Sulconazole-Loaded Solid Lipid Nanoparticles for Enhanced Antifungal Activity: In Vitro and In Vivo Approach
Abstract
:1. Introduction
2. Results
2.1. Characterization of SCZ-SLNs
2.1.1. Particle Size, Polydispersity Index and Zeta Potential
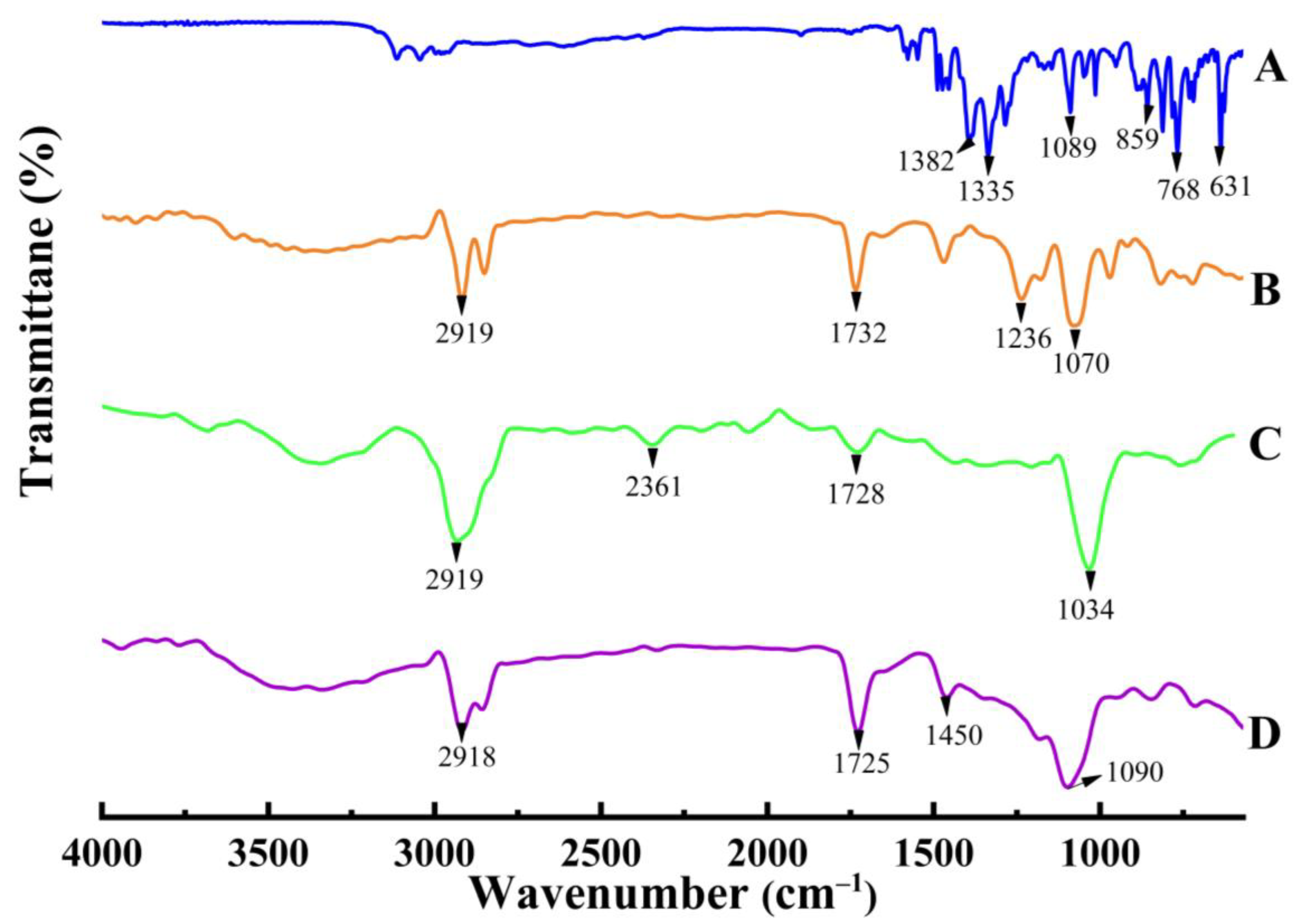
2.1.2. FTIR Spectroscopy
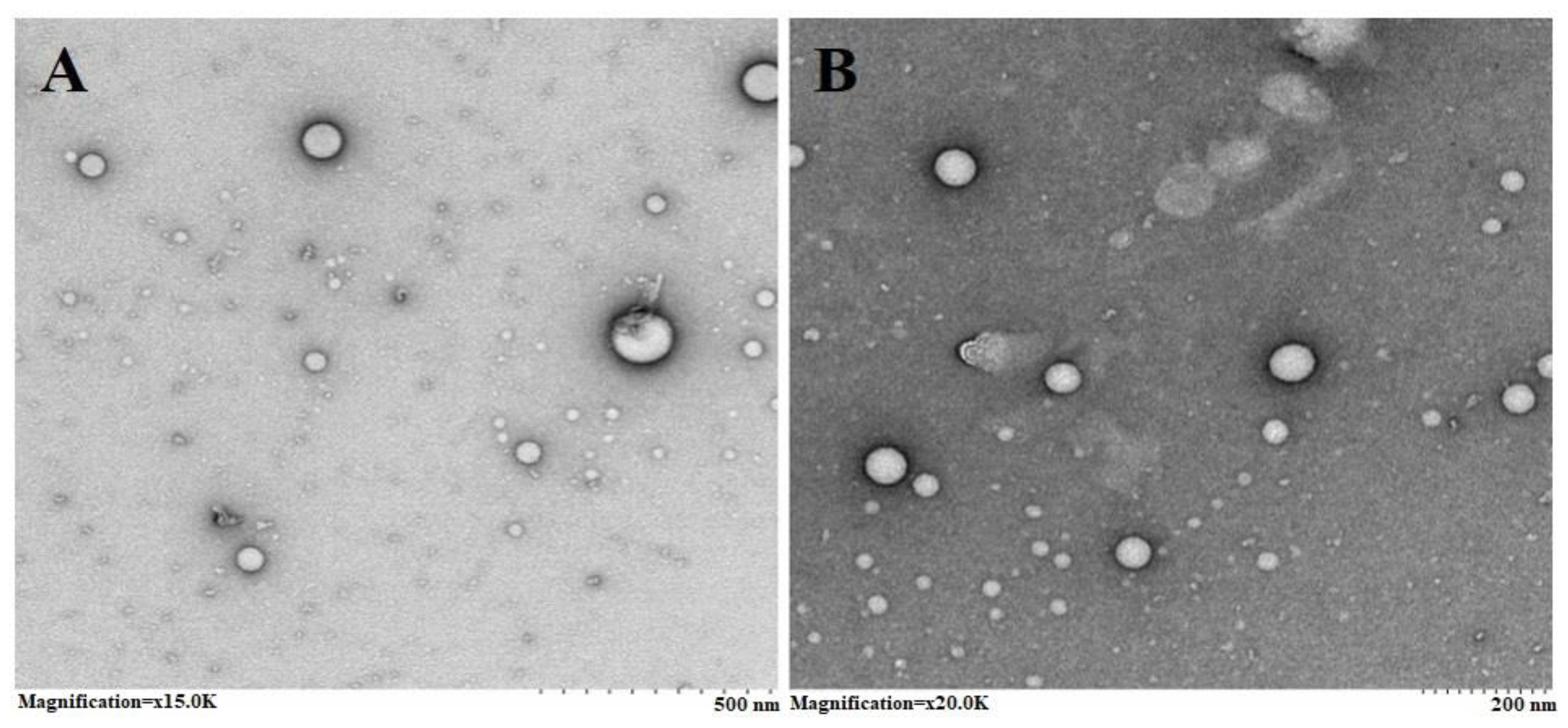
2.1.3. Transmission Electron Microscopy (TEM)
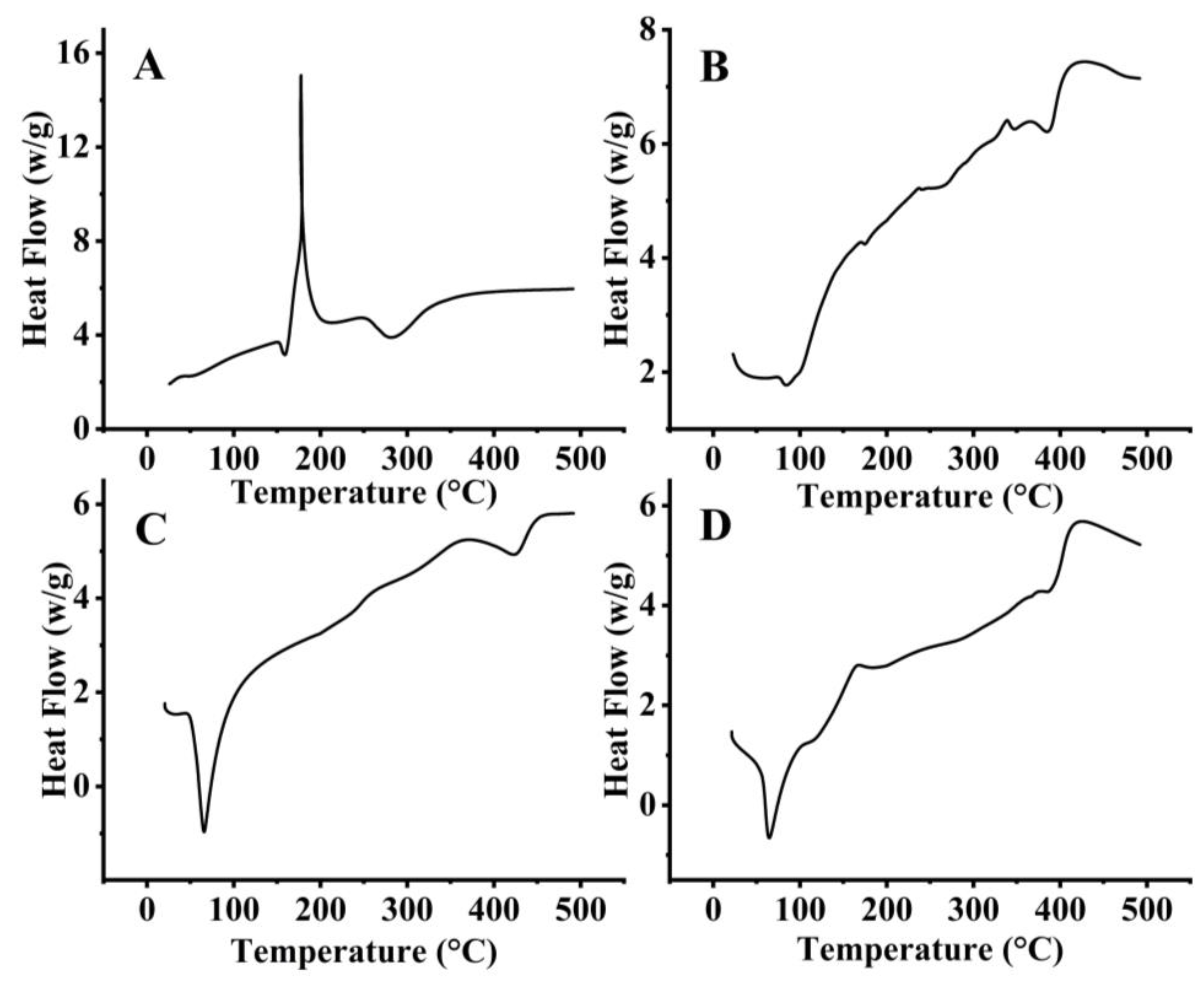
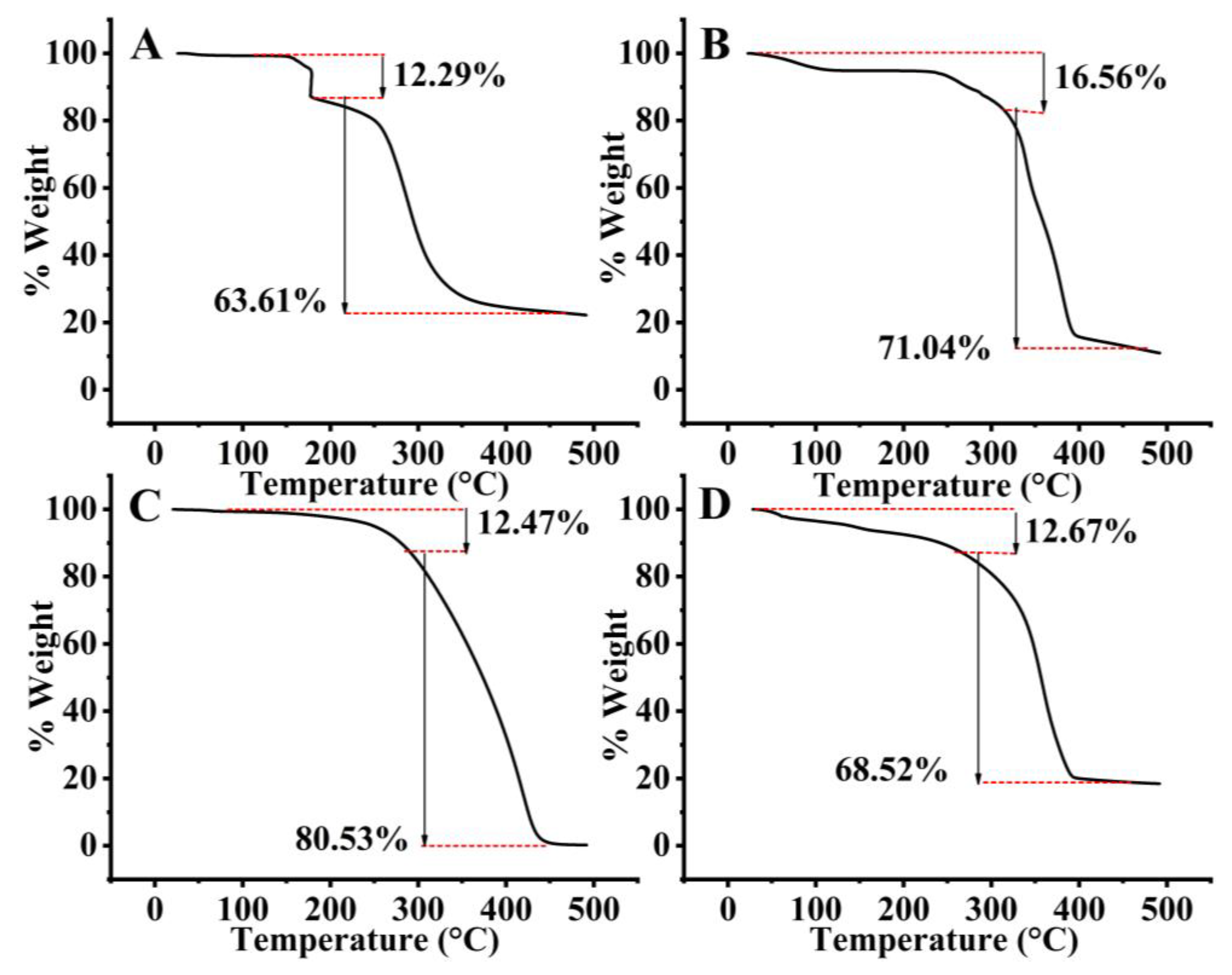
2.1.4. Thermal Analysis
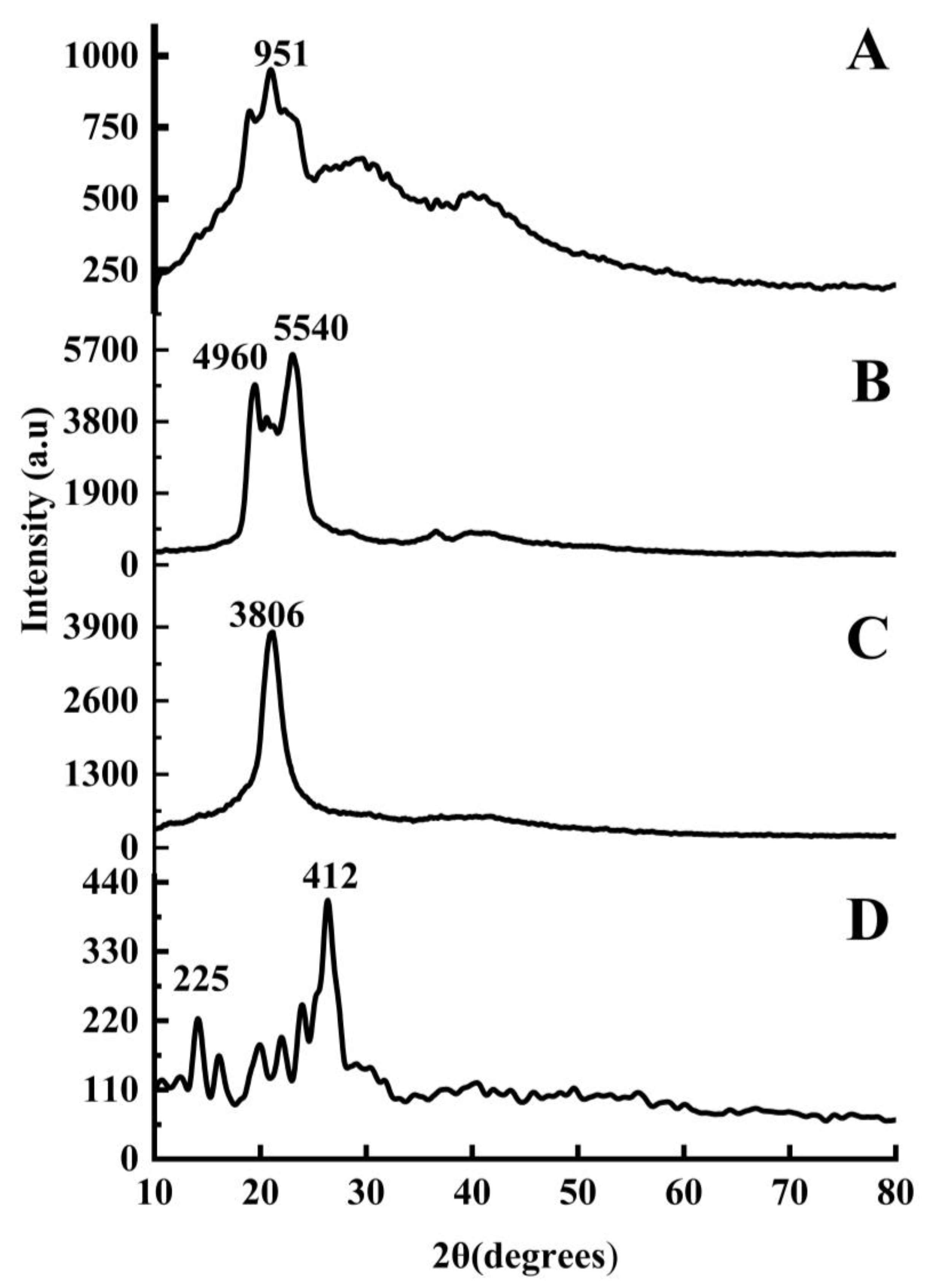
2.1.5. X-ray Diffraction
2.1.6. Encapsulation Efficiency (EE) and % Production Yield
2.2. Characterization of SCZ-SLNs Gel
2.2.1. pH, Homogeneity and Grittiness
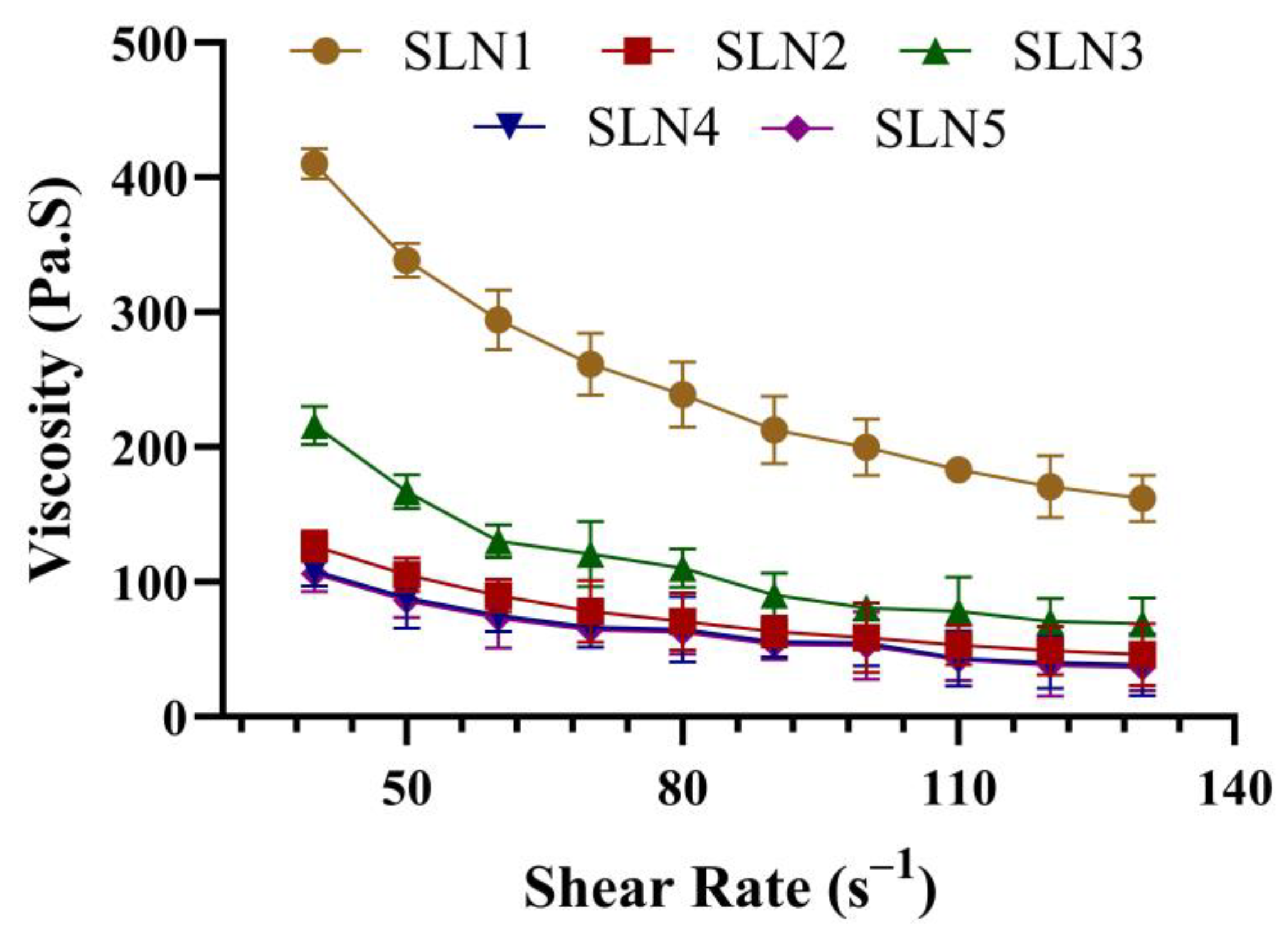
2.2.2. Rheological Studies
2.2.3. Spreadability and Extrudability
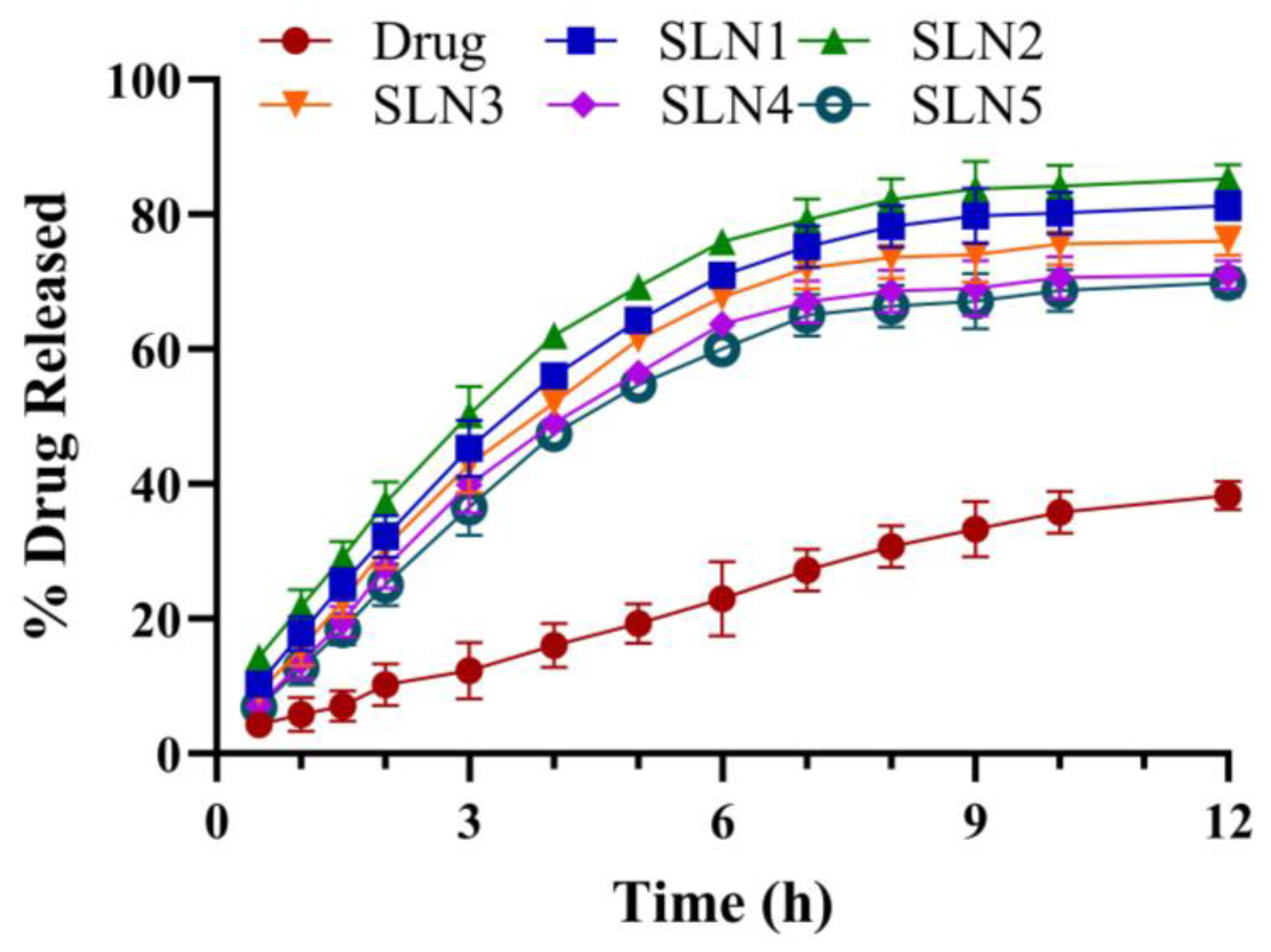
2.3. In Vitro Drug Release and Drug Release Kinetics
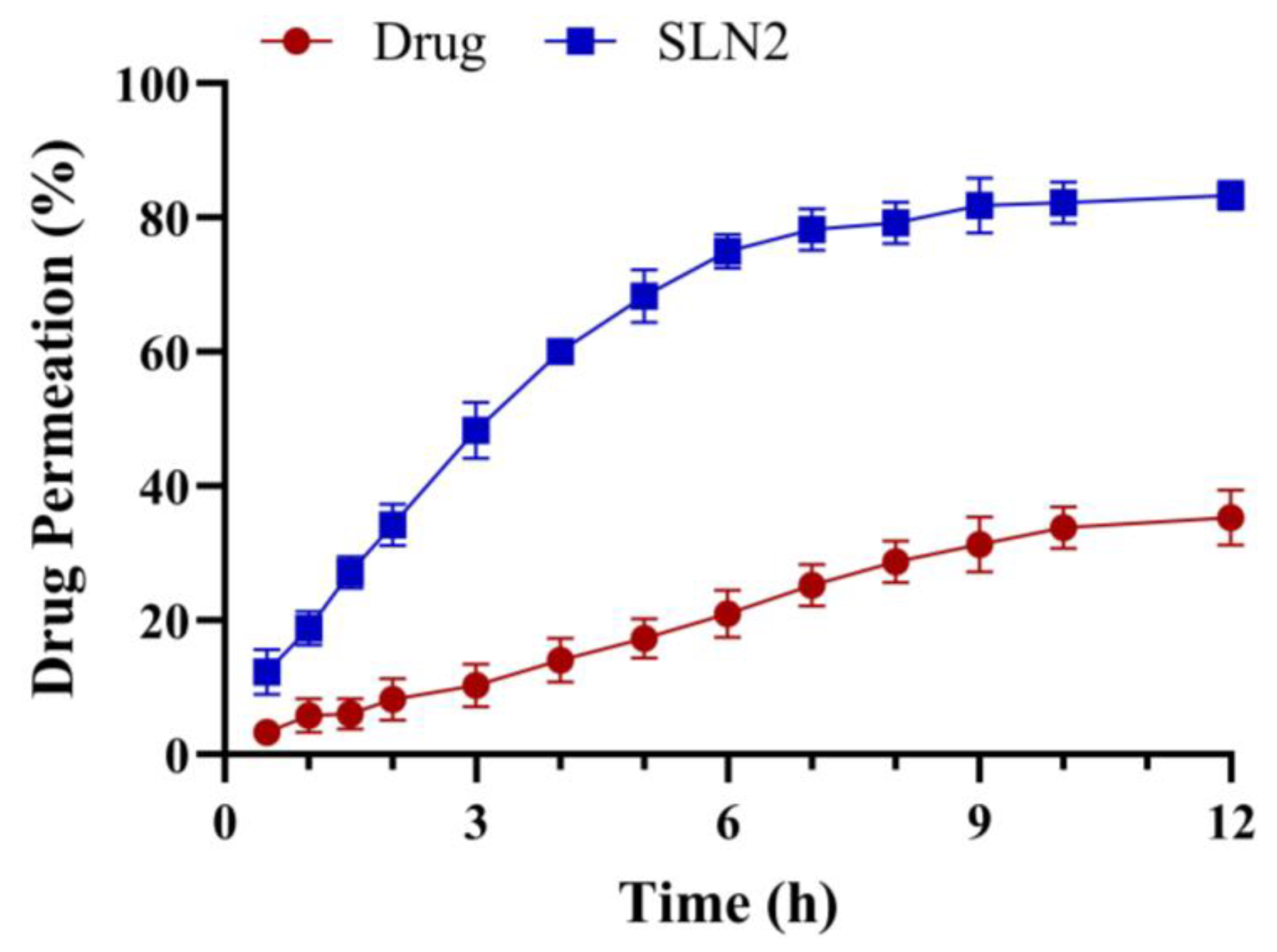
2.4. Ex Vivo Skin Permeation
2.5. Stability Studies
2.6. Antifungal Activity
2.7. In Vivo Study
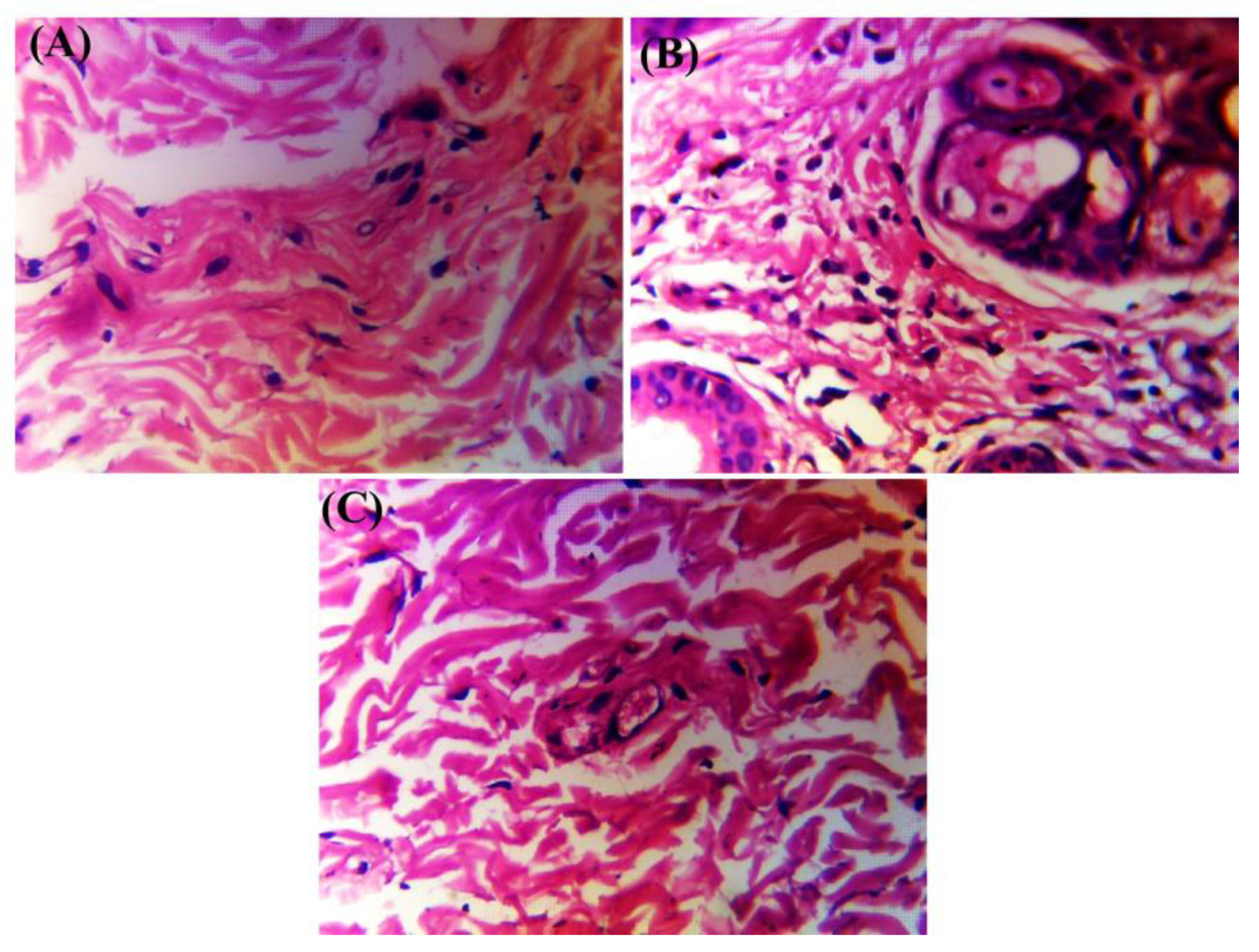
2.8. Histopathology
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of SLNss
4.3. Characterization of SCZ-SLNs
4.3.1. Particle Size, Polydispersity Index (PDI) and Zeta Potential
4.3.2. FTIR Spectroscopy
4.3.3. Transmission Electron Microscopy (TEM)
4.3.4. Thermal Analysis
4.3.5. X-ray Diffraction Analysis (XRD)
4.3.6. Encapsulation Efficiency (EE)
4.3.7. % Production Yield
4.4. Preparation of SCZ-SLN Gel
4.5. Characterization of SCZ-SLNs Gel
4.5.1. pH, Homogeneity and Grittiness
4.5.2. Rheological Studies
4.5.3. Spreadability and Extrudability
4.5.4. Drug Content
4.6. In Vitro Drug Release and Drug Release Kinetics
4.7. Ex Vivo Skin Permeation
4.8. Antifungal Activity
4.9. In Vivo Study
4.10. Histopathology Study
4.11. Stability Studies
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nosanchuk, J.D. Current Status and Future of Antifungal Therapy for Systemic Mycoses. Recent Pat. Anti-Infect. Drug Discov. 2006, 1, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Elfawal, M.A.; Savinov, S.N.; Aroian, R.V. Drug Screening for Discovery of Broad-spectrum Agents for Soil-transmitted Nematodes. Sci. Rep. 2019, 9, 12347. [Google Scholar] [CrossRef] [PubMed]
- Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Suzuki, K.; Ueda, T.; Terauchi, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; et al. Synthesis and antibacterial activity of novel and potent DNA gyrase inhibitors with azole ring. Bioorg. Med. Chem. 2004, 12, 5515–5524. [Google Scholar] [CrossRef]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Marley, J.; Watson, A.; Williams, T. Significance of Non-Dermatophyte Moulds and Yeasts in Onychomycosis. Dermatology 1997, 194 (Suppl. 1), 40–42. [Google Scholar] [CrossRef] [PubMed]
- Kuklová, I.; Kucerová, H. Dermatophytoses in Prague, Czech Republic, between 1987 and 1998. Mycoses 2001, 44, 493–496. [Google Scholar] [CrossRef]
- Pandit, J.; Garg, M.; Jain, N.K. Miconazole nitrate bearing ultraflexible liposomes for the treatment of fungal infection. J. Liposome Res. 2014, 24, 163–169. [Google Scholar] [CrossRef]
- Hainer, B.L. Dermatophyte infections. Am. Fam. Physician 2003, 67, 101–108. [Google Scholar]
- Holmes, A.R.; Cardno, T.S.; Strouse, J.J.; Ivnitski-Steele, I.; Keniya, M.V.; Lackovic, K.; Monk, B.C.; Sklar, L.A.; Cannon, R.D. Targeting efflux pumps to overcome antifungal drug resistance. Futur. Med. Chem. 2016, 8, 1485–1501. [Google Scholar] [CrossRef]
- Choi, F.D.; Juhasz, M.L.; Mesinkovska, N.A. Topical ketoconazole: A systematic review of current dermatological applications and future developments. J. Dermatol. Treat. 2019, 30, 760–771. [Google Scholar] [CrossRef] [PubMed]
- El Zaafarany, G.M.; Awad, G.A.S.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Maibach, H.I. Deep Percutaneous Penetration into Muscles and Joints. J. Pharm. Sci. 2006, 95, 1405–1413. [Google Scholar] [CrossRef]
- Attama, A.A.; Kenechukwu, F.C.; Onuigbo, E.B.; Nnamani, P.O.; Obitte, N.; Finke, J.H.; Pretor, S.; Müller-Goymann, C.C. Solid lipid nanoparticles encapsulating a fluorescent marker (coumarin 6) and antimalarials—Artemether and lumefantrine: Evaluation of cellular uptake and antimalarial activity. Eur. J. Nanomed. 2016, 8, 129–138. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Mohd, S.; Govindaiah, P.; Babu, M.R.; Kumar, R.; Gulati, M.; Gowthamarajan, K.; Madhunapantula, S.V.; Chellappan, D.K.; et al. Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells. Pharmaceutics 2022, 14, 2403. [Google Scholar] [CrossRef]
- Osanlou, R.; Emtyazjoo, M.; Banaei, A.; Hesarinejad, M.A.; Ashrafi, F. Preparation of solid lipid nanoparticles and nanostructured lipid carriers containing zeaxanthin and evaluation of physicochemical properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128588. [Google Scholar] [CrossRef]
- Telange, D.R.; Wavare, K.D.; Patil, A.T.; Umekar, M.J.; Anand, S.; Dave, V.S. Drug-phospholipid complex-loaded matrix film formulation for the enhanced transdermal delivery of quercetin. J. Excip. Food Chem. 2018, 9, 31. [Google Scholar]
- Gardouh, A.R.; Gad, S.; Ghonaim, H.M.; Ghorab, M.M. Design and characterization of glyceryl monostearate solid lipid nanoparticles prepared by high shear homogenization. Br. J. Pharm. Res. 2013, 3, 326–346. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, S.; Gu, Y.; Tang, X.; Wang, T.; Wu, J.; Liu, J. Development of sulconazole-loaded nanoemulsions for enhancement of transdermal permeation and antifungal activity. Int. J. Nanomed. 2019, 14, 3955–3966. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Lee, T.H.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Song, Y.K.; Lee, W.K. Improved anticancer efficacy of methyl pyropheophorbide-a–incorporated solid lipid nanoparticles in photodynamic therapy. Sci. Rep. 2023, 13, 7391. [Google Scholar] [CrossRef] [PubMed]
- Mangrulkar, S.; Shah, P.; Navnage, S.; Mazumdar, P.; Chaple, D. Phytophospholipid Complex of Caffeic Acid: Development, In vitro Characterization, and In Vivo Investigation of Antihyperlipidemic and Hepatoprotective Action in Rats. AAPS PharmSciTech 2021, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, G.A.; Girgis, G.N.; Hamed, M.F.; Soliman, O.A.E.-A.; Gawad, A.E.G.H.A.E. Formulation and Pathohistological Study of Mizolastine–Solid Lipid Nanoparticles–Loaded Ocular Hydrogels. Int. J. Nanomed. 2021, 16, 7775–7799. [Google Scholar] [CrossRef] [PubMed]
- El-Salamouni, N.S.; Farid, R.M.; El-Kamel, A.H.; El-Gamal, S.S. Nanostructured lipid carriers for intraocular brimonidine localisation: Development, in-vitro and in-vivo evaluation. J. Microencapsul. 2018, 35, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Zeb, A.; Shah, K.U.; Rehman, Z.U. Development, in-vitro and in-vivo evaluation of ezetimibe-loaded solid lipid nanoparticles and their comparison with marketed product. J. Drug Deliv. Sci. Technol. 2019, 51, 583–590. [Google Scholar] [CrossRef]
- Kraisit, P.; Hirun, N.; Mahadlek, J.; Limmatvapirat, S. Fluconazole-loaded solid lipid nanoparticles (SLNs) as a potential carrier for buccal drug delivery of oral candidiasis treatment using the Box-Behnken design. J. Drug Deliv. Sci. Technol. 2021, 63, 102437. [Google Scholar] [CrossRef]
- Rudhrabatla, V.S.A.P.; Sudhakar, B.; Reddy, K.V.N.S. In Vitro and In Vivo Assessment of Designed Melphalan Loaded Stealth Solid Lipid Nanoparticles for Parenteral Delivery. BioNanoScience 2019, 10, 168–190. [Google Scholar] [CrossRef]
- Batool, S.; Zahid, F.; Ud-Din, F.; Naz, S.S.; Dar, M.J.; Khan, M.W.; Zeb, A.; Khan, G.M. Macrophage targeting with the novel carbopol-based miltefosine-loaded transfersomal gel for the treatment of cutaneous leishmaniasis: In vitro and in vivo analyses. Drug Dev. Ind. Pharm. 2021, 47, 440–453. [Google Scholar] [CrossRef]
- Kumar, N.; Goindi, S. Development and Optimization of Itraconazole-Loaded Solid Lipid Nanoparticles for Topical Administration Using High Shear Homogenization Process by Design of Experiments: In Vitro, Ex Vivo and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 248. [Google Scholar] [CrossRef]
- Weian, Z.; Wei, L.; Yue’E, F. Synthesis and properties of a novel hydrogel nanocomposites. Mater. Lett. 2005, 59, 2876–2880. [Google Scholar] [CrossRef]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharmacol. 2007, 59, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Harish, N.M.; Prabhu, P.; Charyulu, R.N.; Gulzar, M.A.; Subrahmanyam, E.V.S. Formulation and evaluation of in situ gels containing clotrimazole for oral candidiasis. Indian J. Pharm. Sci. 2009, 71, 421. [Google Scholar] [CrossRef] [PubMed]
- Jamadar, M.J.; Shaikh, R.H. Preparation and evaluation of herbal gel formulation. J. Pharm. Res. Educ. 2017, 1, 201–224. [Google Scholar]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Usman, F.; Naseem, M.; Aati, H.Y.; Ahmad, H.; Manee, S.; Khalil, R.; Khan, K.U.R.; Qureshi, M.I.; Umair, M. Voriconazole Cyclodextrin Based Polymeric Nanobeads for Enhanced Solubility and Activity: In Vitro/In Vivo and Molecular Simulation Approach. Pharmaceutics 2023, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Usman, F.; Zaib, S.; Shah, H.S.; Jamil, Q.A.; Akbar Sheikh, F.; Khan, A.; Rabea, S.; Hagras, S.A.; El-Saber Batiha, G.; et al. Fabrication and evaluation of voriconazole loaded transethosomal gel for enhanced antifungal and antileishmanial activity. Molecules 2022, 27, 3347. [Google Scholar] [CrossRef] [PubMed]
- Rarokar, N.R.; Menghani, S.S.; Kerzare, D.R.; Khedekar, P.B.; Bharne, A.P.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Sreeharsha, N.; Asdaq, S.M.B. Preparation of Terbinafin-Encapsulated Solid Lipid Nanoparticles Containing Antifungal Carbopol® Hydrogel with Improved Efficacy: In Vitro, Ex Vivo and In Vivo Study. Pharmaceutics 2022, 14, 1393. [Google Scholar] [CrossRef]

| Formulation Code | Particle Size | PDI | Zeta Potential (mV) |
|---|---|---|---|
| SLN1 | 60.31 ± 1.33 | 0.266 ± 0.03 | −28.52 ± 1.23 |
| SLN2 | 89.81 ± 2.64 | 0.311 ± 0.07 | −26.98 ± 1.19 |
| SLN3 | 126.32 ± 4.24 | 0.378 ± 0.02 | −19.44 ± 1.63 |
| SLN4 | 178.93 ± 3.55 | 0.406 ± 0.10 | −16.40 ± 1.08 |
| SLN5 | 194.9 ± 2.26 | 0.304 ± 0.13 | −21.14 ± 2.98 |
| Formulation Code | EE (%) | Production Yield (%) |
|---|---|---|
| SLN1 | 83.33 ± 1.45 | 88.33 ± 2.75 |
| SLN2 | 86.52 ± 0.53 | 91.35 ± 1.48 |
| SLN3 | 81.89 ± 1.67 | 85.89 ± 2.38 |
| SLN4 | 79.73 ± 2.45 | 83.73 ± 1.92 |
| SLN5 | 77.16 ± 1.42 | 80.16 ± 1.15 |
| Formulation Code | Zero-Order | First Order | Higuchi Model | Korsmeyer-Peppas Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ko | R2 | K1 | R2 | KH | R2 | KKP | n | R2 | |
| SLN1 | 9.18 | 0.65 | 0.19 | 0.98 | 26.2 | 0.94 | 24.5 | 0.53 | 0.94 |
| SLN2 | 9.74 | 0.53 | 0.22 | 0.98 | 27.9 | 0.94 | 28.4 | 0.49 | 0.94 |
| SLN3 | 8.64 | 0.65 | 0.16 | 0.96 | 24.6 | 0.93 | 22.8 | 0.53 | 0.93 |
| SLN4 | 9.05 | 0.68 | 0.14 | 0.96 | 22.9 | 0.92 | 20.6 | 0.55 | 0.93 |
| SLN5 | 7.79 | 0.72 | 0.13 | 0.96 | 22.0 | 0.93 | 19.1 | 0.57 | 0.93 |
| Formulation Code | % J (μg cm−2 h−1) | Kp (cm h−1) | Enhancement Ratio |
|---|---|---|---|
| Control SCZ gel | 35.29 ± 3.14 | 4.61 ± 2.40 | 2.62 |
| SCZ-SLN2 gel | 83.60 ± 2.95 | 6.57 ± 1.38 |
| Drug Content | Particle Size (nm) | ||
|---|---|---|---|
| Room Temperature | Refrigerator Temperature | Room Temperature | |
| Initial | 94.18 ± 0.46 | 94.11 ± 0.46 | 89.81 ± 2.64 |
| After 1 month | 93.53 ± 0.52 | 94.08 ± 0.09 | 97.03 ± 3.42 |
| After 2 months | 93.10 ± 0.74 | 93.25 ± 0.62 | 103.8 ± 5.21 |
| After 3 months | 92.32 ± 0.95 | 93.07 ± 0.75 | 106.5 ± 4.24 |
| Drug Content | pH | ||
|---|---|---|---|
| Room Temperature | Refrigerator Temperature | Room Temperature | |
| Initial | 94.18 ± 0.25 | 94.18 ± 0.32 | 5.6 ± 0.75 |
| After 1 month | 93.87 ± 0.90 | 93.08 ± 0.46 | 5.8 ± 0.18 |
| After 2 months | 92.06 ± 0.73 | 92.25 ± 0.63 | 6.4 ± 0.65 |
| After 3 months | 90.40 ± 0.52 | 92.07 ± 0.45 | 5.7 ± 0.08 |
| Formulation Code | Zone of Inhibition (ZOI) | |
|---|---|---|
| Candida albicans | Trichophyton rubrum | |
| SCZ-SLNs | 24.3 ± 1.90 | 21.2 ± 2.83 |
| SCZ-SLNs gel | 26.5 ± 3.42 | 23.7 ± 1.45 |
| SCZ-gel | 17.1 ± 1.43 | 16.4 ± 3.5 |
| Blank SLNs | 00 | 00 |
| Formulation Code | SCZ | GMS | Tween 20 | Phospholipon® 90 H |
|---|---|---|---|---|
| SLN1 | 1% | 1% | 2% | 0.75% |
| SLN2 | 1% | 2% | 2% | 0.75% |
| SLN3 | 1% | 3% | 2% | 0.75% |
| SLN4 | 1% | 4% | 2% | 0.75% |
| SLN5 | 1% | 5% | 2% | 0.75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samee, A.; Usman, F.; Wani, T.A.; Farooq, M.; Shah, H.S.; Javed, I.; Ahmad, H.; Khan, R.; Zargar, S.; Kausar, S. Sulconazole-Loaded Solid Lipid Nanoparticles for Enhanced Antifungal Activity: In Vitro and In Vivo Approach. Molecules 2023, 28, 7508. https://doi.org/10.3390/molecules28227508
Samee A, Usman F, Wani TA, Farooq M, Shah HS, Javed I, Ahmad H, Khan R, Zargar S, Kausar S. Sulconazole-Loaded Solid Lipid Nanoparticles for Enhanced Antifungal Activity: In Vitro and In Vivo Approach. Molecules. 2023; 28(22):7508. https://doi.org/10.3390/molecules28227508
Chicago/Turabian StyleSamee, Ayesha, Faisal Usman, Tanveer A. Wani, Mudassir Farooq, Hamid Saeed Shah, Ibrahim Javed, Hassan Ahmad, Riffat Khan, Seema Zargar, and Safina Kausar. 2023. "Sulconazole-Loaded Solid Lipid Nanoparticles for Enhanced Antifungal Activity: In Vitro and In Vivo Approach" Molecules 28, no. 22: 7508. https://doi.org/10.3390/molecules28227508
APA StyleSamee, A., Usman, F., Wani, T. A., Farooq, M., Shah, H. S., Javed, I., Ahmad, H., Khan, R., Zargar, S., & Kausar, S. (2023). Sulconazole-Loaded Solid Lipid Nanoparticles for Enhanced Antifungal Activity: In Vitro and In Vivo Approach. Molecules, 28(22), 7508. https://doi.org/10.3390/molecules28227508







