Effects of Alkaline Hydrogen Peroxide and Cellulase Modifications on the Physicochemical and Functional Properties of Forsythia suspensa Dietary Fiber
Abstract
:1. Introduction
2. Result and Discussion
2.1. Color Analysis
2.2. Structural Analysis
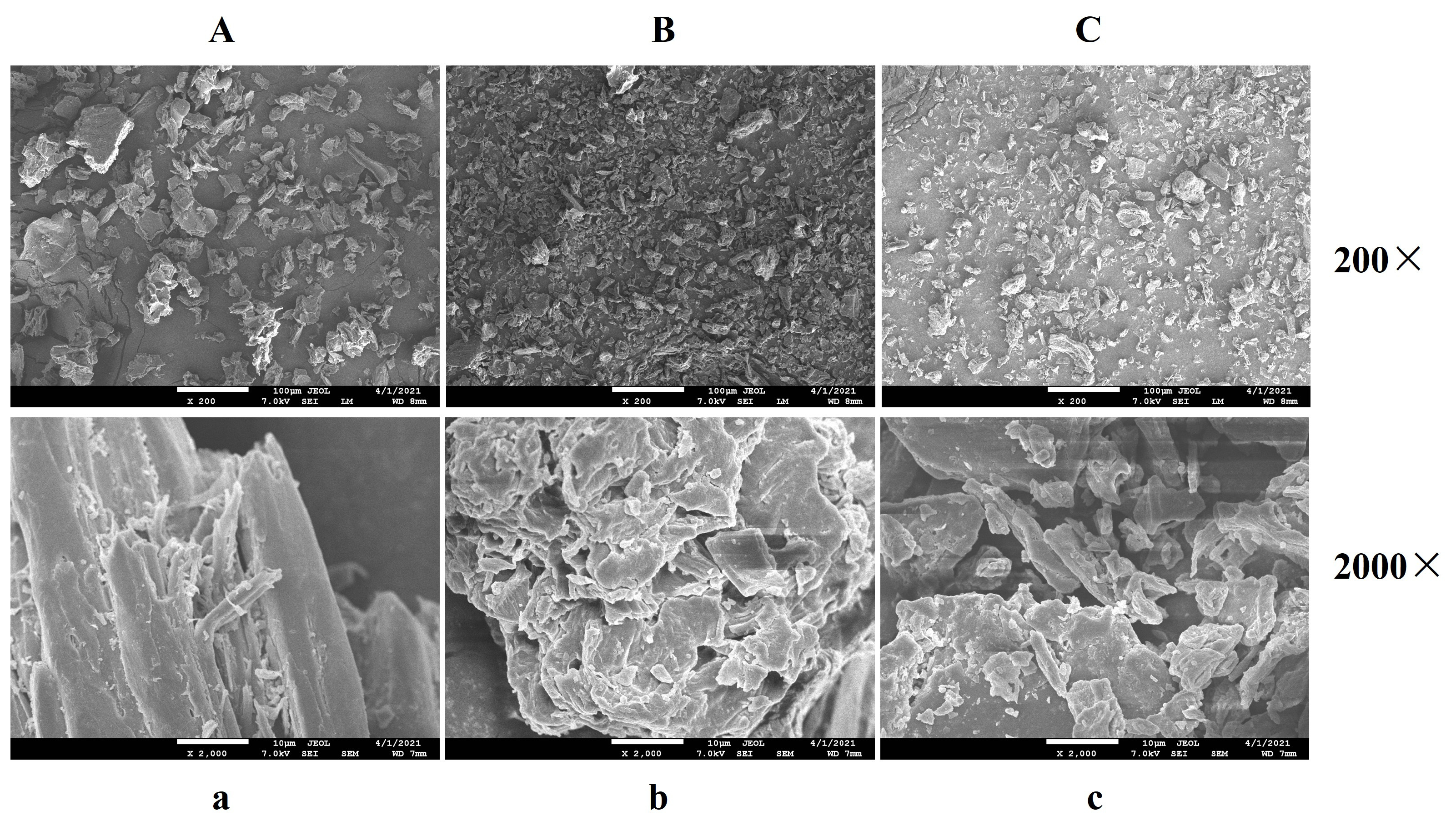
2.2.1. SEM Analysis
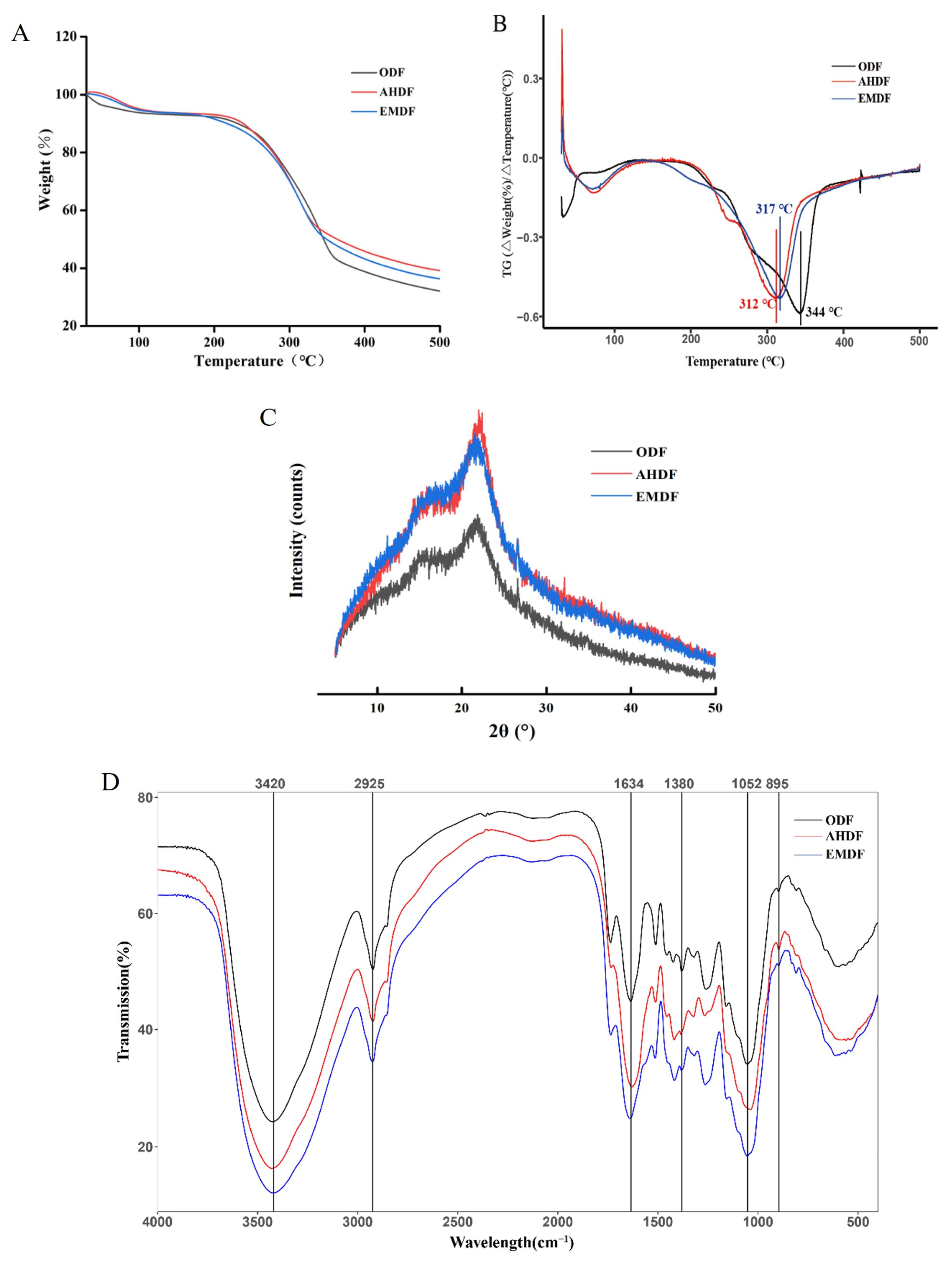
2.2.2. TGA Analysis
2.2.3. XRD Analysis
2.2.4. FT-IR Spectroscopy Analysis
2.3. WHC, WSA, and OHC Analysis
2.4. CEC Analysis
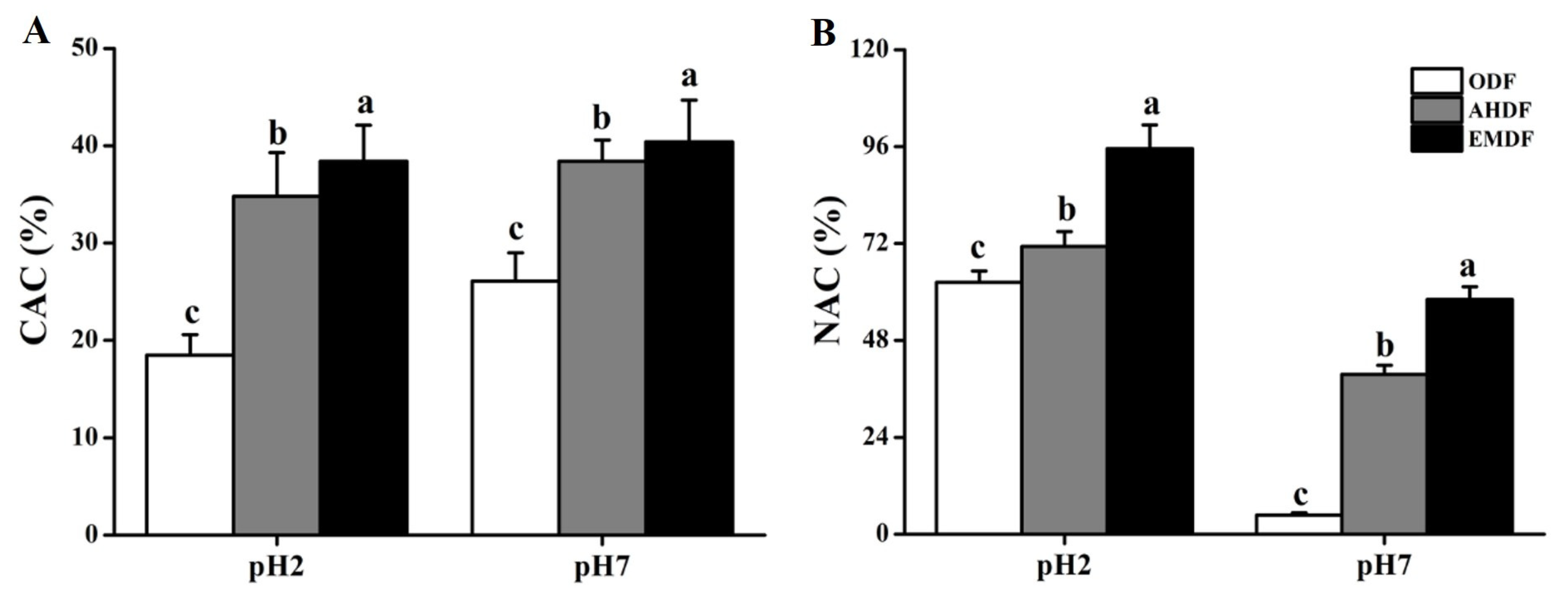
2.5. CAC Analysis
2.6. NAC Analysis
2.7. Hypoglycemic Capacity In Vitro
2.7.1. GAC Analysis
2.7.2. GDRI Analysis
2.7.3. α-Amylase Inhibitory Activity Analysis
2.8. Antioxidant Activities In Vitro
2.8.1. TPC and TFC
2.8.2. Radical Scavenging Capacity and FRAP
3. Materials and Methods
3.1. Materials
3.2. Extraction of Forsythia Suspensa DF
3.3. Modification of Forsythia Suspensa DF
3.4. Chromaticity
3.5. Scanning Electron Microscopy (SEM)
3.6. Thermogravimetric Analysis (TGA)
3.7. X-Ray Diffraction (XRD)
3.8. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.9. Hydration Properties
3.10. Cation Exchange Capacity (CEC)
3.11. Cholesterol Adsorption Capacity (CAC)
3.12. Nitrite Adsorption Capacity (NAC)
3.13. Glucose Adsorption Capacity (GAC)
3.14. Glucose Dialysis Retardation Index (GDRI)
3.15. α-Amylase Inhibitory Activity (AIA)
3.16. Total Phenolic Content (TPC)
3.17. Total Flavonoid Content (TFC)
3.18. Antioxidant Activity
3.19. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zheng, Y.J.; Wang, X.Y.; Tian, H.L.; Li, Y.; Shi, P.Q.; Guo, W.Y.; Zhu, Q.Q. Effect of four modification methods on adsorption capacities and in vitro hypoglycemic properties of millet bran dietary fibre. Food Res. Int. 2021, 147, 110565. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.R.; Si, J.Y.; Chen, Y.; Xie, J.H.; Tian, S.L.; Cheng, Y.N.; Hu, X.B.; Yu, Q. Physicochemical structure and functional properties of soluble dietary fibers obtained by different modification methods from Mesona chinensis Benth. residue. Food Res. Int. 2022, 157, 111489. [Google Scholar] [CrossRef]
- Tang, C.; Wu, L.R.; Zhang, F.S.; Kan, J.Q.; Zheng, J. Comparison of different extraction methods on the physicochemical, structural properties, and in vitro hypoglycemic activity of bamboo shoot dietary fibers. Food Chem. 2022, 386, 132642. [Google Scholar] [CrossRef]
- Li, Y.N.; Yu, Y.S.; Wu, J.J.; Xu, Y.J.; Xiao, G.S.; Li, L.; Liu, H.R. Comparison the structural, physicochemical, and prebiotic properties of litchi pomace dietary fibers before and after modification. Foods 2022, 11, 248. [Google Scholar] [CrossRef]
- Jiang, G.; Ameer, K.; Feng, X.; Wu, Z.; Li, S.; Bai, X.; Zhao, C. Development of wheat bread added with insoluble dietary fiber from ginseng residue and effects on physiochemical properties, in vitro adsorption capacities and starch digestibility. LWT-Food Sci. Technol. 2021, 149, 111855. [Google Scholar] [CrossRef]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Extraction of dietary fiber from kinnow (Citrus reticulata) peels using sequential ultrasonic and enzymatic treatments and its application in development of cookies. Food Biosci. 2023, 54, 102891. [Google Scholar] [CrossRef]
- Mielby, L.H.; Andersen, B.V.; Jensen, S.; Kildegaard, H.; Kuznetsova, A.; Eggers, N.; Brockhoff, P.B.; Byrne, D.V. Changes in sensory characteristics and their relation with consumers’ liking, wanting and sensory satisfaction: Using dietary fibre and lime flavour in Stevia rebaudiana sweetened fruit beverages. Food Res. Int. 2016, 82, 14–21. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, K.Y.; Lee, H.G. Chemical composition and physicochemical properties of barley dietary fiber by chemical modification. Int. J. Biol. Macromol. 2013, 60, 360–365. [Google Scholar] [CrossRef]
- Dutra, E.D.; Santos, F.A.; Alencar, B.R.A.; Reis, A.L.S.; de Souza, R.D.F.R.; Aquino, K.A.D.S.; Morais Jr, M.A.; Menezes, R.S.C. Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: Status and perspectives. Biomass Convers. Bior. 2018, 8, 225–234. [Google Scholar] [CrossRef]
- Meng, X.M.; Liu, F.; Xiao, Y.; Cao, J.W.; Wang, M.; Duan, X.C. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X. 2019, 3, 100029. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.R.; Zeng, W.Q.; Huang, Y.X.; Yang, X.Q. Properties of dietary fiber from citrus obtained through alkaline hydrogen peroxide treatment and homogenization treatment. Food Chem. 2020, 311, 125873. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liao, A.M.; Thakur, K.; Huang, J.H.; Zhang, J.G.; Wei, Z.J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, Y.; Yokoyama, W.; Majeed, H.; Masamba, K.G.; Zhong, F.; Ma, J.G. Cellulosic fraction of rice bran fibre alters the conformation and inhibits the activity of porcine pancreatic lipase. J. Funct. Foods. 2015, 19, 39–48. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Ma, Z.Y.; Wang, W.X.; Mu, J.L.; Liu, Y.Q.; Wang, J.; Stipkovits, L.; Hui, X.D.; Wu, G.; Sun, J.F. The effects of enzymatic modification on the functional ingredient-Dietary fiber extracted from potato residue. LWT-Food Sci. Technol. 2022, 153, 112511. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.Y.; Fu, Y.J.; Zu, Y.G.; Luo, M.; Wang, W.; Zhao, C.J.; Gu, C.B.; Li, J. Application of white-rot fungi treated Fructus forsythiae shell residue as a low-cost biosorbent to enrich forsythiaside and phillygenin. Chem. Eng. Sci. 2012, 74, 244–255. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Li, F.; Hou, G. Study on anti-senile and anti-oxidative activities of Forsythia suspense leaves tea. ACTA Nutr. Sin. 2004, 26, 65–67. (In Chinese) [Google Scholar]
- Li, A.; Gao, H. Product development and active components analysis of Forsythia flowers tea. Agrifood Eng. 2015, 9, 57–61. (In Chinese) [Google Scholar]
- Fen, S.; Fu, L.; Li, C.; Su, X.; Gao, J. Study on the extraction and stability to light and heat of yellow Forsythia flower. Food Ferment. Ind. 2002, 28, 66–70. (In Chinese) [Google Scholar]
- Qi, J.; Yokoyama, W.; Masamba, K.G.; Majeed, H.; Zhong, F.; Li, Y. Structural and physico-chemical properties of insoluble rice bran fiber: Effect of acid–base induced modifications. RSC Adv. 2015, 5, 79915–79923. [Google Scholar] [CrossRef]
- Ma, J.F.; Yang, G.H.; Mao, J.Z.; Xu, F. Characterization of anatomy, ultrastructure and lignin microdistribution in Forsythia suspensa. Ind. Crop Prod. 2011, 33, 358–363. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xia, Q.; Liu, X.; Liu, W.X.; Huang, W.Z.; Mei, X.; Luo, J.; Shon, M.X.; Lin, R.C.; Zou, D.X.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.N.; Zhou, X.J.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y.L. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.). LWT-Food Sci. Technol. 2021, 145, 111313. [Google Scholar] [CrossRef]
- Yoshida, B.Y.; Prudencio, S.H. Alkaline hydrogen peroxide improves physical, chemical, and techno-functional properties of okara. Food Chem. 2020, 323, 126776. [Google Scholar] [CrossRef]
- Huang, J.Y.; Liao, J.S.; Qi, J.R.; Jiang, W.X.; Yang, X.Q. Structural and physicochemical properties of pectin-rich dietary fiber prepared from citrus peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Tem, T.D.; Vasanthan, T. Modification of rice bran dietary fiber concentrates using enzyme and extrusion cooking. Food Hydrocoll. 2019, 89, 773–782. [Google Scholar] [CrossRef]
- Xie, F.Y.; Zhao, T.; Wan, H.C.; Li, M.; Sun, L.N.; Wang, Z.J.; Zhang, S. Structural and physicochemical characteristics of rice bran dietary fiber by cellulase and high-pressure homogenization. Appl. Sci. 2019, 9, 1270. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Li, S.X.; Liu, S.X.; Li, C.F. Modification of coconut residue fiber and its bile salt adsorption mechanism: Action mode of insoluble dietary fibers probed by microrheology. Food Hydrocoll. 2023, 136, 108221. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Li, Y. Physicochemical and functional properties of coconut (Cocos nucifera L.) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef]
- Jia, M.Y.; Chen, J.J.; Liu, X.Z.; Xie, M.Y.; Nie, S.P.; Chen, Y.; Xie, J.H.; Yu, Q. Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation. Food Hydrocoll. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Kutos, T.; Golob, T.; Kac, M.; Plestenjak, A. Dietary fibre content of dry and processed beans. Food Chem. 2003, 80, 231–235. [Google Scholar] [CrossRef]
- Jiang, G.H.; Bai, X.S.; Wu, Z.G.; Li, S.J.; Zhao, C.; Ramachandraiah, K. Modification of ginseng insoluble dietary fiber through alkaline hydrogen peroxide treatment and its impact on structure, physicochemical and functional properties. LWT-Food Sci. Technol. 2021, 150, 111956. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, Y.Y.; Li, Y.B.; Yu, H.S.; Wang, Y.H.; Piao, C.H. Insoluble dietary fibre from okara (soybean residue) modified by yeast Kluyveromyces marxianus. LWT-Food Sci. Technol. 2020, 134, 110252. [Google Scholar] [CrossRef]
- Qiao, H.Z.; Shao, H.M.; Zheng, X.J.; Liu, J.W.; Liu, J.Q.; Huang, J.; Zhang, C.Y.; Liu, Z.; Wang, J.R.; Guan, W.T. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion. Food Chem. 2021, 335, 127522. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Tian, H.L.; Li, Y.; Wang, X.; Shi, P.Q. Effects of carboxymethylation, hydroxypropylation and dual enzyme hydrolysis combination with heating on physicochemical and functional properties and antioxidant activity of coconut cake dietary fibre. Food Chem. 2021, 336, 127688. [Google Scholar] [CrossRef]
- Gu, M.D.; Fang, H.C.; Gao, Y.H.; Su, T.; Niu, Y.G.; Yu, L.L. Characterization of enzymatic modified soluble dietary fiber from tomato peels with high release of lycopene. Food Hydrocoll. 2020, 99, 105321. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Xu, B.F.; Shi, P.Q.; Tian, H.L.; Li, Y.; Wang, X.Y.; Wu, S.; Liang, P.F. The influences of acetylation, hydroxypropylation, enzymatic hydrolysis and crosslinking on improved adsorption capacities and in vitro hypoglycemic properties of millet bran dietary fibre. Food Chem. 2022, 368, 130883. [Google Scholar] [CrossRef]
- Kabir, A.U.; Samad, M.B.; D’Costa, N.M.; Akhter, F.; Ahmed, A.; Hannan, J.M.A. Antihyperglycemic activity of Centella asiatica is partly mediated by carbohydrase inhibition and glucose-fiber binding. BMC Complement. Altern. Med. 2014, 14, 31. [Google Scholar] [CrossRef]
- Hua, M.; Sun, Y.S.; Shao, Z.J.; Lu, J.X.; Lu, Y.S.; Liu, Z.B. Functional soluble dietary fiber from ginseng residue: Polysaccharide characterization, structure, antioxidant, and enzyme inhibitory activity. J. Food Biochem. 2020, 44, e13524. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.D.; Wu, Y.N.; Wang, L.P.; Tan, B.; Shen, W.Y.; Li, X.N.; Liu, Y.X.; Tian, X.H.; Zhang, D.Q. Comparison of six modification methods on the chemical composition, functional properties and antioxidant capacity of wheat bran. LWT-Food Sci. Technol. 2021, 149, 111996. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, P.Y.; Chen, B.C. Ultracentrifugal milling and steam heating pretreatment improves structural characteristics, functional properties, and in vitro binding capacity of cellulase modified soy okara residues. Food Chem. 2022, 384, 132526. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
| a | b | L | ΔE | |
|---|---|---|---|---|
| ODF | −1.22 ± 0.13 a | −2.09 ± 0.17 a | 54.99 ± 0.24 a | — |
| AHDF | −1.79 ± 0.25 b | −3.05 ± 0.08 b | 55.08 ± 0.19 a | 1.13 ± 0.11 a |
| EMDF | −1.87 ± 0.09 b | −3.22 ± 0.12 b | 54.88 ± 0.25 a | 1.31 ± 0.14 a |
| WHC (g/g) | OHC (g/g) | WSA (mL/g) | CEC (mmol/g) | |
|---|---|---|---|---|
| ODF | 3.25 ± 0.0 b | 3.24 ± 0.25 b | 1.17 ± 0.11 b | 0.08 ± 0.02 b |
| AHDF | 4.07 ± 0.05 a | 4.07 ± 0.37 a | 3.56 ± 0.11 a | 0.15 ± 0.03 a |
| EMDF | 2.97 ± 0.02 c | 4.45 ± 0.59 a | 0.91 ± 0.17 b | 0.17 ± 0.05 a |
| GAC (μmol/g) | GDRI (%) | AIA (%) | |
|---|---|---|---|
| ODF | 4.46 ± 0.56 c | 17.9 ± 2.9 c | 11.5 ± 2.1 c |
| AHDF | 13.93 ± 2.35 b | 33.3 ± 3.6 a | 16.5 ± 3.1 b |
| EMDF | 18.28 ± 1.14 a | 27.8 ± 4.3 b | 37.5 ± 0.9 a |
| TPC (mgGAE/g) | TFC (mgRE/g) | DPPH (μgTE/g) | ABTS (μgTE/g) | FRAP (μmoL Fe (II)/g) | |
|---|---|---|---|---|---|
| ODF | 0.28 ± 0.02 a | 1.25 ± 0.08 a | 3.39 ± 0.25 c | 43.42 ± 3.05 a | 6.32 ± 0.54 a |
| AHDF | 0.30 ± 0.02 a | 0.65 ± 0.01 c | 4.36 ± 0.36 a | 27.07 ± 2.54 c | 3.35 ± 0.16 c |
| EMDF | 0.28 ± 0.03 a | 1.13 ± 0.02 b | 4.08 ± 0.27 b | 37.25 ± 1.02 b | 4.84 ± 0.25 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Liu, J.; Yan, W.; Wang, Q.; Huo, Y.; Feng, S.; Zhang, L.; Hu, Q.; Xu, J. Effects of Alkaline Hydrogen Peroxide and Cellulase Modifications on the Physicochemical and Functional Properties of Forsythia suspensa Dietary Fiber. Molecules 2023, 28, 7164. https://doi.org/10.3390/molecules28207164
Yan K, Liu J, Yan W, Wang Q, Huo Y, Feng S, Zhang L, Hu Q, Xu J. Effects of Alkaline Hydrogen Peroxide and Cellulase Modifications on the Physicochemical and Functional Properties of Forsythia suspensa Dietary Fiber. Molecules. 2023; 28(20):7164. https://doi.org/10.3390/molecules28207164
Chicago/Turabian StyleYan, Kejing, Jiale Liu, Wensheng Yan, Qing Wang, Yanxiong Huo, Saisai Feng, Liangliang Zhang, Qingping Hu, and Jianguo Xu. 2023. "Effects of Alkaline Hydrogen Peroxide and Cellulase Modifications on the Physicochemical and Functional Properties of Forsythia suspensa Dietary Fiber" Molecules 28, no. 20: 7164. https://doi.org/10.3390/molecules28207164
APA StyleYan, K., Liu, J., Yan, W., Wang, Q., Huo, Y., Feng, S., Zhang, L., Hu, Q., & Xu, J. (2023). Effects of Alkaline Hydrogen Peroxide and Cellulase Modifications on the Physicochemical and Functional Properties of Forsythia suspensa Dietary Fiber. Molecules, 28(20), 7164. https://doi.org/10.3390/molecules28207164






