. Nanoparticle Res. 2020, 22, 117. [Google Scholar] [CrossRef]
- Martínez, A.; Apip, C.; Meléndrez, M.F.; Domínguez, M.; Sánchez-Sanhueza, G.; Marzialetti, T.; Catalán, A. Dual antifungal activity against Candida albicans of copper metallic nanostructures and hierarchical copper oxide marigold-like nanostructures grown in situ in the culture medium. J. Appl. Microbiol. 2021, 130, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Kon, K.V.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Coping with antibiotic resistance: Combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev. Anti Infect. Ther. 2011, 9, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L. The multifunctional fungal ergosterol. MBio 2018, 9, e01755-18. [Google Scholar] [CrossRef]
- Padmavathi, A.R.; Murthy, S.; Das, A.; Priya, A.; Sushmitha, T.J.; Pandian, S.K.; Toleti, S.R. Impediment to growth and yeast-to-hyphae transition in Candida albicans by copper oxide nanoparticles. Biofouling 2020, 36, 56–72. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Zheng, W.L.; Wang, B.J.; Wang, L.; Shan, Y.P.; Zou, H.; Song, R.L.; Wang, T.; Gu, J.H.; Yuan, Y.; Liu, X.Z.; et al. ROS-mediated cell cycle arrest and apoptosis induced by zearalenone in mouse sertoli cells via ER stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Cuong, H.N.; Pansambal, S.; Ghotekar, S.; Oza, R.; Hai NT, T.; Viet, N.M.; Nguyen, V.H. New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: A review. Environ. Res. 2022, 203, 111858. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.G.; Haseen, U.; Jalal, M.; Khan, R.A.; Alsalme, A.; Ahmad, H.; Khan, H.M. Green Synthesis of Copper Oxide Nanoparticles from the Leaves of Aegle marmelos and Their Antimicrobial Activity and Photocatalytic Activities. Molecules 2023, 28, 7499. https://doi.org/10.3390/molecules28227499
Ali SG, Haseen U, Jalal M, Khan RA, Alsalme A, Ahmad H, Khan HM. Green Synthesis of Copper Oxide Nanoparticles from the Leaves of Aegle marmelos and Their Antimicrobial Activity and Photocatalytic Activities. Molecules. 2023; 28(22):7499. https://doi.org/10.3390/molecules28227499
Chicago/Turabian StyleAli, Syed Ghazanfar, Uzma Haseen, Mohammad Jalal, Rais Ahmad Khan, Ali Alsalme, Hilal Ahmad, and Haris Manzoor Khan. 2023. "Green Synthesis of Copper Oxide Nanoparticles from the Leaves of Aegle marmelos and Their Antimicrobial Activity and Photocatalytic Activities" Molecules 28, no. 22: 7499. https://doi.org/10.3390/molecules28227499
APA StyleAli, S. G., Haseen, U., Jalal, M., Khan, R. A., Alsalme, A., Ahmad, H., & Khan, H. M. (2023). Green Synthesis of Copper Oxide Nanoparticles from the Leaves of Aegle marmelos and Their Antimicrobial Activity and Photocatalytic Activities. Molecules, 28(22), 7499. https://doi.org/10.3390/molecules28227499






