Hydrogen Bonds Stabilize Chloroselenite Anions: Crystal Structure of a New Salt and Donor-Acceptor Bonding to SeO2
Abstract
1. Introduction
2. Results
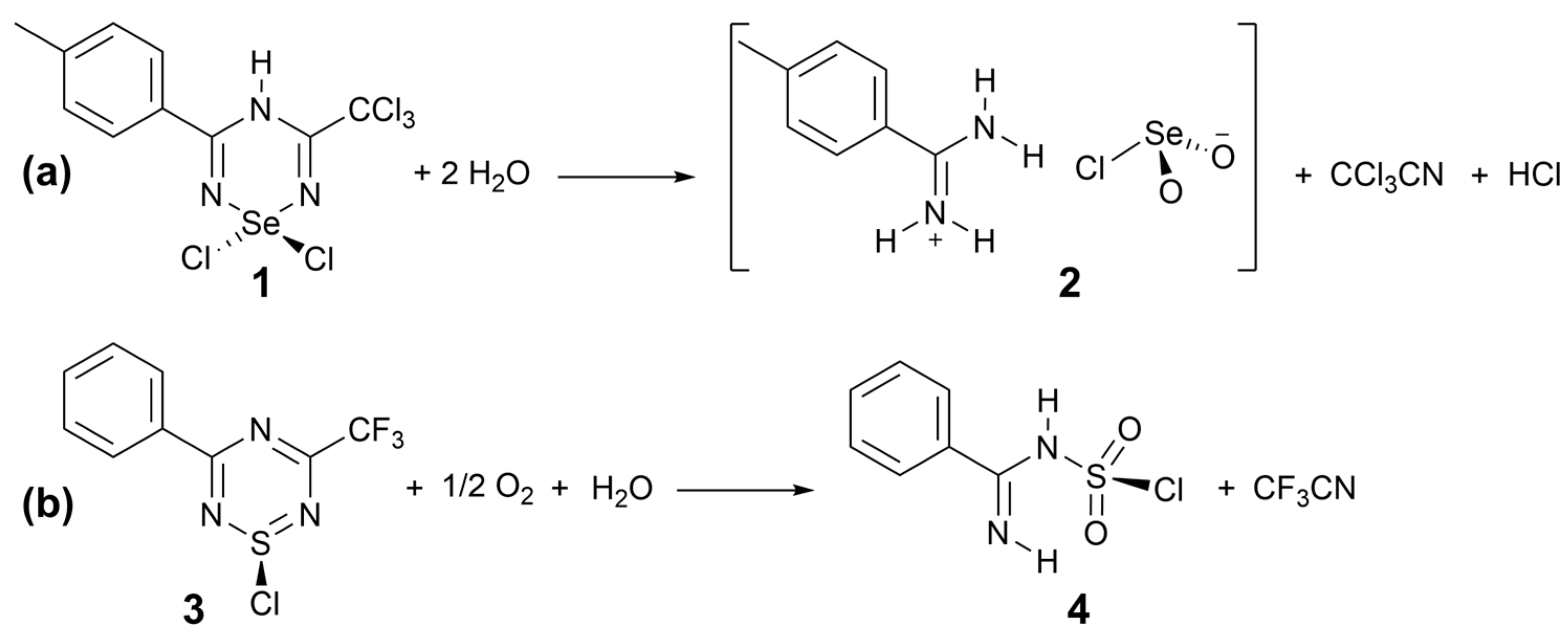
2.1. Formation of the Salt from a Hydrolysis Reaction
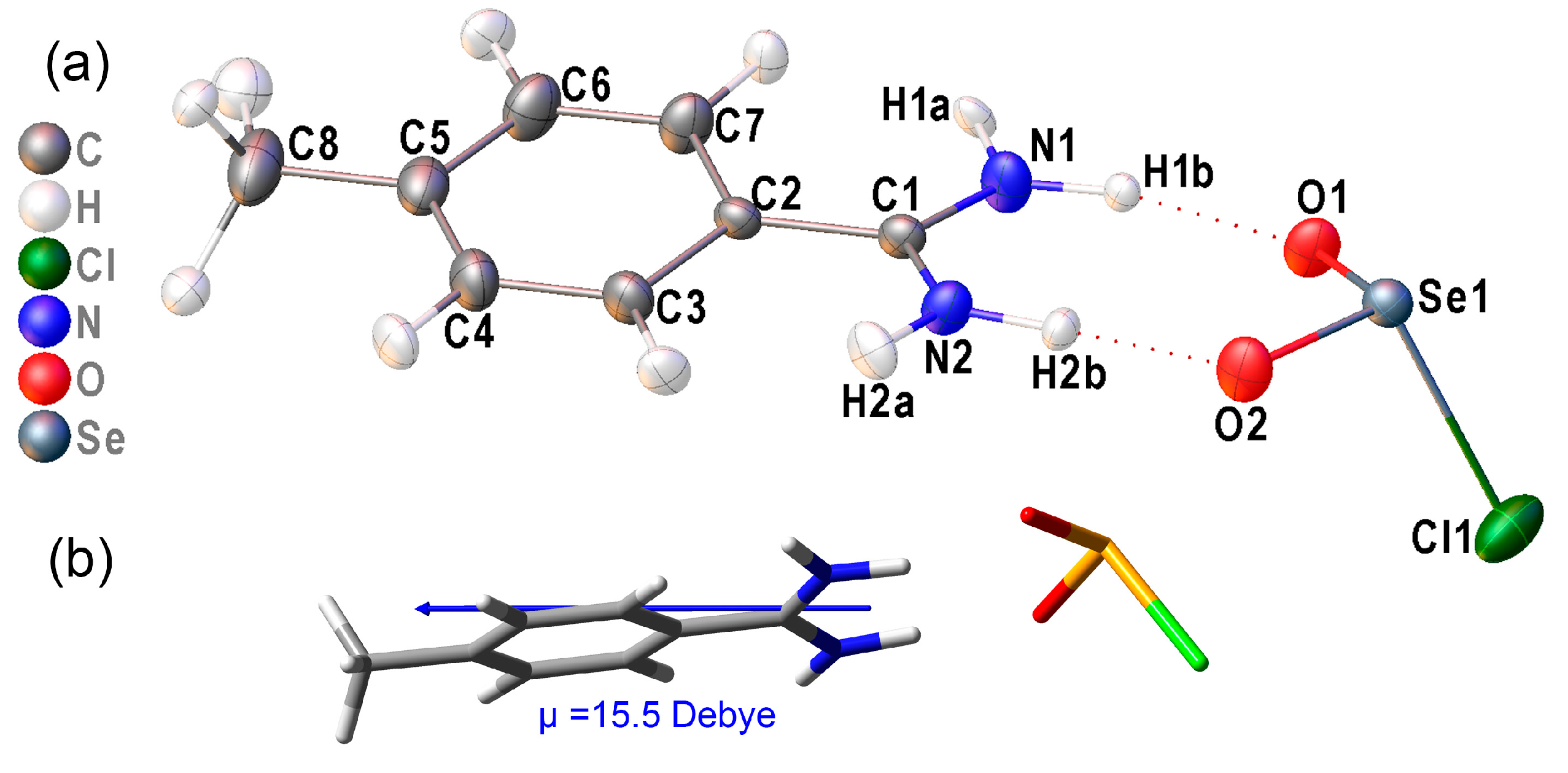
2.2. Crystallographic Characterization
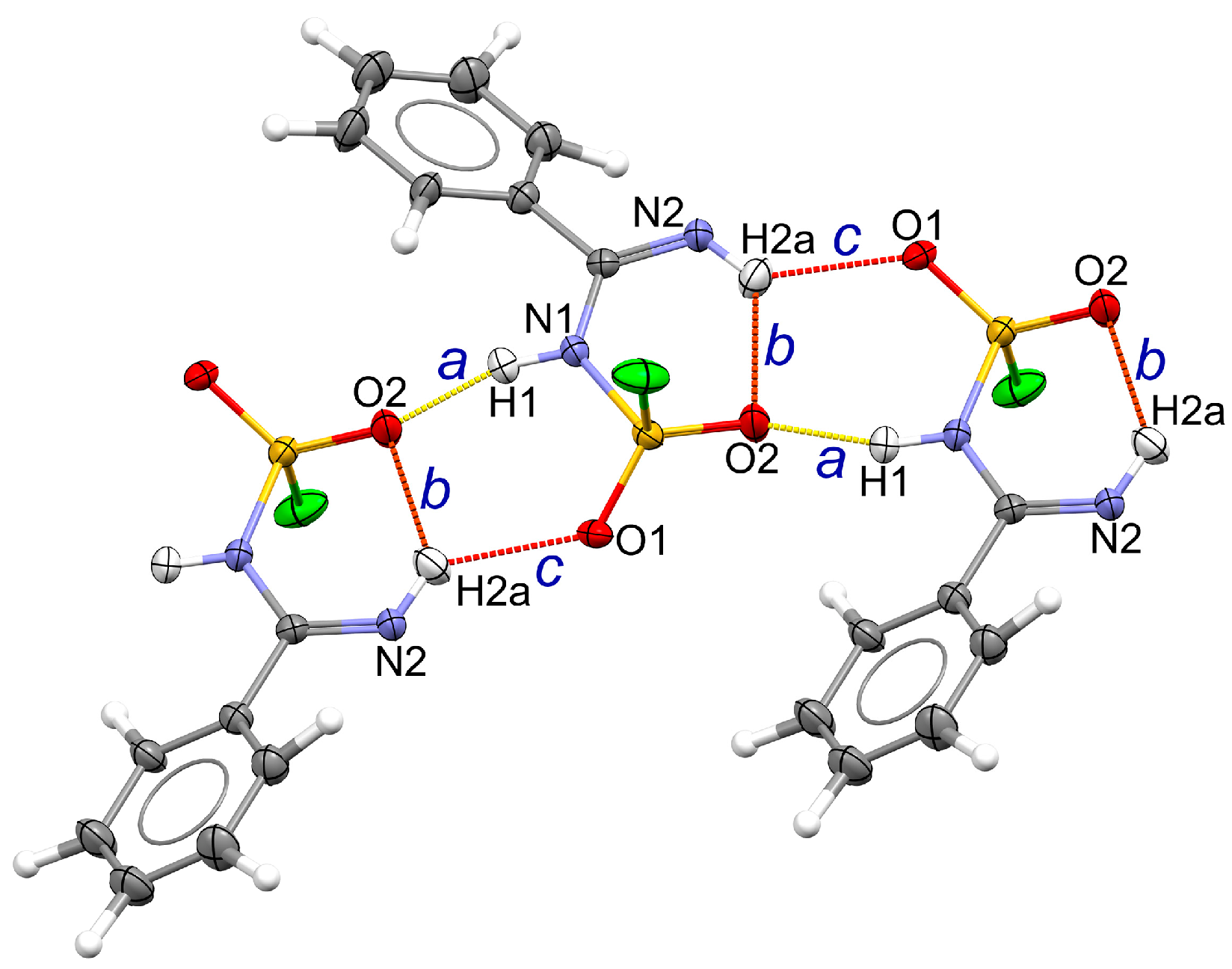
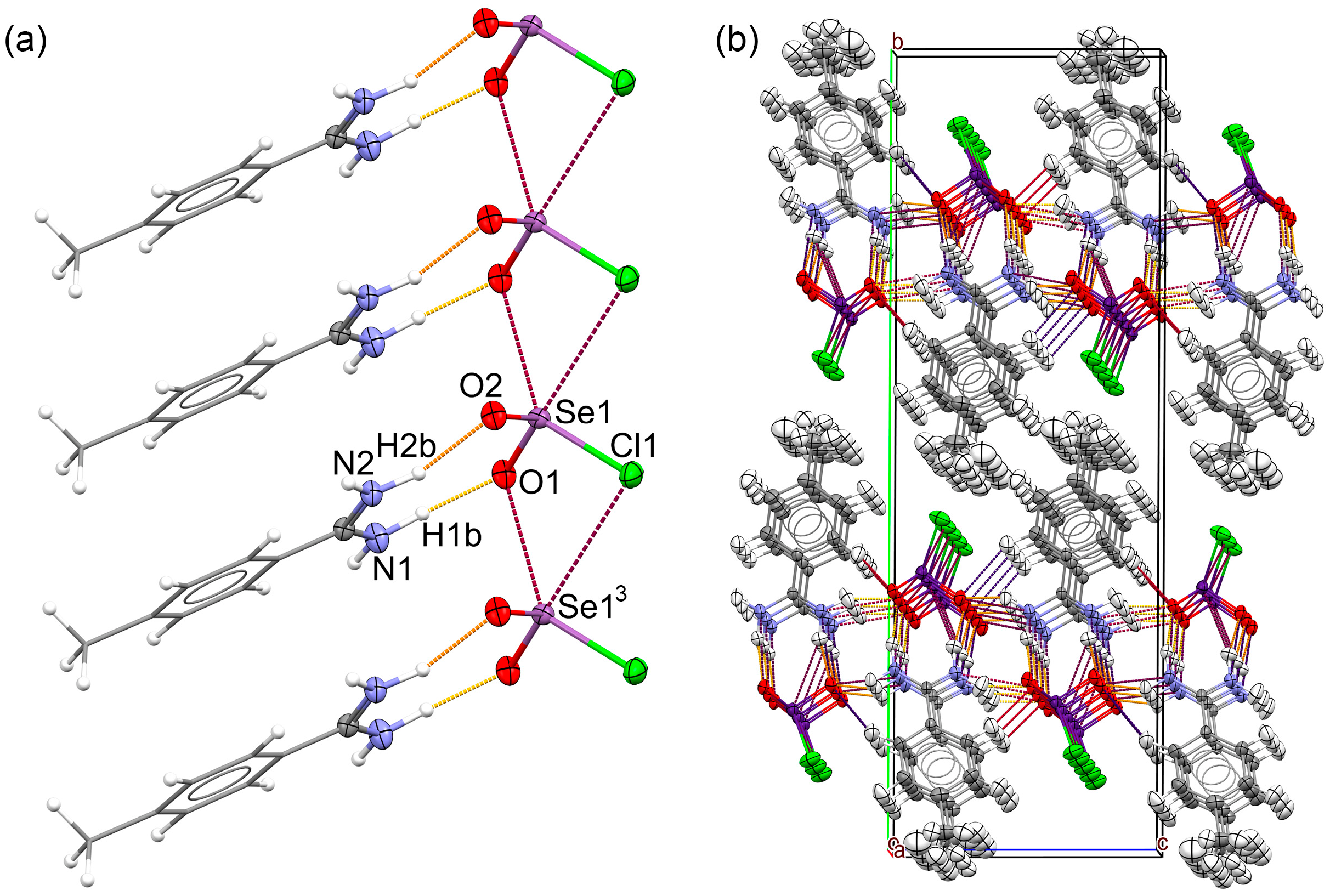
2.3. Hydrogen Bonding in the Solid Lattice of 2
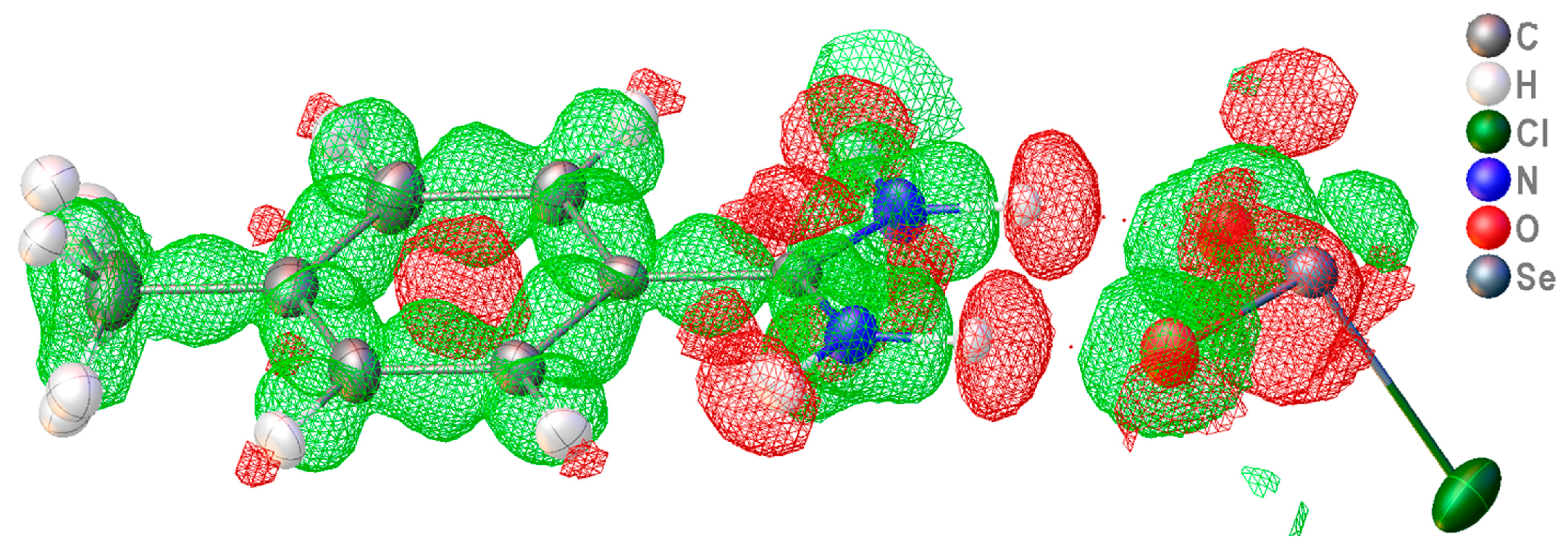
2.4. Intra-Ionic Short Contacts in the Lattice of 2
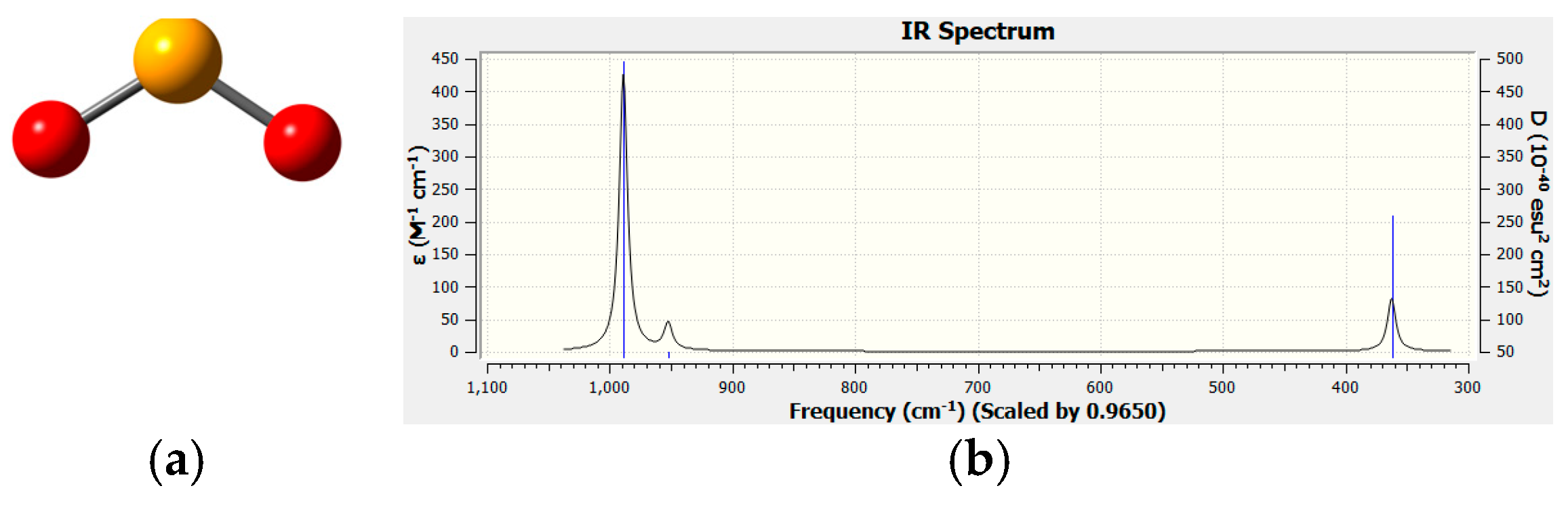
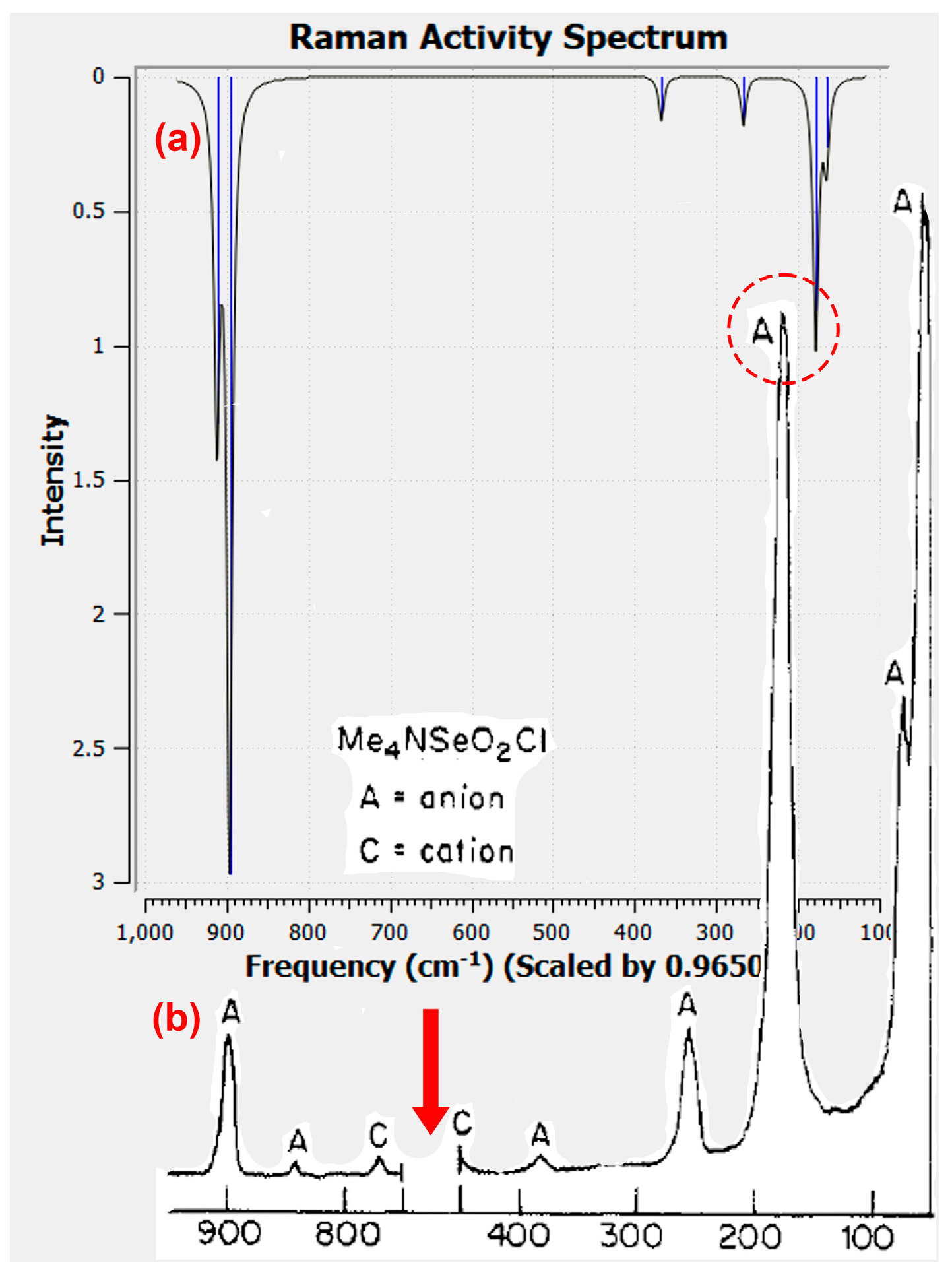
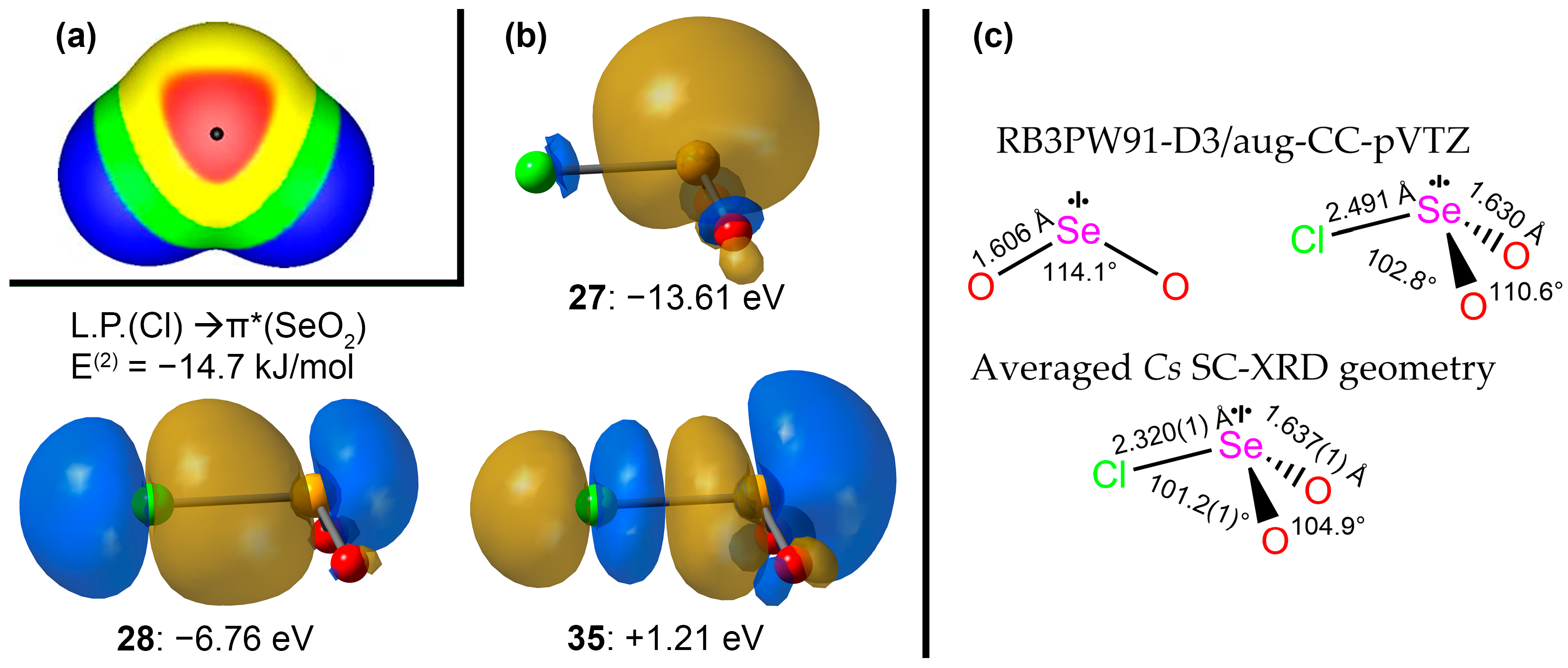
2.5. DFT Computational Investigation of Structure
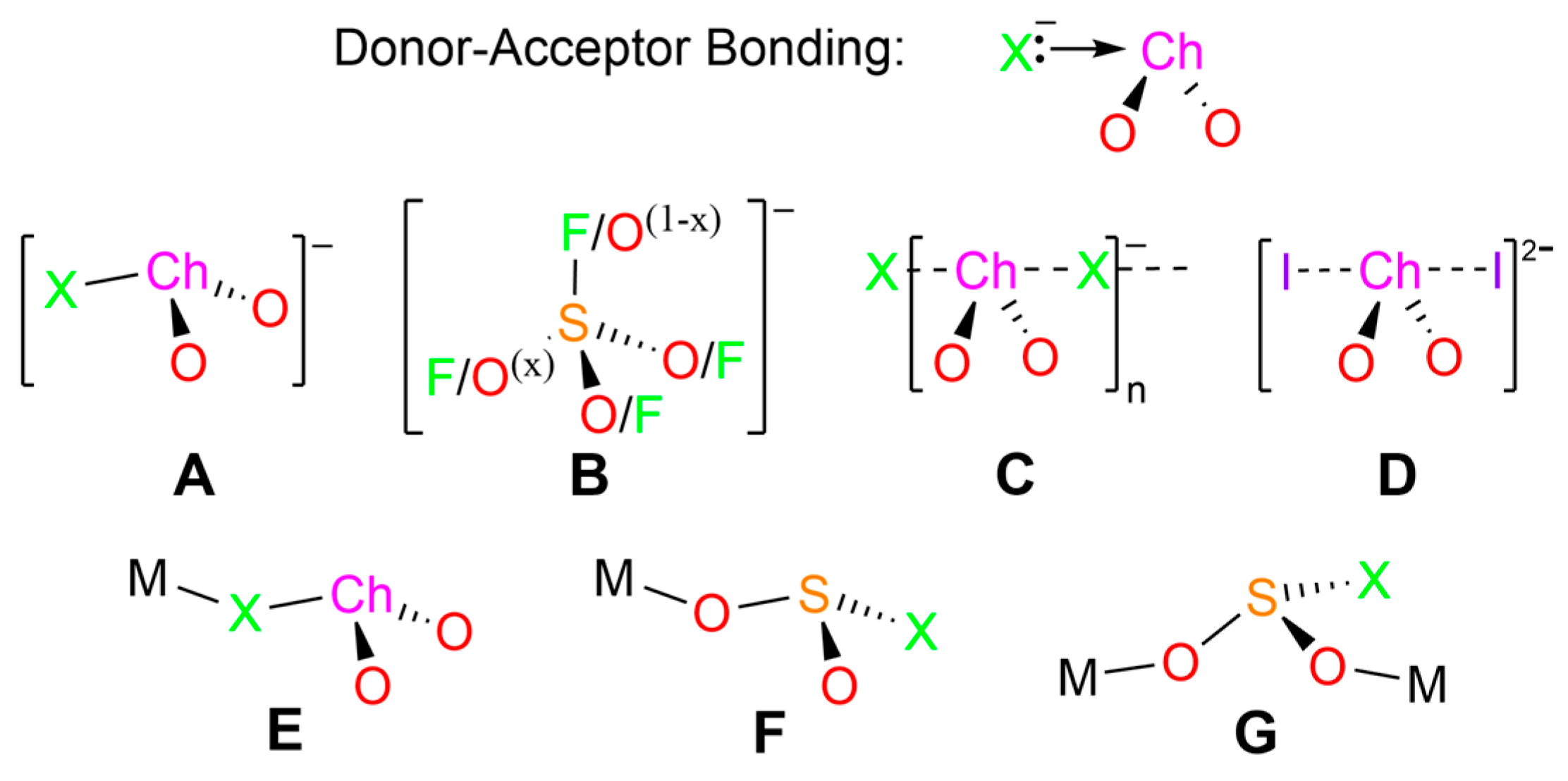
2.6. Donor–Acceptor Bonding in Hypervalent ClSeO2− using the NBO Formalism
3. Experimental
3.1. Chemical Synthesis
3.2. Single-Crystal X-ray Crystallography
3.3. Computational Methods
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Parameter | Strong | Moderate | Weak |
|---|---|---|---|
| Interaction type: | strongly covalent | mostly electrostatic | electrostatic/dispersion |
| d(H···A), Å # | 1.2–1.5 | 1.5–2.2 | >2.2 |
| d(D···A), Å # | 2.2–2.5 | 2.5–3.2 | >3.2 |
| lengthening of D–H | 0.08–0.25 | 0.02–0.08 | <0.02 |
| D–H versus H···A | D–H ≈ H···A | D–H < H···A | D–H << H···A |
| Directionality: | Strong | moderate | weak |
| ∠D–H···A, ° | 170–180 | >130 | >90 |
| Bond energy, kJ mol−1 | 60–160 | 16–60 | <16 |
| IR shift , cm−1 | 25% | 10–25% | <10% |
| 1H downfield shift, ppm | 14–22 | <14 |
References
- LaHaie, P.; Milne, J. Chloro and oxochloro anions of selenium(IV). Inorg. Chem. 1979, 18, 632–637. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Krebs, B.; Hucke, M.; Schäffer, A. Strukturchemie von Selen(IV)-Sauerstoff-Halogenverindinungen. Z. Kristallogr. Kristallgeom. Kristallphys. Kristallchem. 1982, 159, 84–85. [Google Scholar]
- Krebs, B.; Ahlers, F.-P. Developments in chalcogen-halide chemistry. Adv. Inorg. Chem. 1990, 35, 235–317. [Google Scholar]
- Atwood, D.A. Selenium: Inorganic chemistry. In Encyclopedia of Inorganic Chemistry, 2nd ed.; King, R.B., Ed.; Wiley: Chichester, UK, 2005; Volume 3, pp. 4931–4955. [Google Scholar]
- Schäffer, A. Cavity-Enhanced Optical Clocks. Ph.D. Thesis, University of Münster, Münster, Germany, 1984. [Google Scholar]
- Wang, B.-C. Ab Initio and Density Functional Theory Studies of Nuclear Magnetic Resonance and Electron Spin Resonance Parameters of Biomolecules. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 1968. [Google Scholar]
- Collins, M.J.; Ratcliffe, C.I.; Ripmeester, J.A. CP/MAS 77Se NMR in Solids. Chemical Shift Tensors and Isotropic Shifts. J. Magn. Res. 1986, 68, 172–179. [Google Scholar] [CrossRef]
- Wasif, S.; Salama, S.B. Weak complexes of sulfur and selenium. IV. Complex of selenium dioxide and seleninyl dichloride with halide ions. J. Chem. Soc. Dalton Trans. 1975, 21, 2239–2241. [Google Scholar] [CrossRef]
- Milne, J.; LaHaie, P. Chloroselenate(IV) Equilibria in Aqueous Hydrochloric Acid. Inor. Chem. 1979, 18, 3180–3183. [Google Scholar] [CrossRef]
- Milne, J. Haloselenate(IV) formation and selenous acid dissociation equilibria in hydrochloric and hydrofluoric acids. Can. J. Chem. 1987, 65, 316–321. [Google Scholar] [CrossRef]
- Paetzold, R.; Aurich, K. Investigation of selenium-oxygen compounds. XL. Alkali haloselenites and alkali halodiselenites. Z. Chem. 1966, 6, 265. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, X.-Y.; Stoddart, J.F. Weak bonding strategies for achieving regio- and site-selective transformations. Chem 2022, 8, 414–438. [Google Scholar] [CrossRef]
- Narita, H.; Maeda, M.; Tokoro, C.; Suzuki, T.; Tanaka, M.; Shiwaku, H.; Yaita, T. Extraction of Se(IV) and Se(VI) from aqueous HCl solution by using a diamide-containing tertiary amine. RSC Adv. 2023, 13, 17001–17007. [Google Scholar] [CrossRef]
- Xu, W.; Bai, W. The selenium oxygen clusters SeOn (n = 1–5) and their anions: Structures and electron affinities. J. Mol. Struct. THEOCHEM 2008, 863, 1–8. [Google Scholar] [CrossRef]
- Crowther, S.A.; Brown, J.M. The 313 nm band system of SeO2. Part 1: Vibrational structure. J. Mol. Spect. 2004, 225, 196–205. [Google Scholar] [CrossRef]
- Crowther, S.A.; Brown, J.M. The 313 nm band system of SeO2. Part 2: Rotational structure. J. Mol. Spect. 2004, 225, 206–221. [Google Scholar] [CrossRef]
- Grein, F. Theoretical studies on the electronic spectrum of selenium dioxide. Comparison with ozone and sulfur dioxide. Chem. Phys. 2009, 360, 1–6. [Google Scholar] [CrossRef]
- Chulanova, E.A.; Radiush, E.A.; Balmohammadi, Y.; Beckmann, J.; Grabowsky, S.; Zibarev, A.V. New charge-transfer complexes of 1,2,5-chalcogenadiazoles with tetrathiafulvalenes. CrystEngComm 2023, 25, 391–402. [Google Scholar] [CrossRef]
- Rozhkov, A.V.; Zhmykhova, M.V.; Torubaev, Y.V.; Katlenok, E.A.; Kryukov, D.M.; Kukushkin, V.Y. Bis(perfluoroaryl)chalcolanes ArF2Ch (Ch = S, Se, Te) as σ/π-Hole Donors for Supramolecular Applications Based on Noncovalent Bonding. Cryst. Growth Des. 2023, 23, 2593–2601. [Google Scholar] [CrossRef]
- Biswal, H.S.; Sahu, A.K.; Galmés, B.; Frontera, A. Se···O/S and S···O Chalcogen Bonds in Small Molecules and Proteins: A Combined CSD and PDB Study. ChemBioChem 2022, 23, e202100498. [Google Scholar] [CrossRef]
- Burguera, S.; Gomila, R.M.; Bauzá, A.; Frontera, A. Selenoxides as Excellent Chalcogen Bond Donors: Effect of Metal Coordination. Molecules 2022, 27, 8837. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Resnati, G. The Chalcogen Bond in Crystalline Solids: A World Parallel to Halogen Bond. Acc. Chem. Res. 2019, 52, 1313–1324. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Model 2012, 18, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Usoltsev, A.N.; Korobeynikov, N.A.; Kolesov, B.A.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Adonin, S.A. Oxochloroselenate(IV) with Incorporated {Cl2}: The Case of Strong Cl···Cl Halogen Bonding. Chem. Eur. J. 2021, 27, 9292–9294. [Google Scholar] [CrossRef] [PubMed]
- Hermodsson, Y. The crystal structure of (CH3)4NCl∙5SeOCl2. Acta Chim. Scand. 1967, 21, 1328–1342. [Google Scholar] [CrossRef][Green Version]
- Jackson, V.E.; Dixon, D.A.; Christe, K.O. Thermochemical Properties of Selenium Fluorides, Oxides, and Oxofluorides. Inorg. Chem. 2012, 51, 2472–2485. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; McGrady, G.S.; Passmore, J.; Grein, F.; Decken, A. Reversible SO2 Uptake by Tetraalkylammonium Halides: Energetics and Structural Aspects of Adduct Formation Between SO2 and Halide Ions. Z. Anorg. Allg. Chem. 2012, 638, 744–753. [Google Scholar] [CrossRef]
- Lork, E.; Mews, R.; Viets, D.; Watson, P.G.; Borrmann, T.; Vij, A.; Boatz, J.A.; Christe, K.O. Structure of the SO2F− Anion, a Problem Case. Inorg. Chem. 2001, 40, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Kessler, U.; van Wullen, L.; Jansen, M. Structure of the Fluorosulfite Anion: Rotational Disorder of SO2F− in the Alkali Metal Fluorosulfites and Crystal Structures of α- and β-CsSO2F. Inorg. Chem. 2001, 40, 7040–7046. [Google Scholar] [CrossRef]
- Arnold, S.T.; Miller, T.M.; Viggiano, A.A. A Combined Experimental and Theoretical Study of Sulfur Oxyfluoride Anion and Neutral Thermochemistry and Reactivity. J. Phys. Chem. A 2002, 106, 9900–9909. [Google Scholar] [CrossRef]
- Burow, D.F. Spectroscopic Studies of Halosulfinate Ions. Inorg. Chem. 1972, 11, 573–583. [Google Scholar] [CrossRef]
- Milne, J.; LaHaie, P. Raman spectra of haloselenate(IV) ions—The SeO2Br− anion. Spectrochim. Acta 1983, 39, 555–557. [Google Scholar] [CrossRef]
- Zhu, S.-Z.; Huang, Q.-C.; Wu, K. Synthesis and Structure of (Difluoromethyl)triphenylphosphonium Fluorosulfite. Evidence for Formation of Difluorosulfene as an Intermediate. Inorg. Chem. 1994, 33, 4584–4585. [Google Scholar] [CrossRef]
- Kuhn, N.; Bohnen, H.; Fahl, J.; Bläser, D.; Boese, R. Imidazole Derivatives, XIX. Coordination or Reduction? On the Reaction of 1,3-Diisopropyl-4,5-dimethylimidazolylidene with Sulfur Halides and Sulfur Oxygen Halides. Chem. Ber. 1996, 129, 1579–1586. [Google Scholar] [CrossRef]
- Kessler, U.; Jansen, M. Crystal Structures of Monofluorosulfites MSO2F (M = K, Rb). Z. Anorg. Allg. Chem. 1999, 625, 385–388. [Google Scholar] [CrossRef]
- Kuhn, N.; Bohnen, H.; Bläser, D.; Boese, R.; Maulitz, A.H. Selective Reduction of Sulfuric Chloride: The Structure of the Chlorosulfite Ion. J. Chem. Soc. Chem. Commun. 1994, 19, 2283–2284. [Google Scholar] [CrossRef]
- Reuter, K.; Rudel, S.S.; Buchner, M.R.; Kraus, F.; von Hänisch, C. Crown Ether Complexes of Alkali-Metal Chlorides from SO2. Chem. Eur. J. 2017, 23, 9607–9617. [Google Scholar] [CrossRef] [PubMed]
- Awere, E.G.; Burford, N.; Haddon, R.C.; Parsons, S.; Passmore, J.; Waszczak, J.V.; White, P.S. X-ray Crystal Structures of the 1,3,2-Benzodithiazolyl Dimer and 1,3,2-Benzodithiazolium Chloride Sulfur Dioxide Solvate: Comparison of the Molecular and Electronic Structures of the 10-π-Electron C6S2N+ Cation and the C6H4S2N• Radical and Dimer and a Study of the Variable-Temperature Magnetic Behavior of the Radical. Inorg. Chem. 1990, 29, 4821–4830. [Google Scholar]
- Boyle, P.D.; Godfrey, S.M.; Pritchard, R.G. The reaction of N-methylbenzothiazole-2-selone and 1,1-dimethylselenoureawith sulfuryl chloride and dichlorine. J. Chem. Soc. Dalton Trans. 1999, 23, 4245–4250. [Google Scholar] [CrossRef]
- Eller, P.G.; Kubas, G.J. Synthesis, Properties, and Structure of Iodosulfinate Salts. Inorg. Chem. 1978, 17, 894–897. [Google Scholar] [CrossRef]
- Dankert, F.; Feyh, A.; von Hänisch, C. Chalcogen Bonding of SO2 and s-Block Metal Iodides Near Room Temperature: A Remarkable Structural Diversity. Eur. J. Inorg. Chem. 2020, 2020, 2744–2756. [Google Scholar] [CrossRef]
- Eller, P.G.; Kubas, G.J.; Ryan, R.R. Synthesis and Properties of Sulfur Dioxide Adducts of Organophosphinecopper(I) Iodides. Structures of the Dinuclear Compounds Tetrakis(methyldiphenylphosphine)di-μ-iodo-dicopper(I)-Sulfur Dioxide and Tris(triphenylphosphine)di-μ-Li-iodo-dicopper(I). Inorg. Chem. 1977, 16, 2454–2462. [Google Scholar] [CrossRef]
- Snow, M.R.; Ibers, J.A. The Halogen to Sulfur Dioxide Bond. Structure of Iodo(sulfur dioxide)methylbis(triphenyl phosphine)platinum, Pt(CH3)(PPh3)2I-SO2. Inorg. Chem. 1973, 12, 224–229. [Google Scholar] [CrossRef]
- Nagasawa, I.; Amitaa, H.; Kitagawa, H. A new type of iodosulfite ion formulated as I2SO22−. Chem. Commun. 2009, 8, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Boeré, R.T.; Roemmele, T.L.; Yu, X. Unsymmetrical 1λ3-1,2,4,6-Thiatriazinyls with Aryl and Trifluoromethyl Substituents: Synthesis, Crystal Structures, EPR Spectroscopy, and Voltammetry. Inorg. Chem. 2011, 50, 5123–5136. [Google Scholar] [CrossRef] [PubMed]
- Suduweli Kondage, S.; Roemmele, T.L.; Boeré, R.T. Dispersion in Crystal Structures of 1-Chloro-3-aryl-5-trihalomethyl-1λ4,2,4,6-thiatriazines: Towards an Understanding of the Supramolecular Organization of Covalent Thiazyl Chlorides. Synlett 2023, 34, 1113–1121. [Google Scholar]
- Cordes, A.W.; Oakley, R.T.; Reed, R.W. Structure of 1,1-Dichloro-3,5-diphenyl-4H-1,2,4,6-selenatriazine. Acta Cryst. 1986, 42, 1889–1890. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth/Heinemann: Oxford, UK, 1997; pp. 775–779. [Google Scholar]
- Kleemiss, F.; Dolomanov, O.V.; Bodensteiner, M.; Peyerimhoff, N.; Midgley, L.; Bourhis, L.J.; Genoni, A.; Malaspina, L.A.; Jayatilaka, D.; Spencer, J.L.; et al. Accurate crystal structures and chemical properties from NoSpherA2. Chem. Sci. 2021, 12, 1675–1692. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Krebs, B.; Hucke, M.; Hein, M.; Schäffer, A. Monomeric and Dimeric Oxotrihalogenoselenates(IV): Preparation, Structure and Properties of [As(C6H5)4]SeOCl3 and [N(C2H5)4] SeOCl3. Z. Naturforsch. B Chem. Sci. 1983, 38, 20–29. [Google Scholar] [CrossRef]
- Feldmann, C.; Jansen, M. On the Crystal Structures of the Monofluoroselenites MSeO2,F (M = K, Rb, Cs). Chem. Ber. 1994, 127, 2173–2176. [Google Scholar] [CrossRef]
- Cordes, A.W. The crystal structure of 8-hydroxyquinolinium trichlorooxyselenate. Inorg. Chem. 1967, 6, 1204–1208. [Google Scholar] [CrossRef]
- Krebs, B.; Schäffer, A.; Hucke, M. Oxotrihalogenoselenates(IV): Preparation, Structure and Properties of P(C6H5)4SeOCl3, P(C6H5)4SeOBr3 and As(C6H5)4SeOBr3. Z. Naturforsch. B Chem. Sci. 1982, 37, 1410–1417. [Google Scholar] [CrossRef]
- Wang, B.-C.; Cordes, A.W. The Crystal Structure of Dipyridinium(II) Oxytetrachloroselenate(IV), C10H8N2H22+ SeOCl42−. A Highly Coordinated Selenium Compound. Inorg. Chem. 1970, 9, 1643–1650. [Google Scholar] [CrossRef]
- James, M.A.; Knop, O.; Cameron, T.S. Crystal structures of (n-Pr4N)2SnC16, (n-Pr4N)[TeC14(OH)], (n-Pr4N)2[Te2Cl10] (nominal), and (n-Pr4N)2[Se2O2Cl6] with observations on Z2L102n− and Z2L82− dimers in general. Can. J. Chem. 1992, 70, 1795–1821. [Google Scholar] [CrossRef]
- Hasche, S.; Reich, O.; Beckmann, I.; Krebs, B. Stabilization of Oxohalogeno and Halogenochalcogenates(IV), by Proton Acceptors—Synthesis, Structures and Properties of [C4H10NO]2[SeOC14], [C4H10NO]2[Se2Br10] and [(CH3)2CHC(NH2)(OH)][Te3C113] (CH3)2CHCN. Z. Anorg. Allg. Chem. 1997, 623, 724–734. [Google Scholar] [CrossRef]
- Tuononen, H.M.; Suontamo, R.J.; Valkonen, J.U.; Laitinen, R.S.; Chivers, T. Conformations and Energetics of Sulfur and Selenium Diimides. Inorg. Chem. 2003, 42, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Lobring, K.C.; Hao, C.; Forbes, J.K.; Ivanov, M.R.J.; Bachrach, S.M.; Sunderlin, L.S. Bond Strengths in ChCl3− and ChOCl3− (Ch = S, Se, Te): Experiment and Theory. J. Phys. Chem. A 2003, 107, 11153–11160. [Google Scholar] [CrossRef]
- Grabowsky, S.; Luger, P.; Buschmann, J.; Schneider, T.; Schirmeister, T.; Sobolev, A.N.; Jayatilaka, D. The Significance of Ionic Bonding in Sulfur Dioxide: Bond Orders from X-ray Diffraction Data. Angew. Chem. Int. Ed. 2012, 51, 6776–6779. [Google Scholar] [CrossRef]
- Durrant, M.C. A quantitative definition of hypervalency. Chem. Sci. 2015, 6, 6614–6623. [Google Scholar] [CrossRef]
- Landrum, G.A.; Goldberg, N.; Hoffmann, R. Bonding in the trihalides (X3−), mixed trihalides (X2Y−) and hydrogen bihalides (X2H−). The connection between hypervalent, electron-rich three-center, donor–acceptor and strong hydrogen bonding. J. Chem. Soc. Dalton Trans. 1997, 19, 3605–3613. [Google Scholar] [CrossRef]
- Oakley, R.T.; Reed, R.W.; Cordes, A.W.; Craig, S.L.; Graham, J.B. 1,2,4,6-Selenatriazinyl Radicals and Dimers. Preparation and Structural Characterization of l-Chloro-3,5-diphenyl-1,2,4,6-selenatriazine (Ph2C2N3SeCl) and Bis(3,5-diphenyl-1,2,4,6-selenatriazine) ((Ph2C2N3Se)2). J. Am. Chem. Soc. 1987, 109, 7745–7749. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef]
- Boeré, R.T. Crystal structures of CuCl2·2H2O (Eriochalcite) and NiCl2∙6H2O (Nickelbischofite) at low temperature: Full refinement of hydrogen atoms using non-spherical atomic scattering factors. Crystals 2023, 13, 293. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Allen, F.H.; Bruno, I.J. Bond lengths in organic and metalorganic compounds revisited: X-H bond lengths from neutron diffraction data. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2010, 66, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.D.D.; Lilienthal, E.; Bender, C.O.; Boeré, R.T. Accurate Crystal Structures of C12H9CN, C12H8(CN)2, and C16H11CN Valence Isomers Using Nonspherical Atomic Scattering Factors. J. Org. Chem. 2022, 87, 16213–16229. [Google Scholar] [CrossRef]
- Marszaukowski, F.; Boeré, R.T.; Wohnrath, K. Frustrated and Realized Hydrogen Bonding in 4-Hydroxy-3,5-ditertbutylphenylphosphine Derivatives. Cryst. Growth Des. 2022, 22, 2512–2533. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Boeré, R.T. The copper sulfate hydration cycle. Crystal structures of CuSO4 (Chalcocyanite), CuSO4∙H2O (Poitevinite), CuSO4∙3H2O (Bonattite) and CuSO4∙O 5H2O (Chalcanthite) at low temperature using non-spherical atomic scattering factors. New J. Chem. 2022, 46, 5479–5488. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Takeo, H.; Hirota, E.; Morino, Y. Equilibrium structure and potential function of selenium dioxide by microwave spectroscopy. J. Mol. Spectr. 1970, 34, 370–382. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Booij, A.S.; Kovcás, A. The infrared spectra of SeO2 and TeO2 in the gas phase. Chem. Phys. Lett. 1998, 292, 447–453. [Google Scholar] [CrossRef]
- Walsh, A.D. The Electronic Orbitals, Shapes, and Spectra of Polyatomic Molecules. Part II. Non-hydride AB2, and BAC Molecules. J. Chem. Soc. 1955, 2266–2287. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997; Volume 12, p. 330. [Google Scholar]
- Steiner, T. Hydrogen-Bond Distances to Halide Ions in Organic and Organometallic Crystal Structures: Up-to-date Database Study. Acta Cryst. 1998, 54, 456–463. [Google Scholar] [CrossRef]

| Atoms | dExperiment | dComputed | Atoms | ∠Experiment | ∠Computed |
|---|---|---|---|---|---|
| Se1–Cl1 | 2.3202(9) | 2.491 | O1–Se1–Cl1 | 100.86(9) | 102.75 |
| Se1–O1 | 1.645(2) | 1.630 | O2–Se1–Cl1 | 101.55(9) | 102.75 |
| Se1–O2 | 1.629(2) | 1.630 | O2–Se1–O1 | 104.90(11) | 110.58 |
| N1–C1 | 1.323(4) | N2–C1–N1 | 119.1(3) | ||
| N2–C1 | 1.317(4) | C2–C1–N1 | 120.8(3) | ||
| C1–C2 | 1.472(4) | C2–C1–N2 | 120.1(3) | ||
| C2–C3 | 1.404(4) | C3–C2–C1 | 120.5(3) | ||
| C2–C7 | 1.395(4) | C7–C2–C3 | 118.1(3) | ||
| C3–C4 | 1.382(5) | C4–C3–C2 | 120.4(3) | ||
| C4–C5 | 1.395(5) | C5–C4–C3 | 121.3(3) | ||
| C5–C6 | 1.392(5) | C6–C5–C4 | 118.1(3) | ||
| C5–C8 | 1.497(5) | C8–C5–C4 | 120.7(3) | ||
| C6–C7 | 1.384(5) | C8–C5–C6 | 121.2(3) | ||
| C7–C2–C1 | 121.4(3) | ||||
| C7–C6–C5 | 120.9(3) |
| D(–H) | :A | d(D–H)/Å | d(H∙∙∙A)/Å | d−∑rvdW | d(D∙∙∙A)/Å | Angle/° | |
|---|---|---|---|---|---|---|---|
| Hydrogen-bonds | |||||||
| N1–H1a | O2 1 | 1.045(17) | 1.90(3) | −0.82 | 2.852(3) | 150(3) | |
| N1–H1b | O1 | 1.051(18) | 1.843(19) | −0.877 | 2.880(4) | 168(3) | |
| N2–H2a | O1 2 | 1.030(17) | 1.83(3) | −0.89 | 2.792(3) | 153(4) | |
| N2–H2b | O2 | 1.039(18) | 1.95(2) | −0.77 | 2.948(3) | 160(3) | |
| Inter-anion Contacts | |||||||
| O1 | Se1 3 | (angle is Se1–O1∙∙∙Se1 3) | −0.289 | 3.131(2) | 108.8(1) | ||
| Cl1 | Se1 3 | (angle is Se1 3–Cl1 3∙∙∙Se1) | −0.252 | 3.3980(9) | 85.96(3) | ||
| Band | Assignment | Symmetry | Experiment 1 CH3CN Solution | Experiment 1 [Me4N][ClSeO2] | DFT Optimized 2 | DFT X-ray Geom. 3 |
|---|---|---|---|---|---|---|
| ν1 | νsym(SO2) | A′ | 890 (p) | 903 (s) | 896 (vs,p) | 886 (vs,p) |
| ν2 | ν(S–Cl) | A′ | 273 (p) 4 | 267 (s) 4 | 267 (w,p) | 321 (m,p) |
| ν3 | δsciss(SO2) | A′ | 380 (p) | 396 (w) | 367 (w,p) | 382 (w,p) |
| ν4 | δsym(ClSO2−) | A′ | 200 (p) 4 | 193 (vs) 4 | 178 (m,p) | 242 (m,p) |
| ν5 | νasym(SO2) | A″ | 840 (dp) | 841 (w) | 912 (m,dp) | 887 (s,dp) |
| ν6 | δasym(FSO2−) | A″ | not obsv. | not obsv. | 165 (w,dp) | 189 (w,dp) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boeré, R.T. Hydrogen Bonds Stabilize Chloroselenite Anions: Crystal Structure of a New Salt and Donor-Acceptor Bonding to SeO2. Molecules 2023, 28, 7489. https://doi.org/10.3390/molecules28227489
Boeré RT. Hydrogen Bonds Stabilize Chloroselenite Anions: Crystal Structure of a New Salt and Donor-Acceptor Bonding to SeO2. Molecules. 2023; 28(22):7489. https://doi.org/10.3390/molecules28227489
Chicago/Turabian StyleBoeré, René T. 2023. "Hydrogen Bonds Stabilize Chloroselenite Anions: Crystal Structure of a New Salt and Donor-Acceptor Bonding to SeO2" Molecules 28, no. 22: 7489. https://doi.org/10.3390/molecules28227489
APA StyleBoeré, R. T. (2023). Hydrogen Bonds Stabilize Chloroselenite Anions: Crystal Structure of a New Salt and Donor-Acceptor Bonding to SeO2. Molecules, 28(22), 7489. https://doi.org/10.3390/molecules28227489






