A Study of the Bisphosphonic Derivatives from the Pudovik Reaction of Dialkyl α-Oxophosphonates and >P(O)H Reagents: X-ray Structure and Bioactivity
Abstract
1. Introduction
2. Results and Discussion
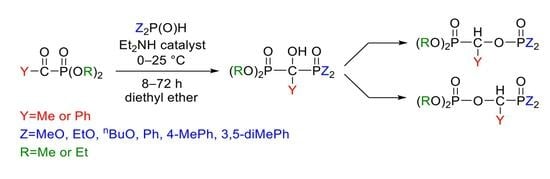
2.1. Synthesis
2.2. X-ray Structure of the Three Adducts

2.3. Bioactivity of the Compounds Prepared
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Tetraalkyl α-Hydroxy-ethylidenebisphosphonates
3.2.1. Tetramethyl α-Hydroxy-ethylidenebisphosphonates (2a)
3.2.2. Diethyl–Dimethyl α-Hydroxy-ethylidenebisphosphonate (2b)
3.2.3. Dibutyl-Dimethyl α-Hydroxy-ethylidenebisphosphonate (2c)
3.3. General Procedure for the Synthesis of Dimethyl 1-Diarylphosphinoyl-1-hydroxy-ethylphosphonate
3.3.1. Dimethyl 1-Diphenylphosphinoyl-1-hydroxy-ethylphosphonate (2d)
3.3.2. Dimethyl 1-Bis(4-methylphenyl)phosphinoyl-1-hydroxy-ethylphosphonate (2e)
3.3.3. Dimethyl 1-Bis(3,5-dimethylphenyl)phosphinoyl-1-hydroxy-ethylphosphonate (2f)
3.4. General Procedure for the Synthesis of Dialkyl 1-(Dialkylphosphonoylethyl)phosphate
3.4.1. Dimethyl 1-(Dimethylphosphonoylethyl)phosphate (3a)
3.4.2. Dimethyl 1-(Diethylphosphonoylethyl)phosphate (3b-1) and Diethyl 1-(Dimethylphosphonoylethyl)phosphate (3b-2)
3.4.3. Dimethyl 1-(Dibutylphosphonoylethyl)phosphate (3c-1) and Dibutyl 1-(Dimethylphosphonoylethyl)phosphate (3c-2)
3.5. General Procedure for Diethyl (Diarylphosphinoyloxybenzyl)phosphonate and Diethyl (Diarylphosphinoylbenzyl)phosphate
3.5.1. Diethyl (Diphenylphosphinoylbenzyl)phosphate (5d-1) and Diethyl (Diphenylphosphinoyloxybenzyl)phosphonate (5d-2)
3.5.2. Diethyl 1-Bis((4-methylphenyl)phosphinoylbenzyl)phosphate (5e-1)
3.5.3. Diethyl 1-Bis((3,5-dimethylphenyl)phosphinoylbenzyl)phosphate (5f-1)
3.6. Single Crystal X-ray Diffraction Studies
3.7. In Vitro Cytotstasis Assays
Cell Lines and Culture Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grün, A.; Bálint, E.; Keglevich, G. Solid–liquid phase C-alkylation of active methylene containing compounds under microwave conditions. Catalysts 2015, 5, 634–652. [Google Scholar] [CrossRef]
- Hays, H.R.; Logan, T.J. gem-Diphosphinoalkanes. Preparation and characterization. J. Org. Chem. 1966, 31, 3391–3394. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Niesor, E.; Bentzen, C.L. gem-Diphosphonate and gem-phosphonate-phosphate compounds with specific high density lipoprotein inducing activity. J. Med. Chem. 1987, 80, 1426–1433. [Google Scholar] [CrossRef]
- Roth, A.G.; Drescher, D.; Reamer, S.; Arenz, C.; Yang, Y.; Uhlig, S. Potent and selective inhibition of acid sphingomyelinase by bisphosphonates. Angew. Chem. Int. Ed. 2009, 48, 7560–7563. [Google Scholar] [CrossRef]
- Kosolopoff, G.M. The chemistry of aliphatic phosphonic acids. I. Alkylation of methanediphosphonic acid. J. Am. Chem. Soc. 1953, 75, 1500–1501. [Google Scholar] [CrossRef]
- Goebel, R.; Richte, F.; Weichmann, H. Synthesis and reactivity of methylene bridged diphosphoryl compounds. Phosphorus Sulfur Silicon Relat. Elem. 1992, 73, 67–80. [Google Scholar] [CrossRef]
- Cotton, F.A.; Schunn, R.A. Metal salts and complexes of dialkoxyphosphonylacetylmethanide ions. J. Am. Chem. Soc. 1963, 85, 2394–2402. [Google Scholar] [CrossRef]
- Greiner, I.; Grün, A.; Ludányi, K.; Keglevich, G. Solid–liquid two-phase alkylation of tetraethyl methylenebisphosphonate under microwave irradiation. Heteroat. Chem. 2011, 22, 11–14. [Google Scholar] [CrossRef]
- Gulyás, K.V.; Keglevich, G. The surprising diacylation of diethyl (ethoxycarbonylmethyl)phosphonate. Mendeleev Commun. 2021, 31, 244–245. [Google Scholar] [CrossRef]
- Breuer, E. The development of bisphosphonates as drugs. In Analogue-Based Drug Discovery; Fischer, J., Ganellin, C.R., Eds.; Wiley-VCH: Weinheim, Germany, 2006; Chapter 15. [Google Scholar]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Grembecka, J.; Mucha, A.; Cierpicki, T.; Kafarski, P. The most potent organophosphorus inhibitors of leucine aminopeptidase. Structure-based design, chemistry, and activity. J. Med. Chem. 2003, 46, 2641–2655. [Google Scholar] [CrossRef] [PubMed]
- Hudson, H.R.; Wardle, N.J.; Bligh, S.W.A.; Greiner, I.; Grün, A.; Keglevich, G. N-Heterocyclic Dronic acids; applications and synthesis. Mini-Rev. Med. Chem. 2012, 12, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.I.; Grün, A.; Greiner, I.; Keglevich, G. The role of phosphorus trichloride and phoshorous acid in the formation of α-hydroxymethylenebisphosphonic acids from the corresponding carboxylic acids—A mechanistic overview. Curr. Org. Chem. 2017, 21, 1567–1578. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Synthesis of hydroxymethylenebisphosphonic acid derivatives in different solvents. Molecules 2016, 21, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. “Greener” synthesis of bisphosphonic/dronic acid derivatives. Green Process. Synth. 2014, 3, 111–116. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Kovács, R.; Garadnay, S.; Greiner, I. Rational synthesis of Ibandronate and Alendronate. Curr. Org. Synth. 2013, 10, 640–644. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Aradi, K.; Garadnay, S.; Greiner, I. Optimized synthesis of N-heterocyclic dronic acids; closing a black-box era. Tetrahedron Lett. 2011, 52, 2744–2746. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Investigation of the effect of medium in the preparation of alendronate: Till now the best synthesis in the presence of an ionic liquid additive. Heteroat. Chem. 2017, 28, e21370. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Pavela, O.; Garadnay, S.; Greiner, I.; Keglevich, G. Efficient synthesis of ibandronate in the presence of an ionic liquid. Lett. Drug Des. Discov. 2018, 15, 713–720. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Sinkovicz, J.; Garadnay, S.; Greiner, I.; Keglevich, G. A study on the synthesis of risedronic acid; The role of ionic liquid additive. Lett. Drug Des. Discov. 2019, 16, 238–244. [Google Scholar] [CrossRef]
- Nagy, D.I.; Grün, A.; Lévay, K.; Garadnay, S.; Greiner, I.; Keglevich, G. Efficient syntheses of zoledronic acid as an active ingredient of a drug against osteoporosis. Synth. Commun. 2018, 48, 663–671. [Google Scholar] [CrossRef]
- Fitch, S.J.; Moedritzer, K. NMR study of the P-C(OH)-P to P-CO-P rearrangement: Tetraethyl 1-hydroxyalkylidenediphosphonates. J. Am. Chem. Soc. 1962, 84, 1876–1880. [Google Scholar] [CrossRef]
- Nicholson, D.A.; Vaughn, H. A general method of preparation of tetramethyl alkyl-1-hydroxy-1,1-diphosphonates. J. Org. Chem. 1971, 36, 3843–3845. [Google Scholar] [CrossRef]
- Turhanen, P.A.; Ahlgren, M.J.; Jarvinen, T.; Vepsalainen, J.J. Bisphosphonate prodrugs. Synthesis and identification of (1-hydroxyetrylidene)-1,1-bisphosphonic acid tetraesters by mass spectrometry, NMR spectroscopy and X-ray crystallography. Phosphorus Sulfur Silicon Relat. Elem. 2001, 170, 115–133. [Google Scholar] [CrossRef]
- Turhanen, P.A.; Ahlgren, M.J.; Jarvinen, T.; Vepsalainen, J.J. Bisphosphonate prodrugs, selective synthesis of (1-hydroxyethylidene)-1,1-bisphosphonate partial esters. Synthesis 2001, 2001, 633–637. [Google Scholar] [CrossRef]
- Grün, A.; Molnár, I.G.; Bertók, B.; Greiner, I.; Keglevich, G. Synthesis of α-hydroxy-methylenebisphosphonates by the microwave-assisted reaction of α-oxophosphonates and dialkyl phosphites under solventless conditions. Heteroat. Chem. 2009, 20, 350–354. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Molnár, I.G.; Greiner, I. Phenyl-, benzyl- and unsymmetrical hydroxy-methylenebisphosphonates as dronic acid ester analogues from α-oxophosphonates by microwave-assisted synthesis. Heteroat. Chem. 2011, 22, 640–648. [Google Scholar] [CrossRef]
- Szalai, Z.; Keglevich, G. Tetraalkyl hydroxymethylenebisphosphonate and dialkyl 1-diphenylphosphinoyl-1-hydroxy-ethylphosphonate derivatives by the Pudovik reaction and their rearranged products. Molecules 2021, 26, 7575. [Google Scholar] [CrossRef]
- Nikander, H.; Heikkila-Hoikka, M.; Pohjala, E.; Hanhijarvi, H.; Lauren, L. New Methylenebisphosphonic Acid Derivatives. U.S. Patent WO9211267, 1992. [Google Scholar]
- DIAMOND, version 3.2i; Crystal Impact GbR: Bonn, Germany, 2014.
- Berek-Nagy, P.J.; Tóth, G.; Bősze, S.; Horváth, L.B.; Darcsi, A.; Csíkos, S.; Knapp, D.G.; Kovács, G.M.; Boldizsár, I. The grass root endophytic fungus Flavomyces fulophazii: An abundant source of tetramic acid and chlorinated azaphilone derivatives. Phytochemistry 2021, 190, 112851. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Rebollar-Ramos, D.; González, M.C.; Raja, H.; Mata, R.; Blanco, E.J.C. Apoptotic activity of xanthoquinodin JBIR-99, from Parengyodontium album MEXU 30054, in PC-3 human prostate cancer cells. Chemio-Biol. Int. 2019, 311, 108798. [Google Scholar] [CrossRef]
- Tromelin, A.; Elmanouni, D.; Burgada, R. α-Ketophosphonates and cyclic esters of hydroxymethylenediphosphonates. Synthesis, structure, and hydrolysis. Phosphorus Sulfur Relat. Elem. 1986, 27, 301–312. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.40.82a; Program package; Rigaku OD: Tokyo, Japan, 2020.
- Sheldrick, G.M. SHELXS-97: Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97: Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON: A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1999. [Google Scholar]
- Cailleau, R.; Olivé, M.; Cruciger, Q.V. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro 1978, 14, 911–915. [Google Scholar] [CrossRef]
- Haigler, H.; Ash, J.F.; Singer, S.J.; Cohen, S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc. Natl. Acad. Sci. USA 1978, 75, 3317–3321. [Google Scholar] [CrossRef] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Fujita, T.; Kiyama, M.; Tomizawa, Y.; Kohno, T.; Yokota, J. Comprehensive analysis of p53 gene mutation characteristics in lung carcinoma with special reference to histological subtypes. Int. J. Oncol. 1999, 15, 927–961. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R.J. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]







 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Y | Catalyst (%) | T (°C) | t | Product Composition (%) [a][b] | Yield (%) | ||
| 2 | 3-1 | 3-2 | ||||||
| 1 | MeO | 5 | 0 | 8 h | 100 | – | 68 (2a) | |
| 2 | MeO | 40 | 0 | 8 h | – | 100 | 75 (3a) | |
| 3 | EtO | 5 | 0 | 8 h | 100 | – | 80 (2b) | |
| 4 | EtO | 40 | 0 | 8 h | 60 | 32 | 8 | – |
| 5 | EtO | 40 | 26 | 3 days | – | 83 | 17 | 87 (3b) |
| 6 | BuO | 5 | 0 | 8 h | 100 | – | 66 (2c) | |
| 7 | BuO | 40 | 0 | 8 h | 50 | 34 | 16 | – |
| 8 | BuO | 40 | 26 | 3 days | – | 81 | 19 | 70 (3c) |
| 9 | Ph | 40 | 0 | 8 h | 100 | – | – | 64 (2d) |
| 10 | 4-MePh | 40 | 0 | 8 h | 100 | – | – | 62 (2e) |
| 11 | 3,5-diMePh | 40 | 0 | 8 h | 100 | – | – | 69 (2f) |
 | ||||
|---|---|---|---|---|
| Entry | Y | Product Composition (%) [a][b] | Yield (%) | |
| 5-1 | 5-2 | |||
| 1 | Ph | 60 | 40 | 70 (5d-1 + 5d-2) |
| 2 | 4-MePh | 88 | 12 | 65 (5e-1) |
| 3 | 3,5-diMePh | 77 | 23 | 72 (5f-1) |
| 2d | 2e | 6 | |||
|---|---|---|---|---|---|
| P1–O1 | P1–O1 | 1.485 (1) | 1.493 (1) | P1–O1 | 1.488 (1) |
| P1–C7 | P1–C8 | 1.809 (2) | 1.801 (2) | P1–C7 | 1.807 (1) |
| P1–C1 | P1–C1 | 1.809 (2) | 1.811 (2) | P1–C1 | 1.809 (1) |
| P1–C13 | P1–C15 | 1.862 (2) | 1.863 (2) | P1–C13 | 1.860 (1) |
| P2–O5 | P2–O3 | 1.466 (1) | 1.463 (1) | P2–O5 | 1.476 (1) |
| P2–O3 | P2–O4A | 1.524 (2) | 1.572 (1) | P2–O3 | 1.568 (1) |
| P2–O4 | P2–O5A | 1.642 (3) | 1.574 (1) | P2–O4 | 1.570 (1) |
| P2–C13 | P2–C15 | 1.834 (2) | 1.831 (2) | P2–C13 | 1.841 (1) |
| O3–C15 | O4A–C17A | 1.425 (5) | 1.440 (2) | O3–C15 | 1.457 (1) |
| O4–C16 | O5A–C18A | 1.415 (7) | 1.438 (2) | O4–C17 | 1.464 (1) |
| C7–C8 | C1–C2 | 1.393 (2) | 1.398 (2) | C1–C2 | 1.394 (1) |
| C7–C12 | C1–C6 | 1.396 (2) | 1.400 (2) | C1–C6 | 1.398 (1) |
| O2–C13 | O2–C15 | 1.424 (2) | 1.424 (2) | O2–C13 | 1.427 (1) |
| Compound | Cytostasis [%] at c = 50 µM | |||
|---|---|---|---|---|
| Cell Line | ||||
| MDA-MB 231 | PC-3 | Ebc-1 | A431 | |
| 2b |  | |||
| 3b | ||||
| 5d | ||||
| 5e-1 | ||||
| 5f-1 | ||||
| 7 | ||||
| Compound | IC50 (µM) a,c | |||
|---|---|---|---|---|
| Cell Line | ||||
| MDA_MB-231 | PC-3 | Ebc-1 | A431 | |
| 2a | >250 | >250 | >250 | >250 |
| 2b | >250 | >250 | >250 | >250 |
| 2c | >250 | >250 | >250 | >250 |
| 2d | >250 | >250 | >250 | >250 |
| 2e | >250 | >250 | >250 | >250 |
| 2f | >250 | >250 | >250 | >250 |
| 3a | >250 | >250 | >250 | >250 |
| 3b | >250 | >250 | >250 | >250 |
| 3c | >250 | >250 | >250 | >250 |
| 5d | 76.7 | >250 | 99.5 | 40.4 |
| 5e-1 | 37.8 | 149.5 | 25.9 | >250 |
| 5f-1 | 100.7 | 115.8 | 94.1 | 110.7 |
| 6 b | n.d. | |||
| 7 | 115.0 | >250 | >250 | >250 |
| 2d | 2e.0.5C3H6O | 6 | |
|---|---|---|---|
| Empirical formula | C16H20O5P2 | C18H24O5P2.0.5C3H6O | C18H24O5P2 |
| Formula mass | 354.26 | 411.35 | 382.31 |
| T [K] | 123 (2) | 123 (2) | 123 (2) |
| Crystal size [mm] | 0.20 × 0.02 × 0.02 | 0.35 × 0.20 × 0.10 | 0.25 × 0.20 × 0.15 |
| Crystal description | colorless rod | colorless block | colorless block |
| Crystal system | monoclinic | monoclinic | triclinic |
| Space group | P21/c | C2/c | P21/n |
| a [Å] | 9.1252 (3) | 13.8562 (3) | 8.6609 (2) |
| b [Å] | 18.1309 (6) | 10.4172 (2) | 9.8169 (2) |
| c [Å] | 10.1680 (4) | 28.5479 (7) | 22.1229 (5) |
| α [°] | 90.0 | 90.0 | 90.0 |
| β [°] | 94.892 (3) | 96.649 (2) | 96.193 (2) |
| γ [°] | 90.0 | 90.0 | 90.0 |
| V [Å3] | 1676.15 (10) | 4092.97 (16) | 1869.98 (7) |
| Z | 4 | 8 | 4 |
| ρcalcd. [g cm−3] | 1.404 | 1.335 | 1.358 |
| μ [mm−1] | 0.281 | 0.242 | 0.258 |
| F (000) | 744 | 1744 | 808 |
| Θ range [°] | 2.24–25.24 | 2.45–25.24 | 2.27–25.24 |
| Index ranges | −12 ≤ h ≤ 12 | −17 ≤ h ≤ 17 | −12 ≤ h ≤ 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalai, Z.; Tóth, B.; Szabó, R.O.; Bősze, S.; Karaghiosoff, K.; Czugler, M.; Drahos, L.; Keglevich, G. A Study of the Bisphosphonic Derivatives from the Pudovik Reaction of Dialkyl α-Oxophosphonates and >P(O)H Reagents: X-ray Structure and Bioactivity. Molecules 2023, 28, 6037. https://doi.org/10.3390/molecules28166037
Szalai Z, Tóth B, Szabó RO, Bősze S, Karaghiosoff K, Czugler M, Drahos L, Keglevich G. A Study of the Bisphosphonic Derivatives from the Pudovik Reaction of Dialkyl α-Oxophosphonates and >P(O)H Reagents: X-ray Structure and Bioactivity. Molecules. 2023; 28(16):6037. https://doi.org/10.3390/molecules28166037
Chicago/Turabian StyleSzalai, Zsuzsanna, Boldizsár Tóth, Rita Oláhné Szabó, Szilvia Bősze, Konstantin Karaghiosoff, Mátyás Czugler, László Drahos, and György Keglevich. 2023. "A Study of the Bisphosphonic Derivatives from the Pudovik Reaction of Dialkyl α-Oxophosphonates and >P(O)H Reagents: X-ray Structure and Bioactivity" Molecules 28, no. 16: 6037. https://doi.org/10.3390/molecules28166037
APA StyleSzalai, Z., Tóth, B., Szabó, R. O., Bősze, S., Karaghiosoff, K., Czugler, M., Drahos, L., & Keglevich, G. (2023). A Study of the Bisphosphonic Derivatives from the Pudovik Reaction of Dialkyl α-Oxophosphonates and >P(O)H Reagents: X-ray Structure and Bioactivity. Molecules, 28(16), 6037. https://doi.org/10.3390/molecules28166037