Mechanistic Study on the Corrosion of (La,Sr)(Co,Fe)O3-δ Cathodes Induced by CO2
Abstract
:1. Introduction
2. Results and Discussion
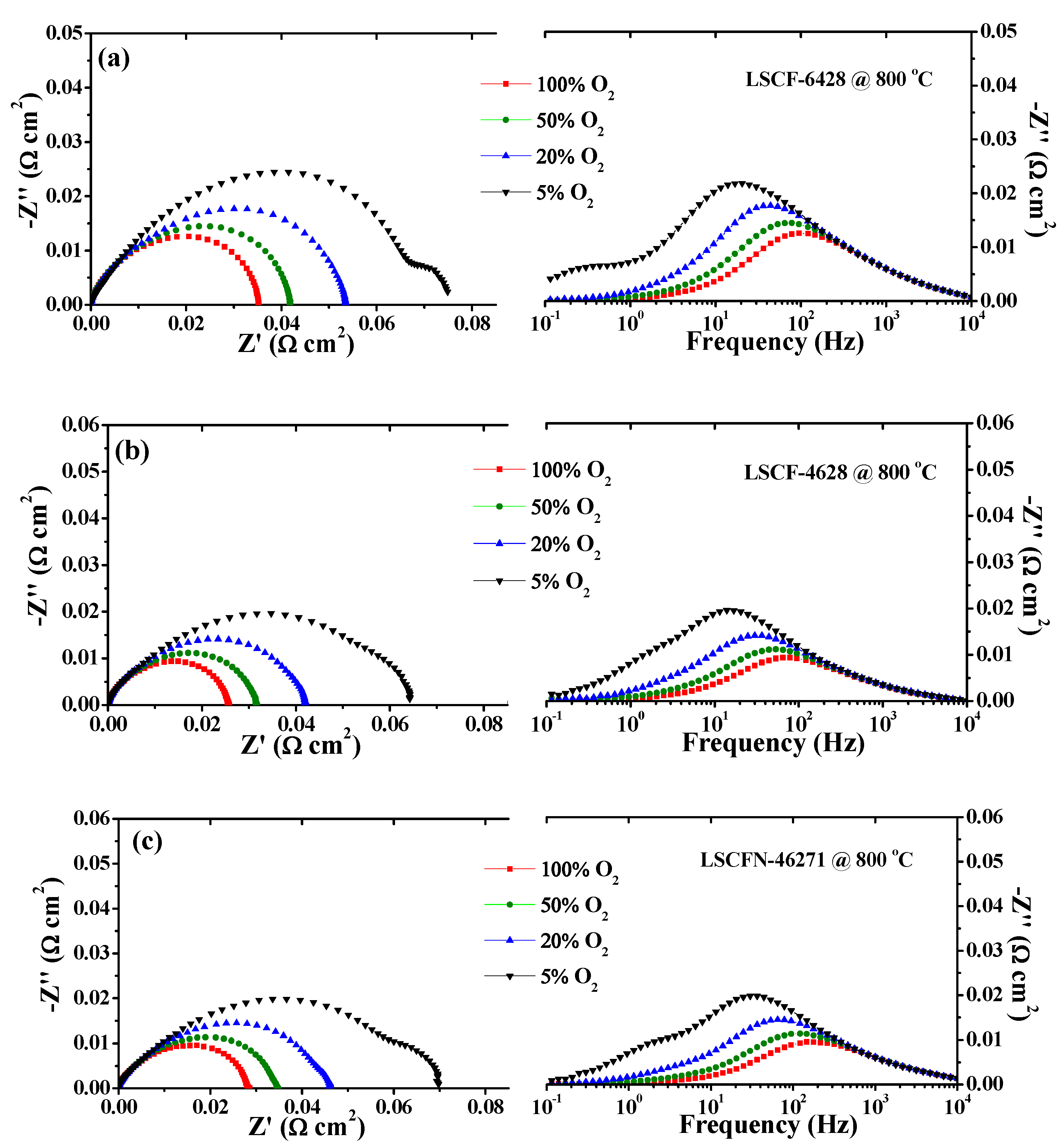
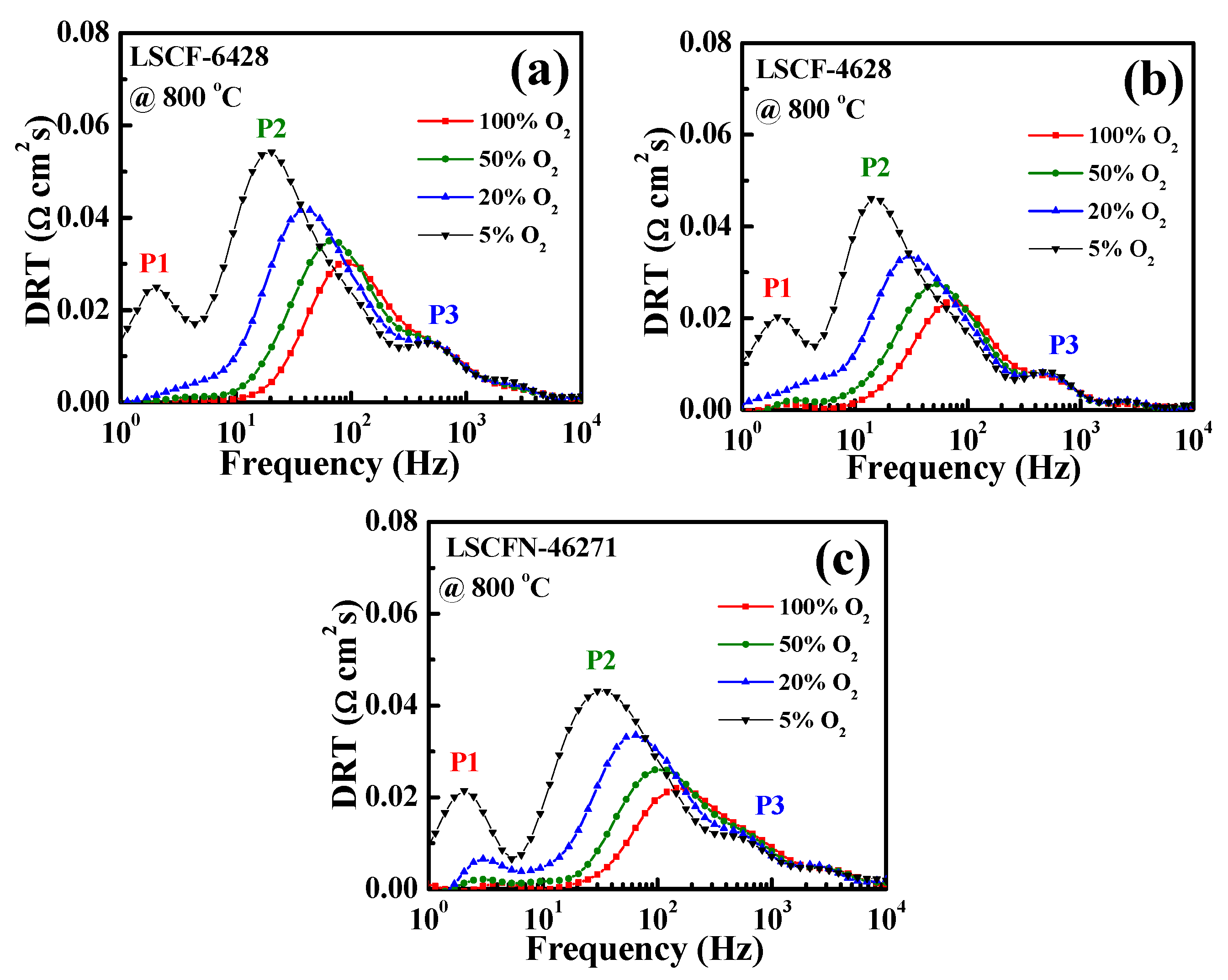
2.1. Impedance and DRT Analysis of Three Electrode Configurations under Various Oxygen Partial Pressures
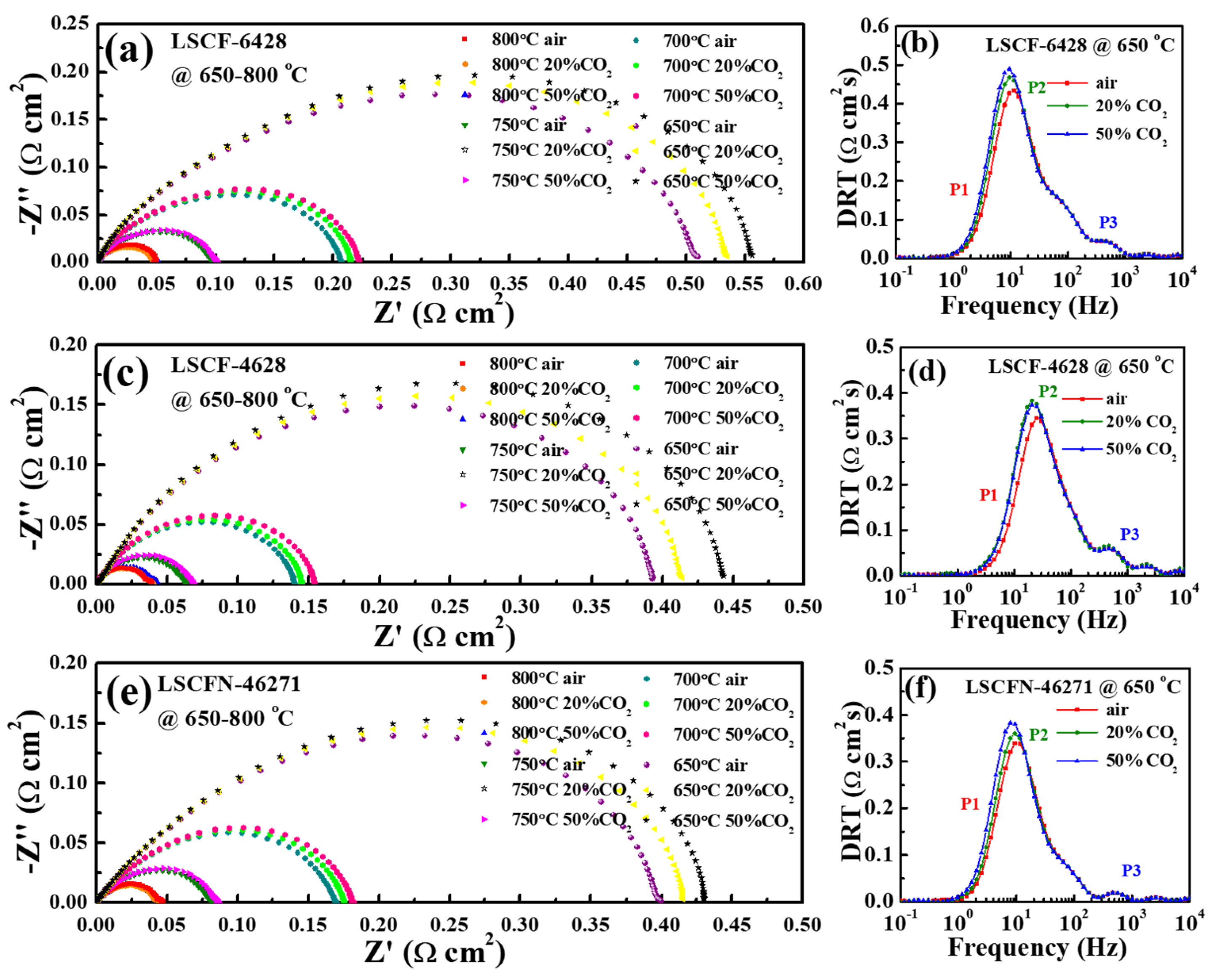
2.2. The Influence of CO2
2.3. Electrode Durability
2.4. XRD Analysis
2.5. SEM Analysis
3. Experimental Section
3.1. Material Synthesis and Electrode Fabrication
3.2. Material Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venkataraman, V.; Pérez-Fortes, M.; Wang, L.G.; Hajimolana, Y.S.; Boigues-Muñoz, C.; Agostini, A.; McPhail, S.J.; Maréchal, F.; Herleb, J.V.; Aravind, P.V. Reversible solid oxide systems for energy and chemical applications-review & perspectives. J. Energy Storage 2019, 24, 100782. [Google Scholar] [CrossRef]
- Yang, X.X.; He, M.; Pan, B.W.; Chen, M.; Yuan, J.L. In-Situ synthesis of Sm0.5Sr0.5Co0.5O3-δ@Sm0.2Ce0.8O1.9 composite oxygen electrode for electrolyte-supported reversible solid oxide cells. Energies 2022, 15, 2178. [Google Scholar] [CrossRef]
- Chen, Y.; Choi, Y.M.; Yoo, S.Y.; Ding, Y.; Yan, Q.Q.; Pei, K.; Qu, C.; Zhang, L.; Chang, L.; Zhao, B.; et al. A Highly Efficient Multi-phase Catalyst Dramatically Enhances the Rate of Oxygen Reduction. Joule 2018, 2, 938–949. [Google Scholar] [CrossRef]
- Zhang, S.W.; Jiang, Y.; Han, H.; Li, Y.H.; Xia, C.R. Perovskite oxyfluoride ceramic within situ exsolved Ni-Fe nanoparticles for direct CO2 electrolysis in solid oxide electrolysis cells. ACS Appl. Mater. Interfaces 2022, 14, 28854–28864. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, C.X.; Gao, J.T.; Li, C.J. Oxidation behavior and interface diffusion of porous metal supported SOFCs with all plasma sprayed functional layers in air at 650 °C. Int. J. Green Energy 2022, 19, 818–826. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Hu, X.Y.; Wan, Y.H.; Xue, S.S.; Zhang, S.W.; Zhang, L.J.; Zhang, B.Z.; Xia, C.R. Electrochemical performance and anode reaction process for Ca doped Sr2Fe15Mo05O6-δ as electrodes for symmetrical solid oxide fuel cells. Electrochim. Acta 2020, 341, 136067. [Google Scholar] [CrossRef]
- Belakry, S.; Rolle, A.; Capoen, E.; Vannier, R.N.; Fasquelle, D. Characterization of Stainless-Steel Grids Dedicated to Cost-Effective Metal-Supported IT-SOFC. ECS J. Solid State Sci. Technol. 2022, 11, 023014. [Google Scholar] [CrossRef]
- Peng, S.J.; Wei, Y.Y.; Xue, J.; Chen, Y.; Wang, H.H. Pr1.8La0.2Ni0.74Cu0.21Ga0.05O4−δ as a potential cathode material with CO2 resistance for intermediate temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2013, 38, 10552–10558. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Sunarso, J.; Zhou, W.; Shao, Z.P. Probing CO2 reaction mechanisms and effects on the SrNb0.1Co0.9−xFexO3−δ cathodes for solid oxide fuel cells. Appl. Catal. B-Environ. 2015, 172, 52–57. [Google Scholar] [CrossRef]
- Yan, A.; Maragou, V.; Arico, A.; Cheng, M.; Tsiakaras, P. Investigation of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ based cathode SOFC: II. The effect of CO2 on the chemical stability. Appl. Catal. B-Environ. 2007, 76, 320–327. [Google Scholar] [CrossRef]
- Shao, Z.P.; Haile, S.M. A high performance cathode for the next generation solid-oxide fuel cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef]
- Yan, A.; Cheng, M.; Dong, Y.L.; Yang, W.S.; Maragou, V.; Song, S.Q.; Tsiakaras, P. Investigation of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ based cathode IT-SOFC: I. The effect of CO2 on the cell performance. Appl. Catal. B-Environ. 2006, 66, 64–71. [Google Scholar] [CrossRef]
- Wang, F.; Nakamura, T.; Yashiro, K.; Mizusaki, J.; Amezawa, K. Effect of Nb doping on the chemical stability of BSCF-based solid solutions. Solid State Ion. 2014, 262, 719–723. [Google Scholar] [CrossRef]
- Yang, Z.B.; Liu, Y.H.; Zhu, T.L.; Chen, Y.; Han, M.F.; Jin, C. Mechanism analysis of CO2 corrosion on Ba0.9Co0.7Fe0.2Nb0.1O3−δ cathode. Int. J. Hydrogen Energy 2016, 41, 1997–2001. [Google Scholar] [CrossRef]
- Yi, J.X.; Schroeder, M.; Weirich, T.; Mayer, J. Behavior of Ba(Co, Fe, Nb)O3−δ perovskite in CO2-containing atmospheres: Degradation mechanism and materials design. Chem. Mater. 2010, 22, 6246–6253. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Zhang, X.; Wu, W.; Tu, B.; Ou, D.; Cheng, M.J. A comparison on effects of CO2 on La0.8Sr0.2MnO3-δ and La0.6Sr0.4CoO3-δ cathodes. J. Power Sources 2013, 222, 542–553. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, B.; Tan, X.Y.; Liu, L.H.; Wang, S.B.; Liu, S.M. CO2-Tolerant ceramic membrane driven by electrical current for oxygen production at intermediate temperatures. J. Am. Ceram. Soc. 2014, 97, 120–126. [Google Scholar] [CrossRef]
- Yang, Z.B.; Guo, M.Y.; Wang, N.; Ma, C.Y.; Wang, J.L.; Han, M.F. A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, T.L.; Yang, Z.B.; Han, M.F. Co-synthesis of LSCFN-GDC electrode for symmetric solid oxide fuel cell running on propane. Electrochim. Acta 2018, 265, 259–264. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, T.L.; Yang, Z.B.; Han, M.F. Fabrication and optimization of La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ electrode for symmetric solid oxide fuel cell with zirconia based electrolyte. J. Mater. Sci. Technol. 2017, 33, 1329–1333. [Google Scholar] [CrossRef]
- Zhang, X.B.; Jin, Y.M.; Li, D.; Zong, X.; Xiong, Y.P. Effects of Gd0.8Ce0.2O1.9-δ coating with different thickness on electrochemical performance and long-term stability of La0.8Sr0.2Co0.2Fe0.8O3-δ cathode in SOFCs. Int. J. Hydrogen Energy 2022, 47, 4100–4108. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, H.; Cetin, D.; Lin, X.; Ludwig, K.; Pal, U. Effect of atmospheric CO2 on surface segregation and phase formation in La0.6Sr0.4Co0.2Fe0.8O3-δ thin films. Appl. Surf. Sci. 2014, 323, 71–77. [Google Scholar] [CrossRef]
- Liu, Y.W.; Cao, Y.C.; Sun, S.S.; Lu, C.L.; Wang, B.; Liu, G.B.; Gao, S.S.; Niu, B.B. Novel CO2-tolerant Co-based double perovskite cathode for intermediate temperature solid oxide fuel cells. J. Eur. Ceram. Soc. 2023, 43, 1028–1038. [Google Scholar] [CrossRef]
- Gui, L.Q.; Wan, Y.H.; Wang, R.R.; Wang, Z.H.; He, B.B.; Zhao, L. A comparison of oxygen permeation and CO2 tolerance of La0.6Sr0.4Co0.2Fe0.6Nb0.2O3-δ and La0.6Sr0.4Fe0.8Nb0.2O3-δ ceramic membranes. J. Alloys Compd. 2015, 644, 788–792. [Google Scholar] [CrossRef]
- Yang, Z.B.; Xu, N.; Han, F.L.; Chen, F.L. Performance evaluation of La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ as both anode and cathode material in solid oxide fuel Cells. Int. J. Hydrogen Energy 2014, 39, 7402–7406. [Google Scholar] [CrossRef]
- Yang, Z.B.; Chen, Y.; Xu, N.; Niu, Y.S.; Han, M.F.; Chen, F.L. Stability investigation for symmetric solid oxide fuel cell with La0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ electrode. J. Electrochem. Soc. 2015, 162, 718–721. [Google Scholar] [CrossRef]
- Świerczek, K. Thermoanalysis, nonstoichiometry and thermal expansion of La0.4Sr0.6Co0.2Fe0.8O3−δ, La0.2Sr0.8Co0.2Fe0.8O3−δ, La0.9Sr0.1Co1/3Fe1/3Ni1/3O3−δ, La0.9Sr0.1 and La0.6Sr0.4Co0.2Fe0.6Ni0.2O3−δ perovskites. Solid State Ion. 2008, 179, 126–130. [Google Scholar] [CrossRef]
- Hanif, M.B.; Gao, J.T.; Shaheen, K.; Wang, Y.P.; Yasir, M.; Li, C.J.; Li, C.X. Highly active and novel A-site deficient symmetric electrode material (Sr0.3La0.7)1-x (Fe0.7Ti0.3)0.9Ni0.1O3-δ and its effect on electrochemical performance of SOFCs. Int. J. Hydrogen Energy 2020, 46, 8778–8791. [Google Scholar] [CrossRef]
- Zheng, Z.W.; Jing, J.M.; Lei, Z.; Wang, Z.X.; Yang, Z.B.; Jin, C.; Peng, S.P. Performance and DRT analysis of infiltrated functional cathode based on the anode supported SOFCs with long-term stability. Int. J. Hydrogen Energy 2022, 47, 18139–18147. [Google Scholar] [CrossRef]
- Im, H.N.; Jeon, S.Y.; Lim, D.K.; Singh, B.; Choi, M.; Yoo, Y.S.; Song, S.J. Steam/CO2 Co-Electrolysis Performance of Reversible Solid Oxide Cell with La0.6Sr0.4Co0.2Fe0.8O3-δ-Gd0.1Ce0.9O2-δ Oxygen Electrode. J. Electrochem. Soc. 2015, 163, F54–F59. [Google Scholar] [CrossRef]
- Osinkin, D.A. An approach to the analysis of the impedance spectra of solid oxide fuel cell using the DRT technique. Electrochim. Acta 2021, 372, 137858. [Google Scholar] [CrossRef]
- Pellegrinelli, C.; Huang, Y.L.; Taillon, J.A.; Salamanca-Riba, L.G.; Wachsman, E.D. Investigating the relationship between operating conditions and SOFC cathode degradation. ECS Trans. 2015, 68, 773–784. [Google Scholar] [CrossRef]
- Liu, R.R.; Kim, S.H.; Taniguchi, S.; Oshima, T.; Shiratori, Y.; Ito, K.; Sasaki, K. Influence of water vapor on long-term performance and accelerated degradation of solid oxide fuel cell cathodes. J. Power Sources 2011, 196, 7090–7096. [Google Scholar] [CrossRef]
- Hanif, M.B.; Motola, M.; Qayyum, S.; Rauf, S.; Khalid, A.; Li, C.J.; Li, C.X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Hanif, M.B.; Gao, J.T.; Shaheen, K.; Wang, Y.P.; Yasir, M.; Zhang, S.l.; Li, C.J.; Li, C.X. Performance evaluation of highly active and novel La0.7Sr0.3Ti0.1Fe0.6Ni0.3O3-δ material both as cathode and anode for intermediate-temperature symmetrical solid oxide fuel cell. J. Power Sources 2020, 472, 228498. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, Y.H.; Jeong, H.; Won, B.R.; Jeon, H.; Myung, J.H. Development of robust YSZ thin-film electrolyte by RF sputtering and anode support design for stable IT-SOFC. Ceram. Int. 2023, 49, 32953–32961. [Google Scholar] [CrossRef]
- Shaheen, K.; Shah, Z.; Gulab, H. Metal oxide nanocomposites as anode and cathode for low temperature solid oxide fuel cell. Solid State Sci. 2020, 102, 106162. [Google Scholar] [CrossRef]
- Graves, C. RAVDAV Data Analysis Software, Version 0.9.7. 2012. Available online: https://www.ele.energy.dtu.dk/research/impedance/impedance-analysis#ravdav (accessed on 3 November 2023).
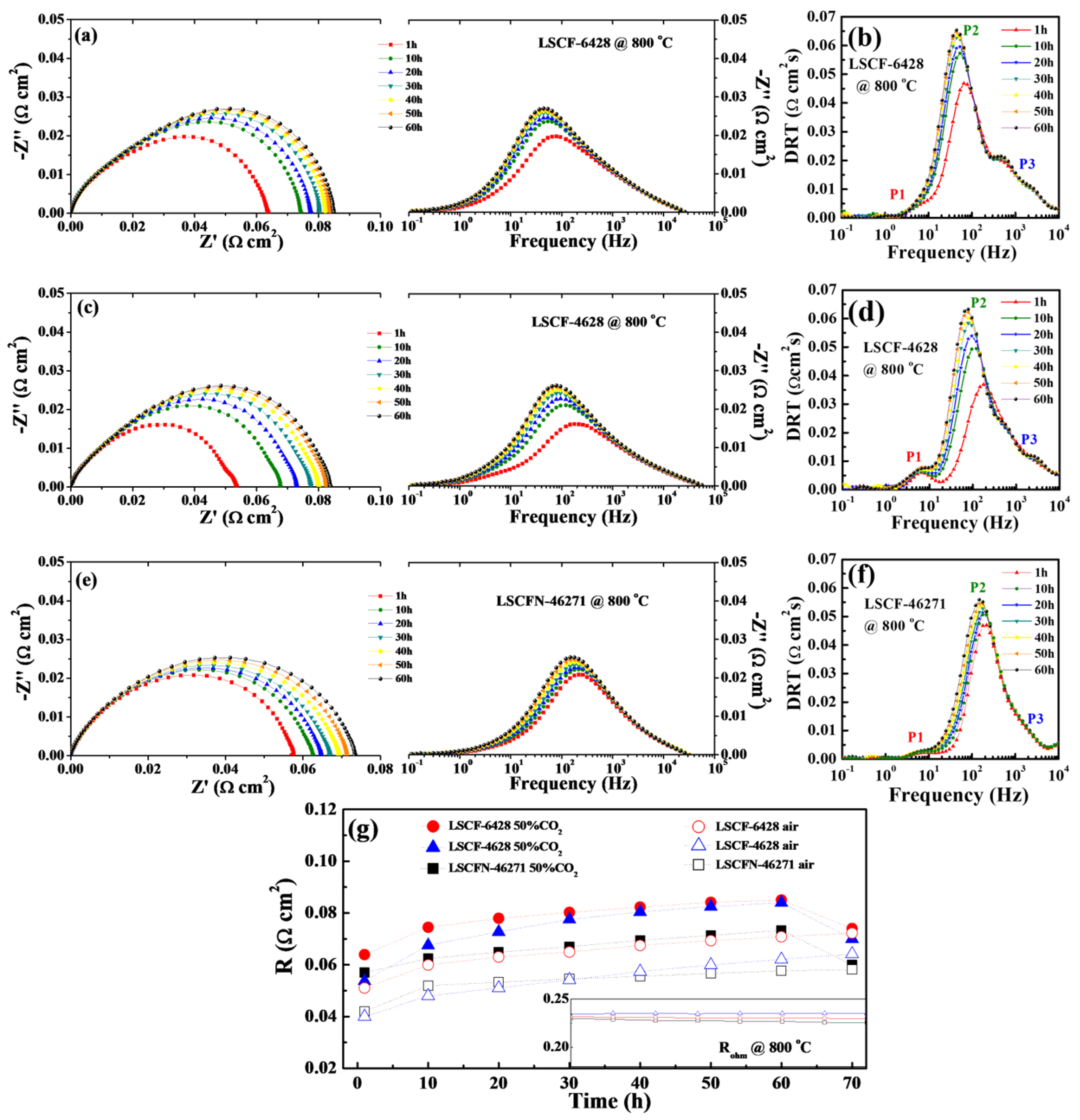
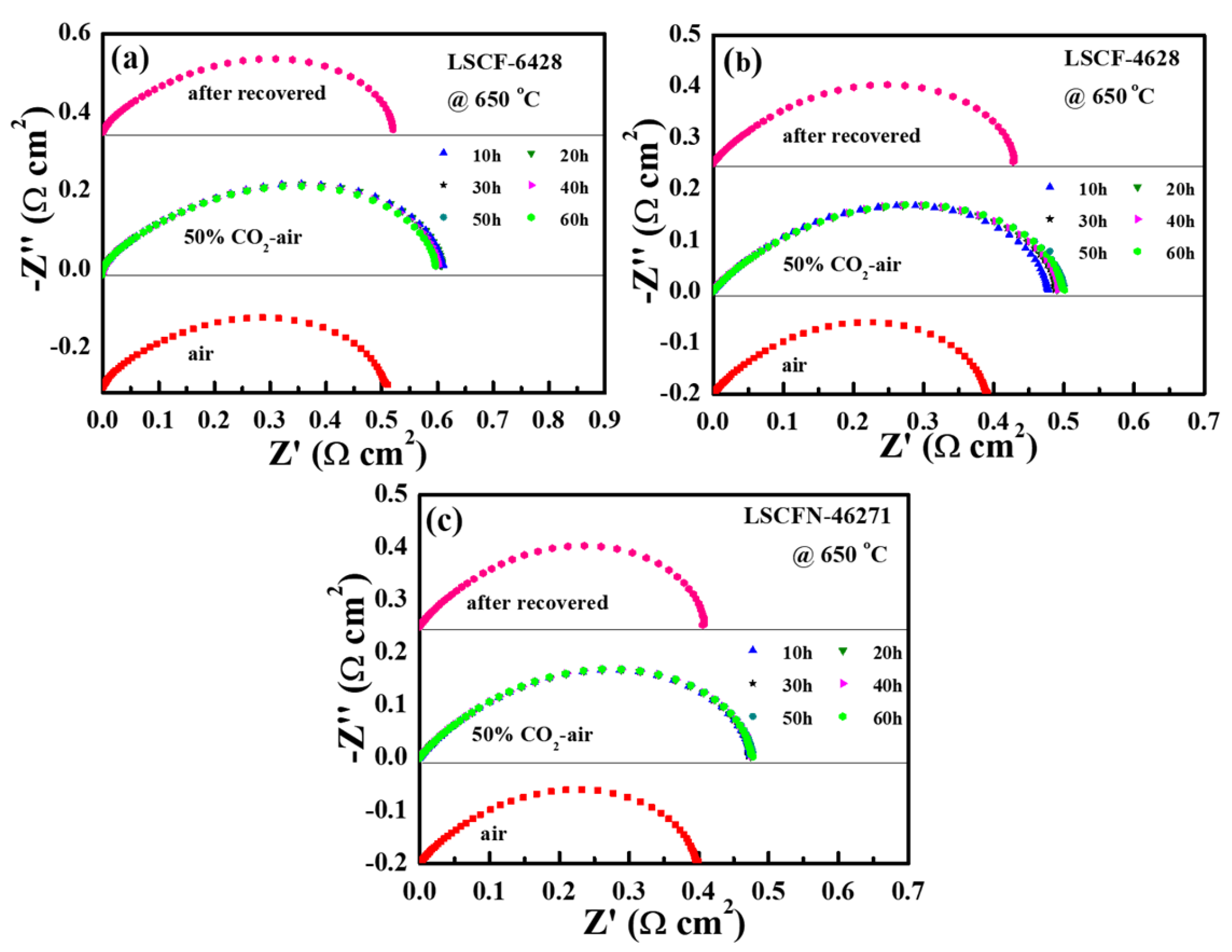
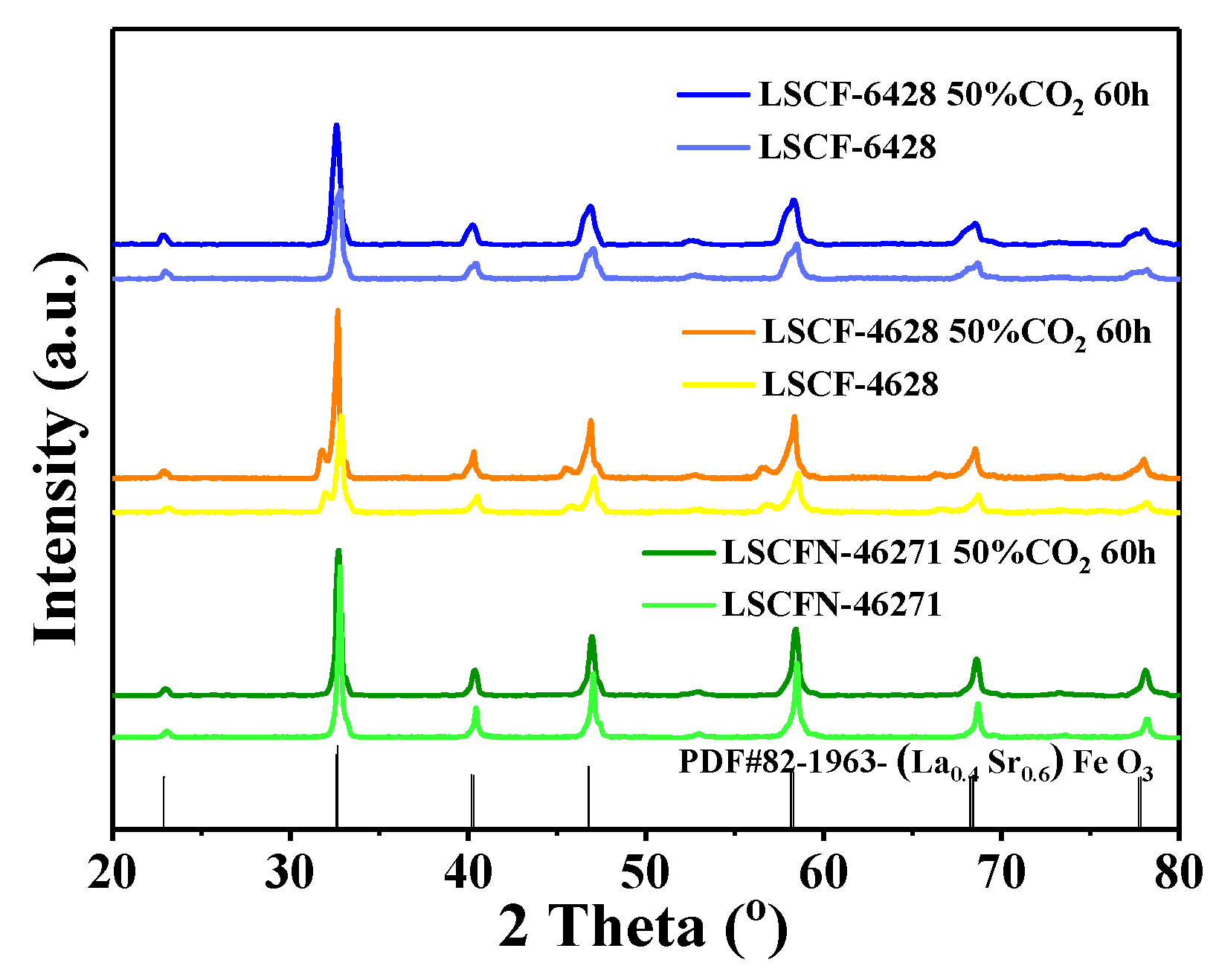
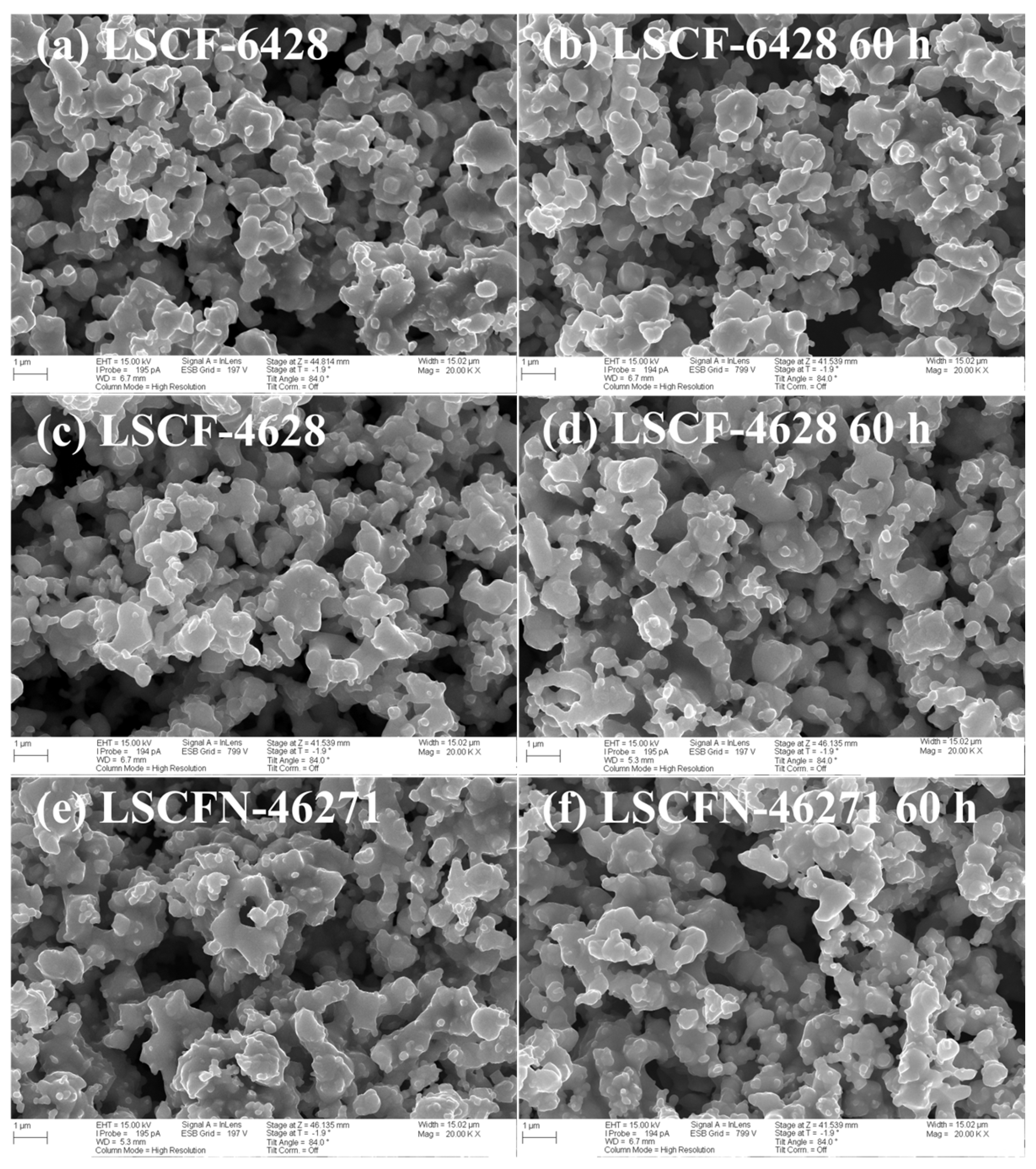
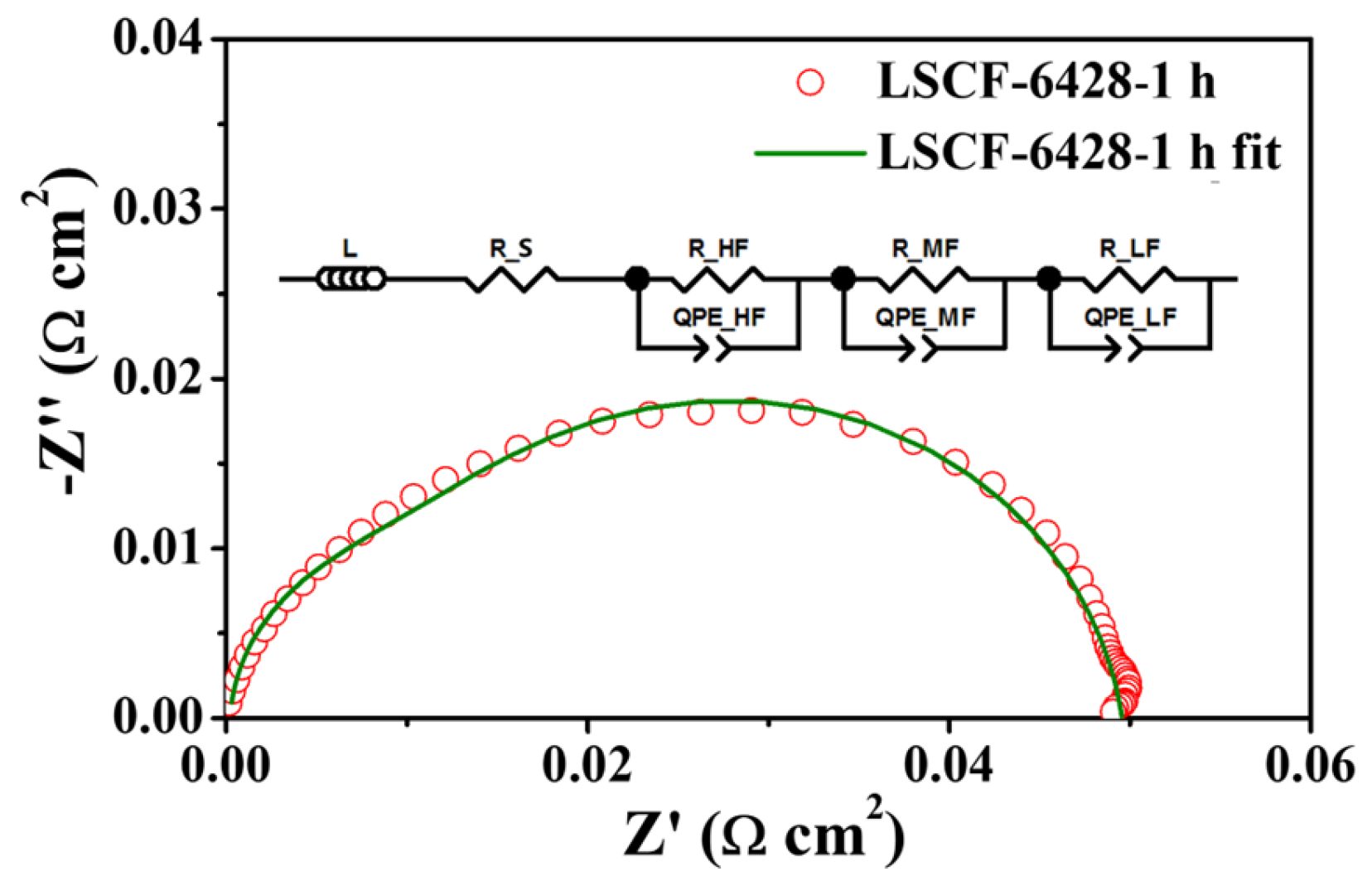
| Electrode | Air/Ω cm2 | 50% CO2-Air 10 h/Ω cm2 | 50% CO2-Air 30 h/Ω cm2 | 50% CO2-Air 60 h/Ω cm2 | After Recovered/ Ω cm2 |
|---|---|---|---|---|---|
| LSCF−6428 | 0.510 | 0.611 | 0.601 | 0.597 | 0.520 |
| LSCF−4628 | 0.392 | 0.478 | 0.488 | 0.501 | 0.427 |
| LSCFN−46271 | 0.400 | 0.475 | 0.472 | 0.478 | 0.406 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Zhang, S.; Zhou, Q.; Wang, H.; Zhao, L.; Xu, Z. Mechanistic Study on the Corrosion of (La,Sr)(Co,Fe)O3-δ Cathodes Induced by CO2. Molecules 2023, 28, 7490. https://doi.org/10.3390/molecules28227490
Xu N, Zhang S, Zhou Q, Wang H, Zhao L, Xu Z. Mechanistic Study on the Corrosion of (La,Sr)(Co,Fe)O3-δ Cathodes Induced by CO2. Molecules. 2023; 28(22):7490. https://doi.org/10.3390/molecules28227490
Chicago/Turabian StyleXu, Na, Shijiao Zhang, Qiongyu Zhou, Hairui Wang, Lina Zhao, and Zhanlin Xu. 2023. "Mechanistic Study on the Corrosion of (La,Sr)(Co,Fe)O3-δ Cathodes Induced by CO2" Molecules 28, no. 22: 7490. https://doi.org/10.3390/molecules28227490
APA StyleXu, N., Zhang, S., Zhou, Q., Wang, H., Zhao, L., & Xu, Z. (2023). Mechanistic Study on the Corrosion of (La,Sr)(Co,Fe)O3-δ Cathodes Induced by CO2. Molecules, 28(22), 7490. https://doi.org/10.3390/molecules28227490








