Superior Valorisation of Juglans regia L. Leaves of Different Maturity through the Isolation of Bioactive Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Obtaining and Characterization of Juglans regia L. Leaves Extracts
2.1.1. Processing of Plant Material
2.1.2. Establishing the Optimal Extraction Parameters
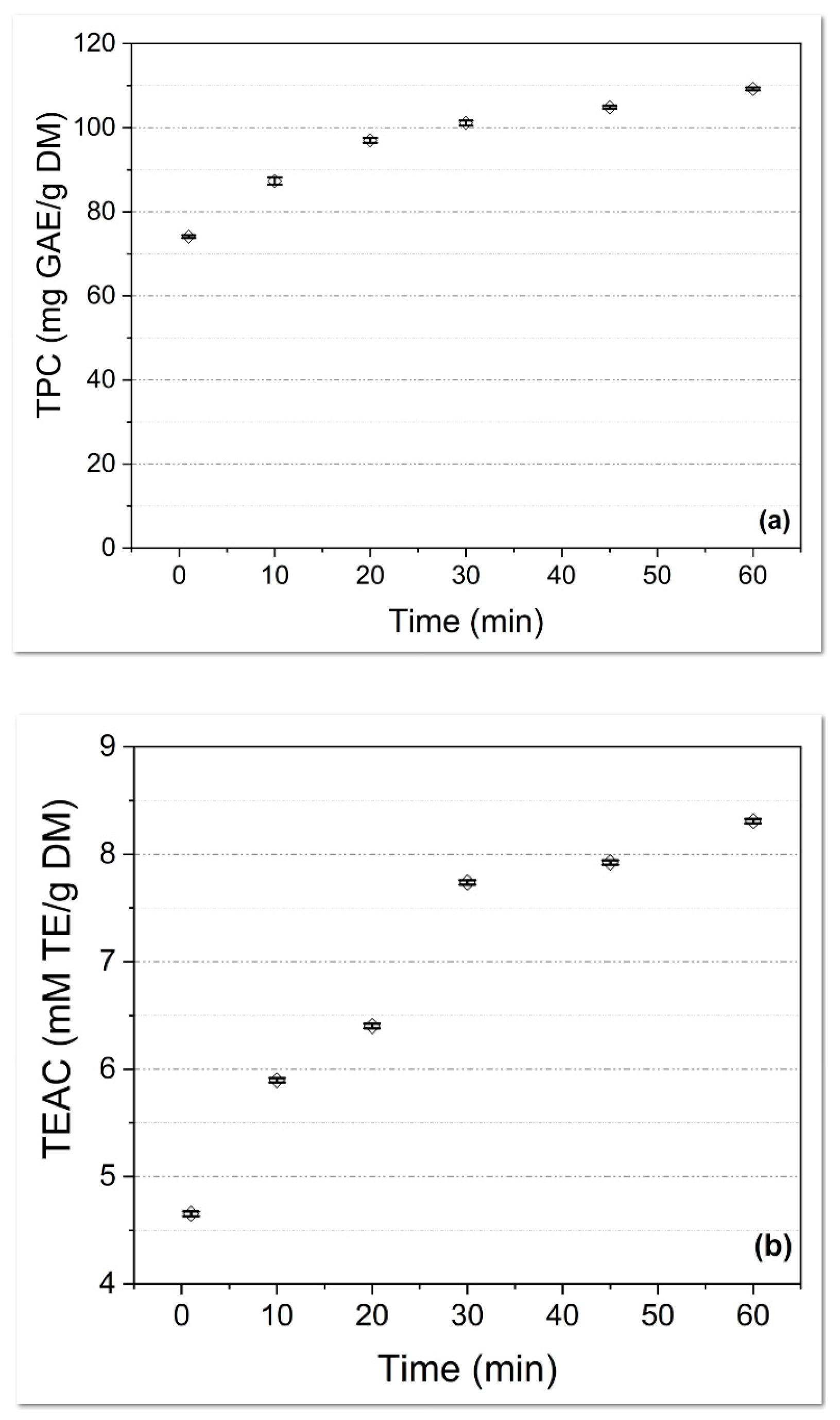
The Influence of the Extraction Time
The Influence of the Extraction Solvent and Processing Method
The Obtaining of Walnut Leaf Extracts (YGL, GL, MGL, YL)—The Influence of the Extraction Solvent
2.2. Phytochemical Characteristics of Walnut Leaves Extracts
Identification of Phytochemical Compounds from Walnut Leaf Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Preparation of Plant Extracts
3.4. Determination of Total Polyphenol Content (TPC) Using Spectrophotometric Method
3.5. Evaluation of Trolox Equivalent Antioxidant Capacity (TEAC) Using the Spectrophotometric Method
3.6. Fourier-Transform Ion-Cyclotron-Resonance High-Resolution Mass Spectrometry (FT-ICR-MS Method)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Besrour, N.; Oludemi, T.; Mandim, F.; Pereira, C.; Dias, M.I.; Sokovic, M.; Stojkovic, D.; Ferreira, O.; Ferreira, I.C.F.R.; Barros, L. Valorization of Juglans regia Leaves as Cosmeceutical Ingredients: Bioactivity Evaluation and Final Formulation Development. Antioxidants 2022, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Medic, A.; Zamljen, T.; Slatnar, A.; Hudina, M.; Grohar, M.C.; Veberic, R. Effect of Juglone and Other Allelochemicals in Walnut Leaves on Yield, Quality and Metabolites of Snack Cucumber (Cucumis sativus L.). Foods 2023, 12, 371. [Google Scholar] [CrossRef]
- Medic, A.; Zamljen, T.; Grohar, M.C.; Slatnar, A.; Hudina, M.; Veberic, R. Using HPLC-MS/MS to Assess the Quality of Beet, Mizuna, Lettuce and Corn Salad after Juglone and Walnut Leaf Extract Treatments. Agronomy 2022, 12, 347. [Google Scholar] [CrossRef]
- Acarsoy Bilgin, N. Morphological Characterization of Pollen in Some Varieties of Walnut (Juglans regia). Int. J. Fruit Sci. 2022, 22, 471–480. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Yeşilyurt, F.; Hacimüftüoğlu, A. Antioxidant and Anticholinesterase Activities of Juglans regia L. Growing in Turkey. J. Fac. Pharm. Ankara 2019, 43, 230–238. [Google Scholar] [CrossRef]
- Bhat, A.A.; Shakeel, A.; Rafiq, S.; Farooq, I.; Malik, A.Q.; Alghuthami, M.E.; Alharthi, S.; Qanash, H.; Alharthy, S.A. Juglans regia Linn.: A Natural Repository of Vital Phytochemical and Pharmacological Compounds. A review. Life 2023, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Amirou, A.; Bnouham, M.; Legssyer, A.; Ziyyat, A.; Aziz, M.; Berrabah, M.; Mekhfi, H. Effects of Juglans regia Root Bark Extract on Platelet Aggregation, Bleeding Time, and Plasmatic Coagulation: In Vitro and Ex Vivo Experiments. Evid. Based Complement. Altern. Med. 2018, 2018, 7313517. [Google Scholar] [CrossRef]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Badaoui, B.; Mourabit, Y.; Salhi, N.; Alshahrani, M.M.; Al Awadh, A.A.; Bouyahya, A.; Goh, K.W.; et al. Comparative Investigation of Chemical Constituents of Kernels, Leaves, Husk, and Bark of Juglans regia L., Using HPLC-DAD-ESI-MS/MS Analysis and Evaluation of Their Antioxidant, Antidiabetic, and Anti-Inflammatory Activities. Molecules 2022, 27, 8989. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Fizesan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.M.; Tomuta, I.; Popa, D.S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Zurek, N.; Pawlowska, A.; Pycia, K.; Grabek-Lejko, D.; Kapusta, I.T. Phenolic Profile and Antioxidant, Antibacterial, and Antiproliferative Activity of Juglans regia L. Male Flowers. Molecules 2022, 27, 2762. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentão, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Vieira, V.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Borros, L.; Ferreira, I.C.F.R. Hydroethanolic extract of Juglans regia L. green husks: A source of bioactive phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.R.; Gheldiu, A.M.; Mocan, A.; Moldovan, C.; Popa, D.S.; Tomuta, I.; Vlase, L. Process Optimization for Improved Phenolic Compounds Recovery from Walnut (Juglans regia L.) Septum: Phytochemical Profile and Biological Activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative-Qualitative Analysis and Chromatographic Purification. Biomolecules 2020, 10, 1363. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G. Impact of the Degree of Maturity of Walnuts (Juglans regia L.) and Their Variety on the Antioxidant Potential and the Content of Tocopherols and Polyphenols. Molecules 2019, 24, 2936. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. A review. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Segelke, T.; von Wuthenau, K.; Kuschnereit, A.; Müller, M.S.; Fischer, M. Origin Determination of Walnuts (Juglans regia L.) on a Worldwide and Regional Level by Inductively Coupled Plasma Mass Spectrometry and Chemometrics. Foods 2020, 9, 1708. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Optimization of ultrasound-assisted hydroalcoholic extraction of phenolic compounds from walnut leaves using response surface methodology. Pharm. Biol. 2016, 54, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Hadis, E.; Mostafa, H.-Z.S.; Saeedeh, N.; Mehdi, M. Study of the effects of walnut leaf on the levels of a number of Blood Biochemical Factors in normal male rats fed with high cholesterol diet. Clin. Biochem. 2011, 44, S331. [Google Scholar] [CrossRef]
- Hosseini, S.; Fallah Huseini, H.; Larijani, B.; Mohammad, K.; Najmizadeh, A.; Nourijelyani, K.; Jamshidi, L. The hypoglycemic effect of Juglans regia leaves aqueous extract in diabetic patients: A first human trial. DARU J. Pharm. Sci. 2014, 22, 19. [Google Scholar] [CrossRef]
- Hosseini, S.; Mehrzadi, S.; Najmizadeh, A.R.; Kazem, M.; Alimoradi, H.; Fallah Huseini, H. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: A randomized double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2014, 152, 451–456. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Eghbali, H.; Hosseini zijoud, S.M.; Pourrashidi, A.; Mohamadi, A.; Borhani, M.; Hassanshahi, G.; Rezaeian, M. Study of the effects of walnut leaf on some blood biochemical parameters in hypercholesterolemic rats. Biochem. Anal. Biochem. 2011, 103, S331. [Google Scholar] [CrossRef]
- Pitschmann, A.; Zehl, M.; Atanasov, A.G.; Dirsch, V.M.; Heiss, E.; Glasl, S. Walnut leaf extract inhibits PTP1B and enhances glucose-uptake in vitro. J. Ethnopharmacol. 2014, 152, 599–602. [Google Scholar] [CrossRef]
- Wianowska, S.; Garbaczewska, A.; Cieniecka-Roslonkiewicz, A.L.; Dawidowicz, A.; Jankowska, A. Comparison of antifungal activity of extracts from different Juglans regia cultivars and juglone. Microb. Pathog. 2016, 100, 263–267. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Lourenço-Lopes, C.; Taofiq, O.; Otero, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. By-Products of Walnut (Juglans regia) as a Source of Bioactive Compounds for the Formulation of Nutraceuticals and Functional Foods. Biol. Life Sci. Forum 2022, 12, 35. [Google Scholar] [CrossRef]
- Qadan, F.; Thewaini, A.J.; Ali, D.A.; Afifi, R.; Elkhawad, A.; Matalka, K.Z. The antimicrobial activities of Psidium guajava and Juglans regia leaf extracts to acne-developing organisms. Am. J. Chin. Med. 2005, 33, 197–204. [Google Scholar] [CrossRef]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Thul, S.T. Phytochemical analysis of the leaf volatile oil of walnut tree (Juglans regia L.) from western Himalaya. Ind. Crops Prod. 2013, 42, 195–201. [Google Scholar] [CrossRef]
- Boukhari, F.; Tigrine-Kordjani, N.; Meklati, B.Y. Phytochemical investigation by microwave-assisted extraction of essential oil of the leaves of walnut cultivated in Algeria. Helv. Chim. Acta 2013, 96, 1168–1175. [Google Scholar] [CrossRef]
- Zurek, N.; Pycia, K.; Pawlowska, A.; Potocki, L.; Kapusta, I.T. Chemical Profiling, Bioactive Properties, and Anticancer and Antimicrobial Potential of Juglans regia L. Leaves. Molecules 2023, 28, 1989. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Durak, A.; Pecio, Ł.; Kowalska, I. Nutraceutical Potential of Tinctures from Fruits, Green Husks, and Leaves of Juglans regia L. Sci. World J. 2014, 2014, 501392. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Majd, A.; Sepahdar, Z.; Azadmanesh, K.; Irian, S.; Ardestaniyan, M.H.; Hedayati, M.H.; Rastkari, N. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm. Biol. 2012, 50, 1416–1422. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Castro-Iglesias, A.; Freire, M.S.; González-Álvarez, J. Optimization of the Extraction of Bioactive Compounds from Walnut (Juglans major 209 x Juglans regia) Leaves: Antioxidant Capacity and Phenolic Profile. Antioxidants 2020, 99, 18. [Google Scholar] [CrossRef]
- Harish, K.; Neha, C.; Varsha, N.; Naveen, K.; Raman, S. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Jan, S.; Fazili, K.M. Bioefficacy Potential of Different Genotypes of Walnut Juglans regia L. J. Food Sci. Technol. 2018, 55, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Untea, A.; Lupu, A.; Saracila, M.; Panaite, T. Comparison of ABTS, DPPH, Phosphomolybdenum Assays for Estimating Antioxidant Activity and Phenolic Compounds in Five Different Plant Extracts. Bull. UASVM Anim. Sci. Biotechnol. 2018, 75, 110. [Google Scholar] [CrossRef]
- Jabli, M.; Sebeia, N.; Boulares, M.; Faidi, K. Chemical Analysis of the Characteristics of Tunisian Juglans regia L. Fractions: Antibacterial Potential, Gas Chromatography–Mass Spectroscopy and a Full Investigation of Their Dyeing Properties. Ind. Crops Prod. 2017, 108, 690–699. [Google Scholar] [CrossRef]
- Sytykiewicz, H.; Chrzanowski, G.; Czerniewicz, P.; Leszczy’nski, B.; Sprawka, I.; Krzyzanowski, R.; Matok, H. Antifungal activity of Juglans regia (L.) leaf extracts against Candida albicans isolates. Pol. J. Environ. Stud. 2015, 24, 1339–1448. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; De-Xing, H.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidant 2019, 8, 479. [Google Scholar] [CrossRef]
- Ivanovic, J.; Tadic, V.; Dimitrijevic, S.; Stamenic, M.; Petrovic, S.; Zizovic, I. Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “Čačanska Bestrna”. Ind. Crops Prod. 2013, 53, 274–281. [Google Scholar] [CrossRef]
- Cristianini, M.; Guillén Sánchez, J.S. Extraction of bioactive compounds from purple corn using emerging technologies: A review. J. Food Sci. 2020, 85, 855–1379. [Google Scholar] [CrossRef] [PubMed]
- Piyapanrungrueang, W.; Chantrapornchai, W.; Haruthaithanasan, V.; Sukatta, U.; Aekatasanawan, C. Comparison of anthocyanin extraction methods from high anthocyanin purple corn cob Hybrid: KPSC 901, and quality of the extract powder. J. Food Process. Preserv. 2016, 40, 1125–1133. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Bäuerl, C.; Cortés-Macías, E.; Calvo-Lerma, J.; Collado, M.C.; Barba, F.J. The impact of liquid-pressurized extracts of Spirulina, Chlorella and Phaedactylum tricornutum on in vitro antioxidant, antiinflammatory and bacterial growth effects and gut microbiota modulation. Food Chem. 2023, 401, 134083. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, M.; Barrada, H.; Zhu, Z.; Grimi, N.; Barba, F.J. Pulsed electric fields (PEF), pressurized liquid extraction (PLE) and combined PEF + PLE process evaluation: Effects on Spirulina microstructure, biomolecules recovery and Triple TOF-LC-MS-MS polyphenol composition. IFSET 2022, 77, 102989. [Google Scholar] [CrossRef]
- Ungureanu, A.R.; Chitescu, C.L.; Luta, E.A.; Morosan, A.; Mihaiescu, D.E.; Mihai, D.P.; Costea, L.; Ozon, E.A.; Fita, A.C.; Balaci, T.D.; et al. Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules 2023, 28, 3668. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.; Ondul Koc, E.; Ozay, Y.; Canli, O.; Ozdemir, S.; Tollu, G.; Dizge, N. Antimicrobial and antioxidant activity of phenolic extracts from walnut (Juglans regia L.) green husk by using pressure-driven membrane process. J. Food Sci. Technol. 2022, 60, 73–83. [Google Scholar] [CrossRef]
- Lockyer, S.; de la Hunty, A.E.; Steenson, S.; Spiro, A.; Stanner, S.A. Walnut consumption and health outcomes with public health relevance-A systematic review of cohort studies and randomized controlled trials published from 2017 to present. Nutr. Rev. 2023, 81, 26–54. [Google Scholar] [CrossRef]
- Almeida, I.; Fernandes, E.; Lima, J.; Costa, P.; Bahia, M. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008, 106, 1014–1020. [Google Scholar] [CrossRef]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef]
- D’Angeli, F.; Malfa, G.A.; Garozzo, A.; Li Volti, G.; Genovese, C.; Stivala, A.; Nicolosi, D.; Attanasio, F.; Bellia, F.; Ronsisvalle, S.; et al. Antimicrobial, Antioxidant, and Cytotoxic Activities of Juglans regia L. Pellicle Extract. Antibiotics 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Arsenie, L.V.; Lacatusu, I.; Oprea, O.; Bordei, N.; Bacalum, M.; Badea, N. Azelaic acid-willow bark extract-panthenol—Loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crops Prod. 2020, 154, 112658. [Google Scholar] [CrossRef]
- Koriem, K.M.M. Caftaric acid: An overview on its structure, daily consumption, bioavailability and pharmacological effects. Biointerface Res. Appl. Chem. 2020, 10, 5616–5623. [Google Scholar] [CrossRef]
- Ortiz, A.L.G.; Berti, F.; Navarini, L.; Crisafulli, P.; Colomban, S.; Forzato, C. Aqueous extracts of walnut (Juglans regia L.) leaves: Quantitative analyses of hydroxycinnamic and chlorogenic acids. J. Chromatogr. Sci. 2018, 56, 753–760. [Google Scholar] [CrossRef]
- Santos, A.; Barros, L.; Calhelha, R.C.; Duenas, M.; Carvalh, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant and antitumor effects. Ind. Crops Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Pasini, A.M.F.; Cominacini, L. Potential Benefits of Antioxidant Phytochemicals on Endogenous Antioxidants Defences in Chronic Diseases. Antioxidants 2023, 12, 890. [Google Scholar] [CrossRef]
- Sebastia, N.; Montoro, A.; León, Z.; Soriano, J.M. Searching trans-resveratrol in fruits and vegetables: A preliminary screening. J. Food Sci. Technol. 2017, 54, 842–845. [Google Scholar] [CrossRef]
- Yang, L.; Ma, S.; Han, Y.; Wang, Y.; Guo, Y.; Weng, Q.; Xu, M. Walnut Polyphenol Extract Attenuates Immunotoxicity Induced by 4-Pentylphenol and 3-methyl-4-nitrophenol in Murine Splenic Lymphocyte. Nutrients 2016, 8, 287. [Google Scholar] [CrossRef]
- Ravanbakhsh, A.; Mahdavi, M.; Jalilzade-Amin, G.; Javadi, S.; Maham, M.; Mohammadnejad, D.; Rashidi, M.R. Acute and Subchronic Toxicity Study of the Median Septum of Juglans regia in Wistar Rats. Adv. Pharm. Bull. 2016, 6, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Manson, J.E.; Willett, W.C.; Hu, F.B. Walnut Consumption Is Associated with Lower Risk of Type 2 Diabetes in Women. J. Nutr. 2013, 143, 512–518. [Google Scholar] [CrossRef] [PubMed]
- ISO 14502-1:2005; Determination of Substances Characteristic of Green and Black Tea. ISO: Geneva, Switzerland, 2005.
- Lungu, L.; Savoiu, M.R.; Manolescu, B.N.; Farcasanu, I.C.; Popa, C.V. Phytotoxic and Antioxidant Activities of Leaf Extracts of Ailanthus altissima Swingle. Rev. Chim. 2016, 67, 1928–1931. [Google Scholar]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef] [PubMed]
| Vegetable Material | Harvest Date | Moisture Content, % |
|---|---|---|
| young green leaves (YGL) | 15 May 2023 | 75.74 ± 0.03 |
| green leaves (GL) | 26 June 2023 | 72.73 ± 0.02 |
| mature green leaves (MGL) | 14 October 2022 | 55.17 ± 0.04 |
| yellow leaves (YL) | 15 November 2022 | 64.64 ± 0.03 |
| Method | Solvent | TPC * | TEAC ** |
|---|---|---|---|
| mg GAE/g DM | mM TE/g DM | ||
| magnetic stirring (MS) | ethanol | 27.76 ± 0.59 a | 2.88 ± 0.02 a |
| water | 76.08 ± 0.90 b | 5.60 ± 0.02 b | |
| water:ethanol | 109.21 ± 0.34 c | 7.53 ± 0.02 c | |
| ultrasounds assisted extraction (UA) | ethanol | 38.40 ± 0.59 a | 3.49 ± 0.02 a |
| water | 82.58 ± 0.90 b | 5.49 ± 0.02 b | |
| water:ethanol | 106.65 ± 0.34 c | 8.27 ± 0.02 c | |
| maceration (M) | ethanol | 42.35 ± 0.90 a | 2.79 ± 0.02 a |
| water | 93.04 ± 0.34 b | 5.28 ± 0.02 b | |
| water:ethanol | 110.39 ± 0.90 c | 6.79 ± 0.02 c |
| No. Exp. | Extraction Method | Solvent | TPC (mg GAE/g DM) | TPCpred (mg GAE/g DM) | ΔTPC (mg GAE/g DM) |
|---|---|---|---|---|---|
| 1 | MS | ethanol | 27.75 | 30.91 | −3.16 |
| 2 | MS | water | 76.08 | 78.64 | −2.56 |
| 3 | MS | water:ethanol | 109.21 | 103.49 | 5.72 |
| 4 | UA | ethanol | 38.40 | 35.77 | 2.63 |
| 5 | UA | water | 82.58 | 83.50 | −0.92 |
| 6 | UA | water:ethanol | 106.65 | 108.36 | −1.71 |
| 7 | M | ethanol | 42.35 | 41.82 | 0.53 |
| 8 | M | water | 93.04 | 89.55 | 3.48 |
| 9 | M | water:ethanol | 110.39 | 114.40 | −4.01 |
| RMSE | 3.134 | ||||
| R2 | 0.990 | ||||
| Adjusted R2 | 0.979 | ||||
| Predicted R2 | 0.950 | ||||
| Adequate Precision | 23.82 | ||||
| F | 94.34 | ||||
| p | 0.0003 |
| No. Exp. | Extraction Method | Solvent | TEAC (mg TE/g DM) | TEACpred (mg TE/g DM) | ΔTEAC (mg TE/g DM) |
|---|---|---|---|---|---|
| 1 | MS | ethanol | 2.88 | 3.04 | −0.1628 |
| 2 | MS | water | 5.60 | 5.44 | 0.1526 |
| 3 | MS | water:ethanol | 7.53 | 7.52 | 0.0102 |
| 4 | UA | ethanol | 3.49 | 3.46 | 0.0315 |
| 5 | UA | water | 5.49 | 5.86 | −0.3687 |
| 6 | UA | water:ethanol | 8.27 | 7.93 | 0.3373 |
| 7 | M | ethanol | 2.79 | 2.66 | 0.1313 |
| 8 | M | water | 5.28 | 5.06 | 0.2162 |
| 9 | M | water:ethanol | 6.79 | 7.13 | −0.3474 |
| RMSE | 0.232 | ||||
| R2 | 0.985 | ||||
| Adjusted R2 | 0.969 | ||||
| Predicted R2 | 0.922 | ||||
| Adequate Precision | 20.30 | ||||
| F | 63.89 | ||||
| p | 0.0007 |
| Type of Leaves | Solvent | TPC * | TEAC ** |
|---|---|---|---|
| mg GAE/g DM | mM TE/g DM | ||
| YGL | ethanol | 1.32 ± 0.34 a | 0.03 ± 0.00 a |
| water | 2.90 ± 0.59 ab | 0.04 ± 0.00 a | |
| water:ethanol | 18.28 ± 0.59 b | 0.09 ± 0.00 a | |
| GL | ethanol | 0.73 ± 0.34 a | 0.03 ± 0.00 a |
| water | 3.49 ± 0.59 ab | 0.03 ± 0.00 a | |
| water:ethanol | 16.90 ± 0.90 b | 0.08 ± 0.00 a | |
| MGL | ethanol | 38.40 ± 0.59 c | 3.50 ± 0.02 b |
| water | 82.58 ± 0.90 cd | 5.50 ± 0.02 b | |
| water:ethanol | 106.65 ± 0.34 c | 8.27 ± 0.02 b | |
| YL | ethanol | 62.66 ± 0.59 c | 4.74 ± 0.02 b |
| water | 110.20 ± 0.34 cd | 7.76 ± 0.02 b | |
| water:ethanol | 146.29 ± 0.90 c | 11.67 ± 0.02 b |
| No. Exp. | Plant Maturity | Solvent | TPC0.5 (mg GAE/g DM) | TPCpred0.5 (mg GAE/g DM) | ΔTPC0.5 (mg GAE/g DM) |
|---|---|---|---|---|---|
| 1 | YGL | ethanol | 12.10 | 12.04 | 0.0531 |
| 2 | YGL | water | 1.70 | 2.34 | −0.6326 |
| 3 | YGL | water:ethanol | 9.09 | 8.50 | 0.5912 |
| 4 | GL | ethanol | 4.28 | 4.25 | 0.0273 |
| 5 | GL | water | 7.92 | 8.34 | −0.4218 |
| 6 | GL | water:ethanol | 0.7297 | 0.4047 | 0.3250 |
| 7 | MGL | ethanol | 4.11 | 4.11 | 0.0023 |
| 8 | MGL | water | 1.15 | 0.5443 | 0.6052 |
| 9 | MGL | water:ethanol | 6.20 | 6.71 | −0.5084 |
| 10 | YL | ethanol | 10.33 | 10.41 | −0.0827 |
| 11 | YL | water | 10.50 | 10.13 | 0.3687 |
| 12 | YL | water:ethanol | 1.87 | 2.20 | −0.3273 |
| RMSE | 0.398 | ||||
| R2 | 0.9895 | ||||
| Adjusted R2 | 0.9808 | ||||
| Predicted R2 | 0.9581 | ||||
| Adequate Precision | 29.15 | ||||
| F | 113.29 | ||||
| p | <0.0001 |
| No. Exp. | Plant Maturity | Solvent | log(TEAC) (mg TE/g DM) | log(TEACpred) (mg TE/g DM) | log(ΔTEAC) (mg TE/g DM) |
|---|---|---|---|---|---|
| 1 | YGL | ethanol | 1.07 | 1.12 | −0.0556 |
| 2 | YGL | water | −1.39 | −1.37 | −0.0168 |
| 3 | YGL | water:ethanol | 0.7399 | 0.6910 | 0.0489 |
| 4 | GL | ethanol | −1.05 | −1.08 | 0.0351 |
| 5 | GL | water | 0.6760 | 0.6750 | 0.0010 |
| 6 | GL | water:ethanol | −1.60 | −1.60 | 0.0051 |
| 7 | MGL | ethanol | −1.07 | −1.15 | 0.0817 |
| 8 | MGL | water | −1.55 | −1.53 | −0.0183 |
| 9 | MGL | water:ethanol | 0.5433 | 0.5311 | 0.0122 |
| 10 | YL | ethanol | 0.9176 | 0.9787 | −0.0612 |
| 11 | YL | water | 0.8896 | 0.8350 | 0.0546 |
| 12 | YL | water:ethanol | −1.53 | −1.44 | −0.0867 |
| RMSE | 0.049 | ||||
| R2 | 0.998 | ||||
| Adjusted R2 | 0.966 | ||||
| Predicted R2 | 0.922 | ||||
| Adequate Precision | 55.98 | ||||
| F | 615.14 | ||||
| p | <0.0001 |
| Compound | Theoretical m/z (ESI+) | Type of Extract | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MGL * | YGL ** | GL *** | MGL * | YL **** | ||||||||||||||||||||||||
| Extraction Method | ||||||||||||||||||||||||||||
| MS (m) | MS | UA | M | UA | ||||||||||||||||||||||||
| Extraction Time (min) | Extraction Solvent (60 min) | Extraction Solvent (60 min) | ||||||||||||||||||||||||||
| 1 | 10 | 20 | 30 | 45 | 60 | e | w | m | e | w | m | e | w | m | e | w | m | e | w | m | e | w | m | e | w | m | ||
| Sample Number | ||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 6 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 9 | 10 | 11 | 21 | 22 | 23 | ||
| Caffeic acid | 181.049535 | − | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | + | − | + | + | − | + | + |
| Caftaric acid+Na | 335.037353 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | − | − | + | − | − | + | + | − | + | − |
| Chlorogenic acid | 355.102359 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Ellagic acid | 303.013544 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − |
| Ferulic acid | 196.065185 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − | + | − | − | + | + | − | + | + |
| Juglone | 175.038971 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | + | − | + | + | − | + | + |
| p-coumaric acid | 165.054621 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − | − | − | − | + | + | − | + | + |
| Sinapate | 225.075750 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − | − | − | − | + | + | − | + | + |
| 3-p-coumaroylquinic acid | 339.107444 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Quercetin 3-o-arabinoside | 435.092188 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Quercetin 3-rhamnoside | 449.107838 | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Quercetin 3-o-pentoside | 567.134447 | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − |
| Quercetin | 303.049929 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Myricetin | 319.044844 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | + | − | + | + | − | + | + |
| Kaempferol | 287.055014 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Kaempferol 3-o-arabinoside | 419.097273 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Kaempferol-3-o-rhamnoside | 433.112923 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Isokaempferide | 301.070665 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | + | − | + | + | − | + | + | − | + | + |
| Catechin hydrate | 309.096879 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Hyperoside | 465.102753 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | + | + | − | − | + | + | − | + | + | − | + | + |
| Luteolin | 287.055014 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Naringenin | 273.075750 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | + | − | + | + | + | + | + | + | + | + |
| Hesperetin | 303.086812 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | + | − | − | + | + | + | + | − | + | + |
| Catechin | 291.086315 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Epigallocatechin | 307.081229 | − | − | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − | − | − | − | + | + | + | + | + |
| Epigallocatechin gallate | 443.097273 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Fisetinidol | 275.091400 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − | + | − | − | + | + | − | + | + |
| Guibourtinidol | 259.096485 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − | + | + | − | + | + | − | + | − |
| Oleanic acid | 457.367622 | + | + | + | + | + | + | + | − | + | + | + | + | + | − | + | + | − | + | + | − | + | + | + | + | + | − | + |
| Stigmasterol | 413.377793 | + | + | + | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + | + | + | + | + |
| Resveratrol | 229.085921 | + | + | + | + | + | + | − | − | + | − | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | + | − |
| Myo-inositol | 181.070665 | − | + | + | + | + | + | − | + | + | − | + | − | − | + | − | − | + | + | − | + | + | − | + | − | − | − | − |
| Taxifolin | 305.065579 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Pelargonidin | 271.060100 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − | + | + | − | + | + |
| Malvidin | 331.081229 | − | − | − | − | − | − | − | + | − | − | + | + | − | + | + | + | + | + | − | + | + | − | + | + | − | + | + |
| Cyanidin-3-glucoside chloride | 485.084516 | + | + | + | + | + | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Quinic acid | 193.070665 | + | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + |
| Citric acid+Na | 215.016223 | + | − | − | − | − | + | − | + | + | − | + | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − |
| Azelaic acid | 189.112135 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| Asiatic acid | 489.357451 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + |
| Total number of compounds | 33 | 34 | 35 | 35 | 35 | 36 | 17 | 34 | 36 | 18 | 36 | 33 | 18 | 32 | 33 | 18 | 23 | 27 | 15 | 26 | 28 | 18 | 36 | 33 | 18 | 34 | 31 | |
| Sample number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 6 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 9 | 10 | 11 | 21 | 22 | 23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tociu, M.; Manolache, F.; Bălănucă, B.; Moroșan, A.; Stan, R. Superior Valorisation of Juglans regia L. Leaves of Different Maturity through the Isolation of Bioactive Compounds. Molecules 2023, 28, 7328. https://doi.org/10.3390/molecules28217328
Tociu M, Manolache F, Bălănucă B, Moroșan A, Stan R. Superior Valorisation of Juglans regia L. Leaves of Different Maturity through the Isolation of Bioactive Compounds. Molecules. 2023; 28(21):7328. https://doi.org/10.3390/molecules28217328
Chicago/Turabian StyleTociu, Mihaela, Fulvia Manolache, Brîndușa Bălănucă, Alina Moroșan, and Raluca Stan. 2023. "Superior Valorisation of Juglans regia L. Leaves of Different Maturity through the Isolation of Bioactive Compounds" Molecules 28, no. 21: 7328. https://doi.org/10.3390/molecules28217328
APA StyleTociu, M., Manolache, F., Bălănucă, B., Moroșan, A., & Stan, R. (2023). Superior Valorisation of Juglans regia L. Leaves of Different Maturity through the Isolation of Bioactive Compounds. Molecules, 28(21), 7328. https://doi.org/10.3390/molecules28217328







