Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium
Abstract
1. Introduction
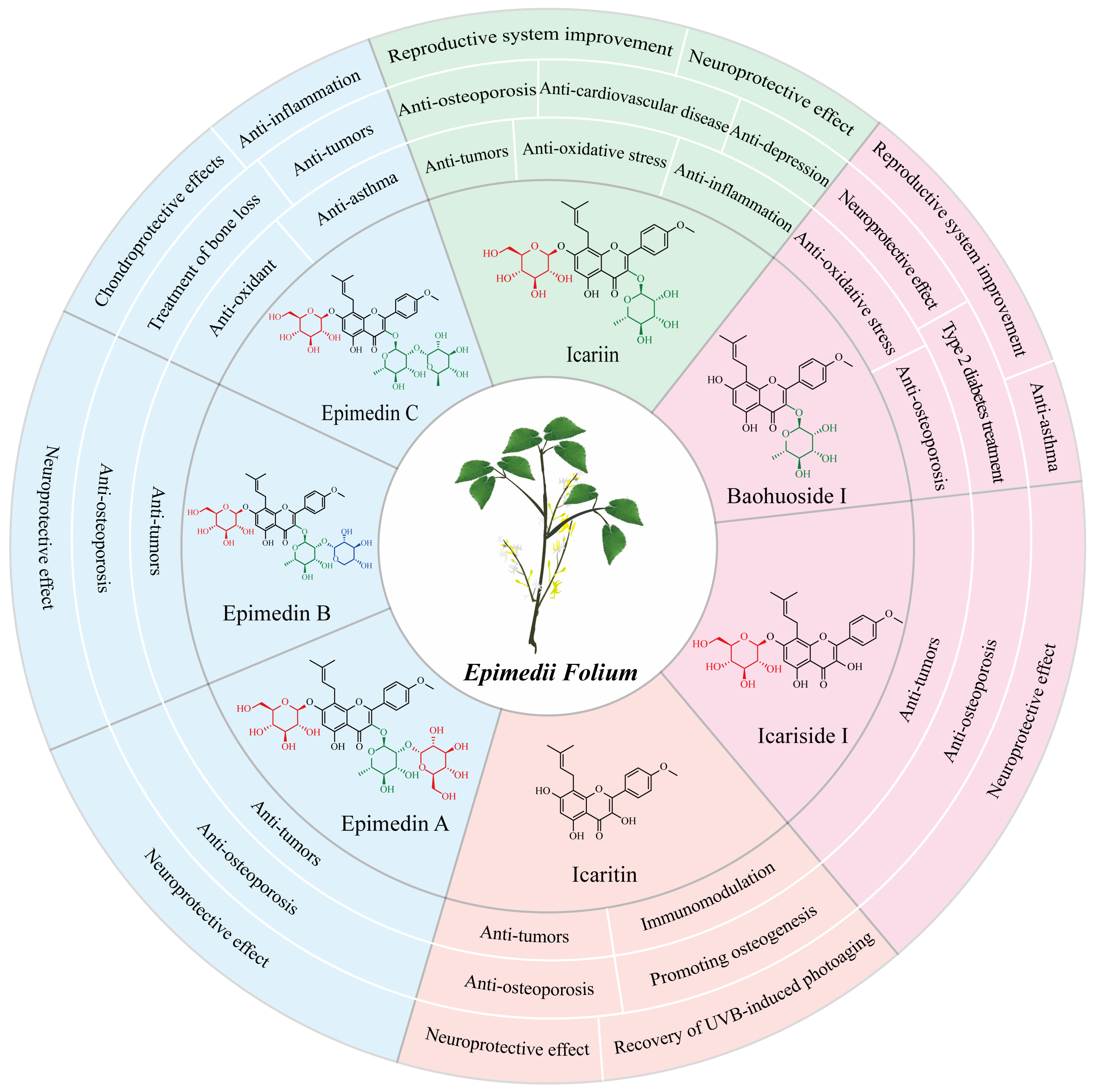
2. The Pharmacological Activities of Major Epimedium Flavonoids
2.1. Icariin and Its Pharmaceutical Effects
2.2. Baohuoside I and Its Pharmaceutical Effects
2.3. Icaritin and Its Pharmaceutical Effects
2.4. Epimedin C and Its Pharmaceutical Effects
2.5. Other Flavonoids and Their Pharmaceutical Effects
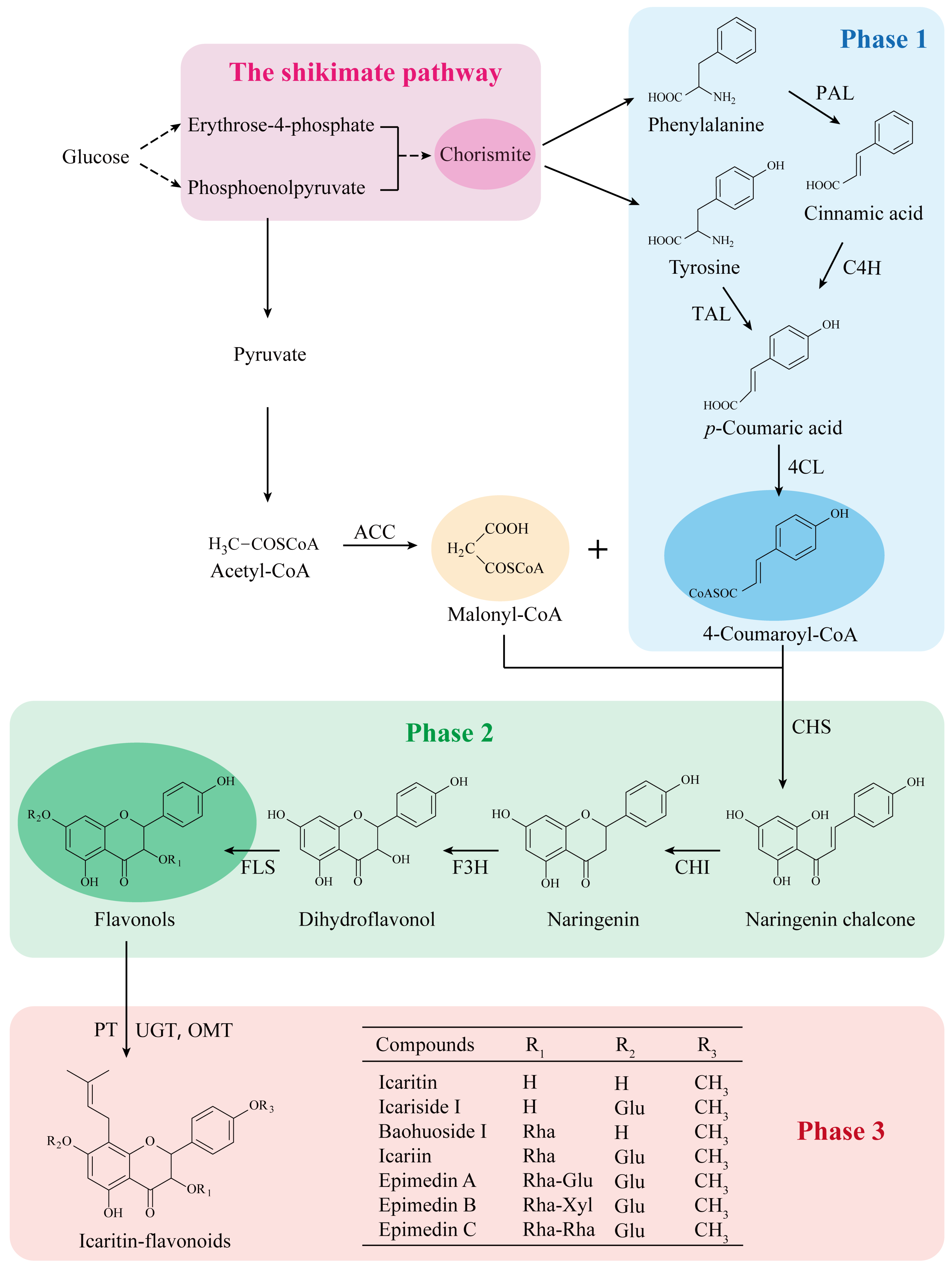
3. The Biosynthetic Pathway of Prenylated Flavonoids in EF
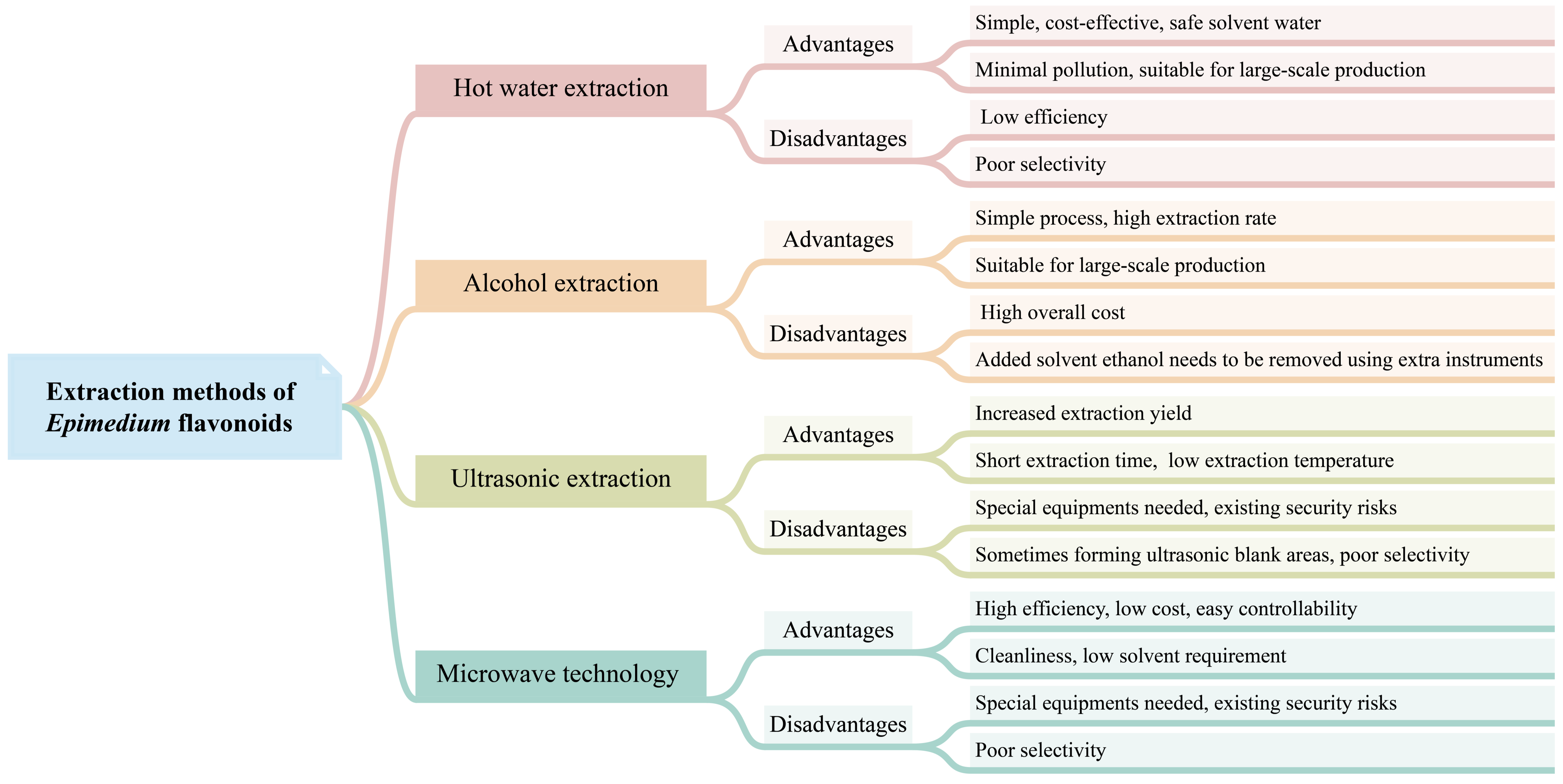
4. Extraction Methods of Epimedium Flavonoids
4.1. Hot Water Extraction
4.2. Alcohol Extraction
4.3. Other Extraction Methods
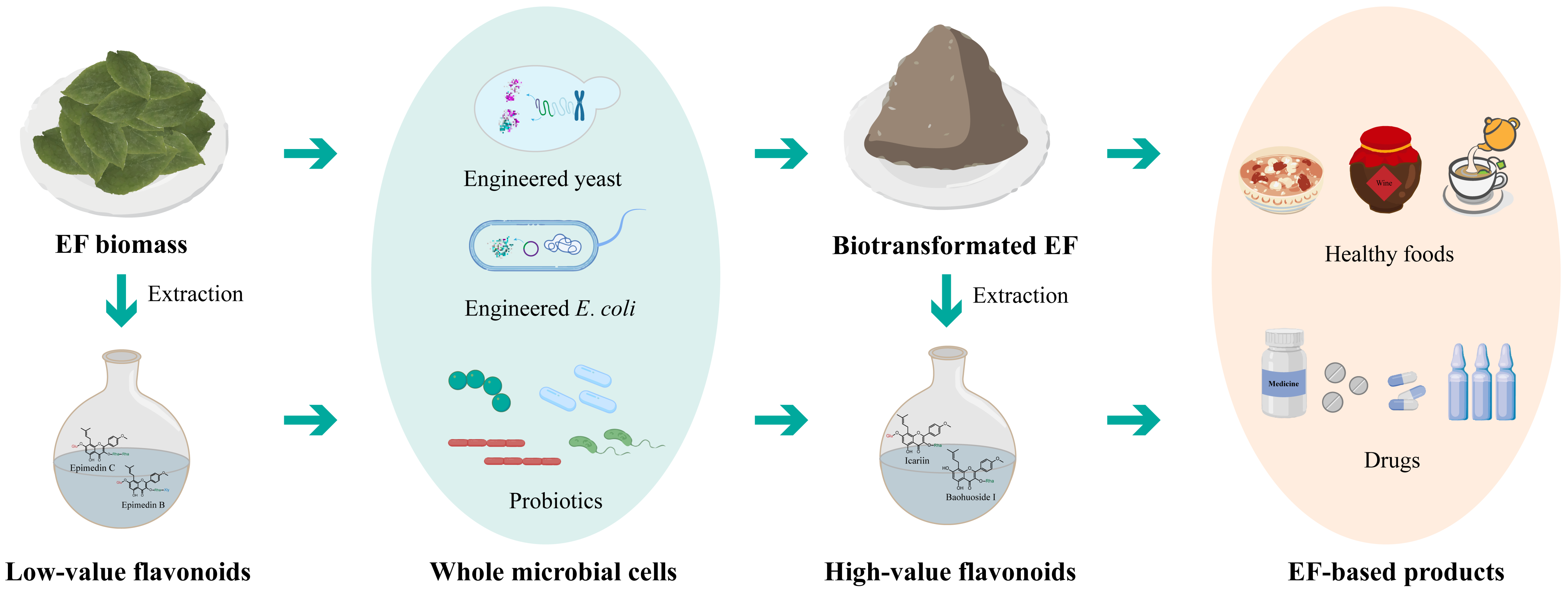
5. Biotransformation and Biosynthesis of Epimedium Flavonoids
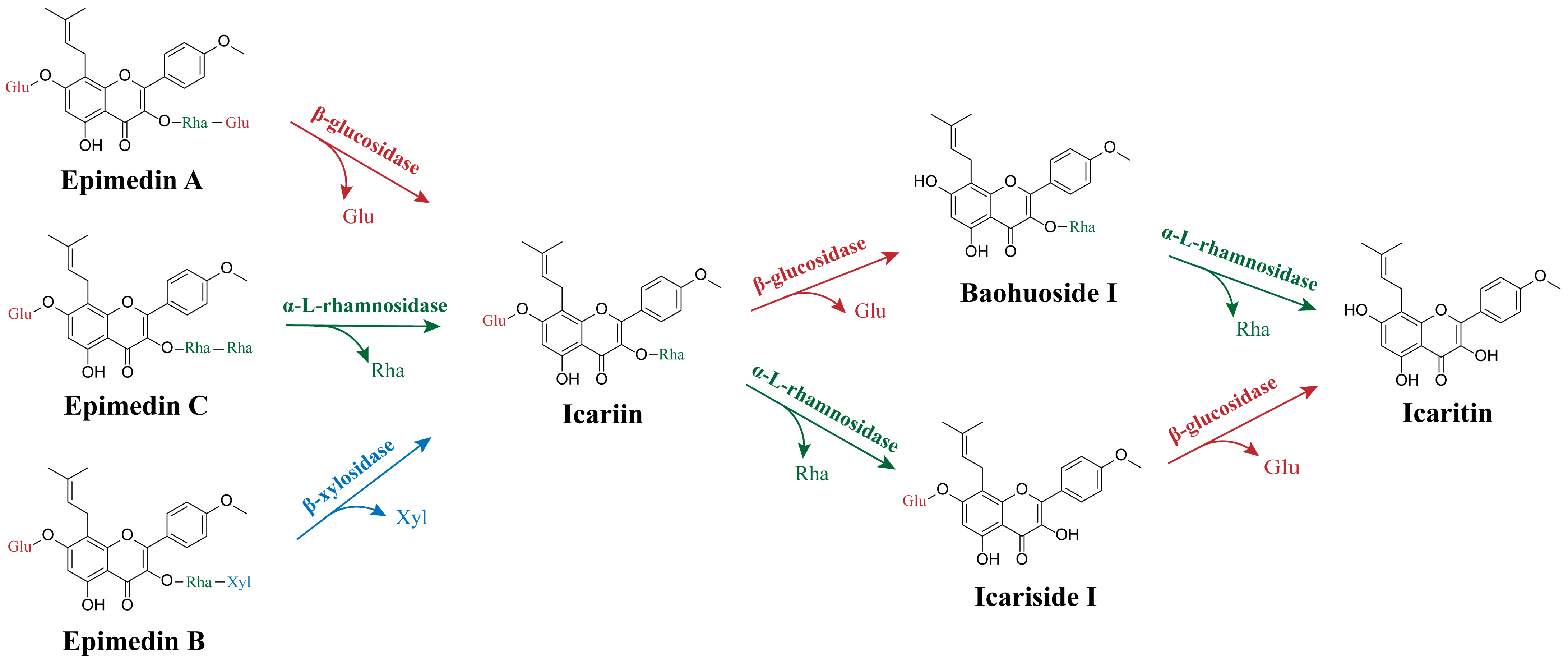
5.1. Biotransformation of Epimedium Flavonoids by Enzymes
| Enzyme Type | Enzyme Name | Enzyme Source | Enzyme Characterization | Enzyme Functions | References |
|---|---|---|---|---|---|
| Rhamnosyl hydrolase | α-l-rhamnosidase (AmRha) | Aspergillus mulundensis | 107.27 kDa, glycoside hydrolase (GH) 78 family; the optimal activity was achieved at 65 °C and pH 5.5, wide application temperature range, high level of enzyme catalytic ability and stability in the range of pH 5.0–7.5, acting on α-1,2-rhamnoside and α-1,6-rhamnoside bonds directly connected with glucose. | Catalyzes the bioconversion of epimedin C to icariin | [112] |
| α-l-rhamnosidase | Papiliotrema laurentii ZJU-L07 | 100 kDa; the optimal activity was achieved at 55° C and pH 7.0, sensitive to temperature, stable at a pH range of 5.5–9.0 with an activity of over 80%, higher selectivity to cleave the α-1,2 glycosidic linkage between glucoside and rhamnoside and the α-1,2 glycosidic linkage between rhamnoside and rhamnoside. | Produces icariin from epimedin C | [118] | |
| α-l-rhamnosidase (PodoRha) | Paenibacillus odorifer | The molecular weight of the monomer was 100.12 kDa, the native recombinant PodoRha was a trimer, GH78 family; the optimal activity was achieved at 45 °C and pH 6.5, a broad range of activity within a pH range of 5.0–8.5, excellent thermostability at 40 °C and 35 °C, high specificity on α-1,2-glycoside in epimedin C. | Converts epimedin C into icariin | [114] | |
| α-l-rhamnosidase (DthRha) | Dictyoglomus thermophilum DSM3960 | 106.96 kDa, GH78 family; the optimal activity was achieved at 95 °C and pH 6.5, stable within the pH range of 4.5–7.5, residual activities exceeded 50% after incubation at 85 °C for 3 h and exceeded 90% after incubation at 75 °C for 3 h. Efficient hydrolyzation of the α-l-rhamnosidic bond of Epimedium flavonoids. | Converts epimedin C into icariin, converts icariin into icariside I, and converts baohuoside I into icaritin | [50] | |
| α-l-rhamnosidase (synAnRhaE) | Aspergillus nidulans | 95 kDa; the optimal activity was achieved at 55 °C and pH 4.5, stable in an acidulous pH range below 55 °C, high specificity on α-1,2 rhamnoside glycosidic bond in epimedin C. | Converts epimedin C into icariin | [119] | |
| α-l-rhamnosidase (SPRHA2) | Novosphingobium sp. GX9 | 120 kDa, GH106 family, when combined with PBGL, the optimal temperature for the reaction was 55 °C, and the highest activity was observed in 200 mM borate saline buffer at pH 8.5. | Catalyzes icariin into icariside I, converts baohuoside I into icaritin | [14] | |
| α-l-rhamnosidase (BtRha) | Bacteroides thetaiotaomicron VPI-5482 | 83.3 kDa, GH78 family; the optimal activity was achieved at 55 °C and pH 6.5, high selectivity to cleave the α-1,2 and α-1,6 glycosidic bond between rhamnoside and rhamnoside, rhamnoside and glycoside, respectively. | Transforms epimedin C to icariin | [120] | |
| α-l-rhamnosidase (Rhase-I) | Talaromyces stollii CLY-6 | 140 kDa, GH106 family; the optimal activity was achieved at 45 °C and pH 4.5, high thermal stability at a temperature lower than 50 °C and superior stability in an acidic environment (pH 2.0–5.0), be activated by Ca2+ and Mg2+, efficiently cleaving both the outer and inner rhamnosidic bonds of epimedin C. | Converts epimedin C into icariin, and converts icariin into icariside I | [121] | |
| α-l-rhamnosidase (AtRha) | Aspergillus terreus CCF3059 | 96.9 kDa, GH78 family; the optimal activity was achieved at 60 °C and pH 6.5, excellent thermal stability and pH stability, hydrolyzed icariin containing the α-1 rhamnoside linkage. | Hydrolyzes icariin to icariside I | [122] | |
| Glucosyl hydrolase | β-glucosidase (Tpebgl3) | Thermomotoga petrophila DSM 13,995 | 81.24 kDa, GH3 family; the optimal activity was achieved at 90 °C and pH 5.0, the thermostability of the enzyme was improved by Ca2+, good stability at high temperatures and organic solvents. | Produces baohuoside I from icariin | [31,123] |
| β-glucosidase (IagBgl1) | Ignisphaera aggregans | The molecular weight of the monomer was 56.36 kDa, the native recombinant IagBgl1 was a trimer, GH1 family; the optimal activity was achieved at 95 °C and pH 6.5, thermostable and glucose-tolerant, retained more than 70% after incubation at 90 °C for 4 h, high catalytic activity towards icariin. | Produces baohuoside I from icariin | [32] | |
| β-glucosidase (PBGL) | Paenibacillus cookii GX-4 | 84 kDa, GH3 family, when combined with SPRHA2, the optimal temperature for the reaction was 55 °C, and the highest activity was observed in 200 mM borate saline buffer at pH 8.5. | Converts icariin into baohuoside I, converts icariside I to icaritin | [14] | |
| β-1,3-glucanase (CtLam55) | Chaetomium thermophilum | 82.7 kDa, GH55 family; the optimal activity was achieved at 60 °C and pH 5.0, thermostable at 50 °C, exo-β-1,3-glucanase activity. | Hydrolyzes icariin to baohuoside I | [113,124] | |
| β-glucosidase (Dth3) | Dictyoglomus thermophilum DSM3960 | 88.7 kDa, GH3 family; the optimal activity was achieved at 90 °C and pH 5.5, highly tolerant to glucose. | Converts epimedin A into baohuoside I, converts icariin into baohuoside I | [112,115] | |
| β-glucosidase | Trichoderma viride | 60 kDa; the optimal activity was achieved at 41 °C and pH 4.0. | Prepares baohuoside I from icariin | [125] | |
| dextranase | - | The optimal activity was achieved at 40 °C and pH 5.4, sensitive to pH. | Hydrolyzes icariin to baohuoside I | [126] | |
| cellulase | - | The optimum conditions for the cellulase were 50 °C and pH 5.0. | Transforms icariin to baohuoside I | [127] | |
| Xylosyl hydrolase | β-xylosidase (BbXyl) | Bifidobacterium breve K-110 | 70 kDa, GH43 family; the optimal activity was achieved at 45 °C and pH 5.5, the residual activity was more than 80% after being incubated at 45 °C for 4 h, showed over 70% of the maximum activity at a pH from 4.5 to 7.0, higher catalytic efficiency and selection specificity. | Converts epimedin B into icariin | [128] |
| β-xylosidase (Dt-2286) | Dictyoglomus turgidum | 85.1 kDa, GH3 family; the optimal activity was achieved at 98 °C and pH 5.0, excellent thermostable/haloduric/organic solvent-tolerance, a multifunctional enzyme with β-xylosidase, α-arabinofuranoside, α-arabinopyranoside and β-glucosidase activities. | Converts epimedin B into sagittatoside B, and converts sagittatoside B into icariside I | [129] | |
| β-xylosidase (Dth3) | Dictyoglomus thermophilum DSM3960 | 88.7 kDa, GH3 family; the optimal activity was achieved at 90 °C and pH 5.5, activity was not affected by xylose in high concentration. | Converts epimedin B into baohuoside I | [112,115] |
5.2. Biotransformation of Epimedium Flavonoids by Whole-Cell Catalysis
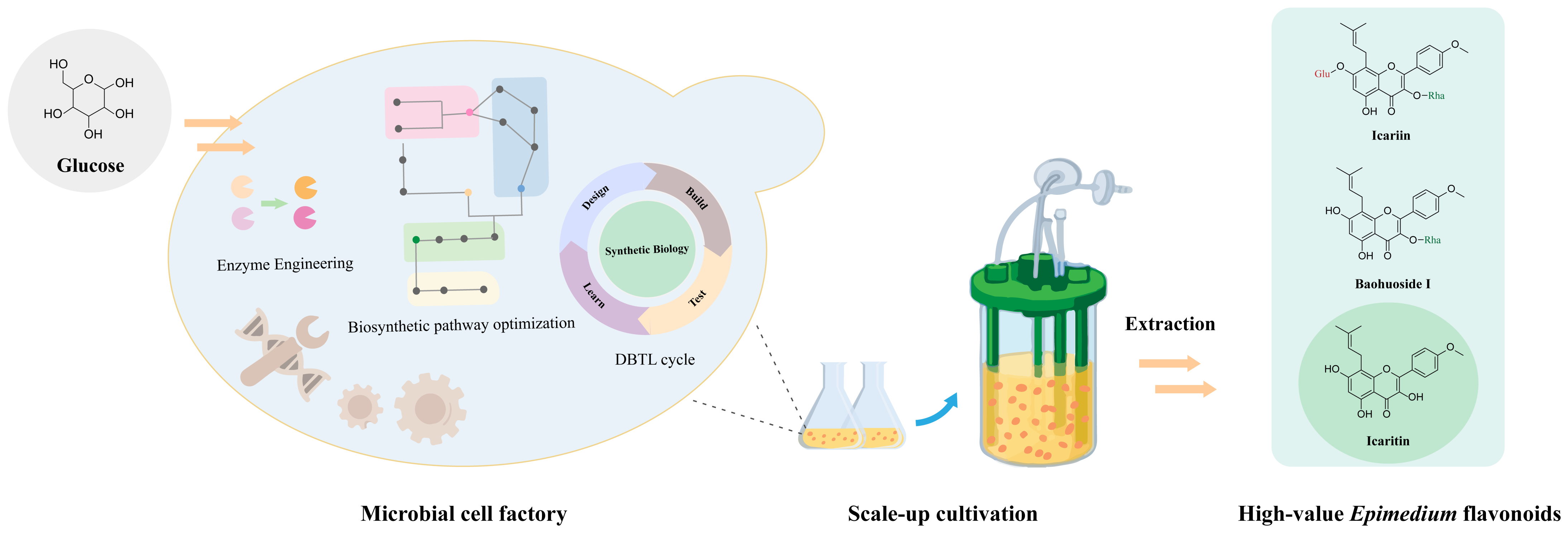
5.3. Complete Biosynthesis of Epimedium Flavonoids
6. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.Q.; Jiang, Y.; Huang, H.; Li, R.Q.; Li, F.Q.; Liu, Y.; Huang, X.F. Taxonomic study of Epimedium L.: Status, issues and prospect. Guihaia 2020, 40, 601–617. [Google Scholar]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Lin, G.; Pan, J.; Liu, J.; Xu, Y.; Cai, Q.; Wang, T.; Luan, Y.; Chen, Y.; Feng, Y.; et al. Deciphering the myth of icariin and synthetic derivatives in improving erectile function from a molecular biology perspective: A narrative review. Transl. Androl. Urol. 2022, 11, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, Z.; Amiri, M.S.; Mohammadzadeh, V.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Mashreghi, M.; Qayoomian, M.; Hashemzadeh, M.R.; Simal-Gandara, J.; Taghavizadeh Yazdi, M.E. Icariin: A promising natural product in biomedicine and tissue engineering. J. Funct. Biomater. 2023, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1. [Google Scholar]
- Punyawudho, B.; Puttilerpong, C.; Wirotsaengthong, S.; Aramwit, P. A randomized, double-blind, placebo-controlled crossover study of Cappra® for the treatment of mild or mild to moderate erectile dysfunction in Thai male. Afr. J. Tradit. Complement. Altern. Med. 2012, 10, 310–315. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Sun, Z.X.; Men, B.; Fu, X.J.; Chen, J.S. Clinical and mechanism research on functional erectile dysfunction treated with moxibustion and qiangshen shugan qiwei decoction. Zhongguo Zhen Jiu 2021, 41, 1325–1330. [Google Scholar]
- Wang, L.; Li, Y.; Guo, Y.; Ma, R.; Fu, M.; Niu, J.; Gao, S.; Zhang, D. Herba Epimedii: An ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Curr. Pharm. Des. 2016, 22, 328–349. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Luo, Q.; Mo, S.; Lyu, Y.; Wei, Y.; Dong, J. The anticancer properties of Herba Epimedii and its main bioactive components icariin and icariside II. Nutrients 2016, 8, 563. [Google Scholar] [CrossRef]
- Zhang, L.B.; Yan, Y.; He, J.; Wang, P.P.; Chen, X.; Lan, T.Y.; Guo, Y.X.; Wang, J.P.; Luo, J.; Yan, Z.R.; et al. Epimedii Herba: An ancient Chinese herbal medicine in the prevention and treatment of rheumatoid arthritis. Front. Chem. 2022, 10, 1023779. [Google Scholar] [CrossRef]
- Cho, J.H.; Jung, J.Y.; Lee, B.J.; Lee, K.; Park, J.W.; Bu, Y. Epimedii Herba: A promising herbal medicine for neuroplasticity. Phytother. Res. 2017, 31, 838–848. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H. Application of Herba Epimedii in food industry: Current status and prospect. Sci. Technol. Food Ind. 2010, 31, 390–393. [Google Scholar]
- Qian, H.Q.; Wu, D.C.; Li, C.Y.; Liu, X.R.; Han, X.K.; Peng, Y.; Zhang, H.; Zhao, B.Y.; Zhao, Y. A systematic review of traditional uses, phytochemistry, pharmacology and toxicity of Epimedium koreanum Nakai. J. Ethnopharmacol. 2024, 318, 116957. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, W.W.; Ding, B.; Guo, M.; Liang, M.; Pang, H.; Wei, Y.T.; Huang, R.B.; Du, L.Q. Highly efficient bioconversion of icariin to icaritin by whole-cell catalysis. Microb. Cell Fact. 2023, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Ji, H.; Zhang, Q.W.; Tu, P.F.; Wang, Y.T.; Guo, B.L.; Li, S.P. A rapid method for simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and ultra-performance liquid chromatography. J. Pharm. Biomed. Anal. 2008, 46, 226–235. [Google Scholar] [CrossRef]
- Chen, X.J.; Guo, B.L.; Li, S.P.; Zhang, Q.W.; Tu, P.F.; Wang, Y.T. Simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2007, 1163, 96–104. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, W.; Tu, C.; Li, P.; Zhao, G.; Xiao, X.; Wang, J.; Bai, Z. Immunostimulatory activity and structure-activity relationship of epimedin B from Epimedium brevicornu Maxim. Front. Pharmacol. 2022, 13, 1015846. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, Z.; Shi, J. Pharmacological effects of icariin. Adv. Pharmacol. 2020, 87, 179–203. [Google Scholar]
- Li, Z.; Li, D.; Chen, R.; Gao, S.; Xu, Z.; Li, N. Cell death regulation: A new way for natural products to treat osteoporosis. Pharmacol. Res. 2023, 187, 106635. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Zeng, Y.; Zhou, G.; Wang, Y.; Zhou, W.; Sun, X.; Wu, M. Icariin, an up-and-coming bioactive compound against neurological diseases: Network pharmacology-based study and literature review. Drug Des. Devel. Ther. 2021, 15, 3619–3641. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiong, Y.; Yang, T.; Wang, Y.; Zeng, J.; Zhou, S.; Luo, Y.; Li, L. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: From effects to molecular mechanisms. Biomed. Pharmacother. 2022, 147, 112642. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.F.; Chen, Y.; Pei, L.J.; Jiang, R. Effect of the icariin on endothelial microparticles, endothelial progenitor cells, platelets, and erectile function in spontaneously hypertensive rats. Andrology 2022, 10, 576–584. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Y.; Tang, Z.; Zu, X.; Qi, L.; Zhang, X.; Wang, G. Icariin improves the sexual function of male mice through the PI3K/AKT/eNOS/NO signalling pathway. Andrologia 2018, 50, e12802. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, D.; Guo, J.; Meng, X.; Zhang, G.; Wang, F. Icariin from Epimedium brevicornum Maxim promotes the biosynthesis of estrogen by aromatase (CYP19). J. Ethnopharmacol. 2013, 145, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and its metabolites as potential protective phytochemicals against Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 271. [Google Scholar] [CrossRef]
- Wang, G.Q.; Li, D.D.; Huang, C.; Lu, D.S.; Zhang, C.; Zhou, S.Y.; Liu, J.; Zhang, F. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front. Mol. Neurosci. 2017, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Xu, Y.; Zhao, Z.; Wu, X.; Du, Y.; Sun, J.; Yi, T.; Dong, J.; Liu, B. Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int. J. Mol. Med. 2016, 38, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.; Wang, X.; Xu, Y.; Zhang, X.; Han, Y.; Yan, H.; Liu, L.; Wang, L.; Ye, H.; et al. Effects of icariin on modulating gut microbiota and regulating metabolite alterations to prevent bone loss in ovariectomized rat model. Front. Endocrinol. 2022, 13, 874849. [Google Scholar] [CrossRef]
- Liang, X.; Hou, Z.; Xie, Y.; Yan, F.; Li, S.; Zhu, X.; Cai, L. Icariin promotes osteogenic differentiation of bone marrow stromal cells and prevents bone loss in OVX mice via activating autophagy. J. Cell Biochem. 2019, 120, 13121–13132. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Du, T.; Chen, K.; Guo, J.; Xiang, W.; Yao, X.; Sun, K.; Ye, Y.; Guo, F. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J. Cell Physiol. 2019, 234, 10123–10137. [Google Scholar] [CrossRef]
- Lu, S.; Zou, K.; Guo, B.; Pei, J.; Wang, Z.; Xiao, W.; Zhao, L. One-step purification and immobilization of thermostable β-glucosidase on Na-Y zeolite based on the linker and its application in the efficient production of baohuoside I from icariin. Bioorg. Chem. 2022, 121, 105690. [Google Scholar] [CrossRef]
- Xie, J.; Xu, H.; Jiang, J.; Zhang, N.; Yang, J.; Zhao, J.; Wei, M. Characterization of a novel thermostable glucose-tolerant GH1 β-glucosidase from the hyperthermophile Ignisphaera aggregans and its application in the efficient production of baohuoside I from icariin and total epimedium flavonoids. Bioorg. Chem. 2020, 104, 104296. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Song, J.; Jia, X.; Zhang, Z. Hyaluronic acid-modified didecyldimethylammonium bromide/ d-a-tocopheryl polyethylene glycol succinate mixed micelles for delivery of baohuoside I against non-small cell lung cancer: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.; Zhang, T.; Lu, L.; Ding, Y.; Zhao, Y. Comparative pharmacokinetics study of icariin and icariside II in rats. Molecules 2015, 20, 21274–21286. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kim, M.; Han, J. Icariin metabolism by human intestinal microflora. Molecules 2016, 21, 1158. [Google Scholar] [CrossRef]
- Ge, P.; Guo, Y.; Shen, J. Icariside II facilitates the differentiation of ADSCs to SCs via let-7i/STAT3 axis to preserve erectile function. Biol. Res. 2019, 52, 54. [Google Scholar] [CrossRef]
- Khan, M.; Maryam, A.; Qazi, J.I.; Ma, T. Targeting apoptosis and multiple signaling pathways with icariside II in cancer cells. Int. J. Biol. Sci. 2015, 11, 1100–1112. [Google Scholar] [CrossRef]
- Ma, M.; Fan, A.Y.; Liu, Z.; Yang, L.Q.; Huang, J.M.; Pang, Z.Y.; Yin, F. Baohuoside I inhibits osteoclastogenesis and protects against ovariectomy-induced bone loss. Front. Pharmacol. 2022, 13, 874952. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, T.; Zhang, W.; Lv, K.; Jia, D.; Yang, F.; Sun, Y.; Lian, J.; Wang, R. Icariside II facilitates the differentiation of ADSCs to schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed. Pharmacother. 2020, 125, 109888. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, T.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Ye, X. Preliminary studies on the anti-osteoporosis activity of Baohuoside I. Biomed. Pharmacother. 2019, 115, 108850. [Google Scholar] [CrossRef]
- Kong, Q.; Ma, M.; Zhang, L.; Liu, S.; He, S.; Wu, J.; Liu, B.; Dong, J. Icariside II potentiates the anti-PD-1 antitumor effect by reducing chemotactic infiltration of myeloid-derived suppressor cells into the tumor microenvironment via ROS-mediated inactivation of the SRC/ERK/STAT3 signaling pathways. Phytomedicine 2023, 110, 154638. [Google Scholar] [CrossRef]
- Peng, Y.G.; Zhang, L. Baohuoside-I suppresses cell proliferation and migration by up-regulating miR-144 in melanoma. Pharm. Biol. 2018, 56, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, N.; Huang, X.; Yang, B.; Zheng, Y.; Zhang, J.; Wang, X.; Lin, Y.; Wang, Z. Baohuoside i suppresses breast cancer metastasis by downregulating the tumor-associated macrophages/C-X-C motif chemokine ligand 1 pathway. Phytomedicine 2020, 78, 153331. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhan, Y.; Xie, Y.; Wang, Y.; Liu, Y. The impact of icariside II on human prostate cancer cell proliferation, mobility, and autophagy via PI3K-AKT-mTOR signaling pathway. Drug Des. Devel. Ther. 2020, 14, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Eun, J.S.; Kim, D.K.; Li, R.H.; Shin, T.Y.; Park, H.; Cho, N.P.; Soh, Y. Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1alpha in human osteosarcoma cells. Eur. J. Pharmacol. 2008, 579, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, H.A.; Sohn, H.S.; Kim, J.W.; Choi, J.S. Potential of icariin metabolites from Epimedium koreanum Nakai as antidiabetic therapeutic agents. Molecules 2017, 22, 986. [Google Scholar] [CrossRef]
- Gao, J.; Ma, C.; Xia, D.; Chen, N.; Zhang, J.; Xu, F.; Li, F.; He, Y.; Gong, Q. Icariside II preconditioning evokes robust neuroprotection against ischaemic stroke, by targeting Nrf2 and the OXPHOS/NF-κB/ferroptosis pathway. Br. J. Pharmacol. 2023, 180, 308–329. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Yu, H.; Shi, H.; Chen, M.; Song, J.; Tang, W.; Teng, F.; Li, C.; Yi, L.; et al. TMT-based quantitative proteomics revealed protective efficacy of Icariside II against airway inflammation and remodeling via inhibiting LAMP2, CTSD and CTSS expression in OVA-induced chronic asthma mice. Phytomedicine 2023, 118, 154941. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Yu, H.; Wu, B.; Huai, B.; Zhuang, Z.; Sun, C.; Xu, L.; Jin, F. Icaritin preparation from icariin by a special Epimedium flavonoid-glycosidase from Aspergillus sp. y848 strain. J. Microbiol. Biotechnol. 2022, 32, 437–446. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J.; Dong, Y.; Wang, Z.; Xiao, W.; Zhao, L. Biotransformation of the total flavonoid extract of epimedium into icaritin by two thermostable glycosidases from Dictyoglomus thermophilum DSM3960. Process Biochem. 2021, 105, 8–18. [Google Scholar] [CrossRef]
- Huong, N.T.; Son, N.T. Icaritin: A phytomolecule with enormous pharmacological values. Phytochemistry 2023, 213, 113772. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Wang, H.; Qi, L.; Lou, Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience 2007, 145, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Lou, Y.J. Inducible effects of icariin, icaritin, and desmethylicaritin on directional differentiation of embryonic stem cells into cardiomyocytes in vitro. Acta Pharmacol. Sin. 2005, 26, 477–485. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, S.Q. Antiosteoporosis effects, pharmacokinetics, and drug delivery systems of icaritin: Advances and prospects. Pharmaceuticals 2022, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, B.; Hu, H.; Xie, C.; Ling, L.; Gao, J.; Cao, Y. Icaritin promotes the osteogenesis of bone marrow mesenchymal stem cells via the regulation of sclerostin expression. Int. J. Mol. Med. 2020, 45, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Y.; Wu, H.; Zhao, M.; Tan, Y.; Li, D.; Long, H.; Dai, Y.; Yung, S.; Chan, T.M.; et al. The role of icaritin in regulating Foxp3/IL17a balance in systemic lupus erythematosus and its effects on the treatment of MRL/lpr mice. Clin. Immunol. 2016, 162, 74–83. [Google Scholar] [CrossRef]
- Hwang, E.; Lin, P.; Ngo, H.T.T.; Gao, W.; Wang, Y.S.; Yu, H.S.; Yi, T.H. Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-κB signaling pathways: A comparative study on UVB-irradiated human keratinocytes. Photochem. Photobiol. Sci. 2018, 17, 1396–1408. [Google Scholar] [CrossRef]
- Yang, X.J.; Xi, Y.M.; Li, Z.J. Icaritin: A novel natural candidate for hematological malignancies therapy. Biomed. Res. Int. 2019, 2019, 4860268. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, X.; Jiang, Y.; Wang, X.; Wang, S. Antitumor effects of icaritin and the molecular mechanisms. Discov. Med. 2020, 29, 5–16. [Google Scholar]
- Fan, Y.; Li, S.; Ding, X.; Yue, J.; Jiang, J.; Zhao, H.; Hao, R.; Qiu, W.; Liu, K.; Li, Y.; et al. First-in-class immune-modulating small molecule Icaritin in advanced hepatocellular carcinoma: Preliminary results of safety, durable survival and immune biomarkers. BMC Cancer 2019, 19, 279. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Jiang, W.; Xue, J.; Cheng, Y.; Wang, J.; Yang, R.; Zhang, X. Icaritin promotes apoptosis and inhibits proliferation by down-regulating AFP gene expression in hepatocellular carcinoma. BMC Cancer 2021, 21, 318. [Google Scholar] [CrossRef]
- Tao, H.; Liu, M.; Wang, Y.; Luo, S.; Xu, Y.; Ye, B.; Zheng, L.; Meng, K.; Li, L. Icaritin induces anti-tumor immune responses in hepatocellular carcinoma by inhibiting splenic myeloid-derived suppressor cell generation. Front. Immunol. 2021, 12, 609295. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, N.; Dong, J.; Wang, X.; Liu, L.; Huang, J. Estrogen receptor-α36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, W.W.; Li, B.; Chen, H.J.; Zhu, W.S.; Yang, G.X.; Chen, M.J.; He, G.Y. Anticancer effect of icaritin on human lung cancer cells through inducing S phase cell cycle arrest and apoptosis. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014, 34, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, M.; Ouyang, Y.; Li, R.; Zhang, X.; Gao, X.; Lin, S.; Wang, X. Icaritin induces ovarian cancer cell apoptosis through activation of p53 and inhibition of Akt/mTOR pathway. Life Sci. 2018, 202, 188–194. [Google Scholar] [CrossRef]
- Jin, Y.B.; Liang, X.C.; Cai, J.H.; Wang, K.; Wang, C.Y.; Wang, W.H.; Chen, X.L.; Bao, S. Mechanism of action of icaritin on uterine corpus endometrial carcinoma based on network pharmacology and experimental evaluation. Front. Oncol. 2023, 13, 1205604. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Lu, R.; Ye, X.J.; Zhang, J.; Tan, Y.Q.; Zhou, G. Icaritin reduces oral squamous cell carcinoma progression via the inhibition of STAT3 signaling. Int. J. Mol. Sci. 2017, 18, 132. [Google Scholar] [CrossRef]
- Wu, T.; Wang, S.; Wu, J.; Lin, Z.; Sui, X.; Xu, X.; Shimizu, N.; Chen, B.; Wang, X. Icaritin induces lytic cytotoxicity in extranodal NK/T-cell lymphoma. J. Exp. Clin. Cancer Res. 2015, 34, 17. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Z.; Zhang, G.; Meng, K.; Kuang, W.; Li, J.; Zhou, X.; Li, R.; Peng, H.; Dai, C.; et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT signalings. PLoS ONE 2011, 6, e23720. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Z.; Li, Z.; Peng, H.; Luo, Y.; Deng, M.; Li, R.; Dai, C.; Xu, Y.; Liu, S.; et al. Icaritin suppresses multiple myeloma, by inhibiting IL-6/JAK2/STAT3. Oncotarget 2015, 6, 10460–10472. [Google Scholar] [CrossRef]
- Liu, X.; Yang, F.; Jia, D.; Dong, X.; Zhang, Y.; Wu, Z. Case report: A case study on the treatment using icaritin soft capsules in combination with lenvatinib achieving impressive PR and stage reduction in unresectable locally progressive pancreatic cancer and a literature review. Front. Genet. 2023, 14, 1167470. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Dong, X.; Jiang, G.; Hong, D.; Liu, X. The synergy of gene targeting drug icaritin soft capsule with immunomodulator and TACE brings new hope for drug combination in patients with advanced liver cancer: A case report and literature review. Cancer Manag. Res. 2023, 15, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Gao, J. Simultaneous preparation and comparison of the osteogenic effects of epimedins A–C and icariin from Epimedium brevicornu. Chem. Biodivers. 2018, 15, e1700578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Yang, T.S.; Li, Z.Z.; Wang, Y. Simultaneous extraction of epimedin A, B, C and icariin from Herba Epimedii by ultrasonic technique. Ultrason. Sonochem. 2008, 15, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Anti-inflammatory and chondroprotective effects of vanillic acid and epimedin C in human osteoarthritic chondrocytes. Biomolecules 2020, 10, 932. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Huang, L.; Han, W.; Shao, Y.; Chen, M.; Zhang, Y.; He, R.; Xie, B. Epimedin C alleviates glucocorticoid-induced suppression of osteogenic differentiation by modulating PI3K/AKT/RUNX2 signaling pathway. Front. Pharmacol. 2022, 13, 894832. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Huang, M.; Feng, J.; Xia, P.; Wang, Y.; Wei, X.; Qiu, L. Effects of icariin and epimedium C on microstructure of bone tissue in glucocorticoid osteoporosis model mice based on Micro-CT technique. Drug Eval. Res. 2020, 43, 1733–1739. [Google Scholar]
- Wei, D.H.; Deng, J.L.; Shi, R.Z.; Ma, L.; Shen, J.M.; Hoffman, R.; Hu, Y.H.; Wang, H.; Gao, J.L. Epimedin C protects H2O2-induced peroxidation injury by enhancing the function of endothelial progenitor HUVEC populations. Biol. Pharm. Bull. 2019, 42, 1491–1499. [Google Scholar] [CrossRef]
- Huang, M.; Wei, Y.; Dong, J. Epimedin C modulates the balance between Th9 cells and Treg cells through negative regulation of noncanonical NF-κB pathway and MAPKs activation to inhibit airway inflammation in the ovalbumin-induced murine asthma model. Pulm. Pharmacol. Ther. 2020, 65, 102005. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, Y.; Chai, L.; Song, L.; Huang, J.; Wang, Q.; Li, Y.; Zhou, K. Development of epimedin A complex drugs for treating the osteoporosis. J. Mater. Sci. Mater. Med. 2021, 32, 17. [Google Scholar] [CrossRef]
- Balaha, M.F.; Ahmed, N.J.; Almalki, Z.S.; Alahmari, A.K.; Alshehri, A.M.; Soliman, G.A.; Hamad, A.M. Epimedin A ameliorates DNFB-induced allergic contact dermatitis in mice: Role of NF-κ B/NLRP3-driven pyroptosis, Nrf2/HO-1 pathway, and inflammation modulation. Life Sci. 2022, 302, 120653. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Wang, L.; Zhou, Y.; Bi, Y.; Zhou, K.; Song, L. The mechanism of Epimedin B in treating osteoporosis as revealed by RNA sequencing-based analysis. Basic Clin. Pharmacol. Toxicol. 2021, 129, 450–461. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Z.F.; Dong, X.L.; Chen, W.F. Epimedin B exerts neuroprotective effect against MPTP-induced mouse model of Parkinson’s disease: GPER as a potential target. Biomed. Pharmacother. 2022, 156, 113955. [Google Scholar] [CrossRef]
- Chen, G.; Huang, J.; Lei, H.; Wu, F.; Chen, C.; Song, Y.; Cao, Z.; Zhang, C.; Zhang, C.; Ma, Y.; et al. Icariside I—A novel inhibitor of the kynurenine-AhR pathway with potential for cancer therapy by blocking tumor immune escape. Biomed. Pharmacother. 2022, 153, 113387. [Google Scholar] [CrossRef]
- Chen, C.; Wu, M.; Lei, H.; Cao, Z.; Wu, F.; Song, Y.; Zhang, C.; Qin, M.; Zhang, C.; Du, R.; et al. A novel prenylflavonoid icariside I ameliorates estrogen deficiency-induced osteoporosis via simultaneous regulation of osteoblast and osteoclast differentiation. ACS Pharmacol. Transl. Sci. 2023, 6, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cai, H. Adverse reactions to Zhuangguguanjie Wan and cause analysis. Advers. Drug React. J. 2000, 2, 15–19. [Google Scholar]
- Du, Q.; Wang, Z.; Yun, N.R.; Huang, Y.H.; Xu, Q.; Wang, B.H. Literature analysis of 185 cases of ADR induced by Xianling Gubao capsule. China Pharm. 2017, 28, 3785–3787. [Google Scholar]
- Wang, Q.; Zhang, P.Y.; Yuan, X.M.; Bi, Y.N.; Zhou, K.; Zhang, Y. Long-term toxicity of different extracts of Epimedium brevicornu maxim in mice. Chin. J. Pharmacovigil. 2018, 15, 65–69. [Google Scholar]
- Zhang, L.; Zhang, J.X.; Fan, Q.Y.; Su, Z.Q.; Chen, C.; Peng, L.; Wang, T. Hepatoxicity of Epimedii folium in rat model based on uniform design and regression analysis. Chin. J. Exp. Tradit. Med. Formulae. 2018, 24, 189–197. [Google Scholar]
- Zhang, L.; Xu, A.L.; Yang, S.; Zhao, B.S.; Wang, T. In vitro screening and toxic mechanism exploring of leading components with potential hepatotoxicity of Herba Epimedii extracts. Toxicol. In Vitro 2020, 62, 104660. [Google Scholar] [CrossRef]
- Song, Z.; Li, Z.; Wen, X.; Liu, R.; Tian, X. UPLC-MS/MS method for simultaneously determining nucleosides and methyl-nucleosides in liver mRNA of Epimedin C-induced liver injury mouse model. Chin. Med. 2021, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Zhao, B.S.; Zhang, J.X.; Yang, S.; Fan, C.L.; Li, P. Effect of 2″-O-rhamnosyl icariside II, baohuoside I and baohuoside II in Herba Epimedii on cytotoxicity indices in HL-7702 and HepG2 Cells. Molecules 2019, 24, 1263. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, L.; Guo, Z.; Kang, Q.; Chen, C.; Liu, X.; Ma, Q.; Zhang, J.; Hu, Y.; Wang, T. Epimedium koreanum Nakai-induced liver injury-A mechanistic study using untargeted metabolomics. Front. Pharmacol. 2022, 13, 934057. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zeng, S.; Xiao, G.; Wei, G.; Liao, S.; Chen, J.; Sun, W.; Lv, H.; Wang, Y. Elucidating the biosynthetic and regulatory mechanisms of flavonoid-derived bioactive components in Epimedium sagittatum. Front. Plant. Sci. 2015, 6, 689. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Luo, Y.; Yao, Y.; Meng, G.; Zhang, Y.; Wang, Y.; Xu, C.; Liu, X.; Zhang, C.; Ding, G.; et al. The discovery of a key prenyltransferase gene assisted by a chromosome-level Epimedium pubescens genome. Front. Plant Sci. 2022, 13, 1034943. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, X.; Shen, G.; Fan, X.; Zhang, Y.; Sun, C.; Suo, F.; Guo, B. Time-series transcriptome provides insights into the gene regulation network involved in the icariin-flavonoid metabolism during the leaf development of Epimedium pubescens. Front. Plant Sci. 2023, 14, 1183481. [Google Scholar] [CrossRef]
- Mir, R.; Jallu, S.; Singh, T.P. The shikimate pathway: Review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit. Rev. Microbiol. 2015, 41, 172–189. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Pandey, R.P.; Parajuli, P.; Koffas, M.A.G.; Sohng, J.K. Microbial production of natural and non-natural flavonoids: Pathway engineering, directed evolution and systems/synthetic biology. Biotechnol. Adv. 2016, 34, 634–662. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Ren, X.X. Optimization of the water-extraction method of total flavonoids from Epimedium sagittatum Maxin. Tianjin Agric. Sci. 2012, 18, 3. [Google Scholar]
- Huang, R.H.; Zhou, Y.C.; Han, W.; Deng, X. Study on water extraction process of Herba epimedii with microwave technology. Zhongguo Zhong Yao Za Zhi. 2005, 30, 107–110. [Google Scholar]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Microwave-assisted extraction of pectin from grape pomace. Sci. Rep. 2022, 12, 12722. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Li, L.; Xue, Y.B.; Zhou, X.X.; Tang, J.H. Flavonoids from Epimedium pubescens: Extraction and mechanism, antioxidant capacity and effects on CAT and GSH-Px of Drosophila melanogaster. PeerJ 2020, 8, e8361. [Google Scholar] [CrossRef] [PubMed]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Su, D.; Hou, F.; Liu, L.; Huang, F.; Dong, L.; Deng, Y.; Zhang, Y.; Wei, Z.; Zhang, M. Optimized ultra-high-pressure-assisted extraction of procyanidins from lychee pericarp improves the antioxidant activity of extracts. Biosci. Biotechnol. Biochem. 2017, 81, 1576–1585. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Wang, Y. Fourteen microsatellite loci for the Chinese medicinal plant Epimedium sagittatum and cross-species application in other medicinal species. Mol. Ecol. Resour. 2008, 8, 640–642. [Google Scholar] [CrossRef]
- Mihaljević, S.; Vršek, I. In vitro shoot regeneration from immature seeds of Epimedium alpinum induced by thidiazuron and CPPU. Sci. Hortic. 2009, 120, 406–410. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Li, X.; Huang, W.; Wang, Y.; Wang, J.; Zhang, Y.; Yang, X.; Yan, X.; Wang, Y.; et al. Complete biosynthesis of the potential medicine icaritin by engineered Saccharomyces cerevisiae and Escherichia coli. Sci. Bull. 2021, 66, 1906–1916. [Google Scholar] [CrossRef]
- Yin, T.; Li, Y.F.; Xu, N.; Meng, F.Y. Current situation and thinking of artificial cultivation of Epimedium. Lishizhen Med. Mater. Medica Res. 2020, 31, 1468–1471. [Google Scholar]
- Zhang, S.; Lu, C.; Cao, S.; Li, Q.; Wu, G.; Zhao, L. Efficient production of icariin and baohuoside I from Epimedium Folium flavonoids by fungal α-l-rhamnosidase hydrolysing regioselectively the terminal rhamnose of epimedin C. Biotechnol. Biofuels Bioprod. 2023, 16, 107. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, M.; Zhou, J.; Chen, Y.; Xu, L.; Wu, M.; Xia, G.; Tam, J.P.; Yu, J.; Teng, X.; et al. Eco-efficient biphasic enzymatic hydrolysis for the green production of rare baohuoside I. Enzyme Microb. Technol. 2019, 131, 109431. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, J.; Zhang, N.; Xu, H.; Yang, J.; Ye, J.; Jiang, J. Efficient production of isoquercitin, icariin and icariside II by a novel thermostable α-l-rhamnosidase PodoRha from Paenibacillus odorifer with high α-1,6-/α-1,2-glycoside specificity. Enzyme Microb. Technol. 2022, 158, 110039. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Zhao, L.; Pei, J.; Wang, Z.; Xiao, W. Highly efficient biotransformation of astragaloside IV to cycloastragenol by sugar-stimulated β-glucosidase and β-xylosidase from Dictyoglomus thermophilum. J. Microbiol. Biotechnol. 2019, 29, 1882–1893. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Z.; Sun, E.; Li, S.; Jia, X. Statistically designed enzymatic hydrolysis of an icariin/β-cyclodextrin inclusion complex optimized for production of icaritin. Acta Pharm. Sin. B 2012, 2, 83–89. [Google Scholar] [CrossRef]
- Liu, C.; Li, R.; Peng, J.; Qu, D.; Huang, M.; Chen, Y. Enhanced hydrolysis and antitumor efficacy of Epimedium flavonoids mediated by immobilized snailase on silica. Process Biochem. 2019, 86, 80–88. [Google Scholar] [CrossRef]
- Lou, H.; Liu, X.; Liu, S.; Chen, Q. Purification and characterization of a novel α-l-rhamnosidase from Papiliotrema laurentii ZJU-L07 and its application in production of icariin from epimedin C. J. Fungi 2022, 8, 644. [Google Scholar] [CrossRef]
- Lyu, Y.; Zeng, W.; Du, G.; Chen, J.; Zhou, J. Efficient bioconversion of epimedin C to icariin by a glycosidase from Aspergillus nidulans. Bioresour. Technol. 2019, 289, 121612. [Google Scholar] [CrossRef]
- Wu, T.; Pei, J.; Ge, L.; Wang, Z.; Ding, G.; Xiao, W.; Zhao, L. Characterization of a α-l-rhamnosidase from Bacteroides thetaiotaomicron with high catalytic efficiency of epimedin C. Bioorg. Chem. 2018, 81, 461–467. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, H.; Cui, H.; Cheng, J.; Wang, W.; Wei, B.; Liu, F.; Liang, H.; Shen, X.; Yuan, Q. A novel α-l-Rhamnosidase renders efficient and clean production of icaritin. J. Clean. Prod. 2022, 341, 130903. [Google Scholar] [CrossRef]
- Li, Q.; Ge, L.; Zheng, D.; Zhang, X.; Zhao, L. Screening and characterization of a GH78 α-l-rhamnosidase from Aspergillus terreus and its application in the bioconversion of icariin to icaritin with recombinant β-glucosidase. Enzyme Microb. Technol. 2022, 153, 109940. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, D.; Zhao, L.; Pei, J.; Xiao, W.; Ding, G.; Wang, Z. Overexpression and characterization of a Ca(2+) activated thermostable β-glucosidase with high ginsenoside Rb1 to ginsenoside 20(S)-Rg3 bioconversion productivity. J. Ind. Microbiol. Biotechnol. 2015, 42, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.C.; Chen, J.; Li, D. Crystal structure and biological implications of a glycoside hydrolase family 55 β-1,3-glucanase from Chaetomium thermophilum. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Yang, J.; Zhang, T.; Yang, Y.S.; Ding, Y. Optimized biotransformation of icariin into icariside II by β-glucosidase from Trichoderma viride using central composite design method. Biomed. Res. Int. 2016, 2016, 5936947. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, L.; Zhang, L.; Xiao, H. Baohuoside I production through enzyme hydrolysis and parameter optimization by using response surface and subset selection. J. Mol. Catal. B. Enzym. 2013, 90, 132–138. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, Z.; Sun, E.; Jia, X.; Qian, Q. Effect of β-cyclodextrin complexation on solubility and enzymatic hydrolysis rate of icariin. J. Nat. Sci. Biol. Med. 2013, 4, 201–206. [Google Scholar] [PubMed]
- Su, J.; Wu, T.; Cao, S.; Pei, J.; Zhao, L. Screening and characterization of a β-xylosidase from Bifidobacterium breve K-110 and its application in the biotransformation of the total flavonoids of epimedium to icariin with α-l-rhamnosidase. Bioorg. Chem. 2023, 132, 106364. [Google Scholar] [CrossRef]
- Tong, X.; Qi, Z.; Zheng, D.; Pei, J.; Li, Q.; Zhao, L. High-level expression of a novel multifunctional GH3 family β-xylosidase/α-arabinosidase/β-glucosidase from Dictyoglomus turgidum in Escherichia coli. Bioorg. Chem. 2021, 111, 104906. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Jia, X.; Tam, J.P.; Yang, H.; Shen, Y.; Li, X. Multidisciplinary strategies to enhance therapeutic effects of flavonoids from Epimedii Folium: Integration of herbal medicine, enzyme engineering, and nanotechnology. J. Pharm. Anal. 2023, 13, 239–254. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- De Carvalho, C.C. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotechnol. 2016, 42, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, L.; Zhang, M.; Wei, Y.; Lin, W. Recent advances on feasible strategies for monoterpenoid production in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 609800. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, M.; Guan, Z.; Jin, C.Y.; Lin, W.; Ji, X.J.; Wei, Y. Metabolic engineering for glycyrrhetinic acid production in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 588255. [Google Scholar] [CrossRef]
- Ro, D.K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef]
- Galanie, S.; Thodey, K.; Trenchard, I.J.; Filsinger Interrante, M.; Smolke, C.D. Complete biosynthesis of opioids in yeast. Science 2015, 349, 1095–1100. [Google Scholar] [CrossRef]
- Chen, X.J.; Tang, Z.H.; Li, X.W.; Xie, C.X.; Lu, J.J.; Wang, Y.T. Chemical constituents, quality control, and bioactivity of Epimedii folium (Yinyanghuo). Am. J. Chin. Med. 2015, 43, 783–834. [Google Scholar] [CrossRef]
- Szabó, R.; Rácz, C.P.; Dulf, F.V. Bioavailability improvement strategies for icariin and its derivates: A review. Int. J. Mol. Sci. 2022, 23, 7519. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, K.; Yue, R.; Lei, M. Therapeutic effects of icariin and icariside II on diabetes mellitus and its complications. Crit. Rev. Food Sci. Nutr. 2023, 1–26. [Google Scholar] [CrossRef]
- Tan, H.L.; Chan, K.G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Lee, L.-H.; Goh, B.-H. Anti-cancer properties of the naturally occurring aphrodisiacs: Icariin and its derivatives. Front. Pharmacol. 2016, 7, 191. [Google Scholar] [CrossRef]
- Yang, X.; Lang, S.; Li, S.; Jiang, C.; Han, J. Preparation of icariside I and icariside II, an exploration of their protective mechanism against cyclophosphamide-induced bone marrow suppression in mice, and their regulatory effects on immune function. Pharmazie 2022, 77, 32–37. [Google Scholar] [PubMed]
- Zhang, D.; Zhao, N.; Wan, C.; Du, J.; Lin, J.; Wang, H. Icariin and icariside II reciprocally stimulate osteogenesis and inhibit adipogenesis of multipotential stromal cells through ERK signaling. Evid. Based Complement. Altern. Med. 2021, 2021, 8069930. [Google Scholar] [CrossRef] [PubMed]
- Son, N.; Shi, L.; Li, Y.; Wang, Q.A. Total synthesis of icaritin via microwave-assistance Claisen rearrangement. Lett. Org. Chem. 2014, 11, 677–681. [Google Scholar]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The application of fermentation technology in traditional Chinese medicine: A review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, 2, e93. [Google Scholar] [CrossRef]
- Xie, Y.; Ya, H.; Zhang, X.; Fan, Y.; Zhao, B.; Wang, R.; Yang, Z. Biotransformation of flavonoid glycosides in Epimedium by Lactobacillus plantarum. China Brew. 2022, 41, 103–109. [Google Scholar]
| Epimedium Flavonoids | Research Systems | Mechanisms | Reference |
|---|---|---|---|
| Alcohol extracts of E. koreanum Nakai and E. wushanense T.S. Ying | SD rats | Compared with the normal group, animal groups treated with EF extracts showed severer hepatotoxicity, which was positively correlated with the dose and course. Additionally, the females experienced more significant damage compared to the males. | [90] |
| Icariside I and sagittatoside A | HL-7702 and HepG2 cells | Icariside I could destroy the cell structure and cause oxidative stress. Sagittatoside A could cause oxidative stress and damage to mitochondria. | [91] |
| Epimedin C | Male Balb/c mice | Epigenetic modification changed in mouse liver after epimedin C treatment with a test dose, and the m6A and m5C may be associated with epimedin C-induced liver injury. | [92] |
| Baohuoside I | HL-7702 and HepG2 cells | The toxicity mechanism(s) of baohuoside I may be involved in increasing oxidative stress and inducing apoptosis. | [93] |
| E. koreanum Nakai ethanol extract | Male Sprague Dawley rats | The mechanism of hepatotoxicity of E. koreanum Nakai was probably related to the induction of ferroptosis in hepatocytes. | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tang, B.; Wen, S.; Wang, Y.; Pan, C.; Qu, L.; Yin, Y.; Wei, Y. Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium. Molecules 2023, 28, 7173. https://doi.org/10.3390/molecules28207173
Zhang X, Tang B, Wen S, Wang Y, Pan C, Qu L, Yin Y, Wei Y. Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium. Molecules. 2023; 28(20):7173. https://doi.org/10.3390/molecules28207173
Chicago/Turabian StyleZhang, Xiaoling, Bingling Tang, Sijie Wen, Yitong Wang, Chengxue Pan, Lingbo Qu, Yulong Yin, and Yongjun Wei. 2023. "Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium" Molecules 28, no. 20: 7173. https://doi.org/10.3390/molecules28207173
APA StyleZhang, X., Tang, B., Wen, S., Wang, Y., Pan, C., Qu, L., Yin, Y., & Wei, Y. (2023). Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium. Molecules, 28(20), 7173. https://doi.org/10.3390/molecules28207173








