Applicability of Titanium-Based Catalysts in the Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzofuran
Abstract
:1. Introduction
2. Results
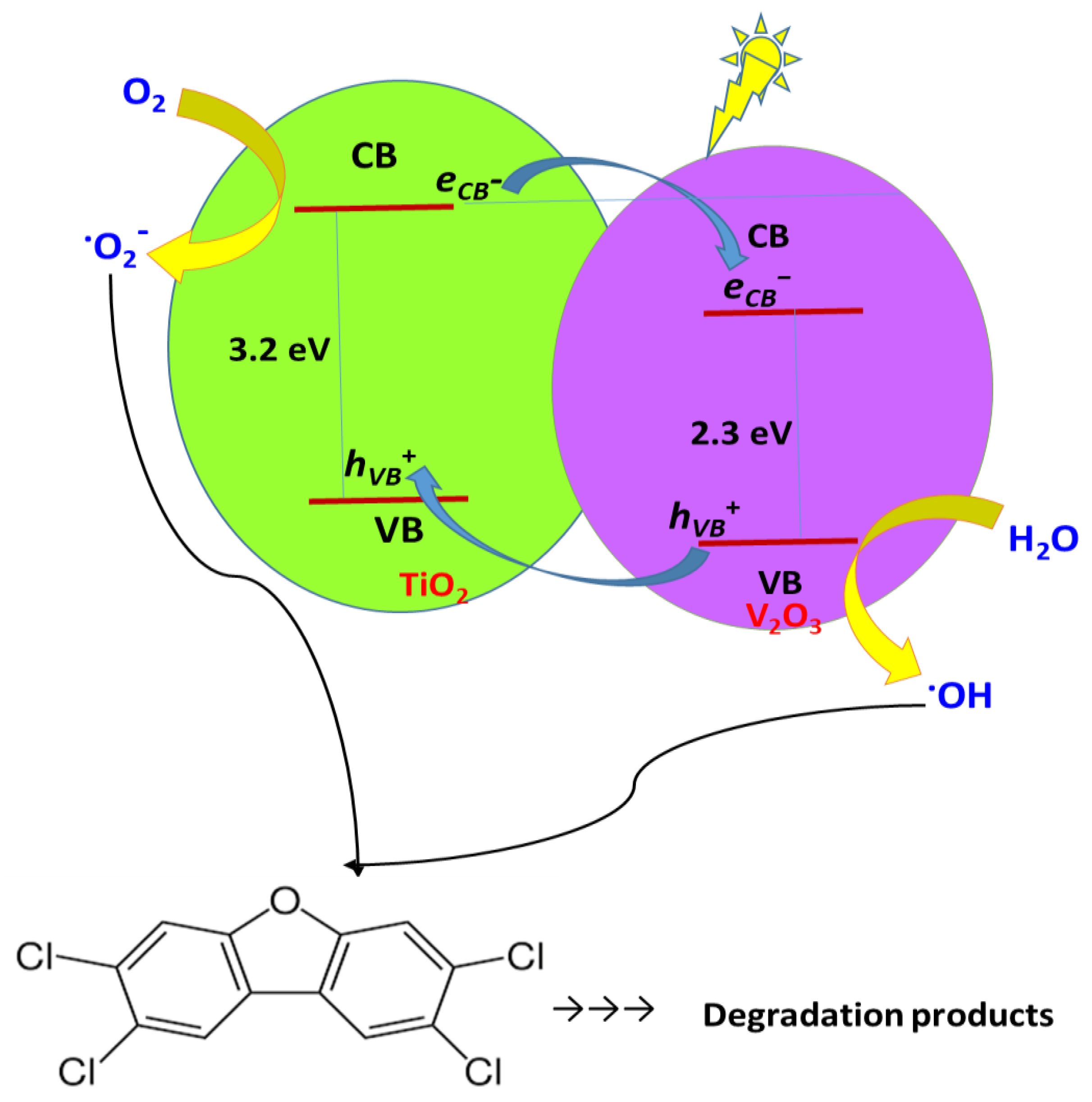
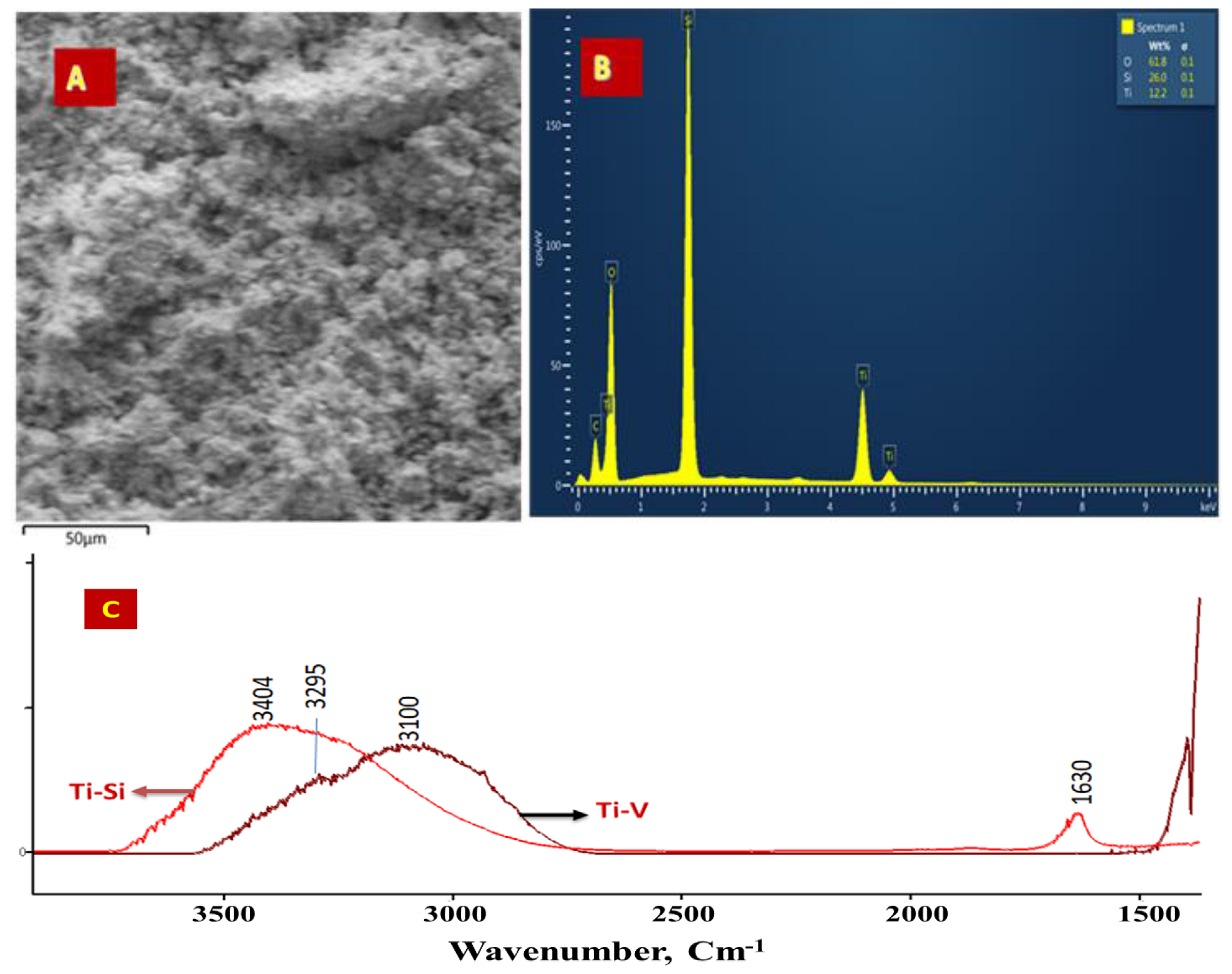
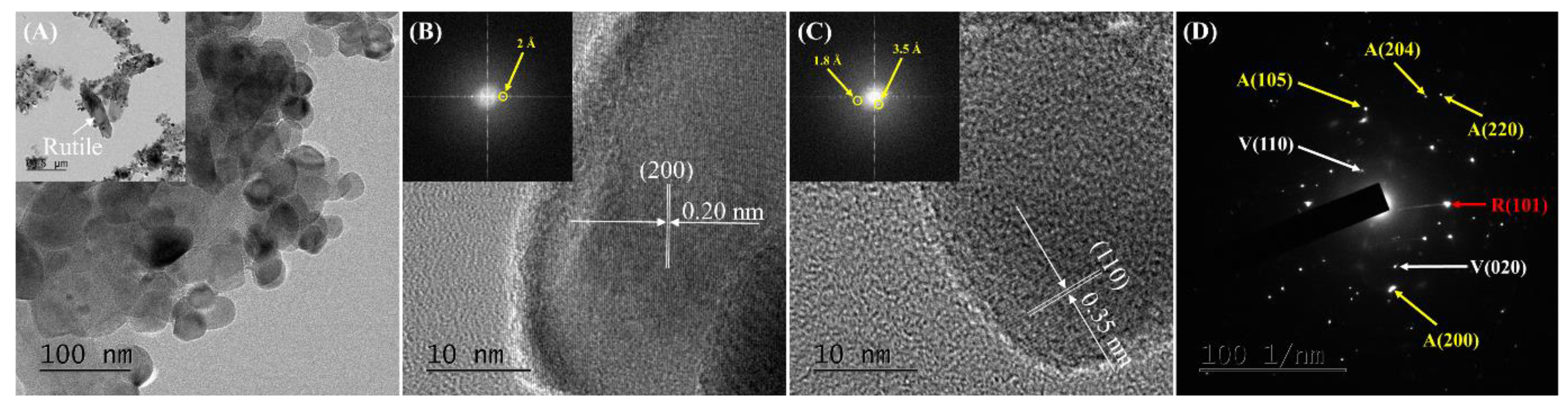
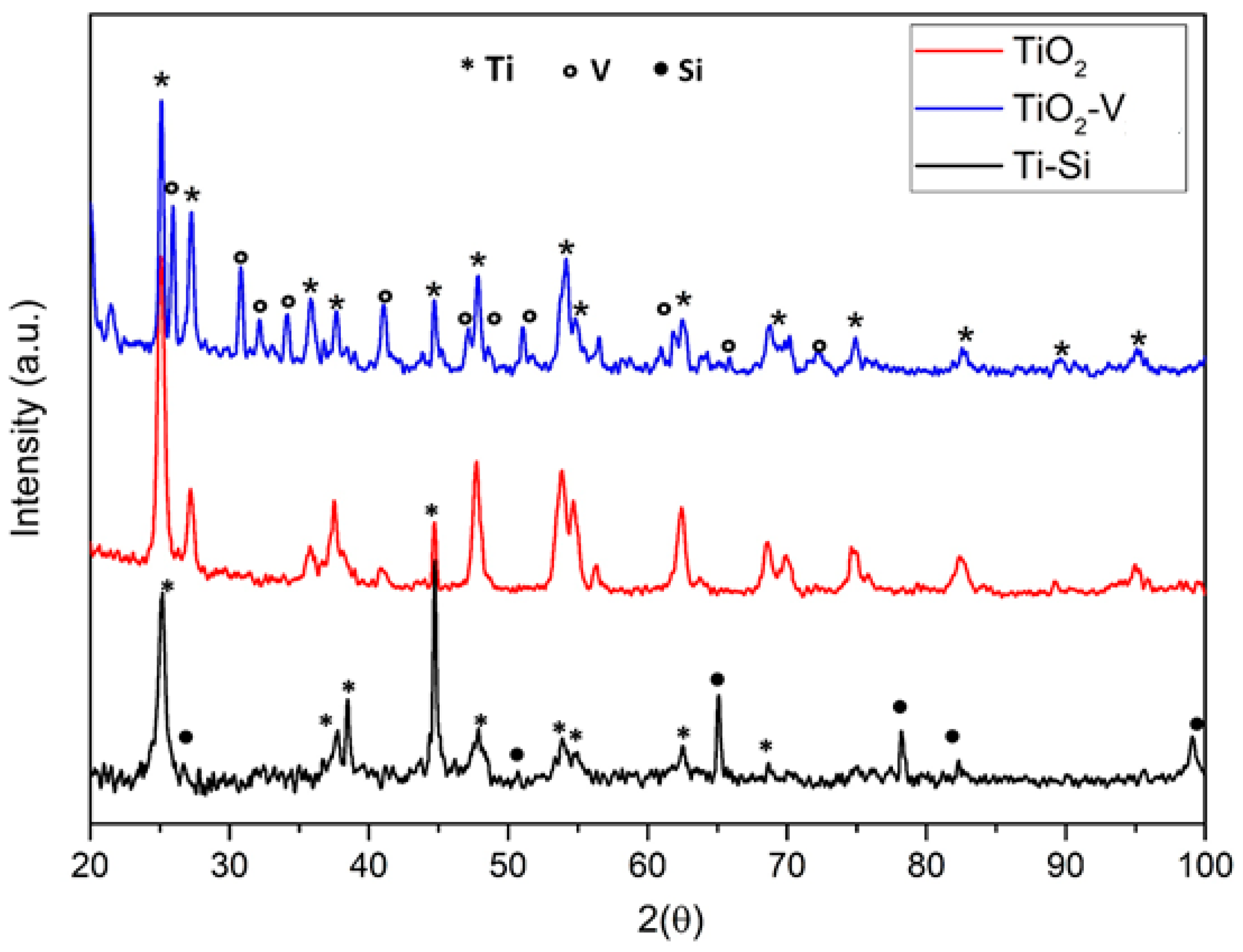
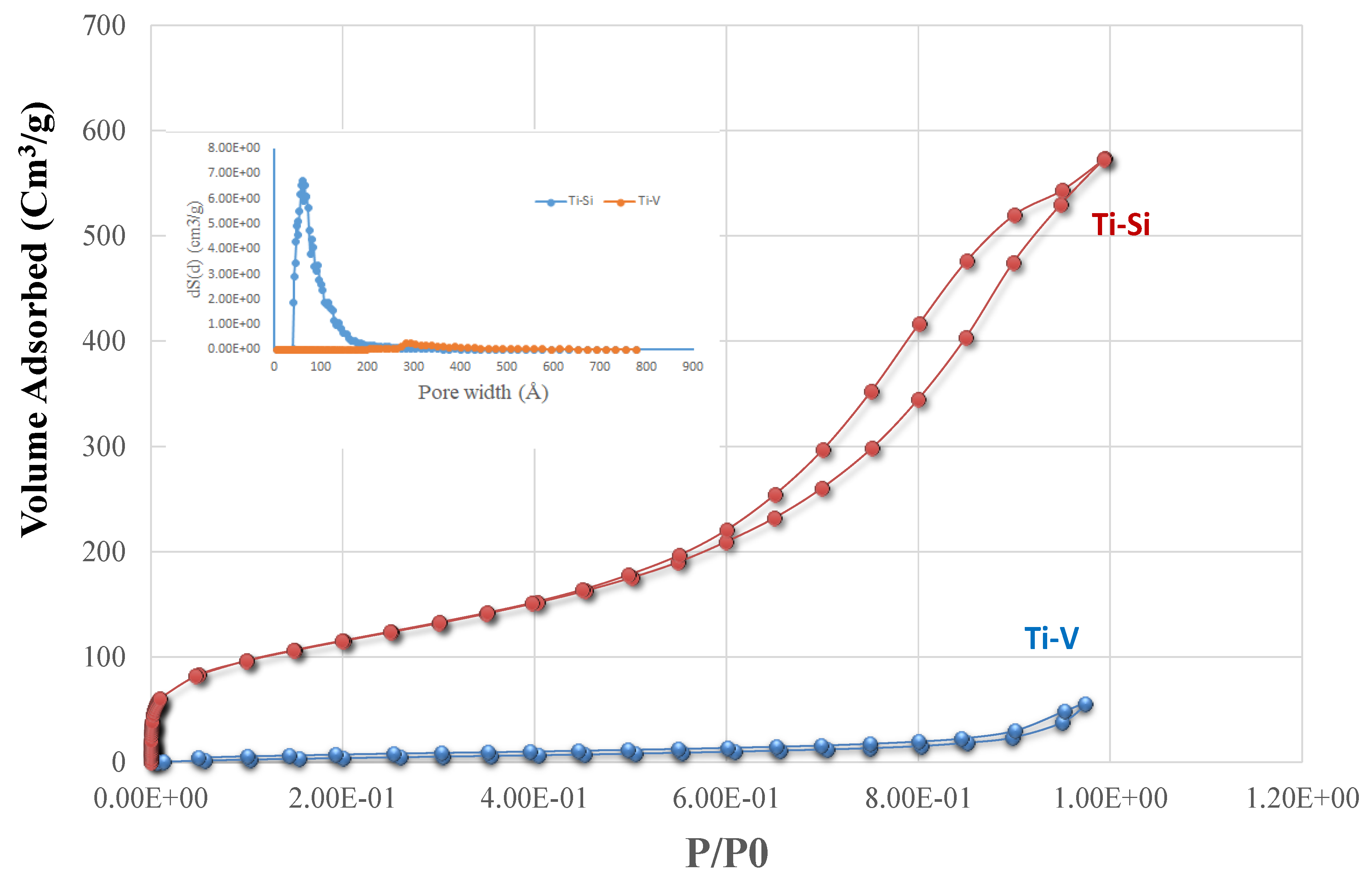
2.1. Characterization of the Catalysts
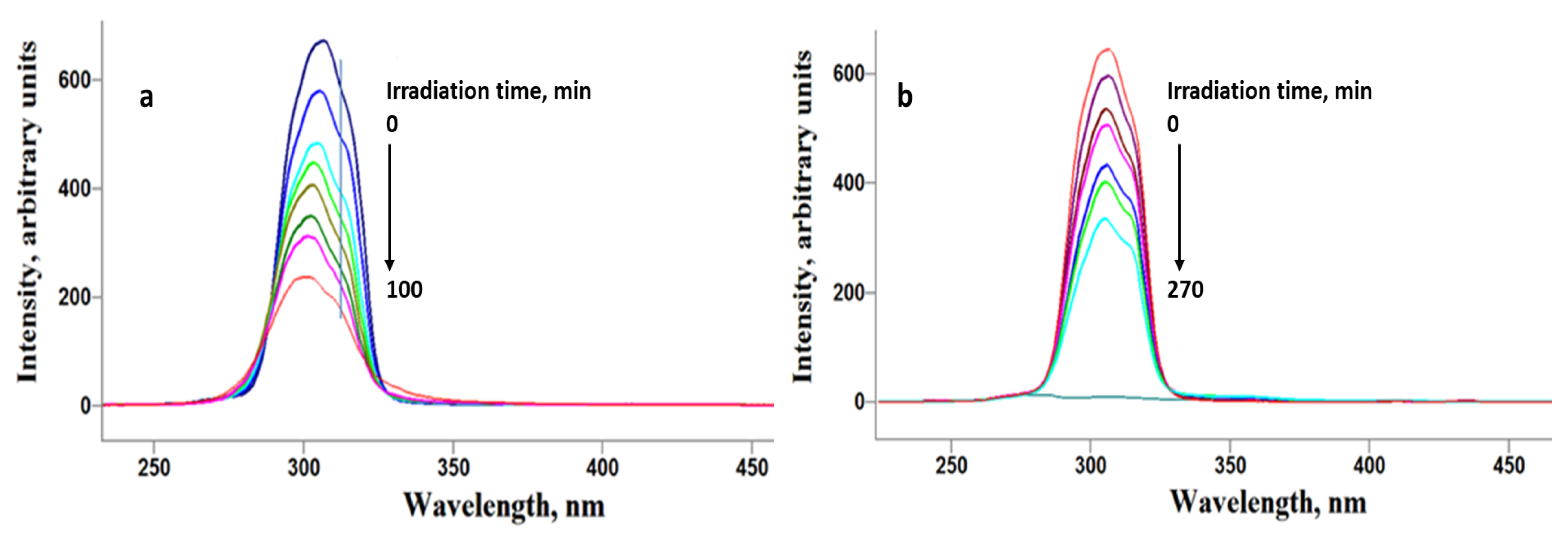
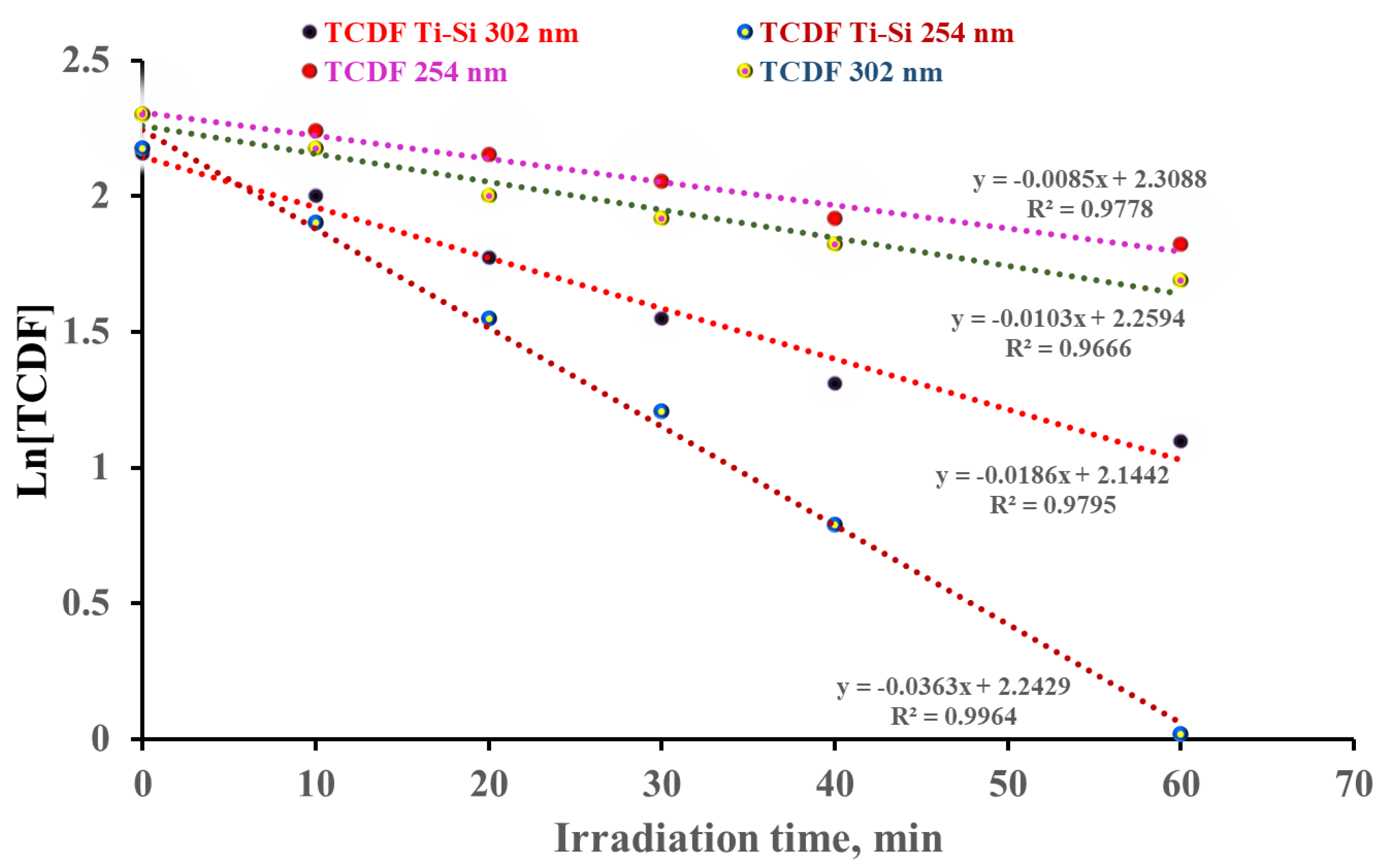
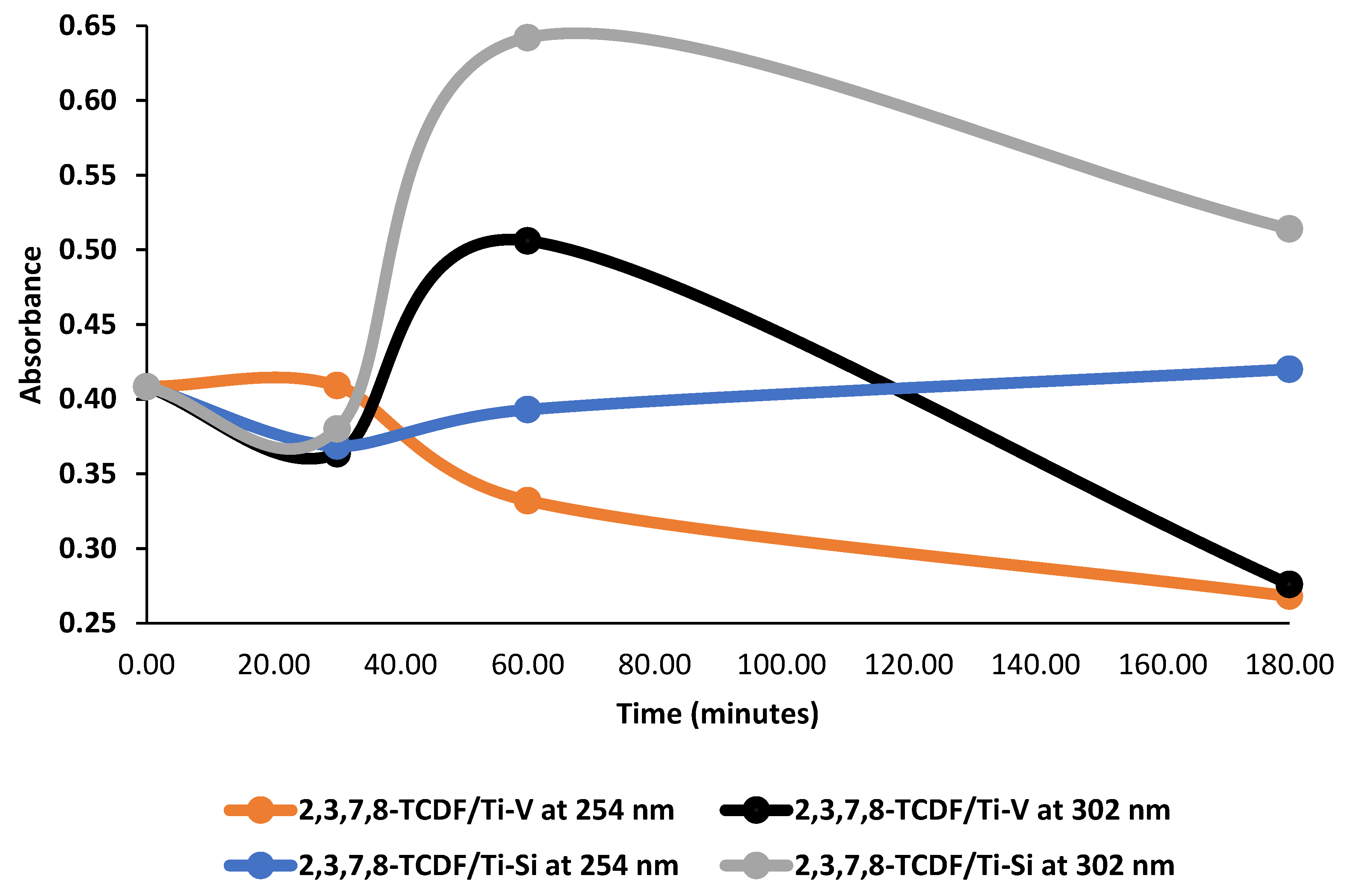
2.2. Photodegradation Experiments
2.3. Toxicity Comparison of Degraded Sample Using Enzyme-Linked Immunosorbent Assay (ELISA)
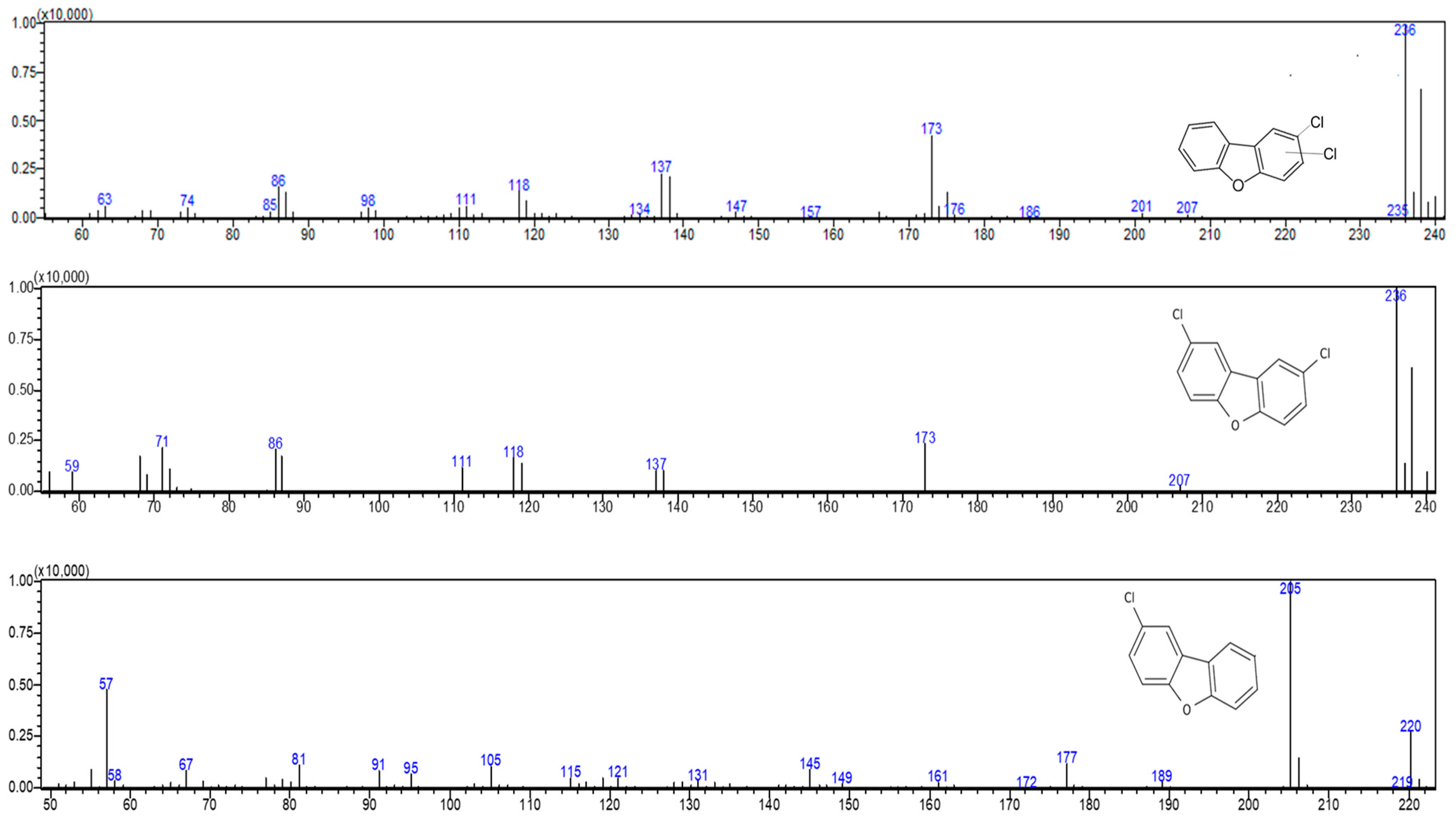
2.4. Gas Chromatography/Mass Spectrometry (GC/MS) Quantification and Identification of Degradation Products
3. Discussion
4. Materials and Methods
4.1. Reagents, Solvents, and Catalysts Preparation
4.2. Photodegradation Irradiation of 2,3,7,8-TCDD and 2,3,7,8-TCDF Experiments
4.3. Fluorescence Spectrometry
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) Experiments
4.5. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samara, F.; Al Shamsi, M.; Kanaan, F.; Kanan, S.M. Photocatalytic UV degradation of 2,3,7,8-tetrachlorodibenzofuran in the presence of silver zeolite. Res. Chem. Intermed. 2017, 46, 1017–1032. [Google Scholar] [CrossRef]
- Persistent Organic Pollutants: A Global Issue, a Global Response|US EPA. 2009. Available online: www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response (accessed on 1 December 2022).
- Vallejo, M.; Román, M.F.S.; Ortiz, I.; Irabien, A. Overview of the PCDD/fs degradation potential and formation risk in the application of advanced oxidation processes (AOPS) to wastewater treatment. Chemosphere 2015, 118, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Kirkok, S.K.; Kibet, J.K.; Kinyanjui, T.K.; Okanga, F.I. A review of persistent organic pollutants: Dioxins, furans, and their associated nitrogenated analogues. SN Appl. Sci. 2020, 2, 1729. [Google Scholar] [CrossRef]
- Urbaniak, M.; Wyrwicka, A. PCDDs/PCDFs and PCBs in Wastewater and Sewage Sludge. In Physico-Chemical Wastewater Treatment and Resource Recovery; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.K.; Osman, O.; Bassem, S.M.; Nassar, H.F.; Temraz, T.A.; Elhaes, H.; Ibrahim, M. Spectroscopic analyses and genotoxicity of dioxins in the aquatic environment of Alexandria. Mar. Pollut. Bull. 2018, 127, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Liu, X.Y.; Shang, Z.Y.; Han, W.S.; Zhang, H. Detection techniques for monitoring dioxin-like compounds: Latest techniques and the comparison. J. Phys. Conf. Ser. 2021, 2045, 012024. [Google Scholar] [CrossRef]
- Samara, F.; Ghalayini, T.; Abu Farha, N.; Kanan, S. The photocatalytic degradation of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the presence of silver–titanium based catalysts. Catalysts 2020, 10, 957. [Google Scholar] [CrossRef]
- Liu, H.; Kong, S.; Liu, Y.; Zeng, H. Pollution Control Technologies of dioxins in municipal solid waste incinerator. Procedia Environ. Sci. 2012, 16, 661–668. [Google Scholar] [CrossRef]
- Robles-Melgarejo, M.; Espino-Valencia, J.; Natividad-Rangel, R.; Guevara-Martínez, S.J.; Rico-Cerda, J.L.; Rangel-Segura, R. Monoliths of TiO2-SiO2: Synthesis, characterization and photocatalytic activity. J. Porous Mater. 2021, 28, 1697–1711. [Google Scholar] [CrossRef]
- Samara, F.; Jermani, E.; Kanan, S.M. Photocatalytic UV-degradation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the presence of silver doped zeolite. Arab. J. Chem. 2019, 12, 1870–1878. [Google Scholar] [CrossRef]
- Moradi, Z.; Jahromi, S.Z.; Ghaedi, M. Design of active photocatalysts and visible light photocatalysis. Photocatal. Fundam. Process. Appl. 2021, 32, 557–623. [Google Scholar] [CrossRef]
- Khan, S.; Park, J.-S.; Ishihara, T. A review of the single-step flame synthesis of defective and heterostructured TiO2 nanoparticles for photocatalytic applications. Catalysts 2023, 13, 196. [Google Scholar] [CrossRef]
- Carneiro, J.T.; Moulijn, J.A.; Mul, G. Photocatalytic oxidation of cyclohexane by titanium dioxide: Catalyst deactivation and regeneration. J. Catal. 2010, 273, 199–210. [Google Scholar] [CrossRef]
- Arlos, M.J.; Liang, R.; Fong, L.C.L.C.; Zhou, N.Y.; Ptacek, C.J.; Andrews, S.A.; Servos, M.R. Influence of methanol when used as a water-miscible carrier of pharmaceuticals in TiO2 photocatalytic degradation experiments. J. Environ. Chem. Eng. 2017, 5, 4497–4504. [Google Scholar] [CrossRef]
- Wang, R.; Ma, X.; Liu, T.; Li, Y.; Song, L.; Tjong, S.C.; Cao, L.; Wang, W.; Yu, Q.; Wang, Z. Degradation aspects of endocrine disrupting chemicals: A review on photocatalytic processes and photocatalysts. Appl. Catal. A Gen. 2020, 597, 117547. [Google Scholar] [CrossRef]
- Yu, X.; Jiajun, L.; Xianglin, Y.; Feng, Z.; Yongjun, L.; Junbo, L. Preparation of graphdiyne-doped TiO2/SiO2 composite for enhanced photocatalytic activity. J. Nanoparticle Res. 2020, 22, 365. [Google Scholar] [CrossRef]
- Uncu, I.; Roata, I.C.; Croitoru, C.; Machedon-Pisu, T. Visible domain photocatalysis performance of Ti-Si Thermal-sprayed coatings. Sustainability 2021, 14, 85. [Google Scholar] [CrossRef]
- Cajes, V. Identification of Photodegradation Products from Commonenvironmental Pollutants Using Gas Chromatography Coupled with High Resolution Mass Spectrometry (Dissertation). 2022. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:oru:diva-100739 (accessed on 1 December 2022).
- Hadamcik, E.; Renard, J.-B.; Rietmeijer, F.; Levasseurregourd, A.; Hill, H.; Karner, J.; Nuth, J. Light scattering by Fluffy Mg–Fe–SIO and C mixtures as cometary analogs (PROGRA2 experiment). Icarus 2007, 190, 660–671. [Google Scholar] [CrossRef]
- Hadamcik, E.; Renard, J.-B.; Levasseur-Regourd, A.; Lasue, J. Light scattering by fluffy particles with the PROGRA2 experiment: Mixtures of materials. J. Quant. Spectrosc. Radiat. Transf. 2006, 100, 143–156. [Google Scholar] [CrossRef]
- Kanan, S.M.; Lu, Z.; Cox, J.K.; Bernhardt, G.; Tripp, C.P. Identification of surface sites on monoclinic wo3 powders by infrared spectroscopy. Langmuir 2002, 18, 1707. [Google Scholar] [CrossRef]
- Lu, Z.; Kanan, S.M.; Tripp, C.P. Synthesis of high surface area monoclinic WO3 particles using organic ligands and emulsion based methods. J. Mater. Chem. 2002, 12, 983. [Google Scholar] [CrossRef]
- Kanan, S.M.; Lu, Z.; Tripp, C.P. A comparative study of the adsorption of chloro- and methyl-containing organophosphonates on WO3. J. Phys. Chem. B 2002, 106, 9576. [Google Scholar] [CrossRef]
- Waghe, A.; Kanan, S.M.; Abu-Yousef, I.; Jensen, B.; Tripp, C.P. Infrared study of uv irradiated tungsten trioxide powders containing adsorbed dimethyl methyl phosphonate and trimethyl phosphate. Res. Chem. Int. 2006, 32, 613–623. [Google Scholar] [CrossRef]
- Berthomieu, C.; Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef] [PubMed]
- IR Spectrum and Characteristic Absorption Bands. Chemistry LibreTexts, Libretexts. 2021. Available online: chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_I_(Liu)/06%3A_Structural_Identification_of_Organic_Compounds-_IR_and_NMR_Spectroscopy/6.03%3A_IR_Spectrum_and_Characteristic_Absorption_Bands#:~:text=The%20absorption%20bands%20in%20IR,show%20more%20intense%20absorption%20band (accessed on 19 October 2023).
- Al-Amin, M.; Dey, S.C.; Rashid, T.U.; Ashaduzzaman, M.; Shamsuddin, S.M. Solar Assisted Photocatalytic Degradation of Reactive Azo Dyes in Presence of Anatase Titanium Dioxide. Int. J. Latest Res. Eng. Technol. 2016, 2, 14–21. [Google Scholar]
- Guide to FT-IR Spectroscopy. Bruker. 2023. Available online: www.bruker.com/en/products-and-solutions/infrared-and-raman/ft-ir-routine-spectrometer/what-is-ft-ir-spectroscopy.html#:~:text=For%20example%2C%20the%20symmetric%20and,those%20energies%20of%20IR%20light (accessed on 19 October 2023).
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. Rutile vs. anatase: What’s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Dai, S.; Wu, Y.; Sakai, T.; Du, Z.; Sakai, H.; Abe, M. Preparation of highly crystalline TiO2 nanostructures by acid-assisted hydrothermal treatment of hexagonal-structured nanocrystalline Titania/Cetyltrimethyammonium bromide Nanoskeleton. Nanoscale Res. Lett. 2010, 5, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative visible light-activated sulfur doped TiO2 films for water treatment. Appl. Catal. B Environ. 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Chen, X.; Pomerantseva, E.; Gregorczyk, K.; Ghodssi, R.; Rubloff, G. Cathodic ald V2O5 thin films for high-rate electrochemical energy storage. RSC Adv. 2013, 3, 4294. [Google Scholar] [CrossRef]
- Topcu, S.; Jodhani, G.; Gouma, P.I. Optimized nanostructured TiO2 photocatalysts. Front. Mater. 2016, 3, 35. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Ene, V.L.; Voicu, B.B.; Bucur, M.A.; Neacsu, I.A.; Vasile, B.S.; Iordache, F. Biocompatible Ag/Fe-enhanced TiO2 nanoparticles as an effective compound in sunscreens. Nanomaterials 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- In-DF1 Kit Insert. Available online: www.cape-tech.com/literature/kits/in_df1a.htm (accessed on 1 August 2022).
- Van Den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Håkansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Julkapli, N.M.; Hamid, S.B.A. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-F.; Lin, X.-Q.; Li, X.-D.; Yan, M.; Prabowo, B.; Li, W.-W.; Chen, T.; Yan, J.-H. Catalytic destruction of PCDD/FS over vanadium oxide-based catalysts. Environ. Sci. Pollut. Res. 2016, 23, 16249–16258. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.-S.; Li, X.-D.; Ren, Y.; Chen, T.; Cen, K.-F.; Ni, M.-J.; Buekens, A. Ozone-enhanced oxidation of PCDD/Fs over V2O5–TiO2-based catalyst. Chemosphere 2013, 92, 265–272. [Google Scholar] [CrossRef]
- Tang, M.; Ye, Q.; Du, C.; Peng, Y.; Makwarimba, C.P.; He, Y.; Lu, S. PCDD/F removal at low temperatures over vanadium-based catalyst: Insight into the superiority of mechanochemical method. Environ. Sci. Pollut. Res. 2021, 29, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Amado-Piña, D.; Roa-Morales, G.; Molina-Mendieta, M.; Balderas-Hernández, P.; Romero, R.; Díaz, C.E.B.; Natividad, R. E-peroxone process of a chlorinated compound: Oxidant species, degradation pathway and phytotoxicity. J. Environ. Chem. Eng. 2022, 10, 108148. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samara, F.; Darra, R.; Mohamed, A.A.; Ahmad, W.; Abu-Farha, N.; Lee, H.; Han, C.; Kanan, S. Applicability of Titanium-Based Catalysts in the Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzofuran. Molecules 2023, 28, 7488. https://doi.org/10.3390/molecules28227488
Samara F, Darra R, Mohamed AA, Ahmad W, Abu-Farha N, Lee H, Han C, Kanan S. Applicability of Titanium-Based Catalysts in the Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzofuran. Molecules. 2023; 28(22):7488. https://doi.org/10.3390/molecules28227488
Chicago/Turabian StyleSamara, Fatin, Rasha Darra, Ahmed A. Mohamed, Waqas Ahmad, Nedal Abu-Farha, Haesung Lee, Changseok Han, and Sofian Kanan. 2023. "Applicability of Titanium-Based Catalysts in the Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzofuran" Molecules 28, no. 22: 7488. https://doi.org/10.3390/molecules28227488
APA StyleSamara, F., Darra, R., Mohamed, A. A., Ahmad, W., Abu-Farha, N., Lee, H., Han, C., & Kanan, S. (2023). Applicability of Titanium-Based Catalysts in the Photocatalytic Degradation of 2,3,7,8-Tetrachlorodibenzofuran. Molecules, 28(22), 7488. https://doi.org/10.3390/molecules28227488







