Global Proteome-Wide Analysis of Cysteine S-Nitrosylation in Toxoplasma gondii
Abstract
1. Introduction
2. Results
2.1. Global Detection of S-Nitrosylated Sites on T. gondii Proteins
2.2. Motifs of S-Nitrosylated Peptides
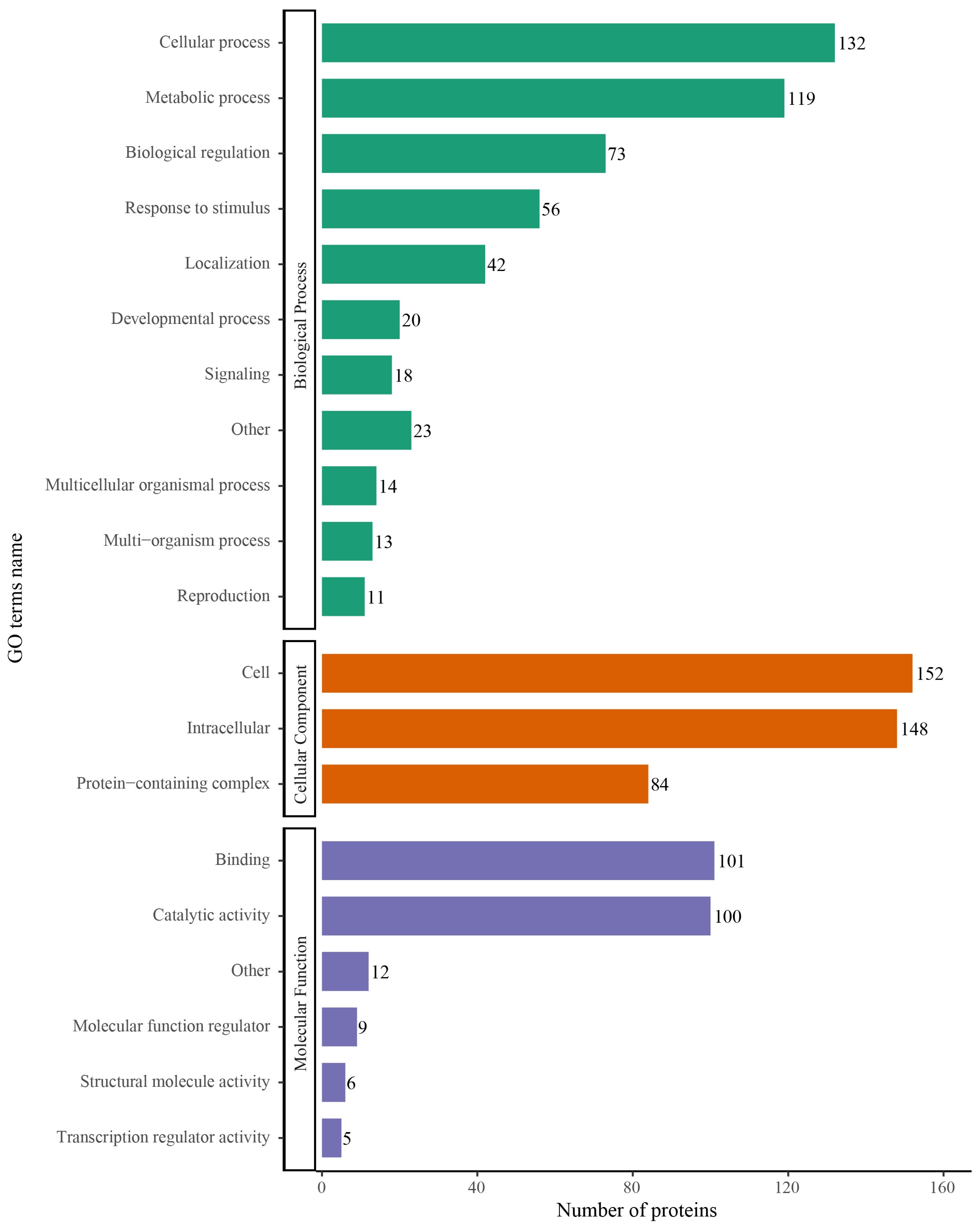
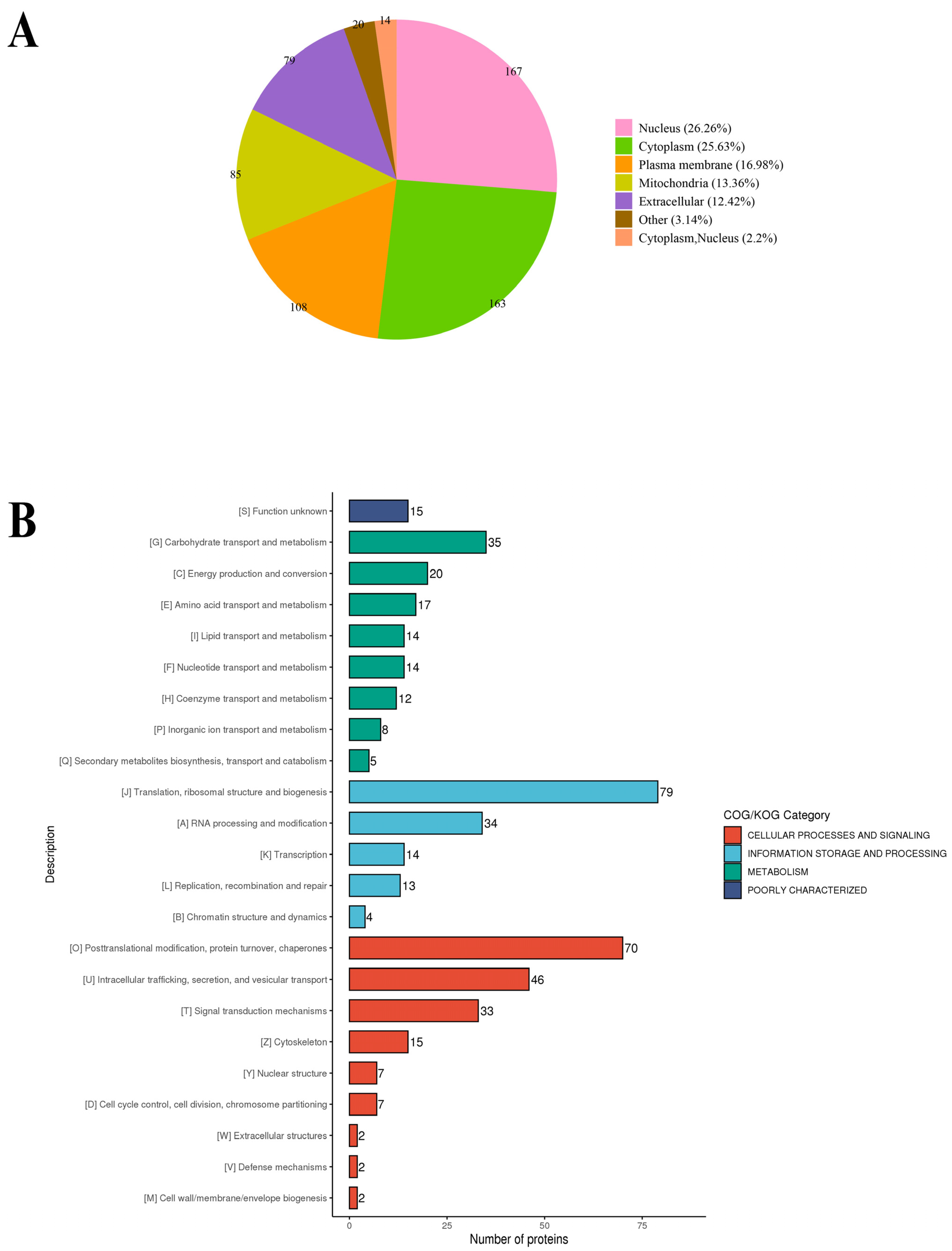
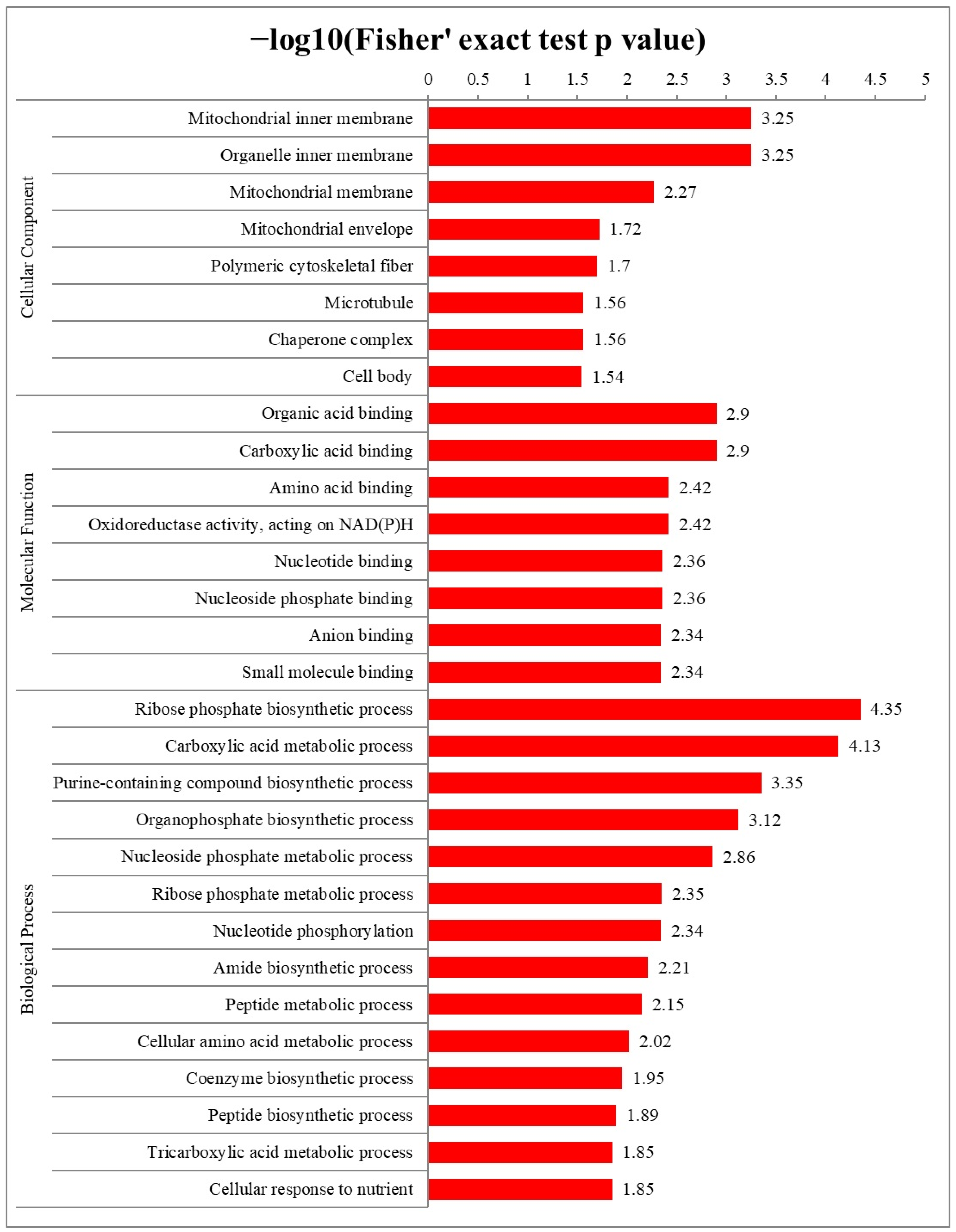
2.3. Functional Annotation of S-Nitrosylated Proteins
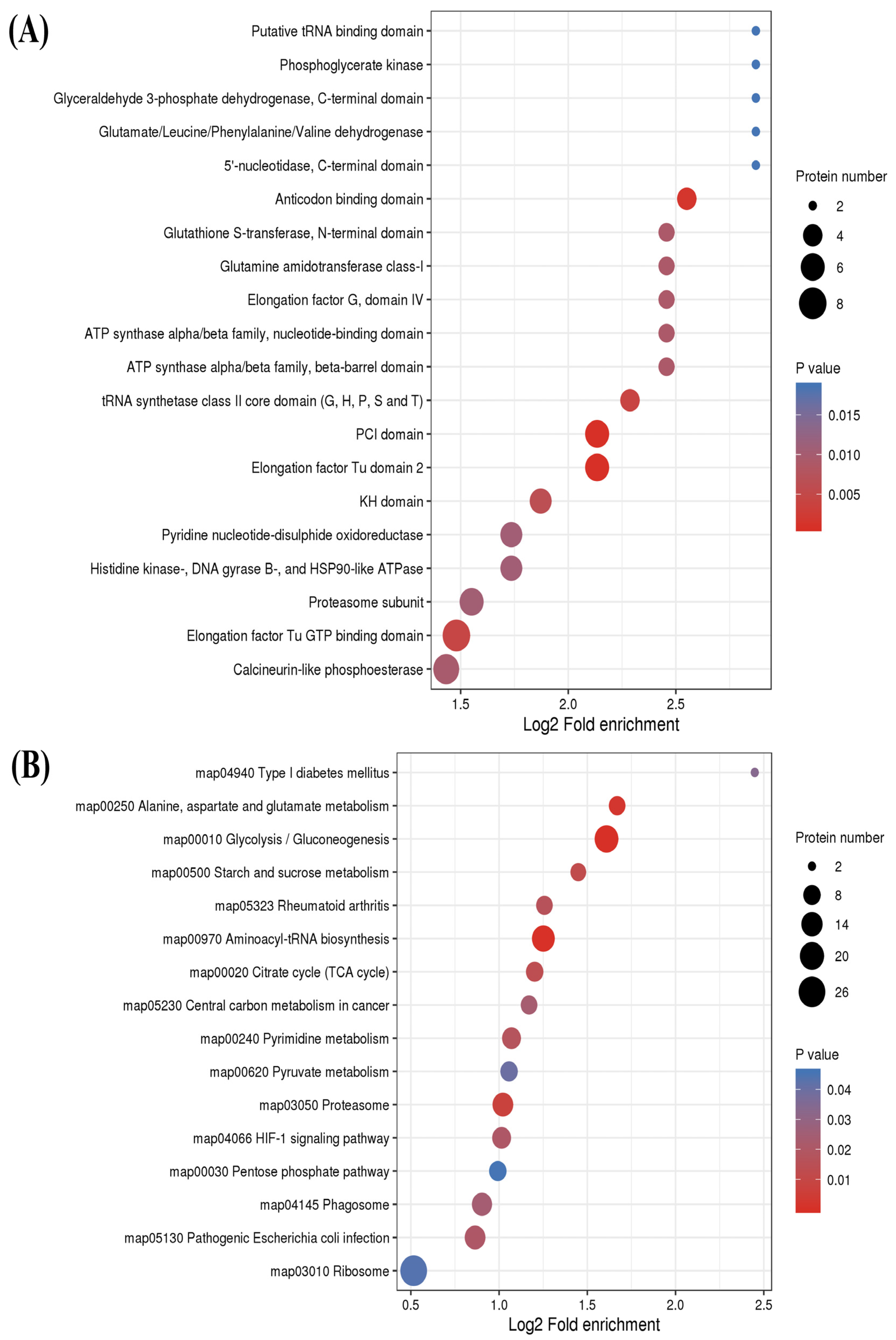
2.4. Functional Enrichment Analysis of S-Nitrosylated Proteins in T. gondii
2.5. Cysteine S-Nitrosylation of the Ribosome
2.6. Cysteine S-Nitrosylation of Microneme Proteins in T. gondii
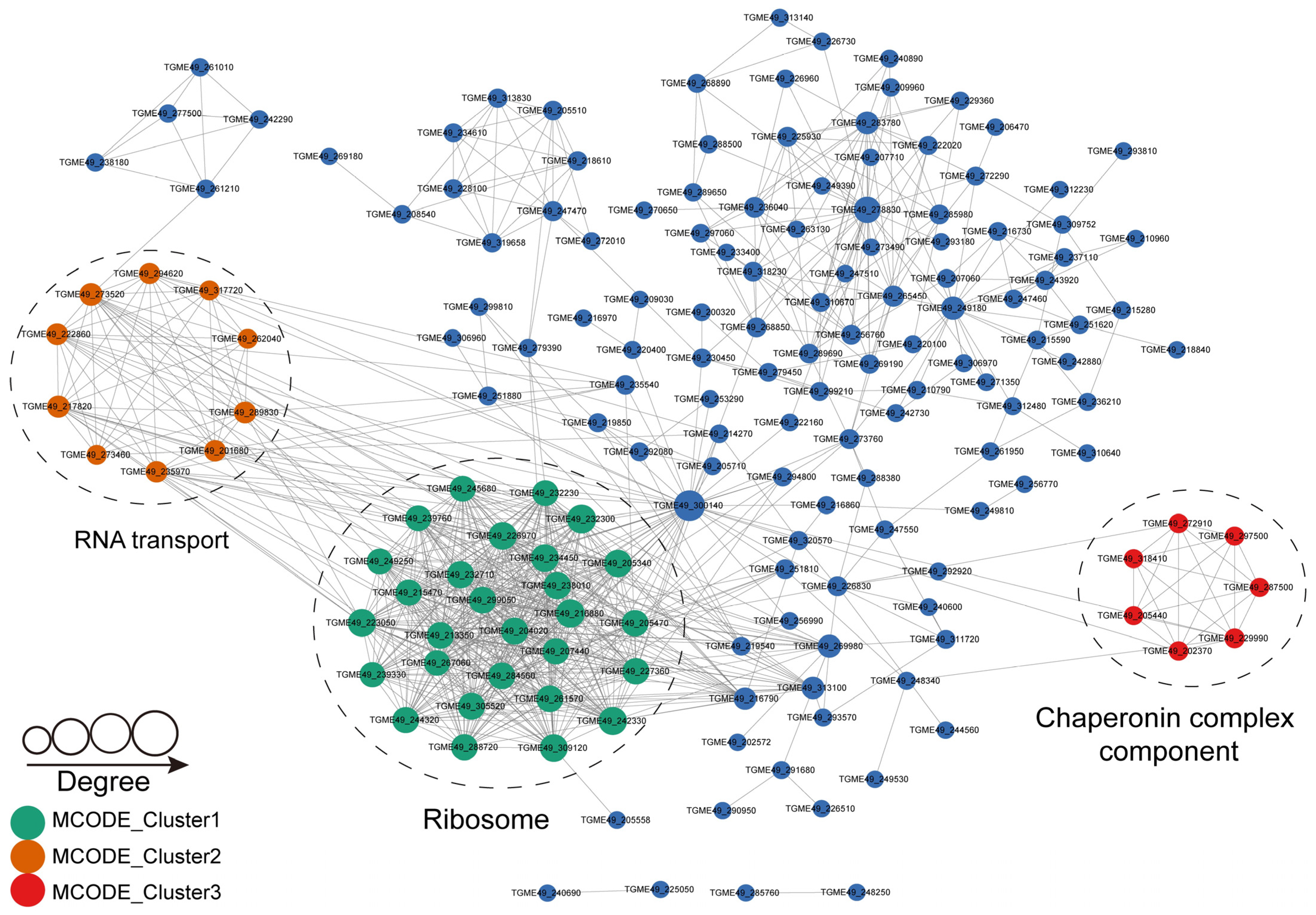
2.7. Protein–Protein Interaction Networks of S-Nitrosylated Proteins in T. gondii
3. Discussion
4. Material and Methods
4.1. Ethics Approval
4.2. Parasite Strains
4.3. Parasite Collection and Purification
4.4. Protein Extraction
4.5. Iodo-TMT Labeling
4.6. Trypsin Digestion
4.7. HPLC Fractionation
4.8. Resin-Assisted Enrichment of S-Nitrosylated Peptides
4.9. LC-MS/MS Analysis of Enriched S-Nitrosylated Peptides
4.10. Data Processing and Peptide Identification
4.11. Bioinformatics Analysis and Results Presentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors 2020, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Lago, E.G.; Gennari, S.M.; Su, C.; Jones, J.L. Toxoplasmosis in humans and animals in Brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 2012, 139, 1375–1424. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Berg, R.; Tagel, M.; Must, K.; Deksne, G.; Enemark, H.L.; Alban, L.; Johansen, M.V.; Nielsen, H.V.; Sandberg, M.; et al. Seroprevalence of Toxoplasma gondii in domestic pigs, sheep, cattle, wild boars, and moose in the Nordic-Baltic region: A systematic review and meta-analysis. Parasite Epidemiol. Control 2019, 5, e00100. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Tenter, A.M. Toxoplasma gondii in animals used for human consumption. Mem. Inst. Oswaldo Cruz 2009, 104, 364–369. [Google Scholar] [CrossRef]
- Stelzer, S.; Basso, W.; Benavides Silván, J.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Conraths, F.J.; Schares, G. Toxoplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, N.; Zhang, T.; Wang, D.; Feng, Y.; Sang, X.; Yang, N.; Chen, Q. Toxoplasma gondii genotype determines Tim-3 expression levels in splenic and circulatory T cells in mice. Front. Microbiol. 2018, 9, 2967. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, W.; Yu, Q.; Xing, T.; Chen, S.; Liu, L.; Yu, L.; Du, J.; Luo, Q.; Shen, J.; et al. Toxoplasma Chinese 1 strain of WH3Δrop16I/III /gra15II genetic background contributes to abnormal pregnant outcomes in murine model. Front. Immunol. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Chen, L.F.; Han, X.L.; Li, F.X.; Yao, Y.Y.; Fang, J.P.; Liu, X.J.; Li, X.C.; Wu, K.; Liu, M.; Chen, X.G. Comparative studies of Toxoplasma gondii transcriptomes: Insights into stage conversion based on gene expression profiling and alternative splicing. Parasites Vectors 2018, 11, 402. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef]
- Dubey, J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009, 39, 877–882. [Google Scholar] [CrossRef]
- Martorelli Di Genova, B.; Wilson, S.K.; Dubey, J.P.; Knoll, L.J. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol. 2019, 17, e3000364. [Google Scholar] [CrossRef]
- Kibe, M.K.; Coppin, A.; Dendouga, N.; Oria, G.; Meurice, E.; Mortuaire, M.; Madec, E.; Tomavo, S. Transcriptional regulation of two stage-specifically expressed genes in the protozoan parasite Toxoplasma gondii. Nucleic Acids Res. 2005, 33, 1722–1736. [Google Scholar] [CrossRef][Green Version]
- Gutierrez-Escobar, A.J.; Gómez-Marin, J.E. Toxoplasma gondii: Identification of a putative nitric oxide synthase motif DNA sequence. Exp. Parasitol. 2005, 111, 211–218. [Google Scholar] [CrossRef]
- Gutierrez-Escobar, A.J.; Arenas, A.F.; Villoria-Guerrero, Y.; Padilla-Londoño, J.M.; Gómez-Marin, J.E. Toxoplasma gondii: Molecular cloning and characterization of a nitric oxide synthase-like protein. Exp. Parasitol. 2008, 119, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.C., Jr.; Augusto, O. Connecting the chemical and biological properties of nitric oxide. Chem. Res. Toxicol. 2012, 25, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.J.; Spratt, H.M.; Soman, K.V.; Stafford, S.; Gupta, S.; Petersen, J.R.; Zago, M.P.; Kuyumcu-Martinez, M.N.; Brasier, A.R.; Wiktorowicz, J.E.; et al. S-Nitrosylation proteome profile of peripheral blood mononuclear cells in human heart failure. Int. J. Proteom. 2016, 2016, 1384523. [Google Scholar] [CrossRef]
- Hlaing, K.H.; Clément, M.V. Formation of protein S-nitrosylation by reactive oxygen species. Free Radic. Res. 2014, 48, 996–1010. [Google Scholar] [CrossRef]
- Mule, S.N.; Manchola, N.C.; de Oliveira, G.S.; Pereira, M.; Magalhães, R.; Teixeira, A.A.; Colli, W.; Alves, M.; Palmisano, G. Proteome-wide modulation of S-nitrosylation in Trypanosoma cruzi trypomastigotes upon interaction with the host extracellular matrix. J. Proteom. 2021, 231, 104020. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Hess, D.T.; Hausladen, A.; Wang, L.; Wang, Y.J.; Stamler, J.S. A multiplex enzymatic machinery for cellular protein S-nitrosylation. Mol. Cell 2018, 69, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation: Determinants of specificity and enzymatic regulation of s-nitrosothiol-based signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.M.; Kohr, M.J.; Murphy, E. S-nitrosylation: Specificity, occupancy, and interaction with other post-translational modifications. Antioxid. Redox Signal. 2013, 19, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.L.; Stomberski, C.T.; Stamler, J.S. Cross talk between S-nitrosylation and phosphorylation involving kinases and nitrosylases. Circ. Res. 2018, 122, 1485–1487. [Google Scholar] [CrossRef]
- Wolhuter, K.; Whitwell, H.J.; Switzer, C.H.; Burgoyne, J.R.; Timms, J.F.; Eaton, P. Evidence against stable protein S-nitrosylation as a widespread mechanism of post-translational regulation. Mol. Cell 2018, 69, 438–450. [Google Scholar] [CrossRef]
- Nie, L.B.; Liang, Q.L.; Du, R.; Elsheikha, H.M.; Han, N.J.; Li, F.C.; Zhu, X.Q. Global Proteomic analysis of lysine malonylation in Toxoplasma gondii. Front. Microbiol. 2020, 11, 776. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Cheng, W.H.; Huang, K.Y.; Ong, S.C.; Ku, F.M.; Huang, P.J.; Lee, C.C.; Yeh, Y.M.; Lin, R.; Chiu, C.H.; Tang, P. Protein cysteine S-nitrosylation provides reducing power by enhancing lactate dehydrogenase activity in Trichomonas vaginalis under iron deficiency. Parasites Vectors 2020, 13, 477. [Google Scholar] [CrossRef]
- Wang, Z.X.; Zhou, C.X.; Elsheikha, H.M.; He, S.; Zhou, D.H.; Zhu, X.Q. Proteomic differences between developmental stages of Toxoplasma gondii revealed by iTRAQ-based quantitative proteomics. Front. Microbiol. 2017, 8, 985. [Google Scholar] [CrossRef]
- Van Poppel, N.F.; Welagen, J.; Vermeulen, A.N.; Schaap, D. The complete set of Toxoplasma gondii ribosomal protein genes contains two conserved promoter elements. Parasitology 2006, 133 Pt 1, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, V.B. Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells. Parasitol. Int. 1999, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Zhu, X.Q.; Elsheikha, H.M.; He, S.; Li, Q.; Zhou, D.H.; Suo, X. Global iTRAQ-based proteomic profiling of Toxoplasma gondii oocysts during sporulation. J. Proteom. 2016, 148, 12–19. [Google Scholar] [CrossRef]
- Bargieri, D.; Lagal, V.; Andenmatten, N.; Tardieux, I.; Meissner, M.; Ménard, R. Host cell invasion by apicomplexan parasites: The junction conundrum. PLoS Pathog. 2014, 10, e1004273. [Google Scholar] [CrossRef] [PubMed]
- Saouros, S.; Edwards-Jones, B.; Reiss, M.; Sawmynaden, K.; Cota, E.; Simpson, P.; Dowse, T.J.; Jäkle, U.; Ramboarina, S.; Shivarattan, T.; et al. A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J. Biol. Chem. 2005, 280, 38583–38591. [Google Scholar] [CrossRef]
- Dodangeh, S.; Daryani, A.; Sharif, M.; Aghayan, S.A.; Pagheh, A.S.; Sarvi, S.; Rezaei, F. A systematic review on efficiency of microneme proteins to induce protective immunity against Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 617–629. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.C.; Zhou, C.X.; Zhu, X.Q. Research advances in interactions related to Toxoplasma gondii microneme proteins. Exp. Parasitol. 2017, 176, 89–98. [Google Scholar] [CrossRef]
- Genestra, M.; de Souza, W.J.; Cysne-Finkelstein, L.; Leon, L.L. Comparative analysis of the nitric oxide production by Leishmania sp. Med. Microbiol. Immunol. 2003, 192, 217–223. [Google Scholar] [CrossRef]
- Gould, N.; Doulias, P.T.; Tenopoulou, M.; Raju, K.; Ischiropoulos, H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J. Biol. Chem. 2013, 288, 26473–26479. [Google Scholar] [CrossRef]
- Jortzik, E.; Wang, L.; Becker, K. Thiol-based posttranslational modifications in parasites. Antioxid. Redox Signal. 2012, 17, 657–673. [Google Scholar] [CrossRef]
- Tryon, J.C.; Weidner, E.; Larson, A.D.; Hart, L.T. A rapid method for isolating purified Toxoplasma tachyzoites from peritoneal exudate and cell culture. J. Parasitol. 1978, 64, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, S.X.; Jiang, X.G.; Fu, X.L.; Shen, Y.J.; Cao, J.P. Separation and purification of Toxoplasma gondii tachyzoites from in vitro and in vivo culture systems. Exp. Parasitol. 2012, 130, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Grigg, M.E. Toxoplasma gondii: Laboratory Maintenance and Growth. Curr. Protoc. Microbiol. 2017, 44, 20C.1.1–20C.1.17. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.J.; Johnson, A.M. Purification of Toxoplasma gondii from host cells. J. Clin. Pathol. 1983, 36, 602–604. [Google Scholar] [CrossRef]
- Li, F.C.; Nie, L.B.; Elsheikha, H.M.; Yin, F.Y.; Zhu, X.Q. Lysine crotonylation is widespread on proteins of diverse functions and localizations in Toxoplasma gondii. Parasitol. Res. 2021, 120, 1617–1626. [Google Scholar] [CrossRef]
- Guo, J.; Gaffrey, M.J.; Su, D.; Liu, T.; Camp, D.G., II; Smith, R.D.; Qian, W.J. Resin-assisted enrichment of thiols as a general strategy for proteomic profiling of cysteine-based reversible modifications. Nat. Protoc. 2014, 9, 64–75. [Google Scholar] [CrossRef]
- Derakhshan, B.; Wille, P.C.; Gross, S.S. Unbiased identification of cysteine S-nitrosylation sites on proteins. Nat. Protoc. 2007, 2, 1685–1691. [Google Scholar] [CrossRef]
- Su, D.; Shukla, A.K.; Chen, B.; Kim, J.S.; Nakayasu, E.; Qu, Y.; Aryal, U.; Weitz, K.; Clauss, T.R.; Monroe, M.E.; et al. Quantitative site-specific reactivity profiling of S-nitrosylation in mouse skeletal muscle using cysteinyl peptide enrichment coupled with mass spectrometry. Free Radic. Biol. Med. 2013, 57, 68–78. [Google Scholar] [CrossRef]
- Yu, L.; Dai, Z.; Zhang, Y.; Iqbal, S.; Lu, S.; Guo, L.; Yao, X. Proteome-wide identification of S-sulfenylated cysteines reveals metabolic response to freezing stress after cold acclimation in Brassica napus. Front. Plant Sci. 2022, 13, 1014295. [Google Scholar] [CrossRef]
- Barbacini, P.; Blottner, D.; Capitanio, D.; Trautmann, G.; Block, K.; Torretta, E.; Moriggi, M.; Salanova, M.; Gelfi, C. Effects of omega-3 and antioxidant cocktail supplement on prolonged bed rest: Results from serum proteome and sphingolipids analysis. Cells 2022, 11, 2120. [Google Scholar] [CrossRef]
- Wijasa, T.S.; Sylvester, M.; Brocke-Ahmadinejad, N.; Kummer, M.P.; Brosseron, F.; Gieselmann, V.; Heneka, M.T. Proteome profiling of s-nitrosylated synaptosomal proteins by isobaric mass tags. J. Neurosci. Methods 2017, 291, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zaman, U.; Richter, F.M.; Hofele, R.; Kramer, K.; Sachsenberg, T.; Kohlbacher, O.; Lenz, C.; Urlaub, H. Dithiothreitol (DTT) acts as a specific, UV-inducible cross-linker in elucidation of protein-RNA interactions. Mol. Cell. Proteom. 2015, 14, 3196–3210. [Google Scholar] [CrossRef] [PubMed]
- Cleland, W.W. Dithiothreitol, a new protective reagent for SH groups. Biochemistry 1964, 3, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, X.; Liu, J.; Niu, Z.; Yang, J.; Shen, B. Essential role of pyrophosphate homeostasis mediated by the pyrophosphate-dependent phosphofructokinase in Toxoplasma gondii. PLoS Pathog. 2022, 18, e1010293. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset, A.E.; Fox, B.A.; Karram, M.H.; Abd Ellah, M.R.; Bzik, D.J.; Igarashi, M. Lactate dehydrogenase in Toxoplasma gondii controls virulence, bradyzoite differentiation, and chronic infection. PLoS ONE 2017, 12, e0173745. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.L.; Elling, R.A.; Wilson, D.K. Structure of Toxoplasma gondii LDH1: Active-site differences from human lactate dehydrogenases and the structural basis for efficient APAD+ use. Biochemistry 2004, 43, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Al-Anouti, F.; Tomavo, S.; Parmley, S.; Ananvoranich, S. The expression of lactate dehydrogenase is important for the cell cycle of Toxoplasma gondii. J. Biol. Chem. 2004, 279, 52300–52311. [Google Scholar] [CrossRef]
- Pérez-Mato, I.; Castro, C.; Ruiz, F.A.; Corrales, F.J.; Mato, J.M. Methionine adenosyltransferase S-nitrosylation is regulated by the basic and acidic amino acids surrounding the target thiol. J. Biol. Chem. 1999, 274, 17075–17079. [Google Scholar] [CrossRef]
- Mnatsakanyan, R.; Markoutsa, S.; Walbrunn, K.; Roos, A.; Verhelst, S.H.L.; Zahedi, R.P. Proteome-wide detection of S-nitrosylation targets and motifs using bioorthogonal cleavable-linker-based enrichment and switch technique. Nat. Commun. 2019, 10, 2195. [Google Scholar] [CrossRef]
- Smith, J.G.; Aldous, S.G.; Andreassi, C.; Cuda, G.; Gaspari, M.; Riccio, A. Proteomic analysis of S-nitrosylated nuclear proteins in rat cortical neurons. Sci. Signal. 2018, 11, eaar3396. [Google Scholar] [CrossRef]
- Seth, D.; Stamler, J.S. The SNO-proteome: Causation and classifications. Curr. Opin. Chem. Biol. 2011, 15, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Toone, E.J.; Lipton, S.A.; Sucher, N.J. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron 1997, 18, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, L.; Xie, Z.; Shao, S.; Wu, Y.; Xu, W.; Gu, B.; Wang, B. An improved sulfur-nitroso-proteome strategy for global profiling of sulfur-nitrosylated proteins and sulfur-nitrosylation sites in mice. J. Chromatogr. A 2023, 1705, 464162. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Delahunty, C.; Prieto, J.H.; Rahlfs, S.; Jortzik, E.; Yates, J.R., 3rd; Becker, K. Protein S-nitrosylation in Plasmodium falciparum. Antioxid. Redox Signal. 2014, 20, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Hertz, R.; Ben Lulu, S.; Shahi, P.; Trebicz-Geffen, M.; Benhar, M.; Ankri, S. Proteomic identification of S-nitrosylated proteins in the parasite Entamoeba histolytica by resin-assisted capture: Insights into the regulation of the Gal/GalNAc lectin by nitric oxide. PLoS ONE 2014, 9, e91518. [Google Scholar] [CrossRef]
- Warner, J.R.; Mcintosh, K.B. How common are extraribosomal functions of ribosomal proteins? Mol. Cell 2009, 34, 3–11. [Google Scholar] [CrossRef]
- Báñez-Vea, M.; Huang, H.; Martínez de Morentin, X.; Pérez, E.; Gato, M.; Zuazo, M.; Arasanz, H.; Fernández-Irigoyen, J.; Santamaría, E.; Fernandez-Hinojal, G.; et al. Characterization of Macrophage endogenous S-nitrosoproteome using a cysteine-specific phosphonate adaptable tag in combination with TiO2 chromatography. J. Proteome Res. 2018, 17, 1172–1182. [Google Scholar] [CrossRef]
- Pereira, M.; Soares, C.; Canuto, G.A.; Tavares, M.F.; Colli, W.; Alves, M.J. Down regulation of NO signaling in Trypanosoma cruzi upon parasite-extracellular matrix interaction: Changes in protein modification by nitrosylation and nitration. PLoS Negl. Trop. Dis. 2015, 9, e0003683. [Google Scholar] [CrossRef]
- Ju, Y.J.; Lee, H.W.; Choi, J.W.; Choi, M.S. The role of protein S-nitrosylation in protein misfolding-associated diseases. Life 2021, 11, 705. [Google Scholar] [CrossRef]
- Foroutan, M.; Zaki, L.; Ghaffarifar, F. Recent progress in microneme-based vaccines development against Toxoplasma gondii. Clin. Exp. Vaccine Res. 2018, 7, 93–103. [Google Scholar] [CrossRef]
- Soldati, D.; Dubremetz, J.F.; Lebrun, M. Microneme proteins: Structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int. J. Parasitol. 2001, 31, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Yuan, Z.G.; Peng, G.H.; Zhou, D.H.; He, X.H.; Yan, C.; Yin, C.C.; He, Y.; Lin, R.Q.; Song, H.Q.; et al. Toxoplasma gondii microneme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitol. Res. 2010, 106, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Chen, J.; Petersen, E.; Zhou, D.H.; Huang, S.Y.; Song, H.Q.; Zhu, X.Q. Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine 2014, 32, 3058–3065. [Google Scholar] [CrossRef]
- Liu, W.G.; Xu, X.P.; Chen, J.; Xu, Q.M.; Luo, S.L.; Zhu, X.Q. MIC16 gene represents a potential novel genetic marker for population genetic studies of Toxoplasma gondii. BMC Microbiol. 2016, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.Y.; Lin, A.; Shin, E.H.; Oh, M.D.; Han, E.T.; Nan, H.W.; Lee, S.H. Laboratory passage and characterization of an isolate of Toxoplasma gondii from an ocular patient in Korea. Korean J. Parasitol. 2003, 41, 147–154. [Google Scholar] [CrossRef]
- Couatarmanach, A.; Andre, P.; Le Minous, D.; Martin, L.; Robert, R.; Deunff, J. In Vitro culture and cloning of Toxoplasma gondii in a newly established cell line derived from TG180. Int. J. Parasitol. 1991, 21, 129–132. [Google Scholar] [CrossRef]
- Zheng, W.Q.; Zhang, Y.; Yao, Q.; Chen, Y.; Qiao, X.; Wang, E.D.; Chen, C.; Zhou, X.L. Nitrosative stress inhibits aminoacylation and editing activities of mitochondrial threonyl-tRNA synthetase by S-nitrosation. Nucleic Acids Res. 2020, 48, 6799–6810. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Z.; Gao, X.; Jin, C.; Wen, L.; Yao, X.; Ren, J. GPS-SNO: Computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PLoS ONE 2010, 5, e11290. [Google Scholar] [CrossRef]
- Zahid, S.; Khan, R.; Oellerich, M.; Ahmed, N.; Asif, A.R. Differential S-nitrosylation of proteins in Alzheimer’s disease. Neuroscience 2014, 256, 126–136. [Google Scholar] [CrossRef]
- Schwartz, D.; Gygi, S.P. An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat. Biotechnol. 2005, 23, 1391–1398. [Google Scholar] [CrossRef]
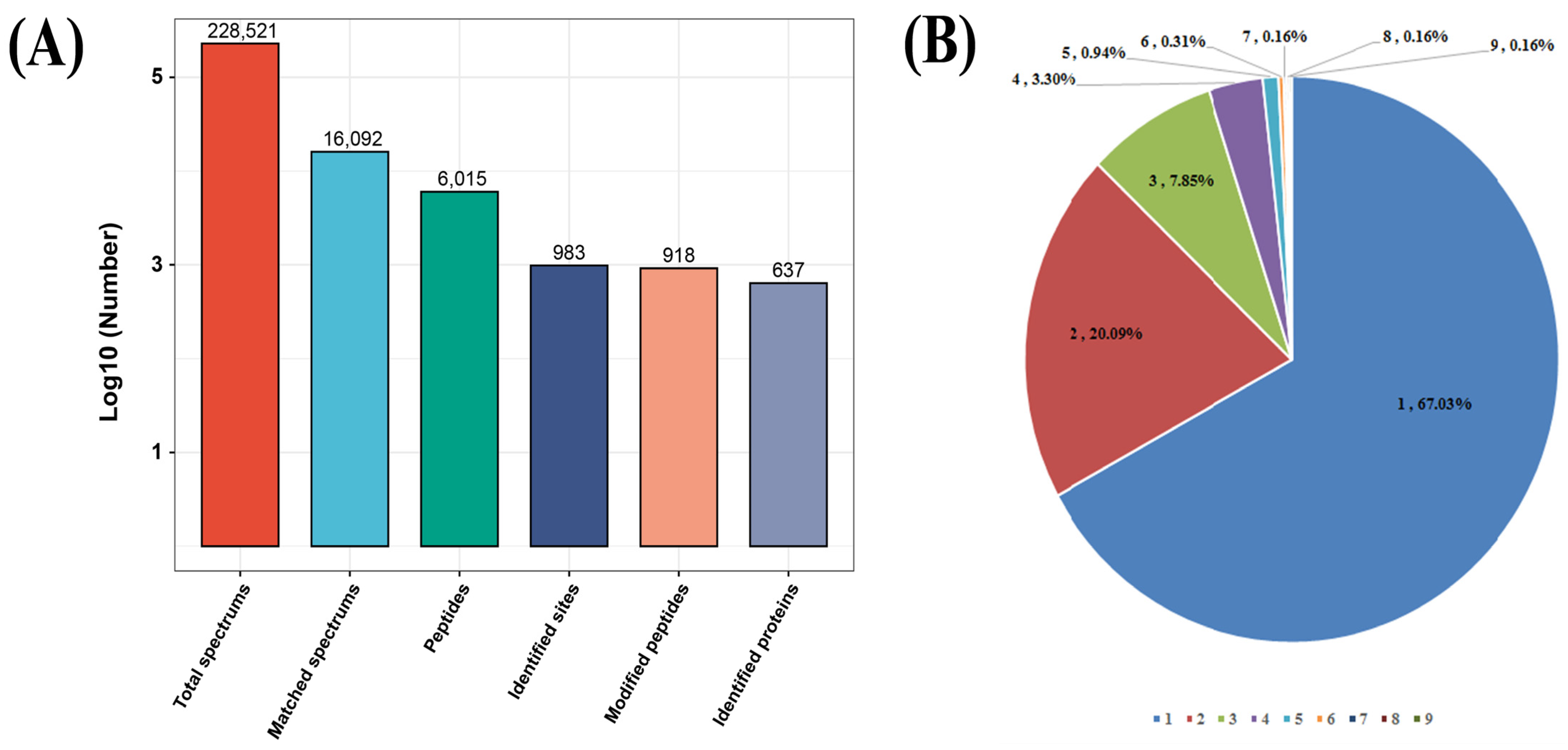
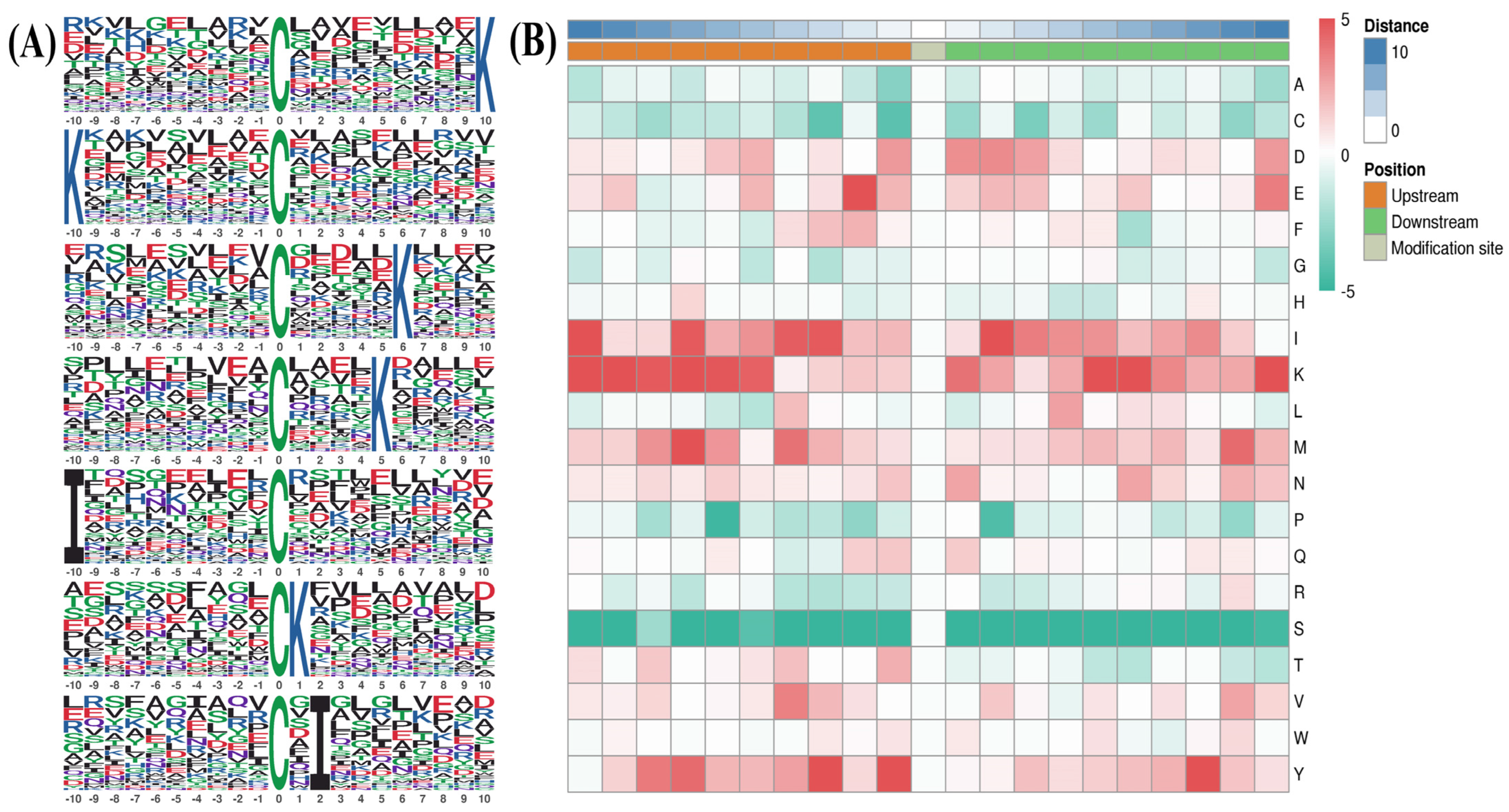
| No.* | Motif | Motif Score | Foreground | Background | Fold Increase | ||
|---|---|---|---|---|---|---|---|
| Matches | Size | Matches | Size | ||||
| 1. | xxxxxxxxxxCxxxxxxxxxK | 8.72 | 76 | 983 | 4810 | 131,270 | 2.1 |
| 2. | KxxxxxxxxxCxxxxxxxxxx | 8.42 | 72 | 907 | 4731 | 126,460 | 2.1 |
| 3. | xxxxxxxxxxCxxxxxKxxxx | 7.02 | 61 | 835 | 4251 | 121,729 | 2.1 |
| 4. | xxxxxxxxxxCxxxxKxxxxx | 7.20 | 58 | 774 | 4070 | 117,478 | 2.2 |
| 5. | IxxxxxxxxxCxxxxxxxxxx | 6.41 | 43 | 716 | 2897 | 113,408 | 2.4 |
| 6. | xxxxxxxxxxCKxxxxxxxxx | 6.15 | 41 | 673 | 2864 | 110,511 | 2.4 |
| 7. | xxxxxxxxxxCxIxxxxxxxx | 6.86 | 38 | 632 | 2475 | 107,647 | 2.6 |
| Gene Product | Gene ID | Number of S-Nitrosylated Site |
|---|---|---|
| ribosomal protein RPL3 | TGME49_227360 | 2 |
| ribosomal protein RPL4 | TGME49_309120 | 2 |
| ribosomal protein RPL7A | TGME49_261570 | 2 |
| ribosomal protein RPL8 | TGME49_204020 | 1 |
| ribosomal protein RPL9 | TGME49_284560 | 1 |
| ribosomal protein RPL10 | TGME49_288720 | 1 |
| ribosomal protein RPL10A | TGME49_215470 | 3 |
| ribosomal protein RPL14 | TGME49_267060 | 1 |
| ribosomal protein RPL17 | TGME49_299050 | 1 |
| ribosomal protein RPL21 | TGME49_245680 | 2 |
| ribosomal protein RPL22 | TGME49_239760 | 1 |
| ribosomal protein RPL23A | TGME49_238010 | 1 |
| ribosomal protein RPL24 | TGME49_244320 | 1 |
| ribosomal protein RPL28 | TGME49_229250 | 1 |
| ribosomal protein RPL30 | TGME49_232230 | 1 |
| ribosomal protein RPL35A | TGME49_249250 | 1 |
| ribosomal protein RPL37 | TGME49_239330 | 1 |
| ribosomal protein RPS2 | TGME49_305520 | 2 |
| ribosomal protein RPS3 | TGME49_232300 | 2 |
| ribosomal protein RPS3A | TGME49_232710 | 2 |
| ribosomal protein RPS4 | TGME49_207440 | 1 |
| ribosomal protein RPS5 | TGME49_242330 | 2 |
| ribosomal protein RPL10 | TGME49_288720 | 1 |
| ribosomal protein RPS11 | TGME49_226970 | 2 |
| ribosomal protein RPS12 | TGME49_205340 | 4 |
| ribosomal protein RPS15 | TGME49_213350 | 1 |
| ribosomal protein RPS15A | TGME49_234450 | 1 |
| ribosomal protein RPS20 | TGME49_223050 | 1 |
| Gene Product | Gene ID | Number of S-Nitrosylated Site |
|---|---|---|
| MIC1 | TGME49_291890 | 2 |
| MIC2 | TGME49_201780 | 4 |
| MIC3 | TGME49_319560 | 5 |
| MIC4 | TGME49_208030 | 3 |
| MIC6 | TGME49_218520 | 1 |
| MIC7 | TGME49_261780 | 2 |
| MIC8 | TGME49_245490 | 3 |
| MIC11 | TGME49_204530 | 1 |
| MIC13 | TGME49_260190 | 2 |
| MIC15 | TGME49_247195 | 2 |
| MIC17A | TGME49_200250 | 1 |
| ROP5 | TGME49_308090 | 1 |
| ROP9 | TGME49_243730 | 1 |
| ROP14 | TGME49_315220 | 1 |
| ROP15 | TGME49_211290 | 1 |
| ROP18 | TGME49_205250 | 1 |
| ROP33 | TGME49_201130 | 1 |
| RON2 | TGME49_300100 | 2 |
| RON3 | TGME49_223920 | 4 |
| RON4 | TGME49_229010 | 1 |
| RON5 | TGME49_311470 | 3 |
| GRA12 | TGME49_288650 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, J.; Yang, Q.; Sun, X. Global Proteome-Wide Analysis of Cysteine S-Nitrosylation in Toxoplasma gondii. Molecules 2023, 28, 7329. https://doi.org/10.3390/molecules28217329
Wang Z, Li J, Yang Q, Sun X. Global Proteome-Wide Analysis of Cysteine S-Nitrosylation in Toxoplasma gondii. Molecules. 2023; 28(21):7329. https://doi.org/10.3390/molecules28217329
Chicago/Turabian StyleWang, Zexiang, Jia Li, Qianqian Yang, and Xiaolin Sun. 2023. "Global Proteome-Wide Analysis of Cysteine S-Nitrosylation in Toxoplasma gondii" Molecules 28, no. 21: 7329. https://doi.org/10.3390/molecules28217329
APA StyleWang, Z., Li, J., Yang, Q., & Sun, X. (2023). Global Proteome-Wide Analysis of Cysteine S-Nitrosylation in Toxoplasma gondii. Molecules, 28(21), 7329. https://doi.org/10.3390/molecules28217329







