Abstract
Plant phenolic compounds have attracted considerable attention because of their health benefits. This study aimed to investigate the composition and antioxidant activity of phenol extracts from Polygonatum zanlanscianense Pamp. steam and leaf (PPP). The FTIR, UPLC-Q-Obtrip-MS, and HPLC−DAD methods were used to analyze the composition of PPP, and 20 phenolic compounds were preliminarily identified. Among them, the contents of hyperin, astragalin, and diosmetin levels were the highest. Treatment with PPP can significantly reduce t-BHP-induced cell damage in HepG2 cells, reactive oxygen species (ROS) accumulation, and malondialdehyde (MDA) content. Meanwhile, the superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), and glutathione peroxidase (GSH−Px) activities can be increased. Moreover, PPP enhanced Nrf2 expression, which was consistent with that of heme oxygenase-1 (HO−1), glutamate–cysteine ligase catalytic subunit (GCLC), and NAD(P)H quinone oxidoreductase-1 (NQO1), whereas the expression of Keap1, the Nrf2 inhibitor, was decreased. All findings indicate that PPP can serve as a natural bioactive substance for preventing oxidative stress.
1. Introduction
In recent years, numerous studies have shown that reactive oxygen species (ROS) are closely related to human health. Under normal physiological conditions, low-level ROS production is considered to be a signal molecule. Numerous chronic and degenerative disorders, including cancer, cardiovascular diseases, diabetes, neurological diseases, and aging, can be brought on by excessive ROS production [1,2]. Due to their safety, potent antioxidant capacity, and few side effects, natural antioxidants are drawing increasing attention. Because of their detrimental health impacts, synthetic antioxidants have drawn attention in the food industry [3]. Therefore, natural antioxidants are urgently needed to replace artificially synthesized antioxidants.
With the rapid development of the traditional Chinese medicine planting industry, many straw resources will be generated during planting, processing, and production. The accumulation and incineration of large amounts of straw will pollute the ecological environment and cause a massive loss of resources. Therefore, the development and implementation of straw resources can result in the recycling of agricultural resources while additionally greatly raising the earning capacity of producers. Phenolic compounds are receiving increasing attention in straw resources. Some studies [4,5,6,7,8] have revealed that the content of phenolic compounds in straw is higher than that in medicinal parts of plants, and that they have several important biological activities, including antioxidant, antitumor, neuroprotective, and antibacterial activities. The antioxidant biological activity exhibited by plant phenolic extracts can serve as an indirect antioxidant that activates the body’s antioxidant mechanism by regulating the expression of intracellular antioxidant enzymes, thereby maintaining the body’s redox homeostasis [9]. Antioxidant and detoxifying enzymes, including NAD(P)H quinone oxidoreductase-1 (NQO1), superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), glutathione (GSH), glutamate–cysteine ligase catalytic subunit (GCLC), glutathione peroxidase (GSH−Px), and heme oxygenase-1 (HO−1), regulate the endogenous antioxidant system of cells. Under oxidative stress damage, they can eliminate excess ROS from the body. Thus, they play an important role in maintaining intracellular redox homeostasis [10]. Nrf2 refers to nuclear factor E2-related factor 2, which regulates the expression of these antioxidant enzymes. When Nrf2 is activated, it dissociates from the Keap1 protein and transfers from the cytoplasm to the nucleus, binding to the antioxidant response element (ARE) in the nucleus. This induces the expression of downstream antioxidant enzymes and plays a role in maintaining redox homeostasis in the body [11].
Polygonatum zanlanscianense Pamp. belongs to the family Polygonatum, widely cultivated in various parts of China [12]. “The Outline of Xinhua Materia Medica” records that “rhizome has the same effect as Polygonatum, which invigorates the spleen, moistens the lung, and kills insects”. “The Dictionary of Traditional Chinese Medicine” records that “the root of Polygonatum zanlanscianense Pamp. has long been used to treat lung diseases, palpitations, stomach discomfort, and diabetes”. At present, research on and applications of the chemical components of Polygonatum zanlanscianense Pamp. mainly focus on the rhizomes, and the stem and leaf parts, which occupy half of the weight of the entire grass, are often discarded and burned. This not only causes great waste of Polygonatum zanlanscianense Pamp. resources, but also causes environmental pollution. The stems and leaves of Polygonatum zanlanscianense Pamp. also have significant medicinal value. Previous studies have shown that Polygonatum zanlanscianense Pamp. leaves are rich in furans, acids, and phenolic substances, and have strong free radical scavenging ability [13]. However, the composition of the stem and leaf components of Polygonatum zanlanscianense Pamp. and its antioxidant mechanism at the cellular level is still unidentified.
Therefore, the aim of this study was to clarify the possible process by which Polygonatum zanlanscianense Pamp. phenols exert antioxidant effects. We used an ultrasonic extraction method to extract phenols from the stems and leaves of Polygonatum zanlanscianense Pamp. and purified them with macroporous resin. The components of the phenolic extract were analyzed using FTIR, UPLC-Q-Obtrip-MS, and HPLC-DAD. The HepG2 cell line is accessible, representative, stable, shows metabolic activity, and demonstrates ease of detection, so it is often used as an oxidative damage model [14,15]. Therefore, the protective execution capabilities of the phenolic extract on t-BHP-induced oxidative damage was studied utilizing a HepG2 cell system.
2. Results
2.1. Qualitative and Quantitative Analysis of the Main Phenols in PPP
2.1.1. FTIR Analysis
Figure 1 shows the infrared characteristic spectrum of PPP. The stretching vibration of O-H caused an absorption peak at the wave number of 3408.56 cm−1. The C-H stretching vibration of -CH2- caused an absorption peak at the wave number of 2918.87 cm−1. The stretching vibration of carbonyl C=O caused an absorption peak at the wave number range of 1640.64 cm−1. The stretching vibration of aromatic compounds caused an absorption peak at the wave number of 1594.84 cm−1. An absorption peak appeared at 1405.37 cm−1, indicating the possibility of phenolic hydroxyl groups.
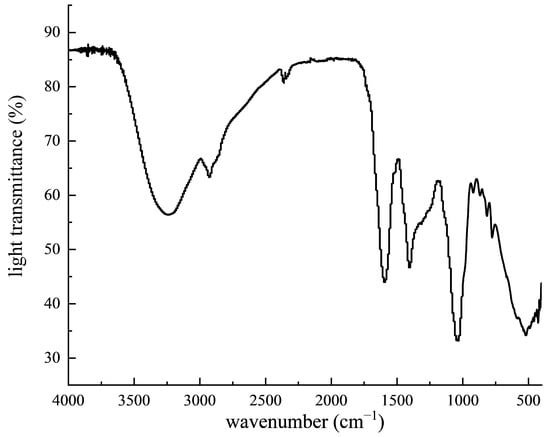
Figure 1.
Infrared spectra of PPP.
2.1.2. UPLC-Q-Obtrip-MS Analysis
Figure 2 shows the UPLC-Q-Obtrip-MS chromatogram of PPP. There were 20 phenolic compounds identified in this study (Table 1), including 3 phenylpropanoic acid compounds: caffeic acid, chlorogenic acid, and ferulic acid, and 17 flavonoids and their glycosides.
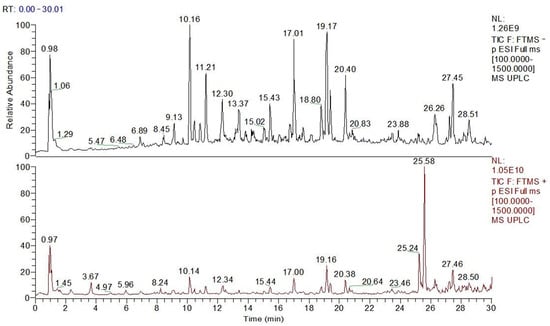
Figure 2.
UPLC-Q-Obtrip-MS chromatogram of PPP.

Table 1.
Phenolic compounds identified in PPP.
2.1.3. HPLC Analysis
The peak time was determined using a single standard sample, and 12 monomeric phenols were used as a mixed standard sample to determine the composition of the purified phenols using HPLC-DAD. The content of the components with a peak area >5% was determined.
Figure 3 shows that the peak time of each peak in the sample was consistent with the time of the standard sample, indicating that PPP contained these 12 phenolic components. Hyperin, astragalin, and diosmetin contents were relatively high at 76.6, 21.4, and 26.4 mg/g, respectively (Table 2).
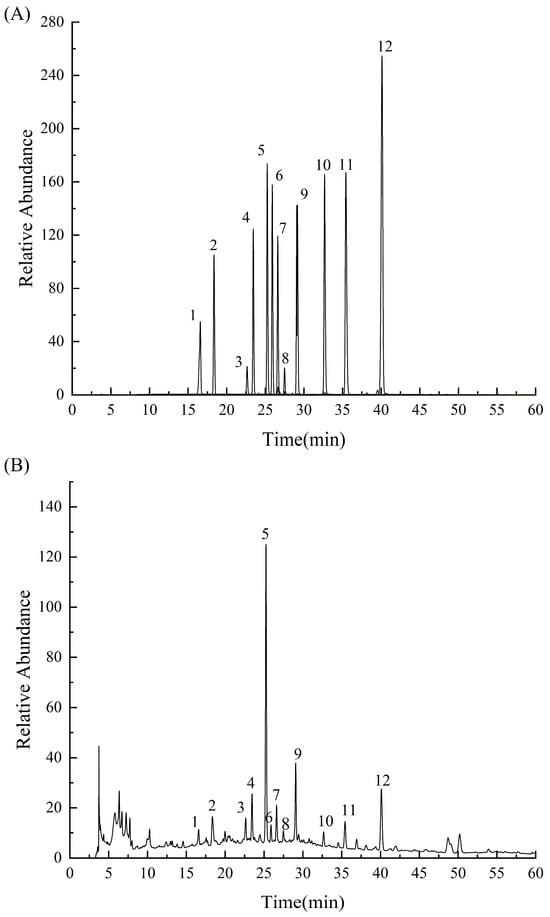
Figure 3.
HPLC results of the mixed phenol standard (A) and PPP (B). Peak1: chlorogenic acid, peak 2: caffeic acid, peak 3: taxifolin, peak 4: ferulic acid, peak 5: hyperin, peak 6: isoquercitrin, peak 7: rutin, peak 8: hesperidin, peak 9: astragalin, peak 10: quercetin, peak 11: luteolin, and peak 12: diosmetin.

Table 2.
Species and monomeric phenol contents of PPP.
2.2. The Effect of PPP and t-BHP on the Proliferation of HepG2 Cells
The effects of PPP and t-BHP on the proliferation and toxicity of HepG2 cells were measured using a CCK-8 assay (Figure 4 and Figure 5). Cytotoxicity was investigated in the presence of PPF alone at different concentrations of 0–100 µg/mL. No cell toxicity was observed at 0–50 µg/mL concentrations, and the cell survival rate was >95%. Subsequently, as the PPP concentration increased, the cell survival rate decreased, indicating that when the concentration exceeded 50 µg/mL, it had a certain toxic effect on HepG2 cells. Based on the above results, three concentrations of 10, 30, and 50 µg/mL were selected to study the protective effect of PPP on t-BHP-induced oxidative damage in HepG2 cells, meaning that the PPP group was divided into a high group (HG), medium group (MG), and low group (LG), respectively. Furthermore, when the t-BHP concentration increased from 0 µg/mL to 1000 µg/mL, the cell survival rate significantly decreased dose-dependently. At low concentrations, t-BHP caused less damage to HepG2 cells, whereas excessive concentrations caused irreversible damage to the cells. When the cell survival rate was between 50% and 70%, the cells underwent oxidative damage and had a certain degree of recovery ability. Therefore, a concentration of 500 µg/mL t-BHP intervention for 2 h was used as the modeling condition for subsequent experiments.
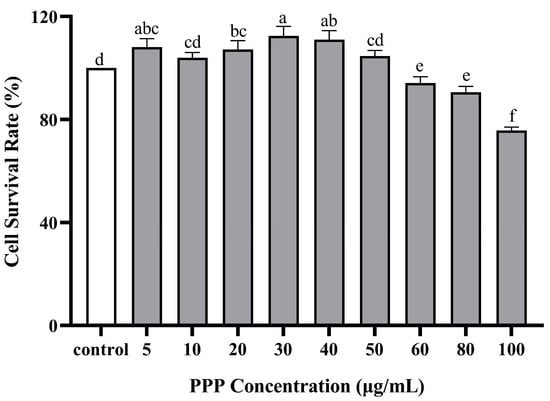
Figure 4.
Effect of PPP on the cell viability of HepG2 detected by CCK-8. Those with the same letter in each column indicate no significant difference (p < 0.05), and those with different letters indicate significant difference (p < 0.05). The farther apart the alphabetical order, the greater the difference.
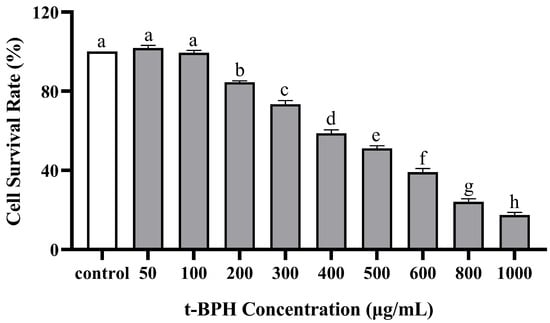
Figure 5.
Effect of t-BHP on the cell viability of HepG2 detected by CCK-8. Those with the same letter in each column indicate no significant difference (p < 0.05), and those with different letters indicate significant difference (p < 0.05). The farther apart the alphabetical order, the greater the difference.
2.3. The Effect of PPP on the Level of Intracellular ROS Induced by t-BHP
ROS in normal cells are maintained at a certain level, and when cells are stimulated by external factors, many ROS are produced, causing several diseases [16]. DCFH−DA is a sensitive and convenient fluorescent probe that can be used to detect ROS in living cells. The higher the fluorescence, the more ROS accumulate and the more severe the degree of oxidative damage. The results are shown in Figure 6. Compared with the control group, the ROS level in the model group increased to 62.72%. After using the positive control drug vitamin C, the ROS level decreased to 16.58%. After using PPP, ROS decreased compared with the model group. The LG, MG, and HG decreased to 33.49%, 20.2%, and 17.47%, respectively.
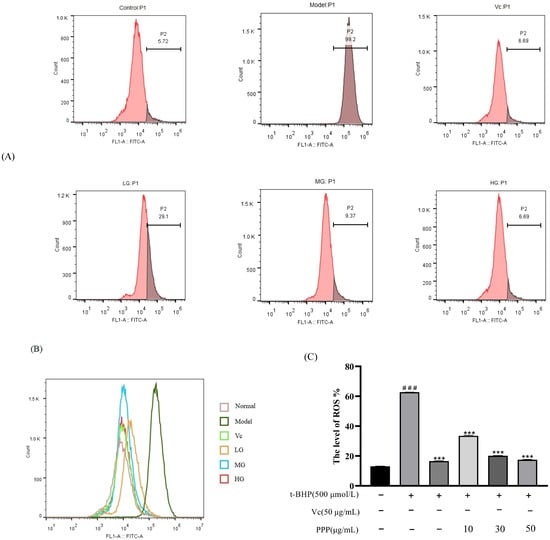
Figure 6.
The average fluorescence intensity was measured using flow cytometry (A,B). Statistical analysis of ROS generation (C). Control group was used as a positive control. Data are presented as mean ± SD. n = 3, ### p < 0.001 vs. control group, *** p < 0.001 vs. model group.
2.4. The Effect of PPP on Antioxidant Enzyme Activity and MDA Content
The levels of intracellular SOD, GSH, GSH-Px, CAT, and MDA can reflect the level of antioxidant activity. Figure 7A−E shows that after t-BHP-induced oxidative damage, intracellular SOD, GSH, GSH-Px, and CAT levels decreased, whereas MDA levels increased, indicating oxidative damage to the cells. After PPP pretreatment, the recovery effect of each indicator level correlated positively with the concentration, and the HG had better recovery effects on GSH and MDA than the Vc group. These results jointly indicated that the protective effect of PPP against t-BHP-induced oxidative stress was primarily attributed to the enhanced activities of CAT, SOD, GSH, and GSH-Px and reduced MDA production in HepG2 cells. The HG showed the strongest antioxidant effect.
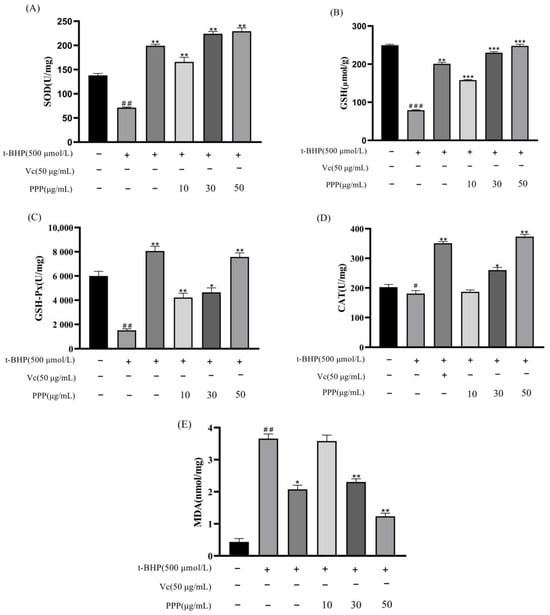
Figure 7.
Effect of PPP on SOD activity (A), GSH activity (B), GSH-Px activity (C), CAT activity (D), and MDA content (E) in HepG2 cells injured by t-BHP. All data are expressed as the mean ± SD according to at least three independent experiments. Compared with the control group, # p < 0.05, ## p < 0.01, ### p < 0.001; compared with the model group, * p < 0.05, ** p < 0.05, *** p < 0.05.
2.5. Effect of PPP on the mRNA Expression of Antioxidant Enzyme Genes Induced by t-BHP
Figure 8A–E shows the effects of PPP on the expression of antioxidant-related genes in HepG2 cells. The levels of intracellular SOD, GSH, GSH-Px, MDA, and CAT can reflect the level of antioxidant activity. Compared with the control group, after adding t-BHP to the cells, the expression levels of Nrf2, HO−1, NQO1, SOD, and GSH-Px were significantly reduced, whereas the expression levels of antioxidant-related genes in the Vc and PPP groups were significantly higher than those in the model group. These results indicated that PPP inhibited oxidative stress by upregulating the expression of genes encoding antioxidant enzymes, and the effect correlated positively with the dose. When the concentration reached 50 µg/mL, the expression level of related genes was the highest, and the effect was close to, or even exceeded, that of the Vc group.
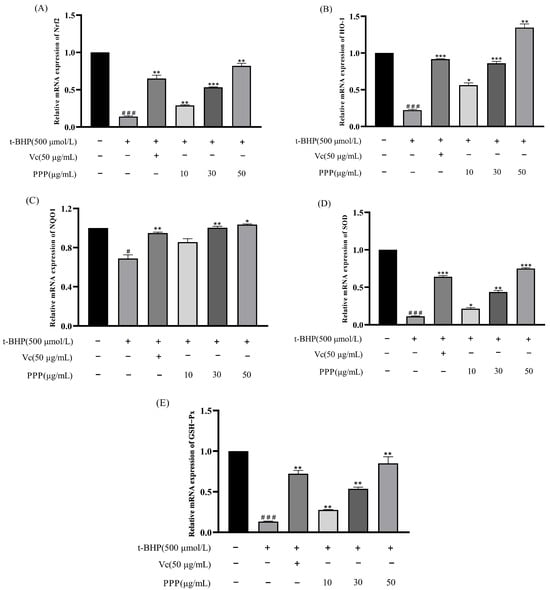
Figure 8.
Effects of PPP on the expression of antioxidant-related genes in HepG2 cells under control and oxidative stress conditions. (A) Nrf2; (B) HO−1; (C) NQO1; (D) SOD; (E) GSH-Px. Values are presented as the mean ± SD (n = 3). Compared with the control group, # p < 0.05, ### p < 0.001; compared with the model group, * p < 0.05, ** p < 0.05, *** p < 0.05.
2.6. Western Blotting
Under normal physiological conditions, Nrf2 mainly binds to its inhibitor Keap1 and is inactive, maintaining a low transcriptional activity. When the ROS in the body rises to a certain level, Nrf2 and Keap1 are uncoupled and activated by stimulation. Keap1 and Nrf2 enter the cell-activated Nrf2/Keap1 signaling pathway. The activated signaling pathway initiates the expression of multiple downstream target proteins. These activated target proteins can regulate the redox balance in the body, allowing the body to recover from oxidative stress to a normal physiological state.
As shown in Figure 9, the results indicated that the protein abundance of Nrf2, HO−1, and GCLC significantly decreased after treatment with 500 µg/mL t-BHP for 2 h compared with the control group. In contrast, the protein abundance of Keap1 increased significantly. However, the protein expression of Nrf2 and antioxidant enzymes (HO−1 and GCLC) in HepG2 cells pretreated with PPP and damaged by t-BHP were increased compared with those in the model groups. In contrast, the protein expression of Keap1 was decreased. The increased Nrf2 protein expression and decreased Keap1 protein expression suggest that the Nrf2/Keap1 signaling pathway was activated, and the abundance of HO−1 and GCLC protein expression was consistent with the abundance of Nrf2 protein expression. Comparing the expression levels of various proteins in the HG, MG, and LG, it was found that the high-dose group had the best antioxidant stress effect.
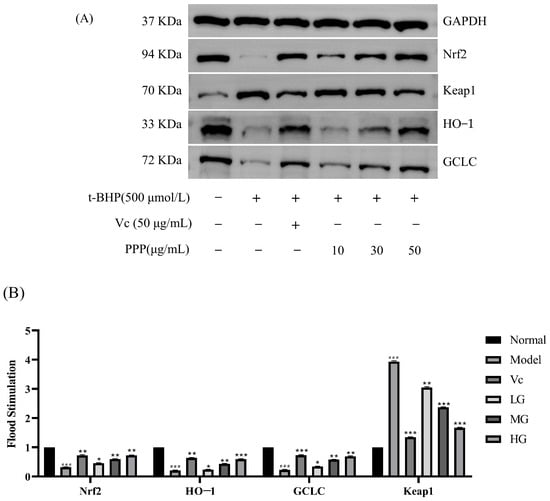
Figure 9.
Effects of PPP on the protein expression of the Nrf2/Keap1 signaling pathway in HepG2 cells. (A) Protein strip; (B) related proteins/-actin relative density. Values are presented as the mean ± SD (n = 3). Compared with the control group, ### p < 0.001; compared with the model group, * p < 0.05, ** p < 0.05, *** p < 0.05.
3. Materials and Methods
3.1. Polygonatum Zanlanscianense Pamp. Stem and Leaf Samples
In mid-March 2022, samples were collected from Group 15, Hongqi Village, Daoming Town, Chongzhou City, Sichuan Province. Associate Professor Long Fei from the Pharmacy Teaching and Research Office of Chengdu University of Traditional Chinese Medicine identified them as the overground part of Polygonatum zanlanscianense Pamp. The stems and leaves were separated, washed, dried in a 60 °C oven to constant weight, crushed, and filtered through a fine sieve of 80 µm mesh. The samples were stored in a refrigerator at 20 °C.
3.2. Sample Extraction
The crude extract was obtained by concentrating 120 mL of 50% ethanol (Chron Chemicals Co., Ltd., Chengdu, China) extract, which was obtained by extracting 3 g of the sample powder at 50 °C and then ultrasonically assisted for 40 min. This extraction process was repeated three times. The concentration of the extract was carried out at 35 °C under reduced pressure until no alcohol odor was present. The crude extract underwent purification using AB-8 macroporous resin (Huayi Chemical Material Technology Co., Ltd., Shanghai, China) at a sample flow rate of 1.0 mL/min, a sample volume of 20 mL, an eluent concentration of 75%, an elution flow rate of 1.0 mL/min, and an elution volume of 200 mL. The purified eluent was evaporated under reduced pressure at 50 °C until no alcohol taste was observed and freeze-dried in a vacuum freeze dryer (Huayi Chemical Material Technology Co., Ltd., Shanghai, China) at −60 °C for three days to eliminate moisture, and the resulting powdered samples were stored at −20 °C for future use.
3.3. Identification and Quantification of Phenolic Compounds
3.3.1. FTIR
According to the solid compression method in the fourth part of the 2020 edition of “The Chinese Pharmacopoeia” [17], the sample PPP was scanned from 4000 to 400 cm−1 and the spectrogram was recorded.
3.3.2. UPLC-Q-Obtrip-MS
The chromatographic conditions were as follows: Mobile phase A was a 0.1% formic acid aqueous solution (Chron Chemicals Co., Ltd., China), B was a methanol solution, gradient elution (Chron Chemicals Co., Ltd., China) sequence: 0–20 min, 5%–70% B (v/v); 20–30 min, 70%–95% B (v/v). The chromatographic column adopted a Thermo Accucore™ C18(100 mm × 3 mm i.d., 2.6 µm) Chromatographic column (Thermo Fisher Scientific, Chengdu, China), with an injection volume of 2 L. The flow rate was 0.4 mL/min, and the column temperature was 28 °C.
The mass spectrum conditions were as follows: An electric spray ion source (ESI) was used. Scan mode: MS/dd MS2 positive and negative ion scanning; scanning range m/z 100–1500; cracking voltage: 3.5 kV (ESI+) and 3.0 kV (ESI−); sheath gas flow rate: 35 arb; auxiliary gas flow rate: 10 arb; ion transfer tube temperature: 320 °C; auxiliary gas temperature: 350 °C; primary resolution: 70,000; secondary resolution: 17,500. In the MS/MS mode, the collision energies in both positive and negative ion modes were set to 20, 40, and 60 eV, respectively.
We imported the raw data collected via UPLC-MS high-resolution mass spectrometry into the Compound Discoverer 3.1 software for peak alignment, peak extraction, and fitting of molecular formulas, and matched fragment ion information results in the mzVault and mzCloud databases to export the matching results. We further analyzed and identified the compounds by referring to the relevant literature and quality deviations.
3.3.3. HPLC-DAD
Chlorogenic acid, caffeic acid, taxifolin, ferulic acid, hyperin, isoquercitrin, rutin, hesperidin, astragalin, quercetin, luteolin, and diosmetin (Desite Biotechnology Co., Ltd., Chengdu, China) were each weighed to 20 mg and dissolved in methanol to form a single standard solution with a mass concentration of 0.5 mg/mL. Then, each, with a concentration of 120 L, was used to form a mixed standard of 12 substances, which were filtered through a 0.22 µm microporous membrane filter. The sample PPP was similarly prepared.
The sample and the mixed standard were analyzed using an HPLC-DAD platform, separated on an Agilent C18 column (250 mm × 4.6 mm, 5 µm). The temperature of the column was maintained at 28 °C, while the flow rate was fixed at 0.8 mL/min. For the mobile phase, a combination of A: water containing 0.3% phosphoric acid and B: pure methanol was used. The injection volume was 10 L, the detection wavelength was 254 nm, and the gradient elution sequence was 0–25 min, 5%–50% B; 25–35 min, 50%–55% B; 35–45 min, 5%–57% B; and 45–60 min, 57%–80% B.
3.4. Cell Culture
HepG2 cells were kindly provided by Procell LifeScience & Technology Co., Ltd. in Wuhan in China. The cells were cultured in a basic medium containing 10% fetal bovine serum, 1% double antibody (Beijing Institute of Biological Products Co., Ltd., Beijing, China), and MEM (Beijing Institute of Biological Products Co., Ltd., Beijing, China) at 37 °C with 5% CO2 in a cell incubator (Wuhan Servicebio Technology Co., Ltd., Wuhan, China). When cells grew to approximately 80% of the bottle wall, they were digested and passaged with trypsin every 3–4 days. HepG2 cells in a good growth state and logarithmic growth phase were subjected to 5 × 104 per well, with 100 L inoculated into a 96-well plate and incubated at 37 °C or 24 h.
3.5. Cell Viability Assay
To determine cell viability, the Cell Counting Kit-8 (Elabscience Biotechnology Co., Ltd., Beijing, China) was utilized. HepG2 cells were exposed to a basic culture medium containing various concentrations of PPP. The PPP concentrations in the wells were 5, 10, 20, 30, 40, 50, 60, 80, and 100 µg/mL. After a 24 h incubation period, 10 µL of CCK-8 solution per 100 µL of medium was added following the manufacturer’s instructions. The cells were then incubated in a 37 °C cell incubator for 2 h. The absorbance was measured at 450 nm using a microplate reader.
HepG2 cells were cultured in a basic medium for 24 h, after which different concentrations of t-BHP (50, 100, 200, 300, 400, 500, 600, 800, and 1000 µg/mL) were introduced. After a 2 h induction period, 10 µL of CCK-8 solution per 100 µL of medium was added according to the manufacturer’s instructions. The cells were incubated in a 37 °C cell incubator for an additional 2 h. The absorbance was subsequently detected at 450 nm using a microplate reader.
In this article, cell viability is characterized by cell survival rate, and the calculation method is shown in Equation (1):
Cell survival rate (%) = (OD experimental group − OD blank group)/(OD control group − OD blank group)
3.6. Intracellular ROS Assay
HepG2 cells with 5 × 104 cells per well in a 96-well plate for 24 h. Control group: an equal amount of basic culture medium was added. Model group: after adding an equal amount of basic culture medium for 24 h, 500 µmol/L t-BHP induction was added for 2 h. Vc group: after 24 h of pre-treatment with an equal amount of vitamin C (5 µg/mL), 500 µmol/L t-BHP induction was added for 2 h. PPP group: after pre-treatment with PPP of three different concentrations (high, medium, and low) for 24 h, 500 µmol/L t-BHP induction was added for 2 h. Cells in each group were cultivated and induced with oxidative stress. Post cultivation, they underwent a washing process with PBS (Wuhan Servicebio Technology Co., Ltd., China), followed by incubation with a DCFH-DA fluorescent probe at a final concentration of 10 µmol/L for 20 min. After three washes with serum-free cell culture medium, the cells were subjected to flow cytometry (CytoFLEX, Beckman coulter, Brea, CA, USA) analysis over time.
3.7. Detection of Cellular Antioxidant Enzyme Activity and Malondialdehyde Content
To evaluate the oxidative stress status and antioxidant defense system in the cellular homogenate, a comprehensive analysis was conducted. Specifically, the levels of CAT, GSH, GSH-Px, SOD, and MDA were quantified using assay kits obtained from Wuhan Elabscience (Wuhan, China). To accurately measure the protein concentration in the sample, a BCA protein assay kit provided by Guangzhou Biosharp (Guangzhou, China) was employed.
3.8. RNA Extraction and Quantitative Real-Time polymerase Chain Reaction (qRT-PCR)
In accordance with the culturing methodology detailed in Section 2.5, total RNA was isolated from the cells using the RNA pure total RNA rapid extraction kit manufactured by Chengdu Foregene (Chengdu, China). The 5× All-In-One MasterMix (with AccuRT Genomic DNA Removal kit) (Biocompete Inc., Shanghai, China) was used for reverse transcription to obtain cDNA, and qRT-PCR analysis was performed using the EvaGreen Express 2 × qPCR MasterMix-No Dye (Biocompete Inc., Shanghai, China). To accurately quantify the expression level of the target gene, it was normalized to the housekeeping gene GAPDH using the 2−ΔΔCt method. To ensure reliability and precision, each sample was evaluated in triplicate. The primers used are shown in Table 3.

Table 3.
The primer sequences for quantitative real-time PCR.
3.9. Western Blotting
Total cell proteins were extracted using RIPA cell lysate (Biosharp, Shanghai, China) with a protease inhibitor PMSF (Biosharp, China). Nuclear proteins were obtained using the nuclear and cytoplasmic protein extraction method from the kit (Biosharp, Shanghai, China). Then, they were incubated at 4 °C and centrifuged at 10,000 r/min for 5 min. The supernatant was obtained, and the protein concentration was measured using a BCA reagent kit (Biosharp, Shanghai, China). The concentration of the separation gel was selected according to the molecular weight of the target protein. First, electrophoresis at a constant pressure of 80 V was performed until the bromophenol blue indicator was linear at the junction of the concentrated and separation gels, and then changed to a constant pressure of 120 V until the bromophenol blue reached the bottom of the gel, which took approximately 90 min. After electrophoresis, the membrane was transferred and sealed in TBST containing 5% skimmed milk powder (Guangzhou Saiguo Biotech Co., Ltd., Guangzhou, China) for 2 h. The first antibody (Histone H3 Monoclonal Antibody, ImmunoWay Biotechnology Co., Ltd., Beijing, China) was incubated overnight at 4 °C, and the second antibody (Goat Anti-Rabbit IgG(H+L) HRP, MultiSciences Biotechnology Co., Ltd., Hangzhou, China) was incubated at room temperature for 2 h. ECL (Biosharp, China) was developed and photographed using GAPDH (Affinity, Shanghai, China) as the internal reference.
3.10. Statistical Analysis
The test data were statistically analyzed using SPSS 20.0 software, and one-way analysis of variance (ANOVA) and Duncan’s multiple comparisons were used to evaluate significance. All experiments were repeated three times except where stated otherwise, and the results are expressed as the mean ± standard deviation (SD).
4. Discussion
Phenolic compounds are an important material basis for the bioactivity of Polygonatum zanlanscianense Pamp. stems and leaves. The chemical components of PPP were isolated and identified using infrared spectroscopy, ultra-high-performance liquid chromatography (UPLC), and high-resolution mass spectrometry, with reference to the information in the mass spectrometry database. To the best of our knowledge, this is the first time that 20 phenolic compounds have been identified from Polygonatum zanlanscianense Pamp. stems and leaves, and the main components were quantified. Previous studies have extracted the phenolic constituents from the whole herb of Polygonatum sibiricum Red. with different solvents of increasing polarity, and it was found that the phenolic constituents of Polygonatum sibiricum Red. have strong antioxidant properties, and their bioactivities correlate positively with the total phenolic content [18]. Other authors have previously identified chlorogenic, ferulic, rutin, and ursolic acids as important constituents of Polygonatum sibiricum Red. leaves [19].
Excessive ROS cause oxidative stress in cells, thus disrupting the original oxidative– metabolic balance of cells, whereas endogenous and exogenous antioxidants can scavenge excessive ROS and maintain cellular redox homeostasis [20]. Previous studies have shown that hyperoside can inhibit H2O2-induced oxidative stress injury in LO2 cells via the Nrf2/Bach1 antioxidant signaling pathway [21]. Similarly, astragalin, kaempferol, ferulic acid, quercetin, and diosmetin, as natural flavonoids, showed strong free radical scavenging ability in vitro [22,23,24,25]. Caffeic acid passing through the Akt/GSK3β/ Nrf2 pathway reduced oxidative stress damage after cerebral ischemia in rats [26,27]. Chlorogenic acid attenuated doxorubicin-induced oxidative stress in cardiomyocytes via Nrf2/HO-1. New findings showed that the antioxidant activity of hesperidin was not only limited to its radical scavenging activity, but it augmented the antioxidant cellular defenses via the ERK/Nrf2 signaling pathway as well [28]. Rutin enhanced cellular antioxidant activities via the Nrf2/HO-1 signaling pathway [29]. As mentioned above, PPP is mainly composed of these phenolic compounds. Therefore, the inhibition of t-BHP-induced ROS overproduction may be mainly attributed to various phenolic compounds. Subsequently, the effects of PPP on antioxidant enzyme activities and MDA content in the cells were determined, and the results showed that PPP increased SOD, CAT, GSH, and GSH-Px activities and decreased MDA content. The results of this study are consistent with the inhibitory effects of other phenolic compounds on oxidative stress. For example, peony petal flavonoid extract reduced H2O2-induced cellular damage, ROS accumulation, and malondialdehyde content and increased antioxidant enzyme activities in BRL3A cells [30]. Additionally, betel palm polyphenolic extract enhanced the Nrf2 and HO-1 expression in oxidatively damaged cells and reduced ROS production in lipopolysaccharide-induced RAW264.7 cells [31].
Under normal physiological conditions, Nrf2 and Keap1 are localized in the cytoplasm as dimers. Once externally stimulated, Nrf2 dissociates from Keap1 and transfers to the nucleus, where it interacts with the ARE to activate the expression of a series of downstream antioxidant enzymes [32]. In this study, we demonstrated that the expression of the Nrf2 transcription factor was significantly upregulated at both gene and protein levels under PPP intervention, which promoted the translocation of Nrf2 into the nucleus and increased its accumulation in the nucleus, thus improving the antioxidant defense capacity of cells. It was shown that the extract of polymethoxy flavonoid components of citrus reticulata Blanco peels could exert antioxidant activities by activating the Nrf2 signaling pathway, which is consistent with the results of this study [33]. Furthermore, PPP significantly enhanced the cytoprotective proteins HO-1, NQO1, SOD, GSH-Px, and GCLC at both the gene and protein levels and downregulated MDA expression. It can be seen that PPP alleviates oxidative stress damage in cells and exerts antioxidant activity by promoting the transfer of Nrf2 into the nucleus, activating the expression of six downstream antioxidant enzymes of the Nrf2-ARE antioxidant signaling pathway, namely NQO1, SOD, CAT, GSH, GCLC, and GSH-Px, at both the mRNA and protein levels, and downregulates the expression of the stress response proteins, MDA, and Keap1.
The HepG2 cell line has good stability and can be cultured in the laboratory for passages while maintaining stable biological characteristics, ensuring the reliability of experimental results. HepG2 cells have certain metabolic activities and can complete normal physiological metabolic processes, including fat metabolism, oxidative stress, and other processes. Therefore, this study used the HepG2 cell model. However, the use of HepG2 cells to evaluate the protective effect of plant phenolic compounds has a major limitation, which is its low universality. HepG2 cells are cancer cells with different characteristics from normal cells. Therefore, using these cells to evaluate the protective effect of polyphenols may not accurately reflect the effect on normal cells. In addition, HepG2 cells are mainly used for liver cancer research and may not be suitable for studying other types of cells or tissues. Future research should be undertaken to verify whether this protective effect applies to other types of liver cells or normal cells.
5. Conclusions
Twenty phenolic compounds were identified from PPP, including three phenylpropanoids, namely caffeic acid, chlorogenic acid, and ferulic acid, and seventeen flavonoids and their glycosides, including kaempferol, dihydroquercetin, kaempferol-3-O-rutoside, fiscone, hesperidin, hyperin, isoquercetin, rutin, quercetin, epicatechin, quercetin, geranium lignin, luteolin, 3-Methoxy-5,7,3, 4-tetrahydroxyflavone, rhododendron, dalspinosin, and genistein B. Among them, chrysoeriol glycoside, zingiberoside, and geranylgeranyl were the main phenolic constituents with higher contents. This study is the first to investigate the composition and application of the stems and leaves of Polygonatum zanlanscianense Pamp. The results of cellular experiments showed that PPP had cytoprotective and antioxidant effects, protecting HepG2 cells from t-BHP-induced oxidative stress by scavenging ROS formation and activating the expression of downstream antioxidant enzyme genes of the Nrf2/Keap1 signaling pathway. These findings provide new insights into the mechanism of antioxidative stress in the stems and leaves of Polygonatum zanlanscianense Pamp., and offer new ideas for the development of natural antioxidants.
Author Contributions
Conceptualization, T.P. and F.L.; methodology, H.C.; validation, J.Y. (Jin Yan); formal analysis, S.T. and J.Y. (Jin Yong); investigation, S.T. and J.Y. (Jin Yan); resources, T.P.; writing—original draft, S.T.; writing—review and editing, F.L.; supervision, T.P. and H.C., funding acquisition, T.P. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Key Project of the Sichuan Science and Technology Department (no. 2021YFN0015 and 2022YFS0444), the Project of Sichuan Traditional Chinese Medicine Administration (no. 2023MS432), and the Chengdu Science and Technology Bureau Technology Innovation Research and Development Project (no. 2021-YF05-02298-SN).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are included in the paper.
Acknowledgments
The authors extend their appreciation to the Department of Science and Technology of Sichuan Province in China for funding this work and the State Key Laboratory of Southwestern Traditional Chinese Medicine Resources for providing test machines.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, D.I.; Griendling, K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Modulation of reactive oxygen species in health and disease. Antioxidants 2019, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Wu, Z.; Shang, X.; Liu, G.; Xie, Y. Comparative analysis of flavonoids, polyphenols and volatiles in roots, stems and leaves of five mangroves. PeerJ 2023, 11, e15529. [Google Scholar] [CrossRef] [PubMed]
- Polat, D.Ç.; Hürkul, M.M. Evaluation of Lonicera etrusca var. etrusca Santi (Caprifoliaceae) Stem and Leaf in Terms of Anatomical Structures and Some Phenolic Compounds. Turk. J. Pharm. Sci. 2022, 19, 636. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Xie, W.; Li, X.; Mei, G.; Xu, J.; Zhao, X.; Teng, H.; Yang, G. Phenolic Compounds from the Stems and Leaves of Berchemia lineata (L.) DC. Front. Chem. 2022, 10, 889441. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.N.; Zhou, X.Y.; Peng, L.P.; Liu, Z.A.; Shu, Q.Y. A comprehensive study of three species of Paeonia stem and leaf phytochemicals, and their antioxidant activities. J. Ethnopharmacol. 2021, 273, 113985. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Govindarajan, V.S.; Nadu, T. Critical reviews in food science and nutrition. Circ. Res. 2022, 62, 3833–3854. [Google Scholar]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem. 2019, 29241, 16817–16824. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2an update. Free. Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Zhang, H.; Zhang, P.F.; Qin, K.; Liu, S.; Peng, D.; Li, L. Prediction of the Potential Distributions and Ecological Suitability of Polygonatum zanlanscianense Pamp. J. Agric. Sci. Technol. 2021, 23, 185–192. [Google Scholar]
- The Chinese Pharmacopoeia; Chinese Medicine Publishing House: Beijing, China, 2020.
- Lee, K.M.; Kwon, T.Y.; Kang, U.; Seo, E.K.; Kim, Y.S. Tussilagonone-induced Nrf2 pathway activation protects Hep G2 cells from oxidative injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 108, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nurhanani, R.; Sarni, M.J.; Azlina, A.A. Polyphenols from the extract and fraction of T indica seeds protected Hep G2 cells against oxidative stress. BMC Complement. Altern. Med. 2015, 15, 438. [Google Scholar]
- Song, G.; Xia, J.; Xiao, Q. GC-MS Analysis and Antioxidant Activities Determination of Volatile Oil from Leaves of Polygonatum zanlanscianense Pamp. J. Hubei Minzu Univ. (Nat. Sci. Ed.) 2022, 40, 374–379+384. [Google Scholar]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.S.; Patra, A. Evaluation of phenolic composition, antioxidant, anti-inflammatory and anticancer activities of Polygonatum verticillatum (L.). J. Integr. Med. 2018, 16, 273–282. [Google Scholar] [CrossRef]
- Yonghua, W.; Jianping, Z.H.A.N.G.; Jiechang, Z.H.A.O. Antioxidantion and composition analysis of purified polygonatum sibiricum polyphenols using macroporous resin. Trans. Chin. Soc. Agric. Eng. 2020, 36, 318–326. [Google Scholar]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Cai, Y.; Li, B.; Peng, D.; Wang, X.; Li, P.; Huang, M.; Chen, J. Crm1-dependent nuclear export of Bach1 is involved in the protective effect of hyperoside on oxidative damage in hepatocytes and CCl4-induced acute liver injury. J. Inflamm. Res. 2021, 14, 551. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, X.; Yang, H.; Lin, C.; Li, L.; Ni, C.; Yan, P. The antioxidant and hypolipidemic effects of mesona chinensis benth extracts. Molecules 2022, 27, 3423. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gong, X.B.; Huang, L.G.; Wang, Z.X.; Wan, R.Z.; Zhang, P.; Zhang, B.S. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723. [Google Scholar] [CrossRef]
- Małgorzata, O.T.; Wianowska, D. Antioxidant Properties of Selected Flavonoids in Binary Mixtures-Considerations on Myricetin, Kaempferol and Quercetin. Int. J. Mol. Sci. 2023, 12, 24. [Google Scholar]
- Song, X.; Liu, C.; Zhang, Y.; Xiao, X.; Han, G.; Sun, K.; Liu, S.; Zhang, Z.; Dong, C.; Li, Y.; et al. Sustainable extraction of ligustilide and ferulic acid from Angelicae Sinensis Radix, for antioxidant and anti-inflammatory activities. Ultrason. Sonochemistry 2023, 94, 106344. [Google Scholar] [CrossRef]
- Shang, N.Y.; Li, X.; Liu, M.Y.; Chen, Q.Y.; Sun, Z.; Lan, J.Q.; Zhang, J.L.; Peng, Y. The protective effect and mechanism of caffeic acid on cerebral ischemic injury. Chin. J. Pharmacol. Toxicol. 2023, 37, 518. [Google Scholar]
- Cicek, B.; Hacimuftuoglu, A.; Yeni, Y.; Danisman, B.; Ozkaraca, M.; Mokhtare, B.; Lazopoulos, G.; Tsarouhas, K.; Tsatsakis, A.; Taghizadehghalehjoughi, A.; et al. Chlorogenic Acid Attenuates Doxorubi-cin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Di-tyrosine Signaling. J. Pers. Med. 2023, 4, 13. [Google Scholar]
- Liu, R.; Li, X.D. Antioxidant and Anti-inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Review of Their Molecular Mechanisms. Chin. J. Med. Guide 2019, 21, 749–752. [Google Scholar]
- Wei, X.; Nan, Z.; Zhang, Z.T. Effects of dietary cyanidin-3-diglucoside-5-glucoside complexes with rutin/Mg(II) against H2O2-induced cellular oxidative stress. Food Res. Int. 2019, 126, 108591. [Google Scholar]
- Liu, L.; Yuan, Y.; Zuo, J.; Tao, J. Composition and antioxidant activity of Paeonia lactiflora petal flavonoid extract and underlying mechanisms of the protective effect on H2O2-induced oxidative damage in BRL3A cells Hortic. Plant J. 2023, 9, 335–344. [Google Scholar] [CrossRef]
- Yi, S.; Zou, L.; Li, Z.; Sakao, K.; Wang, Y.; Hou, D.X. In Vitro Antioxidant Activity of Areca Nut Polyphenol Extracts on RAW264. 7 Cells. Foods 2022, 11, 3607. [Google Scholar] [CrossRef]
- Guo, S.; Szhang, Q. Paeonol protects melanocytes against hydrogen peroxide-induced oxidative stress through activation of Nrf2 signaling pathwa. Drug Dev. Res. 2021, 826, 861–869. [Google Scholar] [CrossRef]
- Ke, Z.; Zhao, Y.; Tan, S.; Chen, H.; Li, Y.; Zhou, Z.; Huang, C. Citrus reticulata Blanco peel extract ameliorates hepatic steatosis, oxidative stress and inflammation in HF and MCD diet-induced NASH C57BL/6 J mice. J. Nutr. Biochem. 2020, 83, 108426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).