Ring-Opening Reaction of 1-Phospha-2-Azanorbornenes via P-N Bond Cleavage and Reversibility Studies
Abstract
1. Introduction
2. Results and Discussion
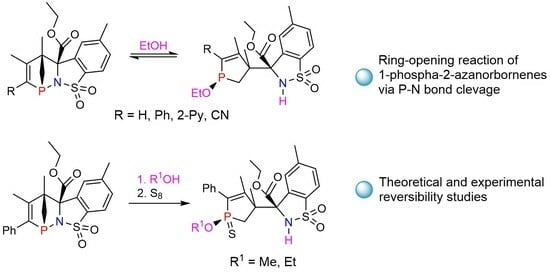
2.1. P-N Bond Cleavage of endo-1b with Alcohols
2.2. Reversibility Studies
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis
4.3. Reversibility Studies
4.4. Computational Details
4.5. X-ray Crystallography Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Li, C.; Geng, D.; Chen, H.; Duan, Z.; Mathey, F. A new versatile route for the conversion of phospholes into phosphinines. Chem. Eur. J. 2010, 16, 10659–10661. [Google Scholar] [CrossRef]
- Luo, H.; Wang, J.; Tian, R.; Duan, Z. 2H-Phosphindole-Enabled Dearomatization and 4+2 Cycloaddition of (Hetero)Arenes. Chem. Eur. J. 2023, e202301898. [Google Scholar] [CrossRef]
- Duffy, M.P.; Delaunay, W.; Bouit, P.-A.; Hissler, M. π-Conjugated phospholes and their incorporation into devices: Components with a great deal of potential. Chem. Soc. Rev. 2016, 45, 5296–5310. [Google Scholar] [CrossRef] [PubMed]
- König, N.; Godínez-Loyola, Y.; Yang, F.; Laube, C.; Laue, M.; Lönnecke, P.; Strassert, C.A.; Hey-Hawkins, E. Facile modification of phosphole-based aggregation-induced emission luminogens with sulfonyl isocyanates. Chem. Sci. 2023, 14, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Mathey, F. The organic chemistry of phospholes. Chem. Rev. 1988, 88, 429–453. [Google Scholar] [CrossRef]
- Johannsen, T.; Golz, C.; Alcarazo, M. α-Cationic Phospholes: Synthesis and Applications as Ancillary Ligands. Angew. Chem. Int. Ed. Engl. 2020, 59, 22779–22784. [Google Scholar] [CrossRef]
- Mathey, F. Transient 2H-phospholes as powerful synthetic intermediates in organophosphorus chemistry. Acc. Chem. Res. 2004, 37, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Möller, T.; Wonneberger, P.; Sárosi, M.B.; Coburger, P.; Hey-Hawkins, E. P-chiral 1-phosphanorbornenes: From asymmetric phospha-Diels-Alder reactions towards ligand design and functionalisation. Dalton Trans. 2016, 45, 1904–1917. [Google Scholar] [CrossRef]
- Wonneberger, P.; König, N.; Kraft, F.B.; Sárosi, M.B.; Hey-Hawkins, E. Access to 1-Phospha-2-azanorbornenes by Phospha-aza-Diels-Alder Reactions. Angew. Chem. Int. Ed. Engl. 2019, 58, 3208–3211. [Google Scholar] [CrossRef]
- Toullec, P.; Ricard, L.; Mathey, F. Hetero-Diels-Alder reactions of 2H-phospholes with aldehydes. J. Org. Chem. 2003, 68, 2803–2806. [Google Scholar] [CrossRef]
- Jia, S.; Ma, M.; Li, E.-Q.; Duan, Z.; Mathey, F. Design of 1-Phosphanorbornene Derivatives as Chiral Organocatalysts for Enantioselective (4 + 2) Annulation Reactions of γ-Benzyl Allenoates. Org. Lett. 2021, 23, 3337–3342. [Google Scholar] [CrossRef]
- Gan, Z.; Zhi, M.; Han, R.; Li, E.-Q.; Duan, Z.; Mathey, F. P-Stereogenic Phosphines Directed Copper(I)-Catalyzed Enantioselective 1,3-Dipolar Cycloadditions. Org. Lett. 2019, 21, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Q.; Wei, D.; Tian, R.; Duan, Z. Hetero-Diels–Alder reactions of 2H-phospholes with allenes: Synthesis and functionalization of 6-methylene-1-phosphanorbornenes. Org. Chem. Front. 2021, 8, 3740–3745. [Google Scholar] [CrossRef]
- Robin, F.; Lelièvre, S.; Mercier, F.; Ricard, L.; Mathey, F. 1-Phosphanorbornadienes in Enantioselective Catalysis. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 1371–1374. [Google Scholar] [CrossRef]
- Holand, S.; Mathey, F. Introducing new phosphorus substituents in terminal phosphinidene complexes. An illustration with [(ethoxycarbonyl)phosphinidene]-, (tert-butoxyphosphinidene)-, and (fluorenylphosphinidene)pentacarbonyltungsten complexes. Organometallics 1988, 7, 1796–1801. [Google Scholar] [CrossRef]
- Holand, S.; Jeanjean, M.; Mathey, F. A Straightforward Access to α-Functional Phospholide Ions. Angew. Chem. Int. Ed. Engl. 1997, 36, 98–100. [Google Scholar] [CrossRef]
- Breque, A.; Mathey, F.; Savignac, P. An Improved One-Pot Synthesis of Phospholes. Synthesis 1981, 1981, 983–985. [Google Scholar] [CrossRef]
- Mathey, F.; Mercier, F.; Robin, F.; Ricard, L. From 2H-phospholes to BIPNOR, a new efficient biphosphine for asymmetric catalysis. J. Organomet. Chem. 1998, 557, 117–120. [Google Scholar] [CrossRef]
- Krishnamurthy, S.S. Phosphazenes and Phosphazanes—The Nature of the P-N Bond. Phosphorus Sulfur Silicon Relat. Elem. 1994, 87, 101–111. [Google Scholar] [CrossRef]
- Emsley, J.; Williams, J.K. The phosphorus–nitrogen bond. Synthesis, characterization, and infrared studies of heterocyclic phosphoryl (phosphetan) amides. J. Chem. Soc. Dalton Trans. 1973, 1576–1581. [Google Scholar] [CrossRef]
- Arias, A.; Forniés, J.; Fortuño, C.; Ibáñez, S.; Martín, A.; Mastrorilli, P.; Gallo, V.; Todisco, S. Addition of nucleophiles to phosphanido derivatives of Pt(III): Formation of P-C, P-N, and P-O bonds. Inorg. Chem. 2013, 52, 11398–11408. [Google Scholar] [CrossRef]
- Marinetti, A.; Carmichael, D. Synthesis and properties of phosphetanes. Chem. Rev. 2002, 102, 201–230. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Cuervo, P.M.; García, M.E.; Ruiz, M.A.; Vega, P. P-C, P-N, and M-N Bond Formation Processes in Reactions of Heterometallic Phosphinidene-Bridged MoMn and MoRe Complexes with Diazoalkanes and Organic Azides to Build Three- to Five-Membered Phosphametallacycles. Inorg. Chem. 2022, 61, 18486–18495. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. Definition of the Pnictogen Bond: A Perspective. Inorganics 2022, 10, 149. [Google Scholar] [CrossRef]
- Bai, Z.; Song, F.; Wang, H.; Cheng, W.; Zhu, S.; Huang, Y.; He, G.; Chen, G. Nitrene-Mediated P–N Coupling Under Iron Catalysis. CCS Chem. 2022, 4, 2258–2266. [Google Scholar] [CrossRef]
- Ding, T.-T.; Qin, C.-C.; Gao, D.-D.; Li, X.-J.; Yan, H.; Liu, R.-M.; Zhao, C.-Q.; Nie, S.-Z. Stereoselective formation of P-N bonds via coupling of H-P species with amines and the addition of Grignard reagents to chiral N-phosphinoylimines. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 102–110. [Google Scholar] [CrossRef]
- Albuerne, I.G.; Alvarez, M.A.; García, M.E.; García-Vivó, D.; Ruiz, M.A.; Vega, P. P-N and N-Mo Bond Formation Processes in the Reactions of a Pyramidal Phosphinidene-Bridged Dimolybdenum Complex with Diazoalkanes and Organic Azides. Inorg. Chem. 2020, 59, 7869–7883. [Google Scholar] [CrossRef]
- Misiura, K.; Silverton, J.V.; Stec, W.J. Stereochemistry of phosphorus-nitrogen bond cleavage. First crystal and structural assignment in cyclic phosphoramidofluoridates. J. Org. Chem. 1985, 50, 1815–1818. [Google Scholar] [CrossRef]
- Gurnani, C.; Đorđević, N.; Muthaiah, S.; Dimić, D.; Ganguly, R.; Petković, M.; Vidović, D. Extending the chemistry of carbones: P-N bond cleavage via an S(N)2′-like mechanism. Chem. Commun. 2015, 51, 10762–10764. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, K.; Stec, W.J. The Stereochemistry of P-N Bond Cleavage in the Staudinger-Wittig-type Reaction of 2-Anilido-3,4-dimethyl-5-phenyl-1,3,2-oxazaphospholidine-2-thiones. Z. Naturforsch. B 1978, 33, 782–785. [Google Scholar] [CrossRef][Green Version]
- Han, Z.S.; Goyal, N.; Herbage, M.A.; Sieber, J.D.; Qu, B.; Xu, Y.; Li, Z.; Reeves, J.T.; Desrosiers, J.-N.; Ma, S.; et al. Efficient asymmetric synthesis of P-chiral phosphine oxides via properly designed and activated benzoxazaphosphinine-2-oxide agents. J. Am. Chem. Soc. 2013, 135, 2474–2477. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, R.; Blanchfield, J.R. The cleavage of phosphorus to nitrogen bonds with hydrogen fluoride. Can. J. Chem. 1966, 44, 501–504. [Google Scholar] [CrossRef]
- Ramazanova, K.; Lönnecke, P.; Hey-Hawkins, E. Facile Synthesis of Enantiomerically Pure P-Chiral 1-Alkoxy-2,3-dihydrophospholes via Nucleophilic P-N Bond Cleavage of a 1-Phospha-2-azanorbornene. Chem. Eur. J. 2023, 29, e202300790. [Google Scholar] [CrossRef] [PubMed]
- Wonneberger, P.; König, N.; Sárosi, M.B.; Hey-Hawkins, E. Reductive Rearrangement of a 1-Phospha-2-azanorbornene. Chem. Eur. J. 2021, 27, 7847–7852. [Google Scholar] [CrossRef] [PubMed]
- Kühl, O. Phosphorus-31 NMR Spectroscopy: A Concise Introduction for the Synthetic Organic and Organometallic Chemist, 2008th ed.; Springer: Berlin, Heidelberg, 2009; ISBN 9783540791188. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts. IUPAC Recommendations 2001. Solid State Nucl. Magn. Reson. 2002, 22, 458–483. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Khattab, I.S.; Bandarkar, F.; Fakhree, M.A.A.; Jouyban, A. Density, viscosity, and surface tension of water+ethanol mixtures from 293 to 323K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package–Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B Condens. Matter 1996, 54, 1703–1710. [Google Scholar] [CrossRef]
- Hartwigsen, C.; Goedecker, S.; Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B Condens. Matter 1998, 58, 3641–3662. [Google Scholar] [CrossRef]
- Krack, M. Pseudopotentials for H to Kr optimized for gradient-corrected exchange-correlation functionals. Theor. Chem. Acc. 2005, 114, 145–152. [Google Scholar] [CrossRef]
- VandeVondele, J.; Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Brehm, M.; Thomas, M.; Gehrke, S.; Kirchner, B. TRAVIS-A free analyzer for trajectories from molecular simulation. J. Chem. Phys. 2020, 152, 164105. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Corporation. CrysAlisPro Software System; Rigaku Oxford Diffraction: Wroclaw, Poland, 1995–2023. [Google Scholar]
- Sheldrick, G.M. SHELXT–integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Crystal Impact GbR, version 4.6.8. DIAMOND 4. Brandenburg, K: Bonn, Germany.





| Reaction | ΔG/kcal mol−1 |
|---|---|
| endo-1a → 6a | −0.2 |
| endo-1b → 6b | 0.8 |
| endo-1c → 6c | 0.4 |
| endo-1d → 6d | −1.1 |
| Compound | Gap/eV | Compound | Gap/eV |
|---|---|---|---|
| endo-1a | 12.0 | 6a | 11.3 |
| endo-1b | 10.8 | 6b | 10.5 |
| endo-1c | 10.4 | 6c | 11.1 |
| endo-1d | 11.8 | 6d | 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramazanova, K.; Müller, A.K.; Lönnecke, P.; Hollóczki, O.; Kirchner, B.; Hey-Hawkins, E. Ring-Opening Reaction of 1-Phospha-2-Azanorbornenes via P-N Bond Cleavage and Reversibility Studies. Molecules 2023, 28, 7163. https://doi.org/10.3390/molecules28207163
Ramazanova K, Müller AK, Lönnecke P, Hollóczki O, Kirchner B, Hey-Hawkins E. Ring-Opening Reaction of 1-Phospha-2-Azanorbornenes via P-N Bond Cleavage and Reversibility Studies. Molecules. 2023; 28(20):7163. https://doi.org/10.3390/molecules28207163
Chicago/Turabian StyleRamazanova, Kyzgaldak, Anna Karina Müller, Peter Lönnecke, Oldamur Hollóczki, Barbara Kirchner, and Evamarie Hey-Hawkins. 2023. "Ring-Opening Reaction of 1-Phospha-2-Azanorbornenes via P-N Bond Cleavage and Reversibility Studies" Molecules 28, no. 20: 7163. https://doi.org/10.3390/molecules28207163
APA StyleRamazanova, K., Müller, A. K., Lönnecke, P., Hollóczki, O., Kirchner, B., & Hey-Hawkins, E. (2023). Ring-Opening Reaction of 1-Phospha-2-Azanorbornenes via P-N Bond Cleavage and Reversibility Studies. Molecules, 28(20), 7163. https://doi.org/10.3390/molecules28207163