Pencil Graphite Electrocatalytic Sensors Modified by Pyrene Coated Reduced Graphene Oxide Decorated with Molybdenum Disulfide Nanoroses for Hydrazine and 4-Nitrophenol Detection in Real Water Samples
Abstract
:1. Introduction
2. Results and Discussion
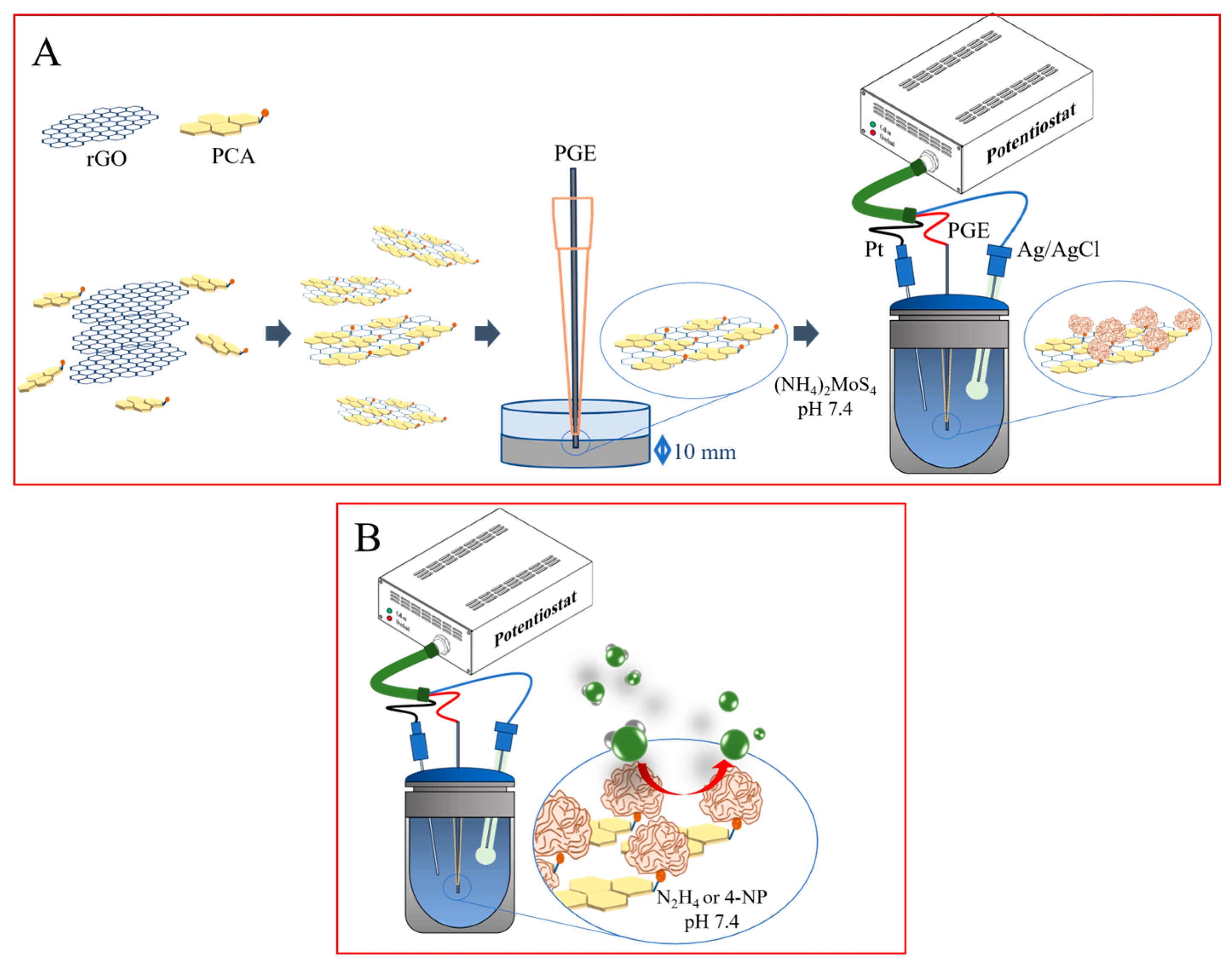
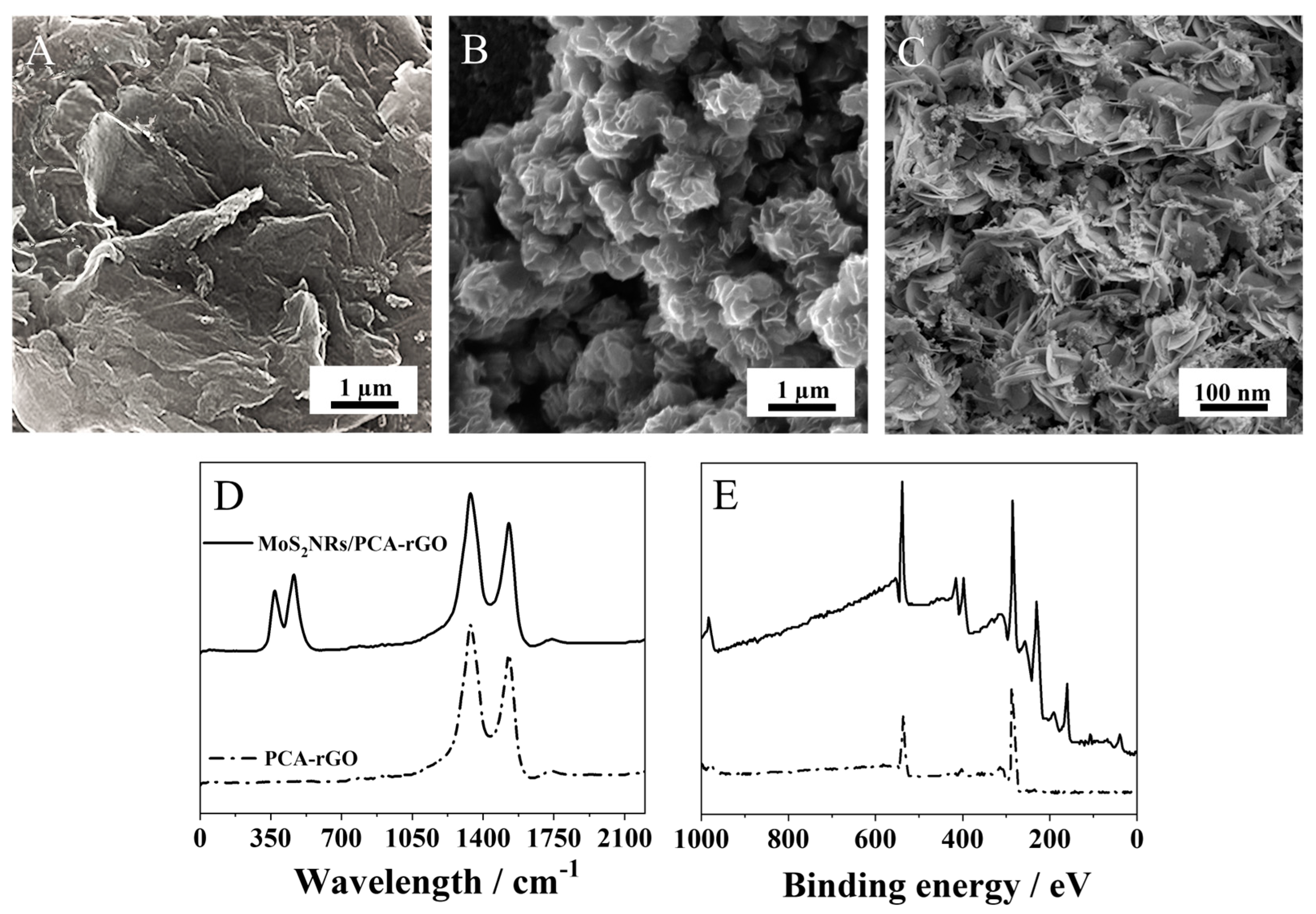
2.1. Decoration of the PCA-rGO/PGEs with MoS2 NRs and Characterization
2.2. Electroanalytical Investigation of the MoS2 NRs/PCA-rGO/PGEs
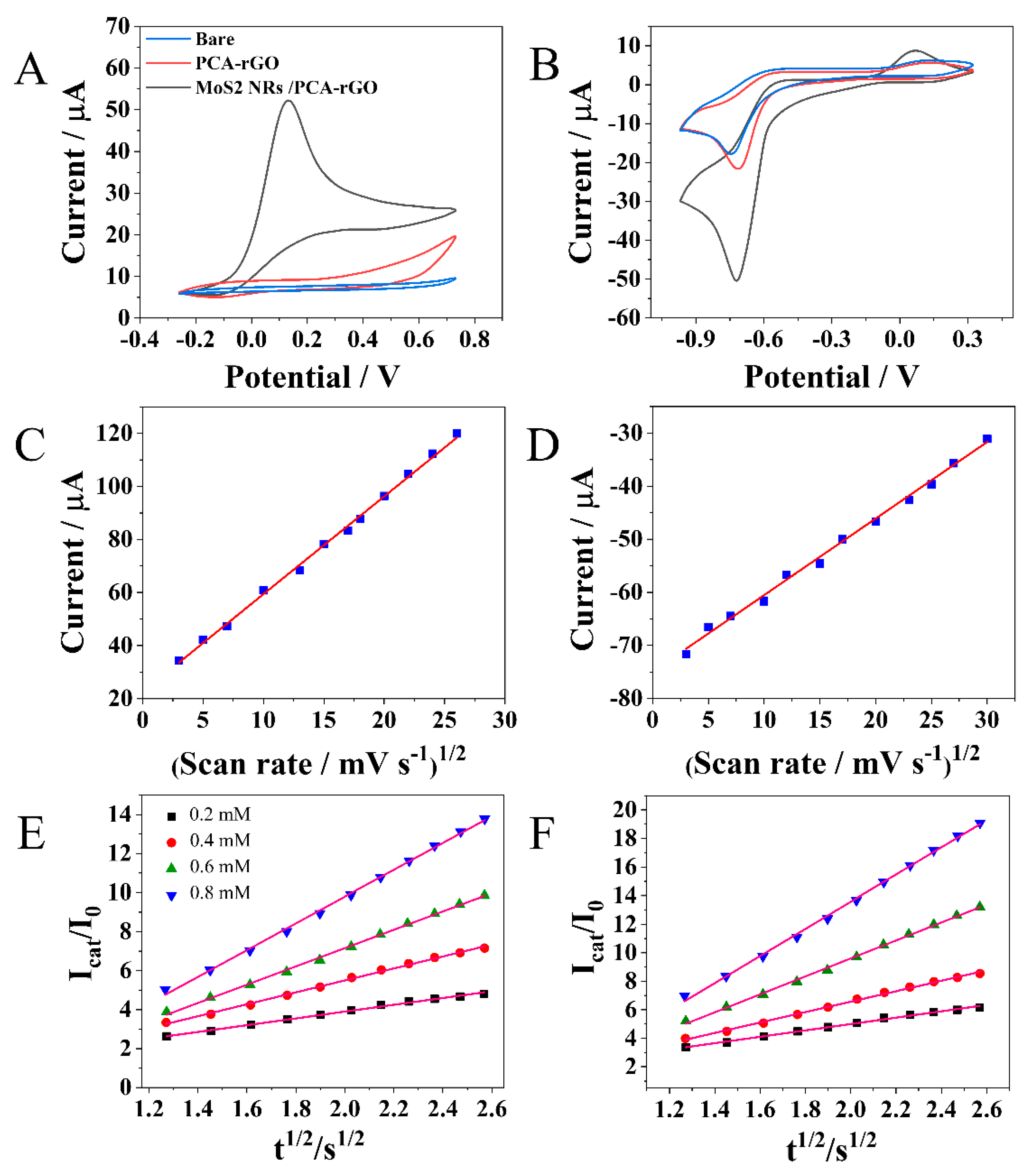
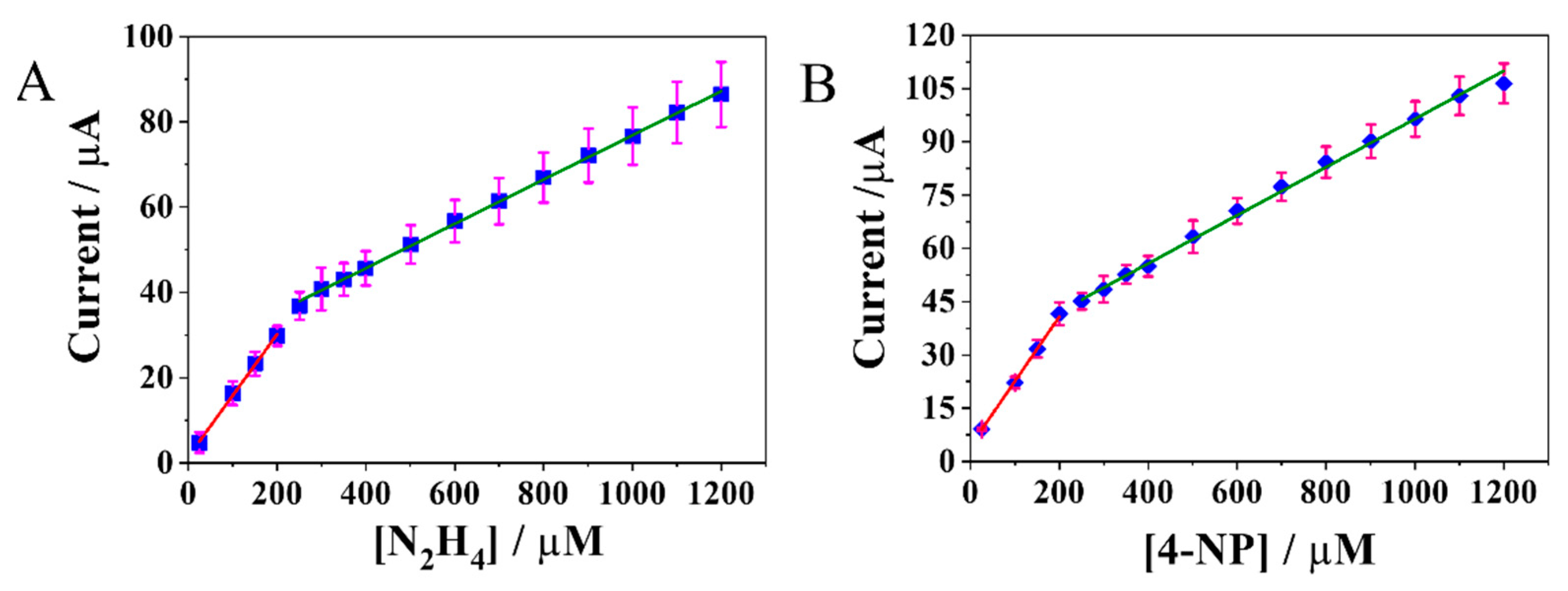
2.2.1. Electrochemical Detection of N2H4 and 4-NP at the MoS2NRs/PCA-rGO/PGEs
2.2.2. Determination of LOD, Repeatability, Reproducibility, and Storage Stability of MoS2NRs/PCA-rGO/PGEs and Interference Effects
2.2.3. Quantification of N2H4 and 4-NP in Real Samples
3. Materials and Methods
3.1. Reagents and Instrumentation
3.2. Exfoliation and Functionalization of Reduced Graphene Oxide (rGO) with 1-Pyrene Carboxylic Acid (PCA)
3.3. Modification of PGEs with PCA-rGO and Decoration with MoS2 Nanoroses
3.4. Electrochemical Investigation of the MoS2 NRs/PCA-rGO/PGEs
3.5. Electroanalytical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of Industries in Water Scarcity and its Adverse Effects on Environment and Human Health. In Environmental Concerns and Sustainable Development, 1st ed.; Shukla, V., Kumar, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Parris, K. Impact of Agriculture on Water Pollution in OECD Countries: Recent Trends and Future Prospects. Int. J. Water Resour. Dev. 2011, 27, 33–52. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Li, C. Theoretical Model and Actual Characteristics of Air Pollution Affecting Health Cost: A Review. Int. J. Environ. Res. Public Health 2022, 19, 3532. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Halden, R.U. Opportunities and limits of wastewater-based epidemiology for tracking global health and attainment of UN sustainable development goals. Environ. Int. 2022, 163, 107217. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Kamble, P.S.; Kamble, A.C. Health effects of water pollution. EPRA Int. J. Econ. Bus. Rev. 2022, 10, 45–50. [Google Scholar] [CrossRef]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Advances in Applications of Voltametric Sensors Modified with Ferrocene and Its Derivatives. ACS Omega 2020, 5, 2049. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, J.X.; Qin, D.G.; Chen, J.; Shan, D.L.; Lu, X.Q. An electrochemical sensor based on polyelectrolyte-functionalized graphene for detection of 4-nitrophenol. J. Electroanal. Chem. 2014, 734, 1–6. [Google Scholar] [CrossRef]
- Watt, G.W.; Chrisp, J.D. Spectrophotometric method for determination of hydrazine. Anal. Chem. 1952, 24, 2006–2008. [Google Scholar] [CrossRef]
- Lv, J.; Huang, J.; Zhang, Z. Determination of hydrazine by flow injection with chemiluminescence. Anal. Lett. 2001, 34, 1323–1330. [Google Scholar] [CrossRef]
- He, J.; Xu, X.; Li, M.; Zhou, S.; Zhou, W. Recent advances in perovskite oxides for non-enzymatic electrochemical sensors: A review. Anal. Chim. Acta 2023, 1251, 341007. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Bahrani, S.; Ramakrishna, S. Integrated polyaniline with graphene oxide-iron tungsten nitride nanoflakes as ultrasensitive electrochemical sensor for precise detection of 4-nitrophenol within aquatic media. J. Electroanal. Chem. 2020, 873, 114406. [Google Scholar] [CrossRef]
- Mphuthi, N.; Sikhwivhilu, L.; Ray, S.S. Functionalization of 2D MoS2 Nanosheets with Various Metal and Metal Oxide Nanostructures: Their Properties and Application in Electrochemical Sensors. Biosensors 2022, 12, 386–431. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lim, J.; Shin, M.; Lee, S.-N.; Choi, J.-W. Graphene/MoS2 Nanohybrid for Biosensors. Materials 2021, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, C.; Corricelli, M.; Disha, A.; Fanizza, E.; Bianco, G.V.; Depalo, N.; Panniello, A.; Agostiano, A.; Striccoli, M.; Curri, M.L. Solvent dispersible nanocomposite based on Reduced Graphene Oxide in situ decorated with gold nanoparticles. Carbon 2019, 152, 777–787. [Google Scholar] [CrossRef]
- Ingrosso, C.; Corricelli, M.; Bettazzi, F.; Konstantinidou, E.; Bianco, G.V.; Depalo, N.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Palchetti, I. Au nanoparticle in situ decorated RGO nanocomposites for highly sensitive electrochemical genosensors. J. Mater. Chem. B 2019, 7, 768–777. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Curcumin graphite pencil electrode modified with molybdenum disulfide nanosheets decorated gold foams for simultaneous quantification of nitrite and hydrazine in water samples. Anal. Chim. Acta 2020, 1137, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.S.; Thakur, N.; Thakur, S.; Singh, D. Electrochemical determination of hydrazine by using MoS2 nanostructure modified gold electrode. Nanofabrication 2021, 6, e002. [Google Scholar] [CrossRef]
- Gharani, M.; Bahari, A.; Ghasemi, S. Preparation of MoS2-reduced graphene oxide/Au nanohybrid for electrochemical sensing of hydrazine. J. Mater. Sci. Mater. Electron. 2021, 32, 7765–7777. [Google Scholar] [CrossRef]
- Jeyapragasam, T.; Devi, M.; Ganesh, V. Molybdenum disulfide-based modifier for electrochemical detection of 4-nitrophenol. Ionics 2018, 24, 4033–4041. [Google Scholar] [CrossRef]
- Nehru, R.; Kumar, B.S.; Chen, C.W.; Dong, C.D. Yolk-shell structured molybdenum disulfide nanospheres as highly enhanced electrocatalyst for electrochemical sensing of hazardous 4-nitrophenol in water. J. Environ. Chem. Eng. 2022, 10, 107663. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, W. Synthesis of MoS2/Graphene Hybrid for Electrochemical Detection and Catalytic Reduction of 4-Nitrophenol. Appl. Mech. Mater. 2017, 872, 149–154. [Google Scholar]
- Zhao, Y.; Kuai, L.; Liu, Y.; Wang, P.; Arandiyan, H.; Cao, S.; Zhang, J.; Li, F.; Wang, Q.; Geng, B.; et al. Well-Constructed Single-Layer Molybdenum Disulfide Nanorose Cross-Linked by Three Dimensional-Reduced Graphene Oxide Network for Superior Water Splitting and Lithium Storage Property. Sci. Rep. 2015, 5, 8722. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. J. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Gołasa, K.; Grzeszczyk, M.; Bożek, R.; Leszczyński, P.; Wysmołek, A.; Potemski, M.; Babiński, A. Resonant Raman scattering in MoS2—From bulk to monolayer. Solid State Commun. 2014, 197, 53–56. [Google Scholar] [CrossRef]
- Liu, G.; Thummavichai, K.; Lv, X.; Chen, W.; Lin, T.; Tan, S.; Zeng, M.; Chen, Y.; Wang, N.; Zhu, Y. Defect-Rich Heterogeneous MoS2/rGO/NiS Nanocomposite for Efficient pH-Universal Hydrogen Evolution. Nanomaterials 2021, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, V.; Testolin, A.; Ingrosso, C.; Curri, M.L.; Palchetti, I.; Falciola, L. Au Nanoparticles Decorated Graphene-Based Hybrid Nanocomposite for As(III) Electroanalytical Detection. Chemosensors 2022, 10, 67. [Google Scholar] [CrossRef]
- Ingrosso, C.; Petrella, A.; Curri, M.L.; Striccoli, M.; Cosma, P.; Cozzoli, P.D.; Agostiano, A. Photoelectrochemical properties of Zn(II) phthalocyanine/ZnO nanocrystals heterojunctions: Nanocrystal surface chemistry effect. Appl. Surf. Sci. 2005, 246, 367–371. [Google Scholar] [CrossRef]
- Su, Q.; Pang, S.; Alijani, V.; Li, C.; Feng, X.; Mullen, K. Composites of Graphene with Large Aromatic Molecules. Adv. Mater. 2009, 21, 3191–3195. [Google Scholar] [CrossRef]
- Pifferi, V.; Barsan, M.M.; Ghica, M.E.; Falciola, L.; Brett, C.M.A. Synthesis, characterization and influence of poly(brilliant green) on the performance of different electrode architectures based on carbon nanotubes and poly(3,4-ethylenedioxythiophene). Electrochim. Acta 2013, 98, 199–207. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Voltammetric simultaneous quantification of p-nitrophenol and hydrazine by using magnetic spinel FeCo2O4 nanosheets on reduced graphene oxide layers modified with curcumin-stabilized silver nanoparticles. Microchim. Acta 2019, 186, 561. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Compton, R.G. The Electro-Oxidation of Hydrazine: A Self-Inhibiting Reaction. J. Phys. Chem. Lett. 2021, 12, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Barman, K.; Changmai, B.; Jasimuddin, S. Electrochemical Detection of Para-nitrophenol using Copper Metal Nanoparticles Modified Gold Electrode. Electroanalysis 2017, 29, 2780–2787. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Reduced graphene oxide nanosheets modified with nickel disulfide and curcumin nanoparticles for non-enzymatic electrochemical sensing of methyl parathion and 4-nitrophenol. Microchim. Acta 2019, 186, 704. [Google Scholar] [CrossRef]
- An, X.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.K.; Talapatra, S.; Kar, S. Stable Aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fan, X.; Lu, Y.; Zhong, L.; Kong, X.; Wang, J. Preparation of an electrochemically modified graphite electrode and its electrochemical performance for pseudo-capacitors in a sulfuric acid electrolyte. Carbon 2010, 48, 3300–3303. [Google Scholar] [CrossRef]
- Ismail, W.N.W.; Mokhtar, S.U. Various Methods for Removal, Treatment, and Detection of Emerging Water Contaminants. In Emerging Contaminants; Nuro, A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Brunetti, B.; Desimoni, E. Voltammetric determination of vitamin B6 in food samples and dietary supplements. J. Food Compos. Anal. 2014, 33, 155–160. [Google Scholar] [CrossRef]
- Soni, R.; Soni, M.; Shukla, D.P. Emerging Techniques and Materials for Water Pollutants Detection. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Advanced Functional Materials and Sensors; Springer: Singapore, 2020. [Google Scholar]
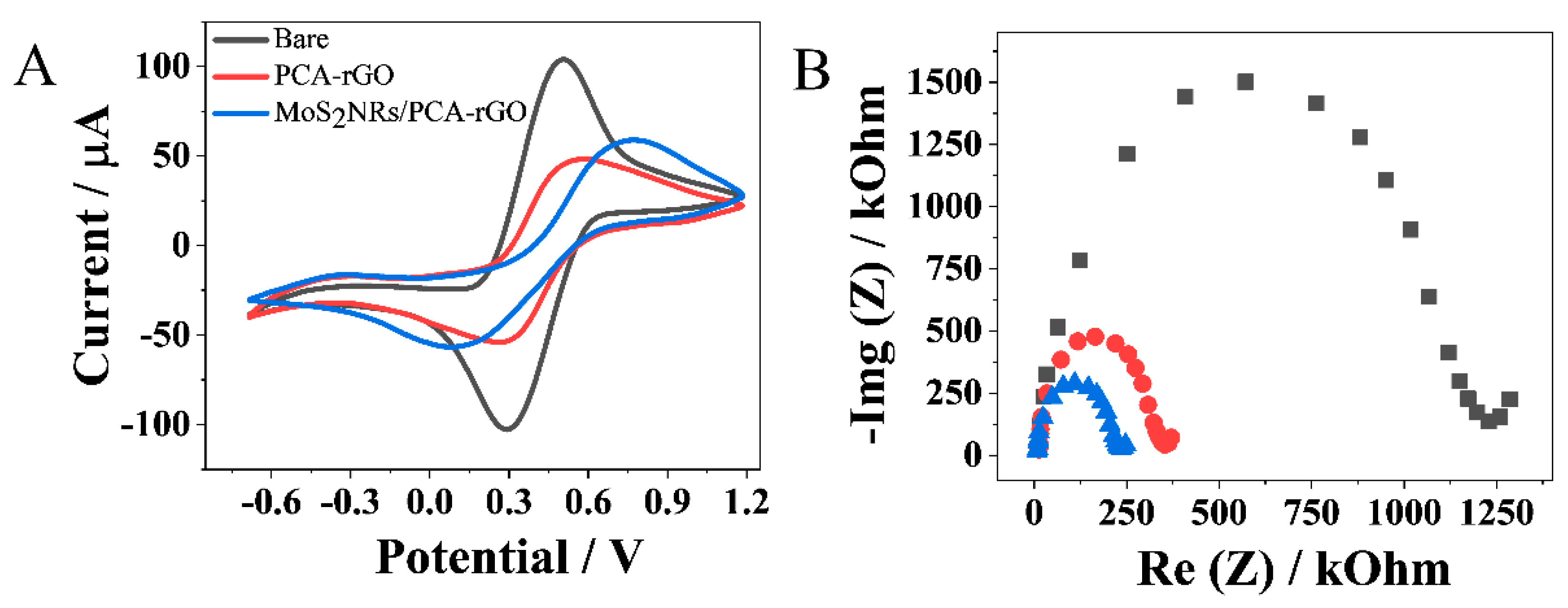
| Aele/mm2 | K0/cm s−1 | Ret/kOhm | |
|---|---|---|---|
| Bare PGE | 1.25 ± 0.03 | 0.004 ± 0.021 | 1193.0 ± 10.7 |
| PCA–rGO/PGE | 1.01 ± 0.02 | 0.006 ± 0.034 | 355.0 ± 4.9 |
| MoS2NRs/PCA-rGO/PGE | 2.19 ± 0.04 | 0.013 ± 0.042 | 237.0 ± 8.2 |
| N2H4 | 4-NP | |
|---|---|---|
| Sensitivities/mA mM−1 | 0.051 | 0.054 |
| LOD/nM | 9.3 | 13.7 |
| LOQ/nM | 30.51 | 45.34 |
| %RSD of repeatability | 3.3 | 3.6 |
| %RSD of reproducibility | 3.4 | 3.7 |
| Storage stability | Decrease by 9.1% | Decrease by 12.6% |
| Samples | Analytes | Standard Concentration (mM) | Concentration Determined by Chronoamperometry (mM) | Recovery Rate % Determined by Chronoamperometry | Concentration Determined by HPLC (mM) | Recovery Rate % Determined by HPLC |
|---|---|---|---|---|---|---|
| Tap water | N2H4 | 300 | 298.2 | 99.4 | 299.7 | 99.9 |
| 500 | 497.4 | 99.5 | 500.7 | 100.1 | ||
| 800 | 802.1 | 100.3 | 801.3 | 100.2 | ||
| River water | 400 | 397.2 | 99.3 | 400.7 | 100.2 | |
| 500 | 501.7 | 100.3 | 499.7 | 99.9 | ||
| 600 | 601.8 | 100.3 | 599.3 | 99.9 | ||
| Wastewater | 500 | 504.7 | 100.9 | 501.4 | 100.3 | |
| 700 | 702.9 | 100.4 | 703.1 | 100.4 | ||
| 900 | 912.1 | 101.3 | 907.3 | 100.8 | ||
| Tap water | 4-NP | 400 | 400.6 | 100.1 | 401.1 | 100.3 |
| 600 | 596.7 | 99.5 | 601.9 | 100.3 | ||
| 800 | 798.5 | 99.8 | 799.2 | 99.9 | ||
| River water | 400 | 400.3 | 100.1 | 398.6 | 99.6 | |
| 500 | 493.7 | 98.7 | 501.3 | 100.3 | ||
| 600 | 599.2 | 99.9 | 601.2 | 100.2 | ||
| Wastewater | 400 | 392.7 | 98.2 | 400.8 | 100.2 | |
| 700 | 704.2 | 100.6 | 701.2 | 100.1 | ||
| 1000 | 989.6 | 99.0 | 996.3 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejri, A.; Mandriota, G.; Hamza, E.; Curri, M.L.; Ingrosso, C.; Mars, A. Pencil Graphite Electrocatalytic Sensors Modified by Pyrene Coated Reduced Graphene Oxide Decorated with Molybdenum Disulfide Nanoroses for Hydrazine and 4-Nitrophenol Detection in Real Water Samples. Molecules 2023, 28, 7311. https://doi.org/10.3390/molecules28217311
Mejri A, Mandriota G, Hamza E, Curri ML, Ingrosso C, Mars A. Pencil Graphite Electrocatalytic Sensors Modified by Pyrene Coated Reduced Graphene Oxide Decorated with Molybdenum Disulfide Nanoroses for Hydrazine and 4-Nitrophenol Detection in Real Water Samples. Molecules. 2023; 28(21):7311. https://doi.org/10.3390/molecules28217311
Chicago/Turabian StyleMejri, Alma, Giacomo Mandriota, Elfil Hamza, Maria Lucia Curri, Chiara Ingrosso, and Abdelmoneim Mars. 2023. "Pencil Graphite Electrocatalytic Sensors Modified by Pyrene Coated Reduced Graphene Oxide Decorated with Molybdenum Disulfide Nanoroses for Hydrazine and 4-Nitrophenol Detection in Real Water Samples" Molecules 28, no. 21: 7311. https://doi.org/10.3390/molecules28217311
APA StyleMejri, A., Mandriota, G., Hamza, E., Curri, M. L., Ingrosso, C., & Mars, A. (2023). Pencil Graphite Electrocatalytic Sensors Modified by Pyrene Coated Reduced Graphene Oxide Decorated with Molybdenum Disulfide Nanoroses for Hydrazine and 4-Nitrophenol Detection in Real Water Samples. Molecules, 28(21), 7311. https://doi.org/10.3390/molecules28217311









