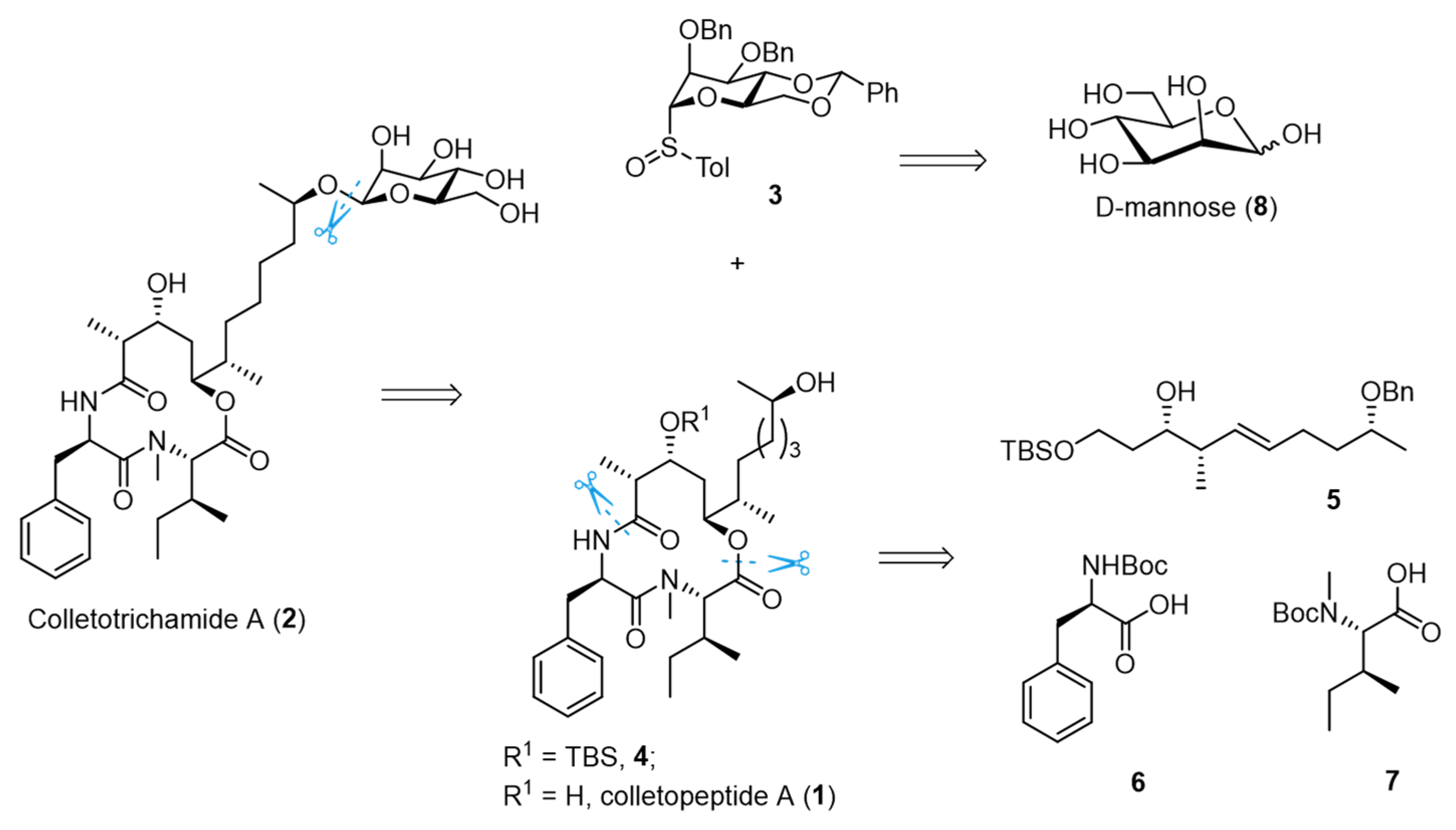
3.2. General Experimental Procedures
3.2.1. (2R,3R,4S,5S,6R)-2-(acetoxymethyl)-6-(p-tolylthio)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9)
To a solution of d-mannose (8) (5.0 g, 27.8 mmol, 1.0 equiv.) and Ac2O (19.7 mL, 208.5 mmol, 7.5 equiv.) in pyridine (80 mL, 0.35 M) at room temperature, DMAP (340 mg, 2.78 mmol, 0.1 equiv.) was added. The reaction mixture was stirred at room temperature for 13 h and then concentrated in vacuo, and the oily residue was redissolved in EtOAc (250 mL) and quenched with an HCl aqueous solution (50 mL, 1 N in H2O). The aqueous layer was extracted with EtOAc (3 × 30 mL) and the combined organic layers were washed with a saturated aqueous solution of NaHCO3 (50 mL) and brine (50 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. The residue was used directly in the next step without further purification.
To a solution of above crude acetate and TolSH (5.2 g, 41.7 mmol, 1.5 equiv.) in DCM (200 mL, 0.14 M) at 0 °C, BF3•OEt2 (10.3 mL, 83.4 mmol, 3.0 equiv.) was added. The mixture was stirred for 9 h at room temperature and then quenched with a saturated aqueous solution of NaHCO3 (50 mL). The aqueous layer was extracted with ethyl acetate (3 × 100 mL). The combined organic layers were washed with H2O (50 mL) and brine (50 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 1/1) to afford 9 (7.6 g, 60%) as a colorless oil. TLC: Rf = 0.4 (hexanes/EtOAc = 2/1), UV & PMA stain. 1H NMR (400 MHz, CDCl3) δ 7.54–7.29 (m, 2H), 7.18–6.97 (m, 2H), 5.46 (dd, J = 2.7, 1.6 Hz, 1H), 5.38 (d, J = 1.7 Hz, 1H), 5.32–5.10 (m, 2H), 4.77–4.45 (m, 1H), 4.26 (dd, J = 12.2, 6.0 Hz, 1H), 4.07 (dd, J = 12.3, 2.4 Hz, 1H), 2.29 (s, 3H), 2.11 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), 1.98 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.5, 169.8, 169.7, 169.7, 138.4, 132.6, 129.9, 128.8, 86.0, 70.9, 69.4, 69.4, 66.4, 62.5, 21.1, 20.8, 20.7, 20.7, 20.6.
3.2.2. (2R,3S,4S,5S,6R)-2-(hydroxymethyl)-6-(p-tolylthio)tetrahydro-2H-pyran-3,4,5-triol (10)
To a solution of acetate 9 (3.0 g, 6.6 mmol, 1.0 equiv.) in MeOH (50 mL, 0.13 M) at room temperature, NaOMe (357 mg, 6.6 mmol, 1.0 equiv.) was added. The reaction mixture was stirred at room temperature for 9 h and then quenched with a saturated aqueous solution of NH4Cl (20 mL). The aqueous layer was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with H2O (30 mL) and brine (30 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (MeOH/DCM = 1/10) to afford 10 (1.7 g, 91%) as a white solid. TLC: Rf = 0.4 (MeOH/DCM = 1/10), UV & PMA stain. 1H NMR (400 MHz, MeOD) δ 7.41 (d, J = 8.2 Hz, 2H), 7.13 (d, J = 7.9 Hz, 2H), 5.35 (d, J = 1.6 Hz, 1H), 4.10–4.04 (m, 1H), 4.03 (dd, J = 5.6, 2.9 Hz, 1H), 3.86–3.73 (m, 2H), 3.75–3.63 (m, 2H), 2.31 (s, 3H). 13C NMR (101 MHz, MeOD) δ 138.9, 133.5, 132.1, 130.7, 90.8, 75.5, 73.7, 73.1, 68.7, 62.6, 21.1.
3.2.3. (2R,4aR,6R,7S,8S,8aR)-7,8-bis(benzyloxy)-2-phenyl-6-(p-tolylthio)hexahydropyrano [3,2-d][1,3]dioxine (12)
To a solution of tetra-ol 10 (1.0 g, 3.5 mmol, 1.0 equiv.) and benzaldehyde dimethyl acetal (0.56 mL, 3.7 mmol, 1.05 equiv.) in MeCN (50 mL, 0.07 M) at room temperature, camphorsulfonic acid (204 mg, 0.88 mmol, 0.25 equiv.) was added. The reaction mixture was heated to reflux under Argon and stirred for 3 h. After being cooled back to room temperature, the reaction mixture was quenched with a saturated aqueous solution of NaHCO3 (20 mL). The aqueous layer was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with H2O (30 mL) and brine (30 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. The residue was used directly in the next step without further purification.
To a suspension of NaH (308 mg, 7.7 mmol, 60% dispersion in mineral oil, 2.2 equiv.) in DMF (50 mL, 0.07 M) at 0 °C, a solution of the above crude diol in DMF (10 mL) was added. A total of 30 min later, BnBr (0.92 mL, 7.7 mmol, 2.2 equiv.) was slowly added at 0 °C. The reaction mixture was warmed up to ambient temperature, stirred for 6 h, and then quenched with a saturated aqueous solution of NH4Cl (30 mL). The aqueous layer was extracted with ethyl acetate (3 × 50 mL) and the combined organic layers were washed with H2O (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 6/1) to afford 12 (1.47 g, 76%) as a white solid. TLC: Rf = 0.5 (hexanes/EtOAc = 6/1), UV & PMA stain. 1H NMR (400 MHz, CDCl3) δ 7.65–7.51 (m, 2H), 7.47–7.29 (m, 15H), 7.19–7.05 (m, 2H), 5.69 (s, 1H), 5.49 (t, J = 1.5 Hz, 1H), 4.87 (dd, J = 12.2, 2.0 Hz, 1H), 4.76 (d, J = 1.9 Hz, 2H), 4.70 (dd, J = 12.3, 2.0 Hz, 1H), 4.36 (qd, J = 6.3, 4.1 Hz, 2H), 4.27 (dd, J = 10.1, 3.6 Hz, 1H), 4.08 (dt, J = 2.9, 1.5 Hz, 1H), 4.03 (dt, J = 9.5, 3.0 Hz, 1H), 3.93 (td, J = 10.1, 8.8, 3.1 Hz, 1H), 2.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 138.5, 138.0, 137.9, 137.7, 132.4, 130.0, 130.0, 129.0, 128.5, 128.5, 128.3, 128.2, 127.9, 127.7, 127.7, 126.2, 101.6, 87.5, 79.2, 78.1, 76.3, 73.2, 73.1, 68.6, 65.5, 21.2. HRMS (ESI) calculated for C34H35O5S+ [M + H]+ 555.2205 found 555.2230.
3.2.4. (2R,4aR,6R,7S,8S,8aR)-7,8-bis(benzyloxy)-2-phenyl-6-(p-tolylsulfinyl)hexahydropyrano[3,2-d][1,3]dioxine (3)
To a solution of thioether 12 (800 mg, 1.4 mmol, 1.0 equiv.) in DCM (30 mL, 0.05M) at –78 °C, m-CPBA (259 mg, 1.5 mmol, 1.1 equiv.) in DCM (5 mL) was added dropwise. The reaction mixture was warmed up to –35 °C, stirred for 2 h, and then quenched with a saturated aqueous solution of NaHCO3 (15 mL) and Na2S2O3 (15 mL). The aqueous layer was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with H2O (20 mL) and brine (20 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 3/1) to afford 3 (695 mg, 87%) as a white solid. TLC: Rf = 0.4 (hexanes/EtOAc = 3/1), UV & PMA stain. 1H NMR (400 MHz, CDCl3) δ 7.52–7.46 (m, 2H), 7.44–7.31 (m, 12H), 7.31–7.18 (m, 5H), 5.61 (s, 1H), 4.81 (d, J = 12.1 Hz, 1H), 4.66 (d, J = 12.1 Hz, 1H), 4.58 (s, 2H), 4.47 (d, J = 1.2 Hz, 1H), 4.40 (dd, J = 3.0, 1.4 Hz, 1H), 4.37–4.25 (m, 2H), 4.19 (dd, J = 10.2, 4.8 Hz, 1H), 4.09 (ddd, J = 13.2, 6.9, 3.1 Hz, 1H), 3.73 (t, J = 10.1 Hz, 1H), 2.41 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 142.2, 138.3, 138.2, 137.4, 137.3, 130.2, 129.0, 128.4, 128.3, 128.3, 128.2, 127.9, 127.8, 127.6, 126.1, 124.4, 101.6, 97.5, 78.1, 76.3, 73.4, 73.2, 72.8, 70.1, 68.2, 21.6. HRMS (ESI) calculated for C34H35O6S+ [M + H]+ 571.2154 found 571.2140.
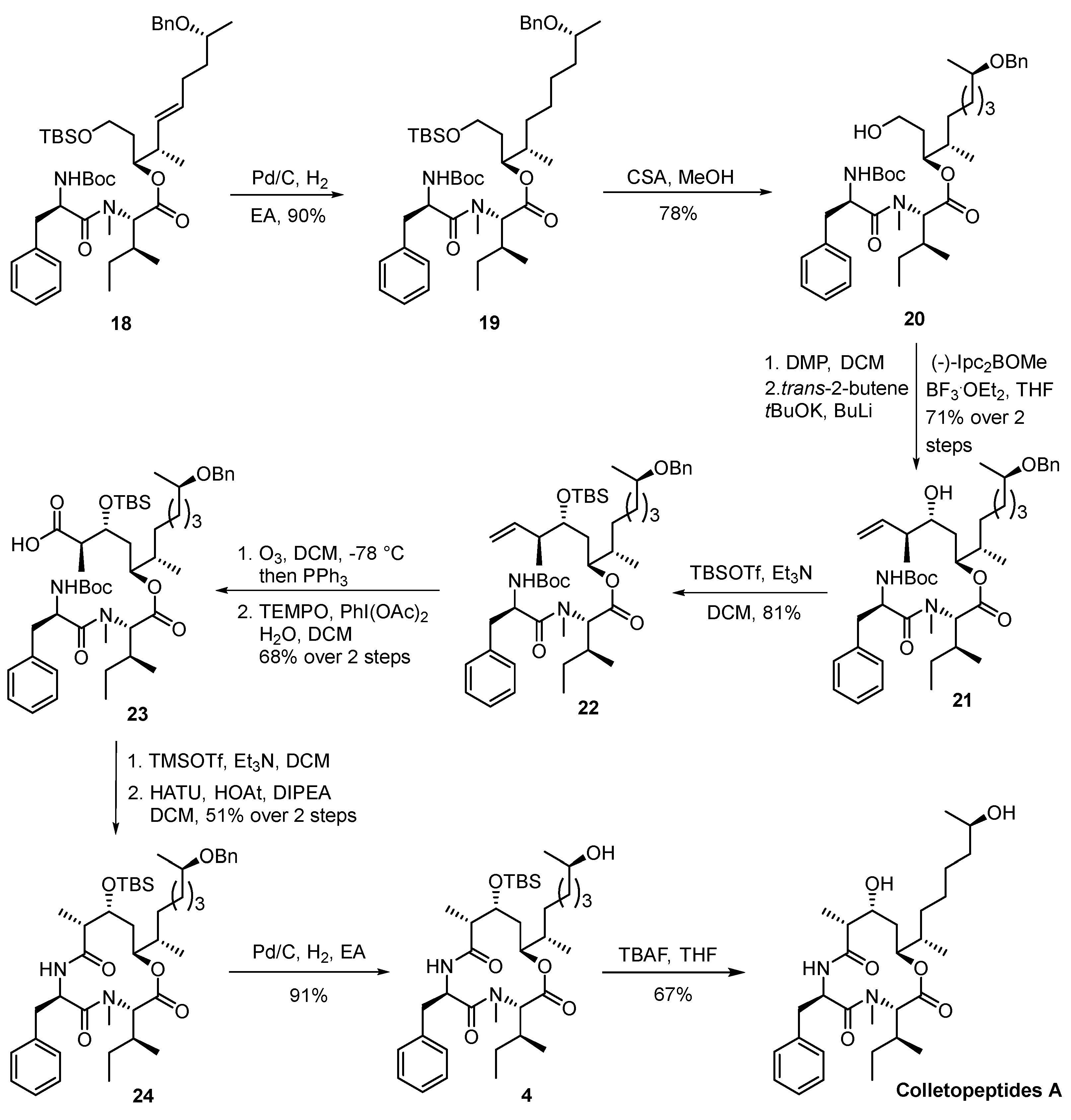
3.2.5. (3S,4S,9R,E)-9-(benzyloxy)-1-((tert-butyldimethylsilyl)oxy)-4-methyldec-5-en-3-ol (5)
To a solution of 14 (500 mg, 2.0 mmol, 1.0 equiv.) and alkene 13 (570 mg, 3.0 mmol, 1.5 equiv.) in DCM (40 mL, 0.05 M) at room temperature, the Grubbs II catalyst (340 mg, 0.4 mmol, 0.2 equiv.) was added. The reaction mixture was heated at 60 °C for 6 h before it was cooled back to room temperature and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 3/1) to afford 5 (658 mg, 81%) as a colorless oil. TLC: Rf = 0.3 (hexanes/EtOAc = 3/1), UV & PMA stain. = −17.7 (c 1.2, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.37–7.29 (m, 4H), 7.30–7.23 (m, 1H), 5.50–5.40 (m, 1H), 5.38–5.29 (m, 1H), 4.57 (d, J = 11.8 Hz, 1H), 4.44 (d, J = 11.7 Hz, 1H), 3.88 (dt, J = 9.9, 4.9 Hz, 1H), 3.78 (ddd, J = 10.0, 8.7, 3.9 Hz, 1H), 3.59 (ddd, J = 8.9, 6.4, 2.1 Hz, 1H), 3.51 (dt, J = 12.2, 6.2 Hz, 1H), 3.33–3.21 (m, 1H), 2.24–2.14 (m, 1H), 2.17–2.03 (m, 2H), 1.75–1.62 (m, 2H), 1.65–1.52 (m, 1H), 1.55–1.44 (m, 1H), 1.19 (d, J = 6.1 Hz, 3H), 1.02 (d, J = 6.8 Hz, 3H), 0.90 (s, 9H), 0.08 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 139.3, 133.0, 130.7, 128.4, 127.8, 127.5, 75.7, 74.5, 70.5, 63.0, 43.2, 36.7, 35.9, 28.9, 26.0, 19.7, 18.3, 16.2, −5.4, −5.4. HRMS (ESI) calculated for C24H43O3Si+ [M + H]+ 407.2981 found 407.2976.
3.2.6. (3S,4S,9R,E)-9-(benzyloxy)-1-((tert-butyldimethylsilyl)oxy)-4-methyldec-5-en-3-yl N-(tert-butoxycarbonyl)-N-methyl-l-isoleucinate (16)
To a solution of alcohol 5 (300 mg, 0.74 mmol, 1.0 equiv.), acid 7 (272 mg, 1.11 mmol, 1.5 equiv.) and Et3N (0.41 mL, 2.96 mmol, 4.0 equiv.) in anhydrous PhMe (15 mL, 0.05 M) at 0 °C under an argon atmosphere was added to a solution of TCBC (0.35 mL, 2.22 mmol, 3.0 equiv.) in anhydrous PhMe (5 mL). The reaction mixture was stirred at room temperature for 30 min, and then a solution of DMAP (542 mg, 4.44 mmol, 6.0 equiv.) in PhMe (5 mL) was added. After being stirred at room temperature for 9 h, the reaction mixture was quenched with a saturated aqueous solution of NH4Cl (10 mL) and extracted with EtOAc (3 × 20 mL). The combined organic layers were washed with a saturated aqueous solution of brine (20 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica gel (hexanes/EtOAc = 6/1) to afford 16 (356 mg, 76%) as a colorless oil. TLC: Rf = 0.4 (hexanes/EtOAc = 6/1), UV & PMA stain. = −51.8 (c 0.75, CHCl3). 1H NMR (500 MHz, CDCl3) (mixture of conformers) δ 7.45–7.29 (m, 4H), 7.26 (dt, J = 7.5, 2.8 Hz, 1H), 5.55–5.37 (m, 1H), 5.32 (dd, J = 15.4, 7.5 Hz, 1H), 4.96–4.83 (m, 1H), 4.56 (d, J = 11.8 Hz, 1H), 4.51 (d, J = 8.0 Hz, 0.5H), 4.43 (d, J = 11.8 Hz, 1H), 4.27 (d, J = 10.7 Hz, 0.5H), 3.68–3.54 (m, 1H), 3.57–3.45 (m, 2H), 2.80 (d, J = 16.3 Hz, 3H), 2.38 (ddp, J = 22.6, 14.1, 6.9 Hz, 1H), 2.23–2.03 (m, 2H), 2.01–1.92 (m, 1H), 1.82–1.73 (m, 1H), 1.67 (dddd, J = 16.5, 9.3, 4.4, 1.7 Hz, 2H), 1.45 (s, 11H), 1.19 (dd, J = 6.4, 2.2 Hz, 3H), 1.07 (ddd, J = 13.8, 8.9, 7.1 Hz, 1H), 0.99–0.94 (m, 3H), 0.93–0.90 (m, 3H), 0.89–0.85 (m, 12H), 0.02 (s, 6H). 13C- NMR (125 MHz, CDCl3) (mixture of conformers) δ 171.1, 170.6, 156.1, 155.6, 139.2, 133.7, 131.5, 131.2, 128.4, 128.0, 127.6, 127. 5, 80.1, 79.9, 75.1, 74.4, 70.4, 63.5, 62.3, 59.8, 59.5, 59.5, 40.5, 40.3, 39.7, 36.6, 34.7, 34.6, 33.3, 33.2, 30.2, 29.9, 28.8, 28.5, 26.0, 26.0, 24.9, 19.7, 19.5, 18.3, 18.3, 16.2, 16.0, 10.8, 10.3, −5.2, −5.3. HRMS (ESI) calculated for C36H64NO6Si+ [M + H]+ 634.4503 found 634.4531.
3.2.7. (3S,4S,9R,E)-9-(benzyloxy)-1-((tert-butyldimethylsilyl)oxy)-4-methyldec-5-en-3-yl N-((tert-butoxycarbonyl)-d-phenylalanyl)-N-methyl-l-isoleucinate (18)
To a solution of 16 (300 mg, 0.47 mmol, 1.0 equiv.) in DCM (10 mL, 0.047 M), Et3N (0.46 mL, 3.29 mmol, 7.0 equiv.) at 0 °C was added, followed by the dropwise addition of trimethylsilyl trifluoromethanesulfonate (0.43 mL, 2.35 mmol, 5.0 equiv.). The reaction mixture was stirred for 3 h at room temperature and then quenched with a saturated aqueous solution of NaHCO3 (10 mL) and extracted with EtOAc (3 × 20 mL). The combined organic layers were washed with a saturated aqueous solution of NH4Cl (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. The residue was used directly in the next step without further purification.
To a solution of crude amine (300 mg, 0.47 mmol, 1.0 equiv.) in DCM (10 mL, 0.047 M) at 0 °C, Boc-d-Phe-OH 6 was added (188 mg, 0.71 mmol, 1.5 equiv.), followed by an addition of DIPEA (0.25 mL, 1.41 mmol, 3.0 equiv.), HATU (357 mg, 0.94 mmol, 2.0 equiv.), and HOAt (97 mg, 0.71 mmol, 1.5 equiv.). The reaction mixture was stirred for 9 h at room temperature and then concentrated in vacuo, where the residue was redissolved in EtOAc (30 mL) and quenched with a 4% aqueous citric acid solution. The aqueous layer was extracted with EtOAc (3 × 20 mL) and the combined organic layers were washed with a saturated aqueous solution of NaHCO3 (15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica gel (hexanes/EtOAc = 6/1) to afford 18 (224 mg, 61%) as a colorless oil. TLC: Rf = 0.4 (hexanes/EtOAc = 6/1), UV & PMA stain. = −46.5 (c 3.0, CHCl3). 1H NMR (400 MHz, CDCl3) (mixture of conformers) δ 7.30 (dd, J = 5.6, 2.6 Hz, 4H), 7.26–7.12 (m, 6H), 5.38 (dt, J = 16.3, 6.6 Hz, 1H), 5.28 (dd, J = 13.7, 8.1 Hz, 1H), 5.17 (dd, J = 9.3, 3.4 Hz, 0.5H), 5.01 (dd, J = 11.6, 3.5 Hz, 0.5H), 4.89 (qd, J = 8.8, 7.4, 4.6 Hz, 1H), 4.77 (d, J = 10.7 Hz, 1H), 4.53 (d, J = 11.7 Hz, 1H), 4.41 (d, J = 11.8 Hz, 1H), 3.55 (ddd, J = 10.9, 6.9, 4.4 Hz, 1H), 3.51–3.40 (m, 2H), 3.03–2.89 (m, 2H), 2.85 (d, J = 11.9 Hz, 3H), 2.34 (q, J = 6.6 Hz, 1H), 2.05 (ddt, J = 16.5, 13.4, 5.7 Hz, 2H), 1.81–1.74 (m, 1H), 1.63 (dddd, J = 12.6, 9.3, 6.2, 1.4 Hz, 2H), 1.50–1.41 (m, 1H), 1.39–1.26 (m, 10H), 1.16 (dd, J = 6.2, 3.2 Hz, 3H), 0.98–0.80 (m, 17H), 0.73 (d, J = 3.6 Hz, 2H), 0.00–0.06 (m, 6H). 13C NMR (100 MHz, CDCl3) (mixture of conformers) δ 172.9, 172.6, 170.5, 155.4, 154.9, 154.8, 139.2, 139.1, 136.7, 136.4, 131.6, 131.4, 131.3, 131.2, 131.1, 129.6, 129.5, 128.5, 128.4, 128.4, 127.7, 127.6, 127.6, 127.5, 126.9, 126.9, 126.7, 79.7, 76.1, 75.5, 74.4, 74.3, 65.4, 60.9, 60.5, 59.7, 59.4, 51.9, 51.8, 51.3, 40.2, 40.1, 39.2, 38.9, 36.5, 34.7, 34.4, 34.0, 33.8, 33.4, 31.3, 29.3, 28.8, 28.4, 28.3, 26.3, 26.0, 26.0, 25.1, 24.4, 19.7, 19.5, 18.3, 18.3, 16.0, 16.0, 14.8, 11.3, 10.8, −5.3, −5.3, −5.4. HRMS (ESI) calculated for C45H73N2O7Si+ [M + H]+ 781.5187 found 781.5182.
3.2.8. (3S,4S,9R)-9-(benzyloxy)-1-((tert-butyldimethylsilyl)oxy)-4-methyldecan-3-yl N-((tert-butoxycarbonyl)-d-phenylalanyl)-N-methyl-l-isoleucinate (19)
To a solution of 18 (300 mg, 0.38 mmol, 1.0 equiv.) in EtOAc (5 mL, 0.076 M), palladium was added on charcoal (30 mg, 10% Pd, 0.013 mmol, 0.034 equiv.) under an argon atmosphere. The reaction flask was evacuated and purged with hydrogen three times. The reaction mixture was stirred under a hydrogen atmosphere at room temperature for 4 h, where the flask was then evacuated and purged with nitrogen three times and the catalyst was removed via filtration using a pad of celite. The filtrate was concentrated under reduced pressure to afford the crude product, which was purified via flash chromatography on silica gel (hexanes/EtOAc = 6/1) to afford 19 (268 mg, 90%) as a colorless oil. TLC: Rf = 0.4 (hexanes/EtOAc = 6/1), UV & PMA stain. = −43.2 (c 1.1, CDCl3). 1H NMR (500 MHz, CDCl3) (mixture of conformers) δ 7.32 (dd, J = 7.3, 3.2 Hz, 4H), 7.29–7.11 (m, 6H), 5.29 (d, J = 9.0 Hz, 0.5H), 5.21 (d, J = 9.2 Hz, 0.5H), 5.01 (dq, J = 8.6, 5.1, 4.5 Hz, 1H), 4.91 (td, J = 8.5, 6.0 Hz, 0.5H), 4.87–4.75 (m, 0.5H), 4.55 (d, J = 11.8 Hz, 1H), 4.44 (d, J = 11.8 Hz, 1H), 3.59 (dt, J = 10.9, 5.6 Hz, 1H), 3.52–3.36 (m, 2H), 3.05–2.93 (m, 2H), 2.88 (d, J = 22.2 Hz, 3H), 1.78–1.62 (m, 3H), 1.57 (tt, J = 6.2, 3.4 Hz, 1H), 1.39 (s, 6H), 1.36–1.25 (m, 9H), 1.24–1.07 (m, 4H), 1.01–0.79 (m, 18H), 0.76 (d, J = 4.0 Hz, 2H), 0.14–0.30 (m, 6H). 13C NMR (125 MHz, CDCl3) (mixture of conformers) δ 172.7, 170.6, 154.9, 139.3, 136.5, 129.6, 129.5, 128.5, 128.3, 127.6, 127.4, 127.4, 126.8, 126.7, 79.6, 75.9, 75.3, 74.9, 74.9, 70.4, 65.4, 60.9, 60.7, 59.8, 59.6, 53.5, 51.9, 51.2, 40.1, 36.7, 36.4, 34.7, 34.3, 34.1, 33.4, 33.1, 33.0, 32.8, 31.3, 29.3, 28.4, 28.3, 27.4, 26.0, 25.9, 25.8, 25.1, 19.7, 19.7, 18.2, 15.8, 15.8, 14.7, 14.6, 11.3, 10.8, −5.3, −5.4. HRMS (ESI) calculated for C45H75N2O7Si+ [M + H]+ 783.5344 found 783.5338.
3.2.9. (3S,4S,9R)-9-(benzyloxy)-1-hydroxy-4-methyldecan-3-yl N-((tert-butoxycarbonyl)-d-phenylalanyl)-N-methyl-l-isoleucinate (20)
To a solution of 19 (250 mg, 0.32 mmol, 1.0 equiv.) in MeOH (5 mL, 0.064 M) at 0 °C, CSA (15 mg, 0.064 mmol, 0.2 equiv.) was added. The reaction mixture was stirred for 3 h at room temperature and then concentrated in vacuo, and the oily residue was redissolved in EtOAc (30 mL) and washed with a saturated aqueous solution of NaHCO3 (15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica gel (hexanes/EtOAc = 3/1) to afford 20 (167 mg, 78%) as a colorless oil. TLC: Rf = 0.4 (hexanes/EtOAc = 3/1), UV & PMA stain. = −52.5 (c 2.9, CHCl3). 1H NMR (500 MHz, CDCl3) (mixture of conformers) δ 7.37–7.28 (m, 4H), 7.28–7.12 (m, 6H), 5.36 (d, J = 9.0 Hz, 1H), 5.07–4.92 (m, 1H), 4.87 (q, J = 8.0 Hz, 1H), 4.82 (d, J = 10.9 Hz, 1H), 4.53 (d, J = 11.8 Hz, 1H), 4.42 (d, J = 11.8 Hz, 1H), 3.52 (dt, J = 10.9, 5.4 Hz, 1H), 3.46 (ddt, J = 12.2, 8.7, 4.0 Hz, 2H), 3.03–2.89 (m, 2H), 2.88 (d, J = 9.4 Hz, 3H), 1.84 (q, J = 8.4 Hz, 1H), 1.71 (h, J = 4.8, 4.0 Hz, 2H), 1.65–1.50 (m, 2H), 1.38 (s, 9H), 1.33 (d, J = 10.3 Hz, 6H), 1.22–1.06 (m, 4H), 0.95–0.78 (m, 9H), 0.77–0.70 (m, 2H).13C NMR (125 MHz, CDCl3) (mixture of conformers) δ 172.9, 171.1, 155.3, 139.1, 136.3, 129.5, 129.5, 129.4, 128.5, 128.4, 128.3, 127.6, 127.6, 127.5, 127.3, 126.8, 126.6, 79.9, 75.5, 74.8, 74.8, 70.3, 65.1, 60.8, 58.9, 58.6, 51.9, 51.2, 39.4, 39.0, 36.7, 36.6, 34.6, 34.2, 33.2, 32.8, 32.7, 30.9, 29.2, 28.3, 28.3, 27.3, 27.2, 26.3, 25.7, 24.7, 19.6, 15.7, 14.8, 14.6, 10.6. HRMS (ESI) calculated for C39H61N2O7+ [M + H]+ 669.4479 found 669.4483.
3.2.10. (3S,4R,6S,7S,12R)-12-(benzyloxy)-4-hydroxy-3,7-dimethyltridec-1-en-6-yl N-((tert-butoxycarbonyl)-d-phenylalanyl)-N-methyl-l-isoleucinate (21)
To a solution of alcohol 20 (235 mg, 0.35 mmol, 1.0 equiv.) in dry DCM (10 mL, 0.035 M) at 0 °C under argon atmosphere, Dess–Martin periodinane was added (225 mg, 0.53 mmol, 1.5 equiv.). After being stirred at room temperature for 4 h, the reaction mixture was quenched with a saturated aqueous solution of Na2SO3 (10 mL) and extracted with EtOAc (3 × 20 mL). The combined organic extracts were washed with a saturated aqueous solution of copper sulfate (15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. The residue was used directly in the next step without further purification.
To a suspension of tBuOK (196 mg, 1.75 mmol, 5.0 equiv.) in anhydrous THF (10 mL, 0.035 M) at −78 °C, trans-2-butene was added (137 mg, 2.45 mmol, 7.0 equiv.), followed by the dropwise addition of nBuLi (1.1 mL, 1.75 mmol, 1.6 M in hexane, 5.0 equiv.). After the addition of nBuLi, the reaction mixture was warmed to −45 °C and stirred for 30 min. After being recooled to −78 °C, a solution of (-)-Ipc2BOMe (664 mg, 2.1 mmol, 6.0 equiv.) in anhydrous THF (5 mL) was added and stirred for 30 min at −78 °C. BF3·Et2O (0.35 mL, 2.8 mmol, 8.0 equiv.) was then added, followed by a solution of the resulting aldehyde (235 mg, 0.35 mmol, 1.0 equiv.) in THF (5 mL). After being stirred at −78 °C for 10 h, the reaction mixture was quenched with MeOH (10 mL) and warmed to room temperature. The solvent was removed in vacuo and the residue was redissolved in THF (20 mL) and H2O (6 mL), and NaBO3.4H2O (377 mg, 2.45 mmol, 7.0 equiv.) was then added. The mixture was stirred at room temperature for 12 h and poured into a mixture of EtOAc (30 mL) and H2O (3 mL). The layers were separated and the aqueous layer was further extracted with EtOAc (2 × 20 mL). The combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 3/1) to afford two separable diastereoisomers (180 mg, 71% total yield, dr > 20:1) with 21 (colorless oil) as the major product. TLC: Rf = 0.3 (hexanes/EtOAc = 3/1), UV & PMA stain. = −30.81 (c 0.57, CHCl3). 1H NMR (500 MHz, CDCl3) (mixture of conformers) δ 7.32 (dd, J = 6.4, 3.8 Hz, 4H), 7.29–7.14 (m, 6H), 5.90–5.69 (m, 1H), 5.44 (d, J = 8.8 Hz, 0.5H), 5.20 (dd, J = 9.2, 6.5 Hz, 0.5H), 5.15–5.04 (m, 2H), 5.02 (q, J = 1.5 Hz, 1H), 5.00–4.94 (m, 1H), 4.88–4.75 (m, 1H), 4.55 (d, J = 11.8 Hz, 1H), 4.44 (d, J = 11.8 Hz, 1H), 3.48 (h, J = 6.0 Hz, 1H), 3.45–3.38 (m, 1H), 3.08–2.92 (m, 2H), 2.89 (d, J = 22.8 Hz, 3H), 2.31–2.19 (m, 1H), 1.90–1.82 (m, 1H), 1.70–1.60 (m, 2H), 1.57 (ddd, J = 12.3, 6.5, 2.3 Hz, 2H), 1.40 (s, 9H), 1.38–1.24 (m, 6H), 1.16 (dd, J = 16.8, 6.1 Hz, 3H), 1.11–1.00 (m, 4H), 0.98–0.83 (m, 9H), 0.82–0.69 (m, 2H). 13C NMR (125 MHz, CDCl3) (mixture of conformers) δ 172.9, 170.8, 155.4, 139.9, 139.7, 139.3, 129.7, 129.6, 129.5, 129.5, 128.5, 128.4, 126.8, 116.6, 115.9, 79.9, 77.3, 75.0, 74.9, 74.7, 73.1, 72.6, 70.4, 61.0, 60.9, 52.4, 44.1, 43.8, 39.5, 36.9, 36.8, 36.3, 33.9, 33.5, 32.8, 32.7, 31.6, 30.9, 28.5, 28.4, 27.5, 25.8, 24.8, 19.8, 19.7, 16.2, 16.1, 15.7, 14.7, 14.2, 10.6. HRMS (ESI) calculated for C43H67N2O7+ [M + H]+ 723.4948 found 723.4943.
3.2.11. (3S,4R,6S,7S,12R)-12-(benzyloxy)-4-((tert-butyldimethylsilyl)oxy)-3,7-dimethyltridec-1-en-6-yl N-((tert-butoxycarbonyl)-d-phenylalanyl)-N-methyl-l-isoleucinate (22)
To a solution of alcohol 21 (230 mg, 0.32 mmol, 1.0 equiv.) in dry DCM (5 mL, 0.064 M) at 0 °C under an argon atmosphere, Et3N was added (89 μL, 0.64 mmol, 2.0 equiv.), followed by TBSOTf (110 μL, 0.48 mmol, 1.5 equiv.). The reaction mixture was warmed up to an ambient temperature, stirred for 2 h, and then quenched with a saturated aqueous solution of NaHCO3 (5 mL). The aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with H2O (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 9/1) to afford silyl ether 22 (217 mg, 81%) as a colorless oil. TLC: Rf = 0.5 (hexanes/EtOAc = 6/1), UV & PMA stain. = −19.33 (c 0.73, CHCl3). 1H NMR (500 MHz, CDCl3) (mixture of conformers) δ 7.37–7.29 (m, 4H), 7.29–7.13 (m, 6H), 5.85–5.61 (m, 1H), 5.33–5.25 (m, 0.5H), 5.19 (t, J = 9.5 Hz, 0.5H), 5.01 (ddt, J = 13.7, 8.4, 2.5 Hz, 2H), 4.98–4.89 (m, 2H), 4.83 (dd, J = 12.9, 6.7 Hz, 1H), 4.60–4.52 (m, 1H), 4.48–4.40 (m, 1H), 3.54 (dtd, J = 13.9, 7.3, 6.6, 3.0 Hz, 1H), 3.52–3.39 (m, 1H), 3.07–2.91 (m, 2H), 2.88 (s, 3H), 2.42–2.24 (m, 1H), 1.70–1.60 (m, 2H), 1.57 (ddt, J = 8.3, 6.5, 4.2 Hz, 2H), 1.38 (d, J = 1.3 Hz, 9H), 1.36–1.24 (m, 6H), 1.18 (dd, J = 6.1, 2.5 Hz, 3H), 0.96 (d, J = 6.9 Hz, 4H), 0.93–0.85 (m, 14H), 0.86–0.79 (m, 4H), 0.78 (td, J = 6.3, 2.5 Hz, 2H), 0.13–0.01 (m, 6H). 13C NMR (125 MHz, CDCl3) (mixture of conformers) δ 173.0, 172.7, 170.8, 170.6, 155.5, 154.9, 140.1, 140.0, 136.6, 129.7, 129.5, 128.6, 128.5, 128.4, 127.7, 127.7, 127.5, 126.9, 126.9, 126.8, 115.3, 79.7, 76.2, 75.3, 75.3, 75.0, 75.0, 74.8, 72.7, 72.5, 70.4, 61.0, 60.8, 52.0, 51.9, 42.3, 42.2, 40.4, 39.0, 37.0, 36.8, 36.8, 36.7, 36.3, 33.8, 33.7, 33.1, 32.8, 31.3, 31.3, 28.5, 28.4, 27.6, 26.5, 26.1, 26.0, 26.0, 26.0, 25.9, 25.3, 24.5, 23.3, 19.8, 19.7, 18.2, 18.2, 17.4, 17.3, 15.9, 14.7, 14.5, 14.4, 11.4, 10.9, −4.1, −4.2, −4.3, −4.5, −4.5. HRMS (ESI) calculated for C49H81N2O7Si+ [M + H]+ 837.5813 found 837.5814.
3.2.12. (6R,9S,12S,14R,15R)-6-benzyl-12-((2S,7R)-7-(benzyloxy)octan-2-yl)-9-((S)-sec-butyl)-14-((tert-butyldimethylsilyl)oxy)-2,2,8,15-tetramethyl-4,7,10-trioxo-3,11-dioxa-5,8-diazahexadecan-16-oic acid (23)
To a solution of alkene 22 (215 mg, 0.26 mmol, 1.0 equiv.) in DCM at –78 °C, ozone was bubbled through the solution until the solution became slightly blue. PPh3 (341 mg, 1.3 mmol, 5.0 equiv.) was added and the resultant solution was stirred at room temperature for 5 h. After concentration, the residue was used directly in the next step without further purification.
To a solution of the above crude aldehyde and PhI(OAc)2 (251 mg, 0.78 mmol, 3.0 equiv.) in DCM (5 mL, 0.052 M) at 0 °C, TEMPO (41 mg, 0.26 mmol, 1.0 equiv.) and H2O (1 mL) were added. The reaction mixture was warmed up to an ambient temperature, stirred for 12 h, and then quenched with a saturated aqueous solution of Na2SO3 (5 mL). The aqueous layer was extracted with DCM (2 × 20 mL) and the combined organic layers were washed with H2O (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 1/1) to afford acid 23 (149 mg, 68%) as a colorless oil. TLC: Rf = 0.4 (hexanes/EtOAc = 3/1), UV & PMA stain. = −32.07 (c 0.92, CHCl3). 1H NMR (500 MHz, CDCl3) (mixture of conformers) δ 8.03 (dd, J = 8.0, 6.4 Hz, 1H), 7.37–7.29 (m, 4H), 7.21 (d, J = 5.9 Hz, 6H), 5.35 (d, J = 9.6 Hz, 1H), 5.13 (dq, J = 12.5, 6.3 Hz, 1H), 4.93 (td, J = 8.5, 6.4 Hz, 1H), 4.74 (t, J = 9.9 Hz, 1H), 4.56 (d, J = 11.8 Hz, 1H), 4.45 (d, J = 11.8 Hz, 1H), 3.97–3.82 (m, 1H), 3.49 (q, J = 6.0 Hz, 1H), 3.10–2.94 (m, 2H), 2.93 (s, 3H), 2.75 (t, J = 5.8 Hz, 1H), 1.78–1.65 (m, 2H), 1.59 (ddd, J = 15.0, 9.7, 5.8 Hz,2H), 1.38 (d, J = 2.2 Hz, 9H), 1.37–1.26 (m, 6H), 1.24–1.15 (m, 2H), 1.15 (dq, J = 8.9, 5.6, 4.2 Hz, 3H), 1.08 (dd, J = 9.1, 6.1 Hz, 2H), 0.95–0.84 (m, 18H), 0.78 (ddd, J = 11.8, 7.4, 5.1 Hz, 2H), 0.10 (d, J = 4.3 Hz, 6H). 13C NMR (125 MHz, CDCl3) (mixture of conformers) δ 176.5, 173.0, 170.9, 170.4, 166.2, 154.9, 139.2, 137.6, 136.4, 132.7, 131.0, 130.3, 129.6, 129.4, 128.6, 128.5, 128.5, 128.3, 127.7, 127.6, 127.4, 126.9, 126.8, 79.8, 75.2, 75.0, 71.6, 71.5, 70.4, 61.0, 52.0, 44.2, 44.0, 40.2, 36.7, 36.6, 36.1, 35.4, 33.6, 32.5, 31.3, 28.4, 28.3, 27.5, 27.3, 26.6, 25.8, 25.8, 25.4, 20.1, 20.1, 19.7, 19.7, 17.9, 15.8, 14.7, 14.1, 14.0, 11.3, 10.7, 10.7, −4.3, −4.8, −4.9. HRMS (ESI) calculated for C48H79N2O9Si+ [M + H]+ 855.5555 found 855.5557.
3.2.13. (3S,6R,9R,10R,12S)-6-benzyl-12-((2S,7R)-7-(benzyloxy)octan-2-yl)-3-((S)-sec-butyl)-10-((tert-butyldimethylsilyl)oxy)-4,9-dimethyl-1-oxa-4,7-diazacyclododecane-2,5,8-trione (24)
To a solution of 23 (150 mg, 0.18 mmol, 1.0 equiv.) in DCM (5 mL, 0.036 M) at 0 °C, Et3N (0.18 mL, 1.26 mmol, 7.0 equiv.) was added, followed by the dropwise addition of trimethylsilyl trifluoromethanesulfonate (0.16 mL, 0.90 mmol, 5.0 equiv.). The reaction mixture was stirred for 6 h at room temperature and then quenched with a saturated aqueous solution of NaHCO3 (5 mL) and extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with a saturated aqueous solution of NH4Cl (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. The residue was used directly in the next step without further purification.
To a solution of above crude amine in DCM (120 mL, 0.0015 M) at 0 °C, DIPEA (0.31 mL, 1.8 mmol, 10.0 equiv.), HATU (342 mg, 0.90 mmol, 5.0 equiv.), and HOAt was added (73 mg, 0.54 mmol, 3.0 equiv.). The reaction mixture was stirred for 9 h at room temperature and then concentrated in vacuo, where the residue was redissolved in EtOAc (30 mL) and quenched with a 4% aqueous citric acid solution. The aqueous layer was extracted with EtOAc (3 × 20 mL) and the combined organic layers were washed with a saturated aqueous solution of NaHCO3 (15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica gel (hexanes/EtOAc = 2/1) to afford 24 (68 mg, 51%) as a white solid. TLC: Rf = 0.3 (hexanes/EtOAc = 1/1), UV & PMA stain. = +7.71 (c 0.64, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.42–7.29 (m, 4H), 7.29–7.16 (m, 6H), 5.76 (d, J = 10.3 Hz, 1H), 5.23 (td, J = 10.9, 4.5 Hz, 1H), 4.93 (d, J = 11.7 Hz, 1H), 4.76 (ddd, J = 11.0, 5.2, 1.7 Hz, 1H), 4.55 (d, J = 11.7 Hz, 1H), 4.45 (d, J = 11.8 Hz, 1H), 4.17 (dd, J = 8.0, 3.2 Hz, 1H), 3.48 (q, J = 5.9 Hz, 1H), 3.22 (t, J = 12.0 Hz, 1H), 2.91 (dd, J = 12.5, 4.5 Hz, 1H), 2.56 (s, 3H), 2.52 (dd, J = 6.7, 3.2 Hz, 1H), 1.92 (dt, J = 7.6, 4.0 Hz, 1H), 1.86 (ddd, J = 11.3, 6.6, 3.8 Hz, 1H), 1.79–1.68 (m, 1H), 1.60 (dt, J = 15.5, 5.0 Hz, 2H), 1.46–1.35 (m, 3H), 1.32–1.22 (m, 2H), 1.18 (d, J = 6.1 Hz, 5H), 1.07 (d, J = 6.6 Hz, 3H), 0.92 (d, J = 3.2 Hz, 9H), 0.87 (d, J = 6.4 Hz, 5H), 0.77–0.66 (m, 6H), 0.22 (s, 3H), 0.15 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 174.5, 174.0, 168.9, 139.2, 136.6, 129.3, 128.7, 128.4, 127.7, 127.4, 127.0, 76.1, 75.0, 70.4, 68.9, 60.6, 50.9, 48.3, 37.3, 36.8, 33.8, 32.7, 32.2, 30.5, 30.1, 26.5, 26.0, 25.7, 23.7, 19.7, 18.2, 15.6, 15.4, 10.0, 7.6, −3.7, −3.8. HRMS (ESI) calculated for C43H69N2O6Si+ [M + H]+ 737.4925 found 737.4924.
3.2.14. (3S,6R,9R,10R,12S)-6-benzyl-3-((S)-sec-butyl)-10-((tert-butyldimethylsilyl)oxy)-12-((2S,7R)-7-hydroxyoctan-2-yl)-4,9-dimethyl-1-oxa-4,7-diazacyclododecane-2,5,8-trione (4)
To a solution of 24 (65 mg, 0.088 mmol, 1.0 equiv.) in EtOAc (3 mL, 0.029 M) under an argon atmosphere, palladium was added on charcoal (20 mg, 10% Pd, 0.0085 mmol, 0.1 equiv.). The reaction flask was evacuated and purged with hydrogen three times. The reaction mixture was stirred under a hydrogen atmosphere at room temperature for 10 h; the flask was then evacuated and purged with nitrogen three times and the catalyst was removed via filtration using a pad of celite. The filtrate was concentrated under reduced pressure to afford the crude product, which was purified via flash chromatography on silica gel (hexanes/EtOAc = 1/1) to afford 4 (52 mg, 91%) as a white solid. TLC: Rf = 0.3 (hexanes/EtOAc = 2/1), UV & PMA stain.= −62.2 (c 0.52, CDCl3). 1H NMR (400 MHz, CDCl3) δ 7.57–6.93 (m, 5H), 5.82 (d, J = 10.3 Hz, 1H), 5.24 (td, J = 11.0, 4.6 Hz, 1H), 4.93 (d, J = 11.7 Hz, 1H), 4.91–4.69 (m, 1H), 4.17 (dd, J = 7.8, 3.1 Hz, 1H), 3.88–3.68 (m, 1H), 3.23 (t, J = 12.0 Hz, 1H), 2.92 (dd, J = 12.5, 4.6 Hz, 1H), 2.58 (s, 3H), 2.53 (dd, J = 6.7, 3.2 Hz, 1H), 1.94 (td, J = 7.2, 3.5 Hz, 1H), 1.91–1.82 (m, 1H), 1.75 (ddd, J = 15.8, 7.9, 1.8 Hz, 1H), 1.62 (dd, J = 15.7, 5.1 Hz, 2H), 1.43 (ddd, J = 17.7, 10.7, 4.2 Hz, 3H), 1.36–1.24 (m, 3H), 1.19 (d, J = 6.1 Hz, 5H), 1.08 (d, J = 6.7 Hz, 3H), 0.93 (s, 9H), 0.88 (d, J = 6.4 Hz, 3H), 0.86–0.68 (m, 7H), 0.23 (s, 3H), 0.16 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 174.6, 174.1, 169.0, 136.6, 129.3, 128.7, 127.1, 76.1, 69.0, 68.2, 60.7, 51.0, 48.3, 39.5, 37.3, 33.8, 32.7, 32.3, 30.6, 30.2, 26.8, 26.1, 25.8, 23.8, 23.7, 18.2, 15.7, 15.5, 10.1, 7.6, −3.6, −3.8. HRMS (ESI) calculated for C36H63N2O6Si+ [M + H]+ 647.4455 found 647.4448.
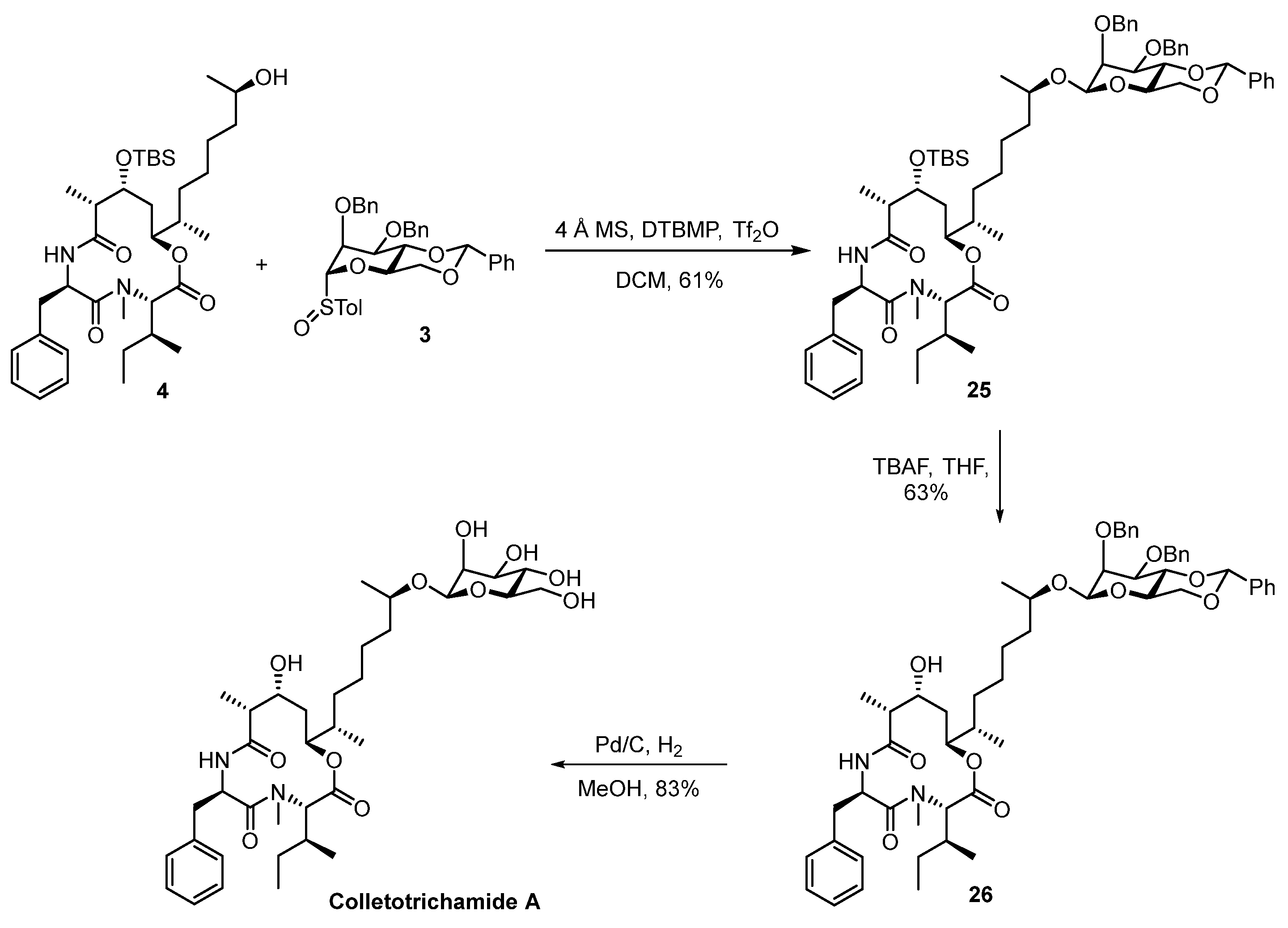
3.2.15. (3S,6R,9R,10R,12S)-6-benzyl-12-((2S,7R)-7-(((2R,4aR,6R,7S,8S,8aR)-7,8-bis(benzyloxy)-2-phenylhexahydropyrano [3,2-d][1,3]dioxin-6-yl)oxy)octan-2-yl)-3-((S)-sec-butyl)-10-((tert-butyldimethylsilyl)oxy)-4,9-dimethyl-1-oxa-4,7-diazacyclododecane-2,5,8-trione (25)
Glycosyl donor 3 (55 mg, 0.096 mmol, 1.2 equiv.) and 2,6-tert-butyl-4-methyl pyridine (43 mg, 0.21 mmol, 2.6 equiv.) were dried under a high vacuum for 1 h prior to being dissolved in DCM (5 mL, 0.016M). After a 4Å Molecular Sieve (200 mg, powder) was added, the suspension was stirred at room temperature for 1 h and then cooled to –78 °C. Tf2O (17 μL, 0.10 mmol, 1.3 equiv.) was added at –78 °C, and, 10 min later, a solution of glycosyl acceptor 4 (52 mg, 0.080 mmol, 1.0 equiv.) in DCM (2 mL) was dropwise added at –78 °C. The reaction mixture was stirred at –78 °C for 4 h and then quenched with water (3 mL). The reaction mixture was filtered, and the filtrate was diluted with EtOAc (30 mL), washed with H2O (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 6/1) to afford 25 (53 mg, 61%) as a white solid. TLC: Rf = 0.4 (hexanes/EtOAc = 6/1), UV & PMA stain.= −57.67 (c 0.57, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.63–7.45 (m, 4H), 7.43–7.06 (m, 16H), 5.82 (d, J = 10.4 Hz, 1H), 5.62 (s, 1H), 5.24 (td, J = 10.9, 4.5 Hz, 1H), 4.99 (d, J = 12.4 Hz, 1H), 4.93 (d, J = 11.7 Hz, 1H), 4.90–4.83 (m, 1H), 4.83–4.75 (m, 1H), 4.72–4.65 (m, 1H), 4.63–4.57 (m, 1H), 4.54 (s, 1H), 4.29 (dd, J = 10.5, 4.8 Hz, 1H), 4.25–4.19 (m, 1H), 4.18 (dd, J = 8.0, 3.1 Hz, 1H), 3.95 (t, J = 10.3 Hz, 1H), 3.90–3.78 (m, 2H), 3.60 (dd, J = 9.9, 3.0 Hz, 1H), 3.32 (td, J = 9.7, 4.9 Hz, 1H), 3.23 (t, J = 12.0 Hz, 1H), 2.92 (dd, J = 12.6, 4.6 Hz, 1H), 2.58 (s, 3H), 2.53 (dd, J = 6.7, 3.1 Hz, 1H), 2.00–1.91 (m, 1H), 1.87 (dt, J = 11.8, 6.7 Hz, 1H), 1.75 (dd, J = 15.8, 7.8 Hz, 1H), 1.63 (dd, J = 15.5, 5.3 Hz, 2H), 1.45 (dt, J = 16.8, 8.1 Hz, 2H), 1.41–1.28 (m, 3H), 1.25–1.16 (m, 3H), 1.12 (d, J = 6.1 Hz, 2H), 1.08 (dd, J = 6.7, 3.4 Hz, 4H), 0.93 (s, 9H), 0.88 (d, J = 6.4 Hz, 3H), 0.81–0.65 (m, 7H), 0.23 (s, 3H), 0.16 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 174.7, 174.1, 173.5, 169.0, 138.9, 138.7, 137.9, 136.7, 129.4, 129.0, 128.7, 128.7, 128.4, 128.3, 128.2, 127.7, 127.6, 127.5, 127.0, 126.4, 126.3, 101.6, 99.7, 78.9, 78.4, 76.9, 76.1, 74.9, 74.2, 72.5, 69.0, 68.9, 67.9, 60.8, 51.1, 48.4, 37.4, 33.9, 32.7, 32.3, 30.6, 30.1, 29.9, 26.3, 26.1, 25.6, 23.8, 19.2, 18.2, 15.8, 15.6, 10.0, 7.6, −3.7, −3.8. HRMS (ESI) calculated for C63H89N2O11Si+ [M + H]+ 1077.6236 found 1077.6238.
3.2.16. (3S,6R,9R,10R,12S)-6-benzyl-12-((2S,7R)-7-(((2R,4aR,6R,7S,8S,8aR)-7,8-bis(benzyloxy)-2-phenylhexahydropyrano [3,2-d][1,3]dioxin-6-yl)oxy)octan-2-yl)-3-((S)-sec-butyl)-10-hydroxy-4,9-dimethyl-1-oxa-4,7-diazacyclododecane-2,5,8-trione (26)
To a solution of 25 (52 mg, 0.048 mmol, 1.0 equiv.) in THF (3 mL, 0.016 M), TBAF was added (0.58 mL, 0.58 mmol, 12.0 equiv., 1.0 M in THF) at 0 °C. The reaction mixture was warmed up to an ambient temperature, stirred for 6 h, and then quenched with a saturated aqueous solution of NH4Cl (3 mL). The aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with H2O (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 3/1) to afford alcohol 26 (29 mg, 63%) as a colorless oil. TLC: Rf = 0.3 (hexanes/EtOAc = 3/1), UV & PMA stain. = −32.07 (c 0.92, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.66–7.44 (m, 4H), 7.44–7.11 (m, 16H), 6.52 (d, J = 10.2 Hz, 1H), 5.74 (s, 1H), 5.29 (td, J = 10.8, 4.7 Hz, 1H), 5.00 (d, J = 12.3 Hz, 1H), 4.91 (t, J = 12.4 Hz, 2H), 4.82 (ddd, J = 10.7, 4.4, 2.0 Hz, 1H), 4.72 (d, J = 12.5 Hz, 1H), 4.63 (d, J = 12.6 Hz, 1H), 4.56 (s, 1H), 4.31 (dd, J = 10.4, 4.8 Hz, 1H), 4.25 (t, J = 9.6 Hz, 1H), 4.20–4.10 (m, 1H), 4.00 (t, J = 10.3 Hz, 1H), 3.92–3.79 (m, 2H), 3.63 (dd, J = 9.9, 3.2 Hz, 1H), 3.34 (td, J = 9.8, 4.9 Hz, 1H), 3.23 (t, J = 11.9 Hz, 1H), 2.93 (dd, J = 12.6, 4.7 Hz, 1H), 2.67 (s, 3H), 2.53 (dd, J = 6.8, 3.3 Hz, 1H), 2.00 (qd, J = 9.7, 7.9, 2.9 Hz, 1H), 1.90 (dt, J = 11.5, 7.1 Hz, 1H), 1.82–1.56 (m, 3H), 1.54–1.37 (m, 3H), 1.39–1.28 (m, 2H), 1.28 (s, 3H), 1.11 (dd, J = 23.9, 6.4 Hz, 6H), 0.89 (d, J = 6.3 Hz, 4H), 0.85–0.70 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 174.5, 174.0, 168.8, 138.6, 138.5, 137.8, 136.7, 129.3, 128.9, 128.7, 128.7, 128.7, 128.4, 128.3, 128.3, 127.7, 127.6, 127.0, 126.2, 101.5, 99.8, 78.8, 78.2, 76.4, 75.0, 74.6, 72.4, 68.8, 68.2, 67.8, 60.8, 50.7, 48.3, 37.2, 34.1, 32.0, 31.9, 30.5, 30.3, 29.8, 26.2, 25.3, 23.8, 19.5, 15.9, 15.6, 10.1, 7.7. HRMS (ESI) calculated for C57H75N2O11+ [M + H]+ 963.5371 found 963.5371.
3.2.17. Colletotrichamide A
To a solution of 26 (25 mg, 0.026 mmol, 1.0 equiv.) in MeOH (3 mL, 0.009 M), palladium was added on charcoal (25 mg, 10% Pd, 0.011 mmol, 0.423 equiv.) under an argon atmosphere. The reaction flask was evacuated and purged with hydrogen three times. The reaction mixture was stirred under a hydrogen atmosphere at room temperature for 12 h, where the flask was then evacuated and purged with nitrogen three times and the catalyst was removed via filtration using a pad of celite. The filtrate was concentrated under reduced pressure to afford the crude product, which was performed via flash chromatography on silica gel (MeOH/DCM = 1/10) to afford colletotrichamide A (15 mg, 83%) as a colorless amorphous solid. TLC: Rf = 0.3 (MeOH/DCM = 1/10), UV & PMA stain.= −78.10 (c 1.0, MeOH). 1H NMR (400 MHz, DMSO) δ 8.58 (d, J = 9.5 Hz, 1H), 7.26 (d, J = 7.1 Hz, 2H), 7.23 (d, J = 4.0 Hz, 2H), 7.23–7.14 (m, 1H), 5.06 (dt, J = 9.4, 6.2 Hz, 1H), 4.77 (d, J = 11.6 Hz, 1H), 4.71 (s, 1H), 4.64 (d, J = 10.0 Hz, 1H), 4.53 (s, 1H), 4.50 (d, J = 5.1 Hz, 1H), 4.43 (s, 1H), 4.35 (t, J = 5.5 Hz, 1H), 4.17 (d, J = 4.3 Hz, 1H), 3.88 (d, J = 8.9 Hz, 1H), 3.77 (q, J = 6.2 Hz, 1H), 3.67 (ddd, J = 11.5, 5.3, 2.2 Hz, 1H), 3.54 (s, 1H), 3.42–3.47 (m, 1H), 3.32–3.22 (m, 2H), 3.08 (dd, J = 12.9, 9.4 Hz, 1H), 3.01 (td, J = 6.5, 3.0 Hz, 1H), 2.84 (dd, J = 12.8, 6.2 Hz, 1H), 2.66 (s, 3H), 2.58 (dt, J = 6.9, 4.0 Hz, 1H), 1.95–1.75 (m, 2H), 1.61–1.47 (m, 3H), 1.44 (d, J = 17.1 Hz, 1H), 1.34 (d, J = 6.8 Hz, 1H), 1.32–1.24 (m, 2H), 1.23 (s, 1H), 1.22–1.11 (m, 1H), 1.06 (d, J = 6.1 Hz, 3H), 0.98 (s, 1H), 0.97–0.89 (m, 1H), 0.84 (d, J = 6.8 Hz, 3H), 0.79 (d, J = 6.4 Hz, 3H), 0.71 (d, J = 6.5 Hz, 3H), 0.71–0.62 (m, 4H). 13C NMR (150 MHz, DMSO) δ 173.5, 173.0, 168.6, 137.7, 129.1, 128.2, 126.4, 97.5, 77.4, 76.0, 73.9, 72.4, 71.3, 67.3, 67.0, 61.5, 59.8, 49.6, 46.3, 36.9, 35.8, 33.5, 31.7, 31.5, 29.8, 29.8, 26.1, 25.3, 23.3, 19.2, 15.6, 15.3, 9.7, 7.5. HRMS (ESI) calculated for C36H59N2O11+ [M + H]+ 695.4119 found 695.4121.
3.2.18. Colletopeptide A
To a solution of 4 (12 mg, 0.019 mmol, 1.0 equiv.) in THF (3 mL, 0.006 M) at 0 °C, TBAF was added (0.23 mL, 0.23 mmol, 12.0 equiv., 1.0 M in THF). The reaction mixture was warmed up to an ambient temperature, stirred for 6 h, and then quenched with a saturated aqueous solution of NH4Cl (3 mL). The aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with H2O (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo. Purification of the crude product was performed via flash chromatography on silica (hexanes/EtOAc = 4/1) to afford colletopeptide A (7 mg, 67%) as a colorless amorphous solid. TLC: Rf = 0.3 (hexanes/EtOAc = 3/1), UV & PMA stain. = −62.03 (c 0.8, MeOH). 1H NMR (500 MHz, MeOD) δ 7.35–7.21 (m, 4H), 7.25–7.15 (m, 1H), 5.26 (dd, J = 10.7, 5.3 Hz, 1H), 4.85 (s, 1H), 4.76 (ddd, J = 10.6, 4.3, 2.2 Hz, 1H), 4.55 (s, 1H), 4.04 (dd, J = 8.3, 3.6 Hz, 1H), 3.78–3.57 (m, 1H), 3.22 (dd, J = 12.8, 10.7 Hz, 1H), 2.91 (dd, J = 12.8, 5.3 Hz, 1H), 2.71 (s, 3H), 2.70–2.60 (m, 1H), 2.05–1.92 (m, 1H), 1.94–1.83 (m, 1H), 1.69 (ddd, J = 15.8, 8.4, 2.2 Hz, 1H), 1.67–1.60 (m, 1H), 1.63–1.54 (m, 1H), 1.52–1.42 (m, 1H), 1.42–1.34 (m, 3H), 1.36–1.25 (m, 2H), 1.14 (d, J = 6.1 Hz, 3H), 1.10–1.01 (m, 1H), 1.01 (d, J = 6.9 Hz, 3H), 0.98–0.92 (m, 1H), 0.86 (d, J = 6.5 Hz, 3H), 0.80 (d, J = 6.6 Hz, 3H), 0.79–0.69 (m, 1H), 0.74 (d, J = 3.5 Hz, 3H). 13C NMR (100 MHz, MeOD) δ 176.4, 175.2, 170.1, 138.4, 130.3, 129.6, 127.9, 77.9, 69.1, 68.6, 62.1, 51.8, 48.7, 40.2, 37.4, 35.3, 33.3, 32.7, 31.7, 30.9, 27.6, 27.1, 24.9, 23.5, 16.3, 15.9, 10.3, 7.8. HRMS (ESI) calculated for C30H49N2O6+ [M + H]+ 533.3591 found 533.3578.