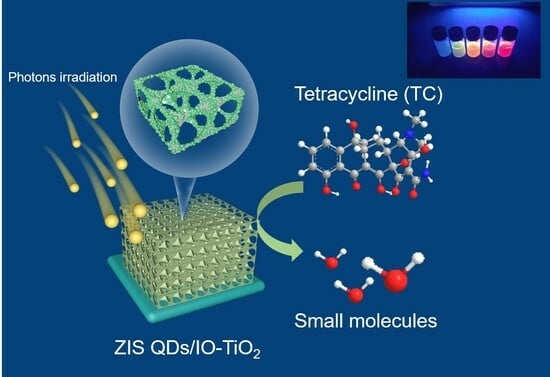
Enhancing the Photocatalytic Performance of Antibiotics Using a Z-Scheme Heterojunction of 0D ZnIn2S4 Quantum Dots and 3D Hierarchical Inverse Opal TiO2
Abstract
:1. Introduction
2. Results and Discussion
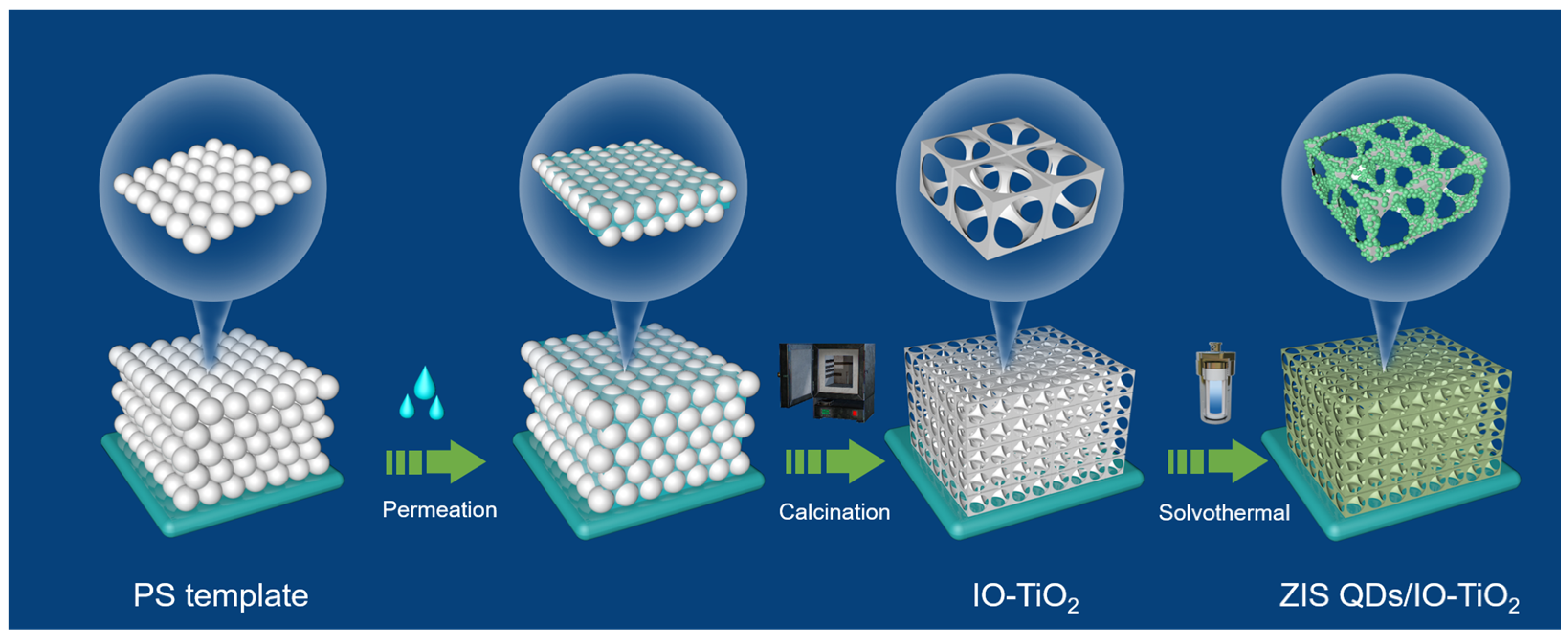
2.1. Fabrication Procedure of As-Prepared IO-TiO2
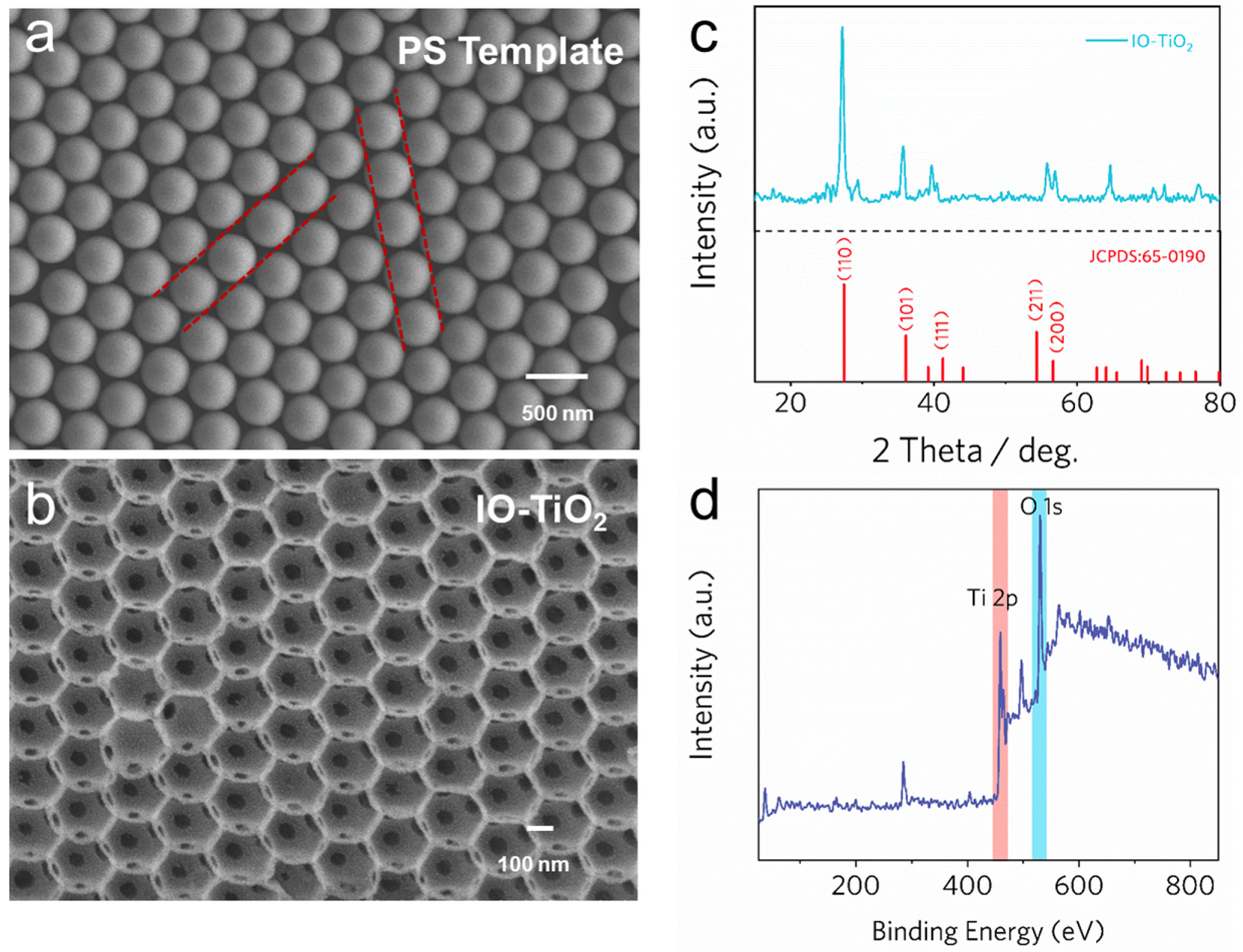
2.2. Characterizations of As-Prepared IO-TiO2
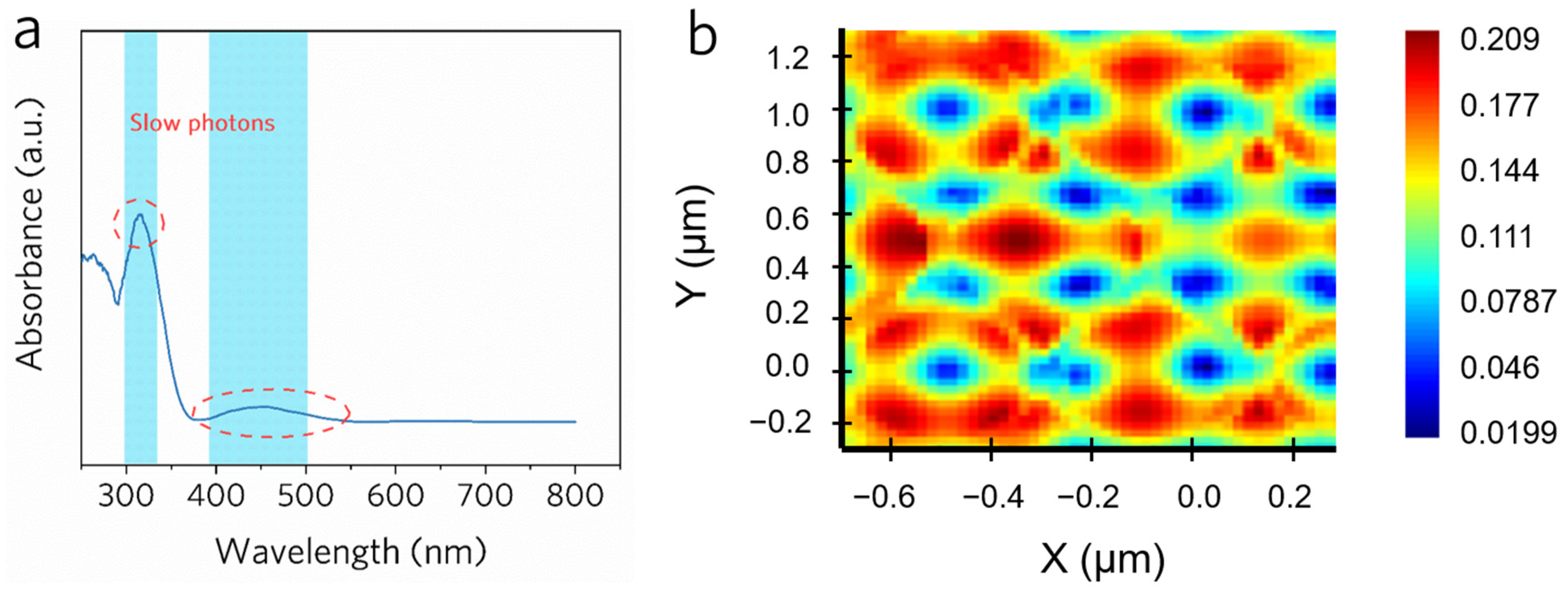
2.3. Proof of the Slow-Photon Effect
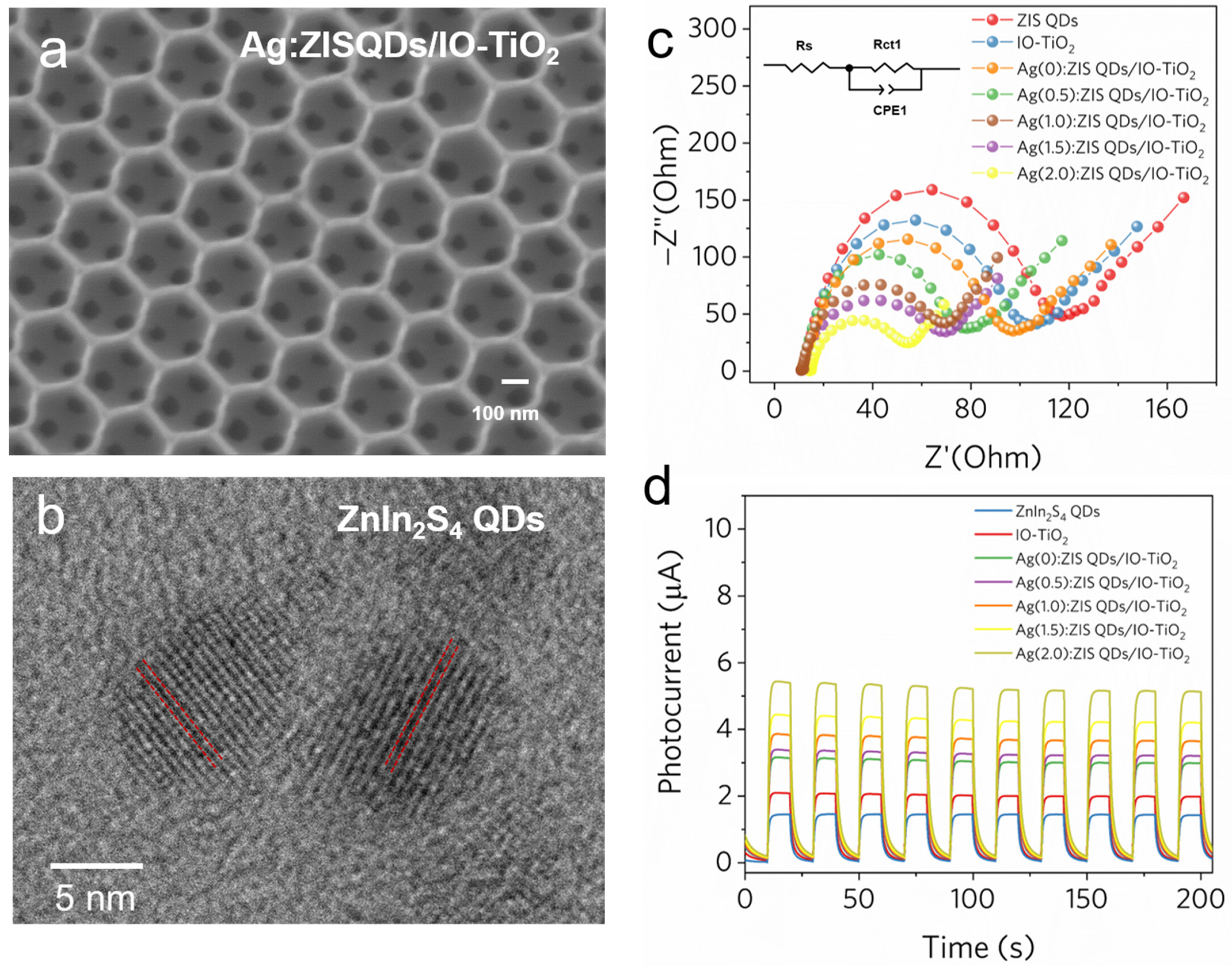
2.4. Characterizations of As-Prepared Ag-ZIS QDs/IO-TiO2
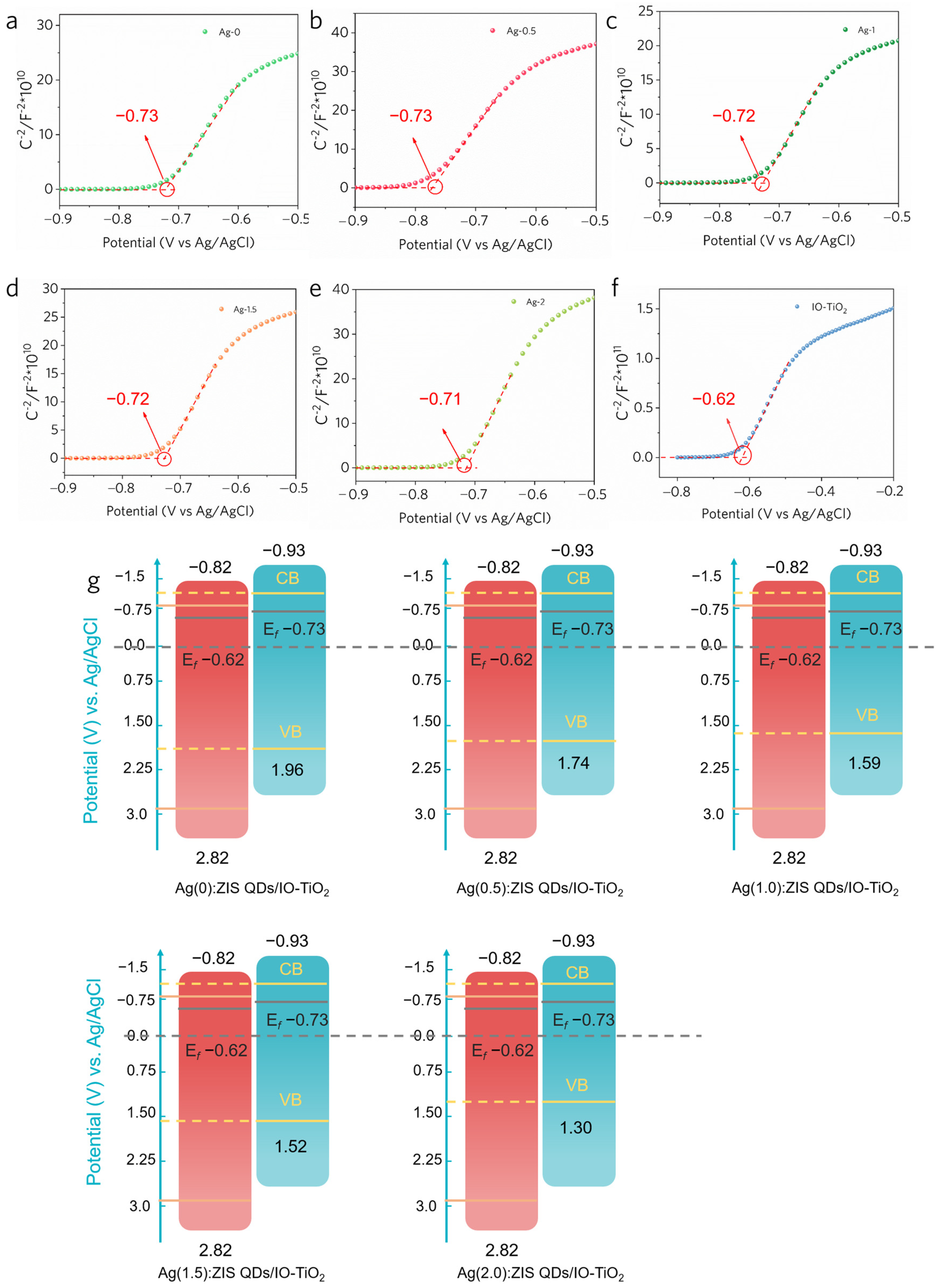
2.5. Band Structure of an Internal Electric Field
2.6. Photocatalytic Activity
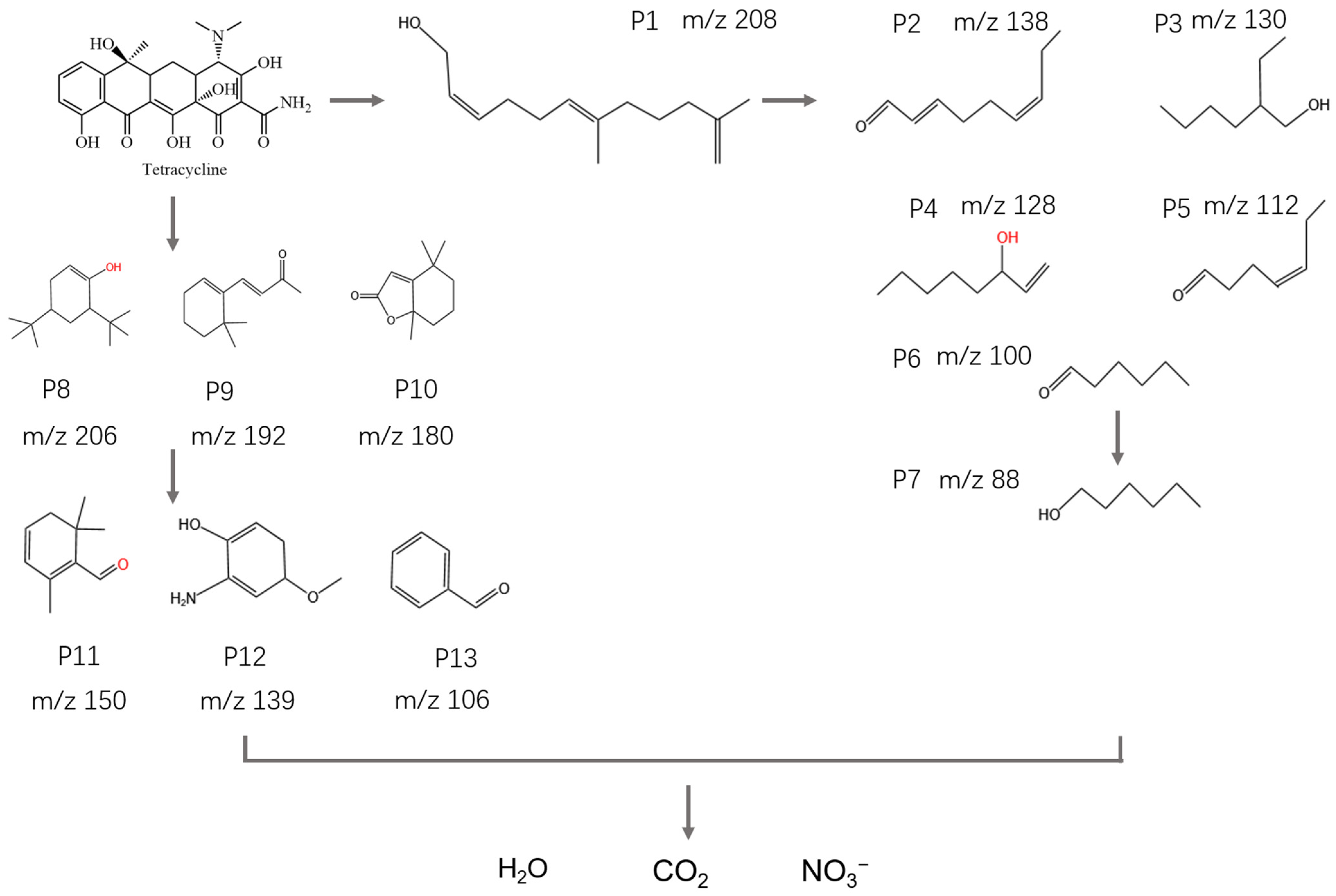
2.7. A Possible TC Photo-Degradation Pathway
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Synthesis of Ag: ZnIn2S4 Quantum Dots (Ag: ZIS QDs)
3.3. Synthesis of PS Microspheres
3.4. Fabrication of Ordered PS Array Film Templates
3.5. Synthesis of IO-TiO2 Structure
3.6. Photocatalytic Activity Test
3.7. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Xie, W.; Du, A.; Pan, D.; Guo, Z. Overview of electrocatalytic treatment of antibiotic pollutants in wastewater. Catal. Rev. 2023, 65, 569–619. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Hau Ng, Y.; Sik Ok, Y. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wang, L.; Cheng, X.; Shang, Q. Rapid removal of phenol/antibiotics in water by Fe-(8-hydroxyquinoline-7-carboxylic)/TiO2 flower composite: Adsorption combined with photocatalysis. Chem. Eng. J. 2020, 402, 126260. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Z.; Yu, G.; Wu, H.; Chen, H.; Zhou, L.; Zhang, Y.; Su, Y.; Tan, S.; Yang, L.; et al. A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere 2021, 272, 129501. [Google Scholar] [CrossRef]
- Lei, J.; Chen, B.; Zhou, L.; Ding, N.; Cai, Z.; Wang, L.; In, S.-I.; Cui, C.; Zhou, Y.; Liu, Y.; et al. Efficient degradation of antibiotics in different water matrices through the photocatalysis of inverse opal K-g-C3N4: Insights into mechanism and assessment of antibacterial activity. Chem. Eng. J. 2020, 400, 125902. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Wang, H.; Chen, Y.; Luo, C.; Liang, D.; Hu, B.; Qiu, R.; Yan, K. 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl. Catal. B 2020, 277, 119171. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dai, R.; Chen, M.; Khan, S.J.; Wang, Z. Applications of membrane bioreactors for water reclamation: Micropollutant removal, mechanisms and perspectives. Bioresour. Technol. 2018, 269, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Huang, J.; Zeng, G.; Shi, L.; Shi, Y.; Yi, K. Fate of pharmaceuticals during membrane bioreactor treatment: Status and perspectives. Bioresour. Technol. 2018, 268, 733–748. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Yang, X.; Zhang, T.; Tang, H. 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B 2018, 232, 562–573. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. A review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Mustafa, F.S. Bismuth-based nanostructured photocatalysts for the remediation of antibiotics and organic dyes. Beilstein J. Nanotechnol. 2023, 14, 291–321. [Google Scholar] [CrossRef]
- Azalok, K.A.; Oladipo, A.A.; Gazi, M. UV-light-induced photocatalytic performance of reusable MnFe-LDO–biochar for tetracycline removal in water. J. Photochem. Photobiol. A 2021, 405, 112976. [Google Scholar] [CrossRef]
- Lyu, J.; Shao, J.; Wang, Y.; Qiu, Y.; Li, J.; Li, T.; Peng, Y.; Liu, F. Construction of a porous core-shell homojunction for the photocatalytic degradation of antibiotics. Chem. Eng. J 2019, 358, 614–620. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Zhan, Z.; Wang, H.; Huang, Q.; Li, S.; Yi, X.; Tang, Q.; Wang, J.; Tan, B. Grafting Hypercrosslinked Polymers on TiO2 Surface for Anchoring Ultrafine Pd Nanoparticles: Dramatically Enhanced Efficiency and Selectivity toward Photocatalytic Reduction of CO2 to CH4. Small 2022, 18, 2105083. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Wu, S.; Tan, X.; Lei, J.; Chen, H.; Wang, L.; Zhang, J. Ga-Doped and Pt-Loaded Porous TiO2–SiO2 for Photocatalytic Nonoxidative Coupling of Methane. J. Am. Chem. Soc. 2019, 141, 6592–6600. [Google Scholar] [CrossRef]
- Ran, L.; Qiu, S.; Zhai, P.; Li, Z.; Gao, J.; Zhang, X.; Zhang, B.; Wang, C.; Sun, L.; Hou, J. Conformal Macroporous Inverse Opal Oxynitride-Based Photoanode for Robust Photoelectrochemical Water Splitting. J. Am. Chem. Soc. 2021, 143, 7402–7413. [Google Scholar] [CrossRef]
- Xu, L.; Aumaitre, C.; Kervella, Y.; Lapertot, G.; Rodríguez-Seco, C.; Palomares, E.; Demadrille, R.; Reiss, P. Increasing the Efficiency of Organic Dye-Sensitized Solar Cells over 10.3% Using Locally Ordered Inverse Opal Nanostructures in the Photoelectrode. Adv. Funct. Mater. 2018, 28, 1706291. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Wu, M.; Van der Schueren, B.; Li, Y.; Deparis, O.; Ye, J.; Ozin, G.A.; Hasan, T.; Su, B.-L. Slow Photons for Photocatalysis and Photovoltaics. Adv. Mater. 2017, 29, 1605349. [Google Scholar] [CrossRef]
- Eftekhari, E.; Broisson, P.; Aravindakshan, N.; Wu, Z.; Cole, I.S.; Li, X.; Zhao, D.; Li, Q. Sandwich-structured TiO2 inverse opal circulates slow photons for tremendous improvement in solar energy conversion efficiency. J. Mater. Chem. A 2017, 5, 12803–12810. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, R.; Yin, L. Slow-Photon-Effect-Induced Photoelectrical-Conversion Efficiency Enhancement for Carbon-Quantum-Dot-Sensitized Inorganic CsPbBr3 Inverse Opal Perovskite Solar Cells. Adv. Mater. 2017, 29, 1703682. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jiao, J.; Zhao, Z.; Liu, J.; Li, J.; Jiang, G.; Wang, Y.; Duan, A. Fabrication of inverse opal TiO2-supported Au@CdS core–shell nanoparticles for efficient photocatalytic CO2 conversion. Appl. Catal. B 2015, 179, 422–432. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef]
- Luo, Z.; Ye, X.; Zhang, S.; Xue, S.; Yang, C.; Hou, Y.; Xing, W.; Yu, R.; Sun, J.; Yu, Z.; et al. Unveiling the charge transfer dynamics steered by built-in electric fields in BiOBr photocatalysts. Nat. Commun. 2022, 13, 2230. [Google Scholar] [CrossRef]
- Zikalala, N.; Parani, S.; Tsolekile, N.; Oluwafemi, O.S. Facile green synthesis of ZnInS quantum dots: Temporal evolution of their optical properties and cell viability against normal and cancerous cells. J. Mater. Chem. C 2020, 8, 9329–9336. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Si, Y.; Ma, C.; Yu, J.; Liu, Y.-T.; Ding, B. Boron-induced sulfur vacancies in ZnIn2S4 nanosheets coupled to TiO2 nanofibers enhance the hydrogen evolution performance. Compos. Commun. 2021, 27, 100903. [Google Scholar] [CrossRef]
- Alias, S.S.; Harun, Z.; Azhar, F.H.; Ibrahim, S.A.; Johar, B. Comparison between commercial and synthesised nano flower-like rutile TiO2 immobilized on green super adsorbent towards dye wastewater treatment. J. Clean. Prod. 2020, 251, 119448. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Q.; Lv, K.; Li, M. Heterojunction construction between TiO2 hollowsphere and ZnIn2S4 flower for photocatalysis application. Appl. Surf. Sci. 2017, 398, 81–88. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, K.; Wang, X.; Guo, Q.; Wu, Y.; Du, Y.; Zhang, L.; Xia, J.; Tang, H.; Zhang, X.; et al. 0D/2D Schottky junction synergies with 2D/2D S-scheme heterojunction strategy to achieve uniform separation of carriers in 0D/2D/2D quasi CNQDs/TCN/ZnIn2S4 towards photocatalytic remediating petroleum hydrocarbons polluted marine. Appl. Catal. B 2023, 325, 122387. [Google Scholar] [CrossRef]
- Wang, T.; Nie, C.; Ao, Z.; Wang, S.; An, T. Recent progress in g-C3N4 quantum dots: Synthesis, properties and applications in photocatalytic degradation of organic pollutants. J. Mater. Chem. A 2020, 8, 485–502. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, T.; Zhao, X.; Yao, W.; Fang, R.; Shen, K.; Li, Y. Ordered Macroporous Carbonous Frameworks Implanted with CdS Quantum Dots for Efficient Photocatalytic CO2 Reduction. Adv. Mater. 2021, 33, 2102690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-B.; Bao, N.; Zhang, Q.; Ding, S.-N. Synergistically Enhanced Photocatalytic Degradation by Coupling Slow-Photon Effect with Z-Scheme Charge Transfer in CdS QDs/IO-TiO2 Heterojunction. Molecules 2023, 28, 5437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, X.; Qu, Y.; Zhou, F.; Wang, Z.; Wang, W.; Zhao, C.; Wang, H.; Li, L.; Yao, Y.; et al. A hierarchical heterostructure of CdS QDs confined on 3D ZnIn2S4 with boosted charge transfer for photocatalytic CO2 reduction. Nano Res. 2021, 14, 81–90. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, S.; Chang, X.; Hu, J.; Wang, X. Effect of the combination of inverse opal photon superficial area crystal and heterojunction on active free radicals and its effect on the mechanism of photocatalytic removal of organic pollutants. Ceram. Int. 2023, 49, 27107–27116. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Chougule, S.S.; Jung, N.; Yu, Y.-J.; Oh, J.-E.; Kim, M.-D. Plasmonic Pt nanoparticles triggered efficient charge separation in TiO2/GaN NRs hybrid heterojunction for the high-performance self-powered UV photodetectors. Appl. Surf. Sci. 2022, 594, 153474. [Google Scholar] [CrossRef]
- Guo, P.; Zhao, F.; Hu, X. Fabrication of a direct Z-scheme heterojunction between MoS2 and B/Eu-g-C3N4 for an enhanced photocatalytic performance toward tetracycline degradation. J. Alloys Compd. 2021, 867, 159044. [Google Scholar] [CrossRef]
- Gong, G.; Liu, Y.; Mao, B.; Tan, L.; Yang, Y.; Shi, W. Ag doping of Zn-In-S quantum dots for photocatalytic hydrogen evolution: Simultaneous bandgap narrowing and carrier lifetime elongation. Appl. Catal. B 2017, 216, 11–19. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Xi, Z.; Wang, L.; Zhang, J. Enhanced photocatalytic performance of TiO2 based on synergistic effect of Ti3+ self-doping and slow light effect. Appl. Catal. B 2014, 160–161, 621–628. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.-B.; Ding, S.-N. Enhancing the Photocatalytic Performance of Antibiotics Using a Z-Scheme Heterojunction of 0D ZnIn2S4 Quantum Dots and 3D Hierarchical Inverse Opal TiO2. Molecules 2023, 28, 7174. https://doi.org/10.3390/molecules28207174
Zhu L-B, Ding S-N. Enhancing the Photocatalytic Performance of Antibiotics Using a Z-Scheme Heterojunction of 0D ZnIn2S4 Quantum Dots and 3D Hierarchical Inverse Opal TiO2. Molecules. 2023; 28(20):7174. https://doi.org/10.3390/molecules28207174
Chicago/Turabian StyleZhu, Li-Bang, and Shou-Nian Ding. 2023. "Enhancing the Photocatalytic Performance of Antibiotics Using a Z-Scheme Heterojunction of 0D ZnIn2S4 Quantum Dots and 3D Hierarchical Inverse Opal TiO2" Molecules 28, no. 20: 7174. https://doi.org/10.3390/molecules28207174
APA StyleZhu, L.-B., & Ding, S.-N. (2023). Enhancing the Photocatalytic Performance of Antibiotics Using a Z-Scheme Heterojunction of 0D ZnIn2S4 Quantum Dots and 3D Hierarchical Inverse Opal TiO2. Molecules, 28(20), 7174. https://doi.org/10.3390/molecules28207174