Tunable Electronic Transport of New-Type 2D Iodine Materials Affected by the Doping of Metal Elements
Abstract
:1. Introduction
2. Results and Discussion
2.1. Equilibrium Electron Transport
2.2. Non-Equilibrium Electron Transport
3. Computational Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Glavin, N.R.; Rao, R.; Varshney, V.; Bianco, E.; Apte, A.; Roy, A.; Ringe, E.; Ajayan, P.M. 2D Materials: Emerging Applications of Elemental 2D Materials (Adv. Mater. 7/2020). Adv. Mater. 2020, 32, 2070052. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Duan, X. Van der Waals integration before and beyond two-dimensional materials. Nature 2019, 567, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kang, J.S.; Nguyen, H.D.; Wu, H.; Aoki, T.; Hu, Y. Anisotropic Thermal Boundary Resistance across 2D Black Phosphorus: Experiment and Atomistic Modeling of Interfacial Energy Transport. Adv. Mater. 2019, 31, 1901021. [Google Scholar] [CrossRef]
- Kim, S.; Cui, J.; Dravid, V.P.; He, K. Orientation-Dependent Intercalation Channels for Lithium and Sodium in Black Phosphorus. Adv. Mater. 2019, 31, 1904623. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Andreeva, D.V.; Ren, W.; Shan, G. Graphene and other two-dimensional materials. Front. Phys. 2019, 14, 13301. [Google Scholar] [CrossRef]
- Molle, A.; Goldberger, J.; Houssa, M.; Xu, Y.; Zhang, S.-C.; Akinwande, D. Buckled two-dimensional Xene sheets. Nat. Mater. 2017, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.-F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Castro Neto, A.H. Phosphorene: From theory to applications. Nat. Rev. Mater. 2016, 1, 16061. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, J.; Xia, Y.; Zhao, C.; Jiang, M.; Ma, J.; Tie, Z.; Jin, Z. 2D Arsenene and Arsenic Materials: Fundamental Properties, Preparation, and Applications. Small 2022, 18, 2104556. [Google Scholar] [CrossRef]
- Niu, T.; Meng, Q.; Zhou, D.; Si, N.; Zhai, S.; Hao, X.; Zhou, M.; Fuchs, H. Large-Scale Synthesis of Strain-Tunable Semiconducting Antimonene on Copper Oxide. Adv. Mater. 2020, 32, 1906873. [Google Scholar] [CrossRef]
- Reis, F.; Li, G.; Dudy, L.; Bauernfeind, M.; Glass, S.; Hanke, W.; Thomale, R.; Schäfer, J.; Claessen, R. Bismuthene on a SiC substrate: A candidate for a high-temperature quantum spin Hall material. Science 2017, 357, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Qiu, G.; Jian, J.; Zhou, H.; Yang, L.; Charnas, A.; Zemlyanov, D.Y.; Xu, C.-Y.; Xu, X.; Wu, W.; et al. Controlled Growth of a Large-Size 2D Selenium Nanosheet and Its Electronic and Optoelectronic Applications. ACS Nano 2017, 11, 10222–10229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, G.; Wang, R.; Huang, S.; Wang, Q.; Liu, Y.; Du, Y.; Goddard, W.A.; Kim, M.J.; Xu, X.; et al. Field-effect transistors made from solution-grown two-dimensional tellurene. Nat. Electron. 2018, 1, 228–236. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Jia, Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Chen, M.; Wang, J.; Liu, X.; Wei, B.; Wang, Z.; Li, J.; Gu, L.; Zhang, Q.; et al. Few-Layer Bismuthene with Anisotropic Expansion for High-Areal-Capacity Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1807874. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Gusmão, R.; Sofer, Z.; Bouša, D.; Pumera, M. Pnictogen (As, Sb, Bi) Nanosheets for Electrochemical Applications Are Produced by Shear Exfoliation Using Kitchen Blenders. Angew. Chem. Int. Edit. 2017, 56, 14417–14422. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, J.; Ding, P.; Shen, B.; Yin, J.; Xu, F.; Xia, Y.; Liu, Z. Synthesis of Easily Transferred 2D Layered BiI3 Nanoplates for Flexible Visible-Light Photodetectors. ACS Appl. Mater. Interfaces 2018, 10, 21527–21533. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Huang, L.; Deng, H.-X.; Wang, X.; Li, B.; Wei, Z.; Li, J. Flexible photodetectors based on phase dependent PbI2 single crystals. J. Mater. Chem. C 2016, 4, 6492–6499. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Zhang, Y.; Nie, A.; Zhao, W.; Wang, D.; Huang, F.; Zhai, T. Honeycomb RhI3 Flakes with High Environmental Stability for Optoelectronics. Adv. Mater. 2020, 32, 2001979. [Google Scholar] [CrossRef]
- Hulkko, E.; Kiljunen, T.; Kiviniemi, T.; Pettersson, M. From Monomer to Bulk: Appearance of the Structural Motif of Solid Iodine in Small Clusters. J. Am. Chem. Soc. 2009, 131, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Xu, Z.; Wang, Z.; Wei, B.; Wang, H.; Hu, S.; Liu, L.M.; Guo, L. Realizing Few-Layer Iodinene for High-Rate Sodium-Ion Batteries. Adv. Mater. 2020, 32, 2004835. [Google Scholar] [CrossRef] [PubMed]
- Huran, A.W.; Wang, H.-C.; San-Miguel, A.; Marques, M.A.L. Atomically Thin Pythagorean Tilings in Two Dimensions. J. Phys. Chem. Lett. 2021, 12, 4972–4979. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, G.; Yan, Y.; Jia, G.; Liu, C.; Wang, B.; Yin, H. Strain-tunable phase transition and doping-induced magnetism in iodinene. Appl. Phys. Lett. 2021, 119, 102403. [Google Scholar] [CrossRef]
- Bafekry, A.; Stampfl, C.; Faraji, M.; Mortazavi, B.; Fadlallah, M.M.; Nguyen, C.V.; Fazeli, S.; Ghergherehchi, M. Monoelemental two-dimensional iodinene nanosheets: A first-principles study of the electronic and optical properties. J. Phys. D Appl. Phys. 2022, 55, 135104. [Google Scholar] [CrossRef]
- Kuang, C.; Zeng, W.; Qian, M.; Liu, X. Liquid-Phase Exfoliated Few-Layer Iodine Nanosheets for High-Rate Lithium-Iodine Batteries. ChemPlusChem 2021, 86, 865–869. [Google Scholar] [CrossRef]
- You, Y.; Zhu, Y.-X.; Jiang, J.; Chen, Z.; Wu, C.; Zhang, Z.; Lin, H.; Shi, J. Iodinene Nanosheet-to-Iodine Molecule Allotropic Transformation for Antibiosis. J. Am. Chem. Soc. 2023, 145, 13249–13260. [Google Scholar] [CrossRef]
- Liu, H.; Cao, S.; Chen, L.; Zhao, K.; Wang, C.; Li, M.; Shen, S.; Wang, W.; Ge, L. Electron acceptor design for 2D/2D iodinene/carbon nitride heterojunction boosting charge transfer and CO2 photoreduction. Chem. Eng. J. 2022, 433, 133594. [Google Scholar] [CrossRef]
- Wen, T.; Wang, Y.; Li, N.; Zhang, Q.; Zhao, Y.; Yang, W.; Zhao, Y.; Mao, H.-k. Pressure-Driven Reversible Switching between n- and p-Type Conduction in Chalcopyrite CuFeS2. J. Am. Chem. Soc. 2019, 141, 505–510. [Google Scholar] [CrossRef]
- Bu, K.; Luo, H.; Guo, S.; Li, M.; Wang, D.; Dong, H.; Ding, Y.; Yang, W.; Lü, X. Pressure-Regulated Dynamic Stereochemical Role of Lone-Pair Electrons in Layered Bi2O2S. J. Phys. Chem. Lett. 2020, 11, 9702–9707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Li, N.; Wang, B.; Huang, Q.; Wang, L.; Zhang, D.; Wang, Y.; Yang, W. Pressure-driven chemical lock-in structure and optical properties in Sillen compounds PbBiO2X (X = Cl, Br, and I). J. Mater. Chem. A 2020, 8, 13610–13618. [Google Scholar] [CrossRef]
- Duan, D.; Jin, X.; Ma, Y.; Cui, T.; Liu, B.; Zou, G. Effect of nonhydrostatic pressure on superconductivity of monatomic iodine: An ab initio study. Phys. Rev. B 2009, 79, 064518. [Google Scholar] [CrossRef]
- Sakai, N.; Takemura, K.-i.; Tsuji, K. Electrical properties of high-pressure metallic modification of iodine. J. Phys. Soc. Japan 1982, 51, 1811–1816. [Google Scholar] [CrossRef]
- Kenichi, T.; Kyoko, S.; Hiroshi, F.; Mitsuko, O. Modulated structure of solid iodine during its molecular dissociation under high pressure. Nature 2003, 423, 971–974. [Google Scholar] [CrossRef]
- San Miguel, A.; Libotte, H.; Gaspard, J.P.; Gauthier, M.; Itié, J.P.; Polian, A. Bromine metallization studied by X-ray absorption spectroscopy. Eur. Phys. J. B Condens. Matter Complex Syst. 2000, 17, 227–233. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Liu, N.; Du, M.; Jin, X.; Li, Q.; Du, Y.; Guo, L.; Liu, B. Pressure Engineering for Extending Spectral Response Range and Enhancing Photoelectric Properties of Iodine. Adv. Opt. Mater. 2021, 9, 2101163. [Google Scholar] [CrossRef]
- Song, D.; Liu, K.; Li, J.; Zhu, H.; Sun, L.; Okulov, A. Mechanical tensile behavior-induced multi-level electronic transport of ultra-thin SiC NWs. Mater. Today Commun. 2023, 36, 106528. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Liu, Q.; Song, D.; Xie, X.; Duan, Y.; Wang, Y.; Li, J. Superior electron transport of ultra-thin SiC nanowires with one impending tensile monatomic chain. Vacuum 2022, 199, 110950. [Google Scholar] [CrossRef]
- Mlinar, V. Electronic and optical properties of nanostructured MoS2 materials: Influence of reduced spatial dimensions and edge effects. Phys. Chem. Chem. Phys. 2017, 19, 15891–15902. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Liu, R.; Li, H.; Okulov, A. Non-equilibrium electronic properties of ultra-thin SiC NWs influenced by the tensile strain. J. Mater. Res. Technol. 2023, 26, 6955–6965. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Zhou, Y.; Wu, W.; Zhang, L.; Li, H. Distinctive electron transport on pyridine-linked molecular junctions with narrow monolayer graphene nanoribbon electrodes compared with metal electrodes and graphene electrodes. Phys. Chem. Chem. Phys. 2016, 18, 28217–28226. [Google Scholar] [CrossRef]
- Cao, J.; Dong, J.; Saglik, K.; Zhang, D.; Solco, S.F.D.; You, I.J.W.J.; Liu, H.; Zhu, Q.; Xu, J.; Wu, J.; et al. Non-equilibrium strategy for enhancing thermoelectric properties and improving stability of AgSbTe2. Nano Energy 2023, 107, 108118. [Google Scholar] [CrossRef]
- Brandbyge, M.; Mozos, J.-L.; Ordejón, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165401. [Google Scholar] [CrossRef]
- Taylor, J.; Guo, H.; Wang, J. Ab initio modeling of quantum transport properties of molecular electronic devices. Phys. Rev. B 2001, 63, 245407. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, J.; Shen, L.; Fletcher, C.; Li, H. Taper-shaped carbon-based spin filter. Appl. Surf. Sci. 2019, 495, 143501. [Google Scholar] [CrossRef]
- Landauer, R. Electrical resistance of disordered one-dimensional lattices. Philos. Mag. 1970, 21, 863–867. [Google Scholar] [CrossRef]
- Reed, M.A.; Zhou, C.; Muller, C.; Burgin, T.; Tour, J. Conductance of a molecular junction. Science 1997, 278, 252–254. [Google Scholar] [CrossRef]
- Datta, S. Electronic Transport in Mesoscopic Systems; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
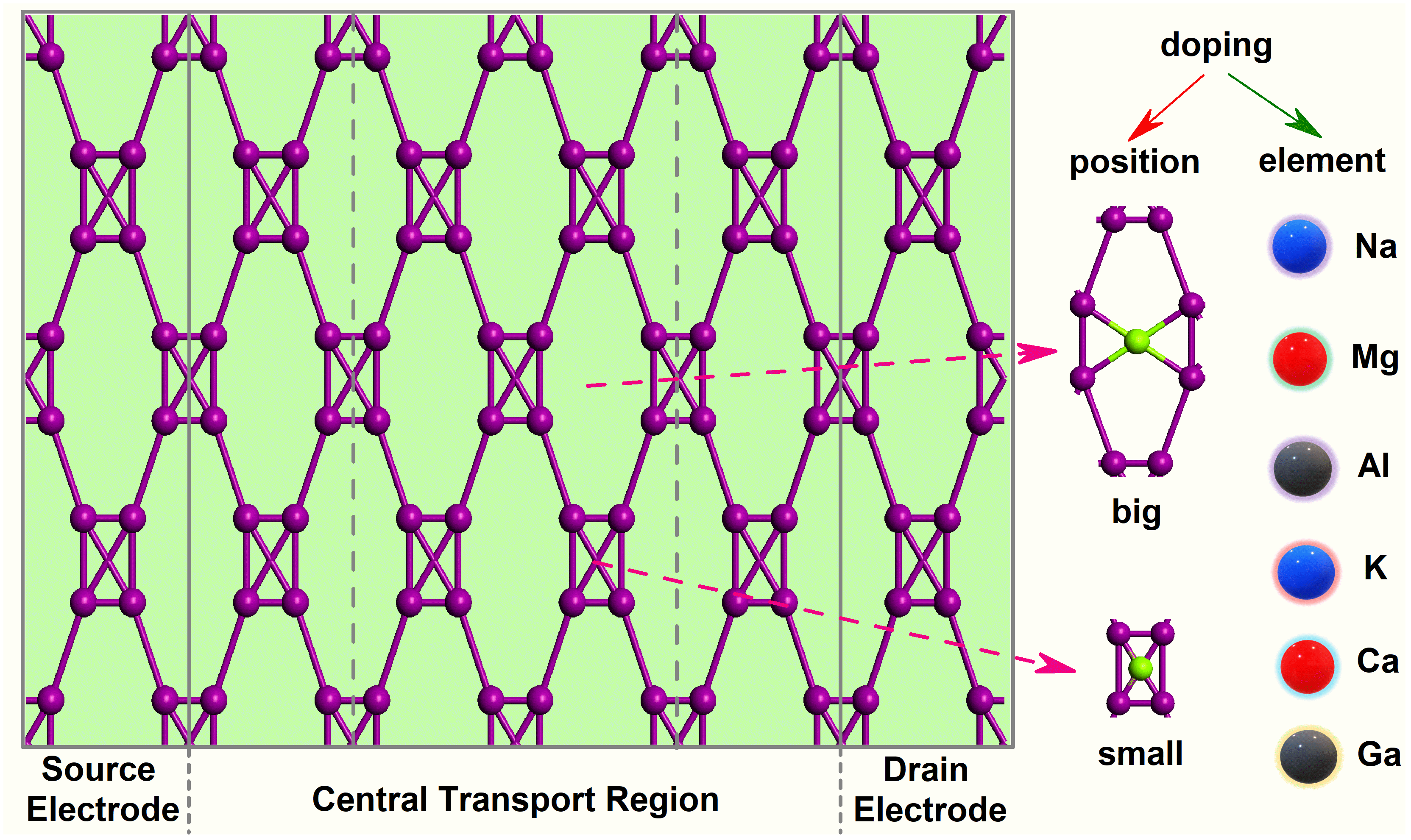
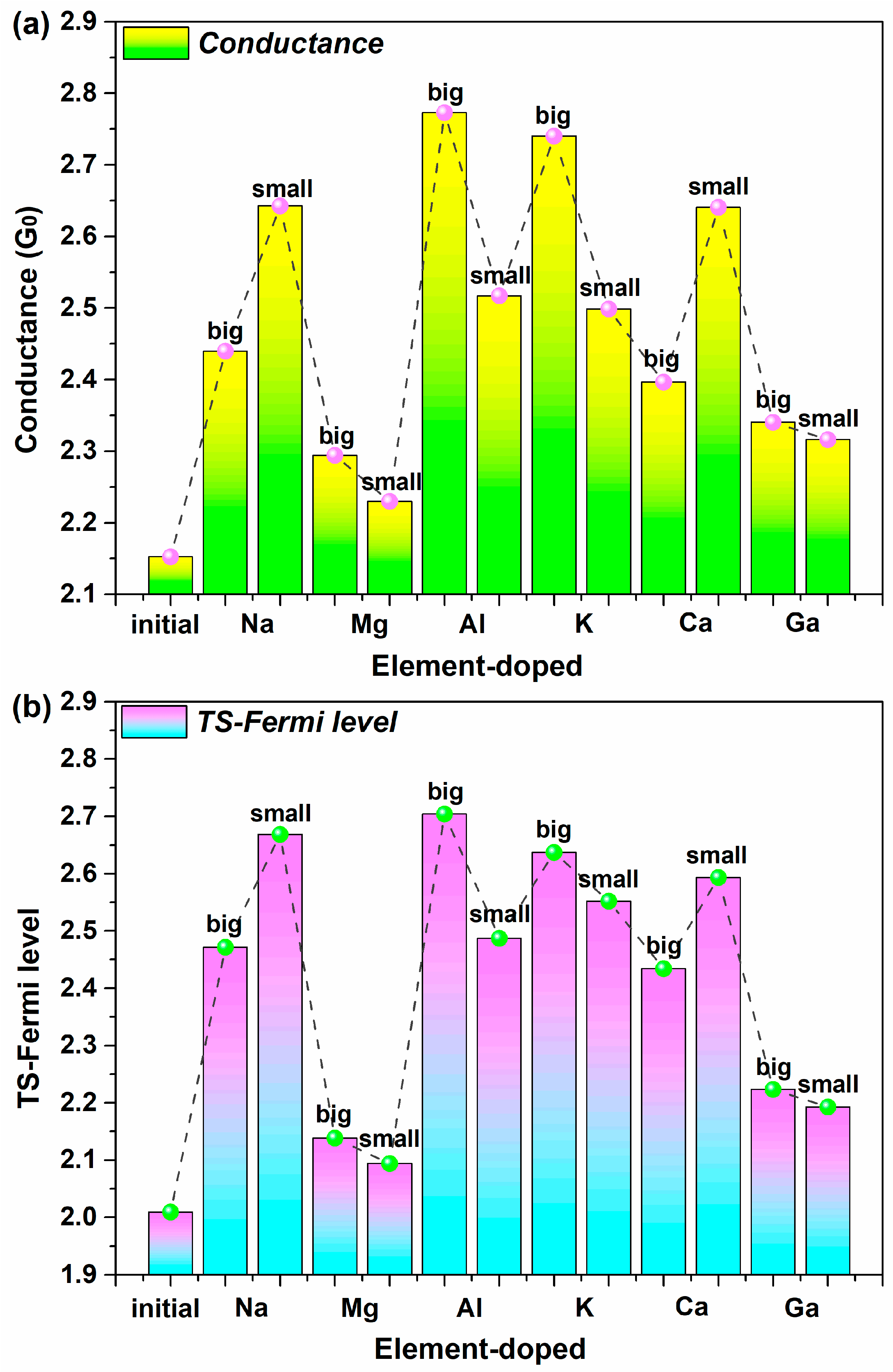
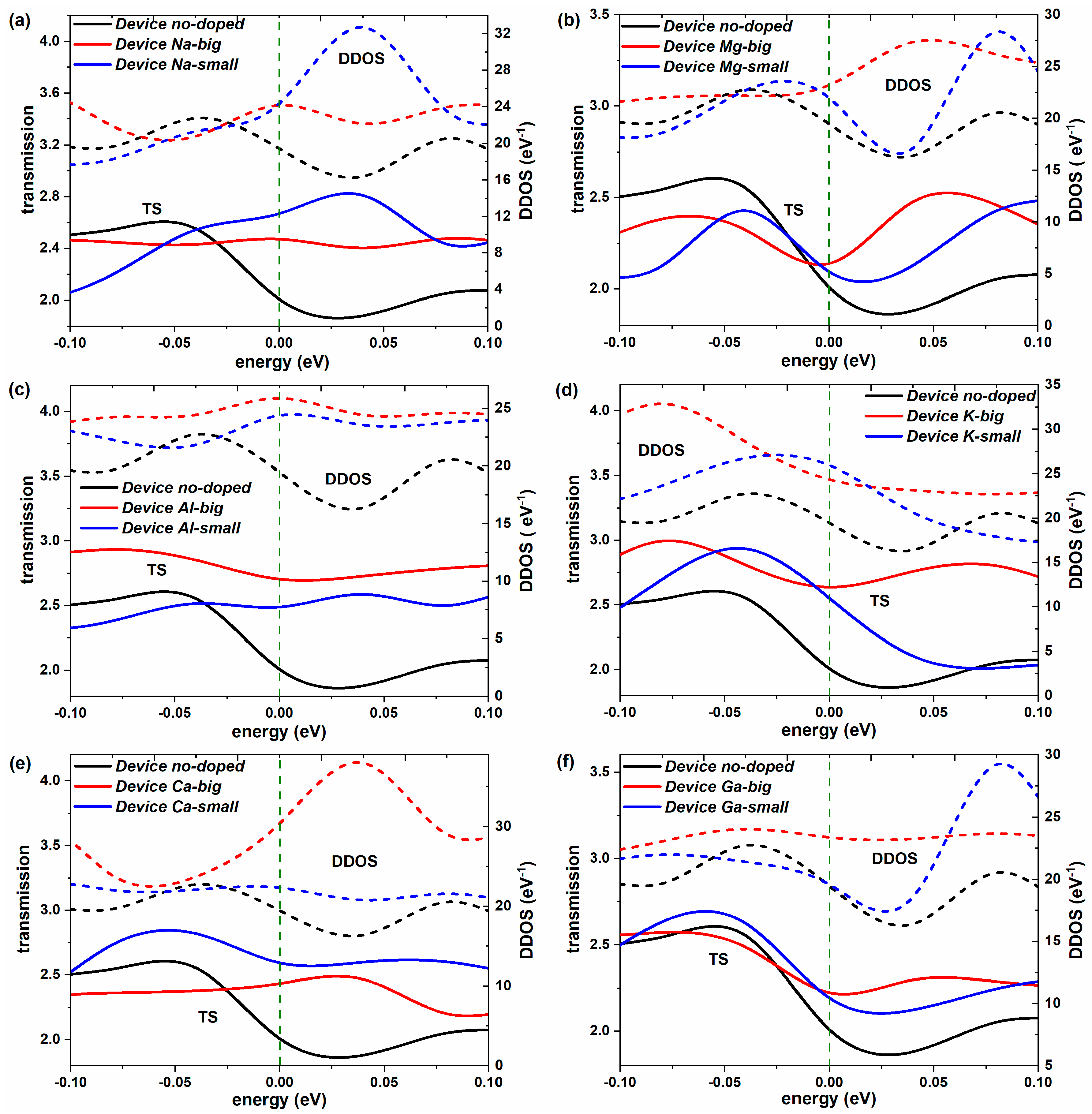
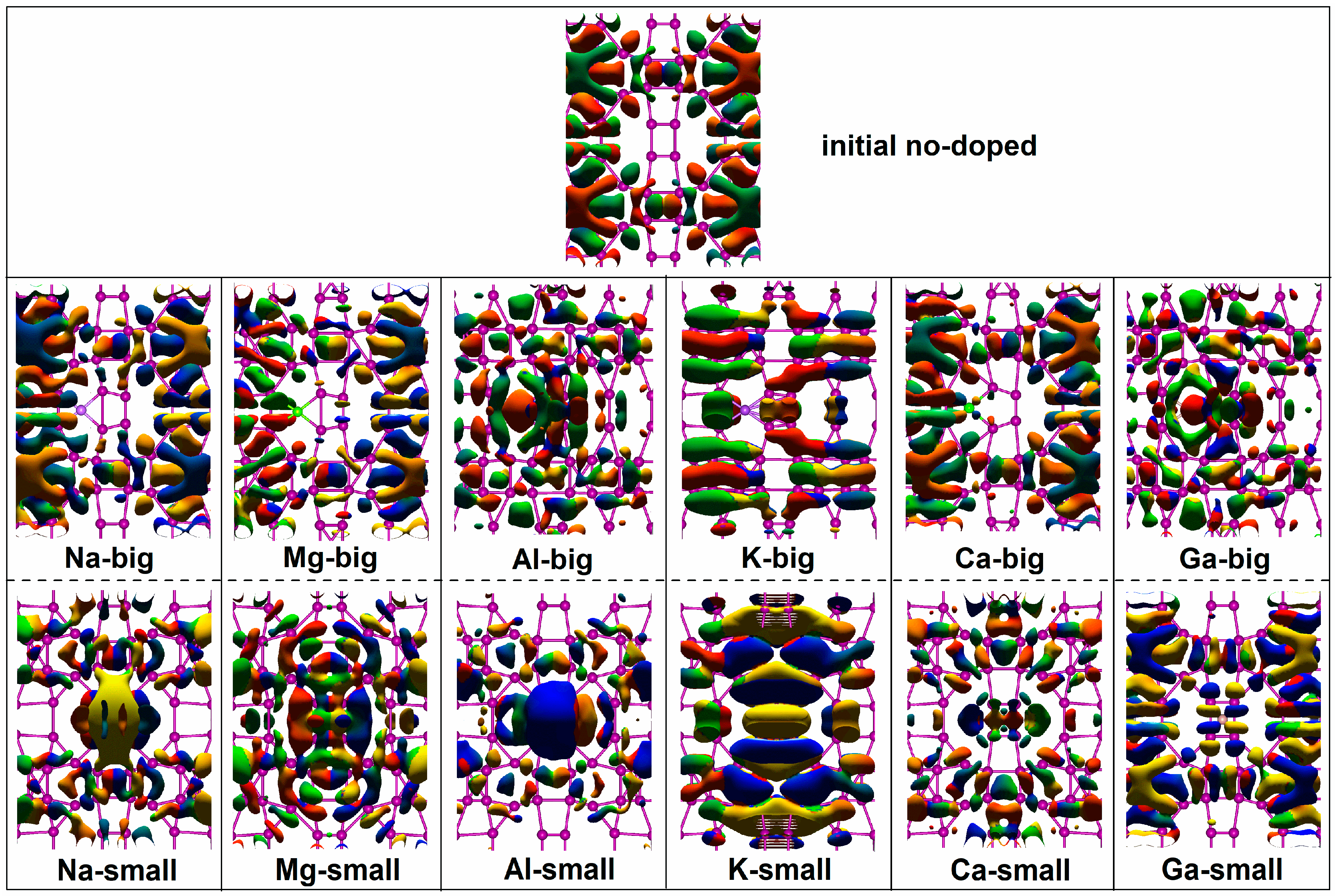
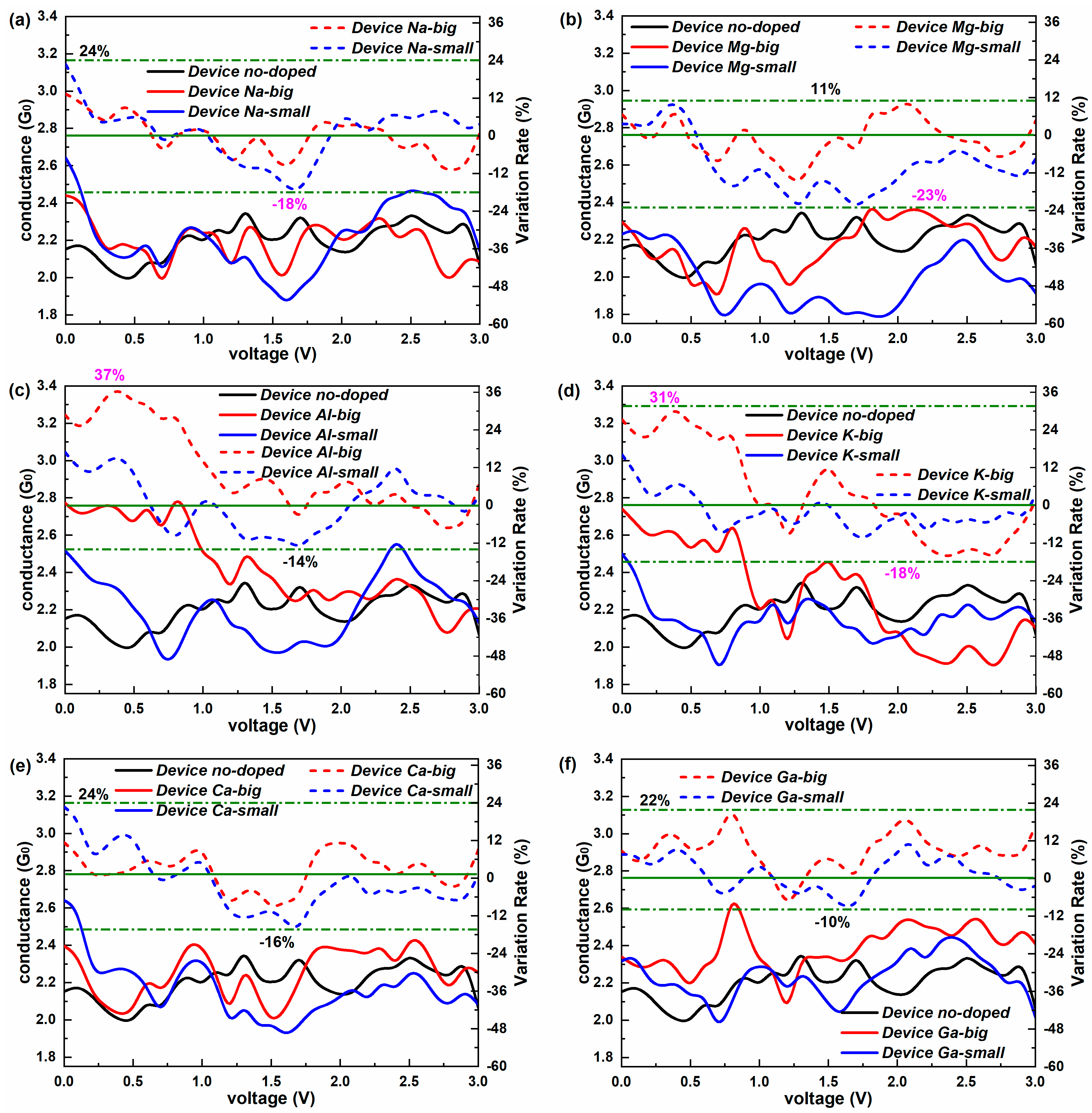
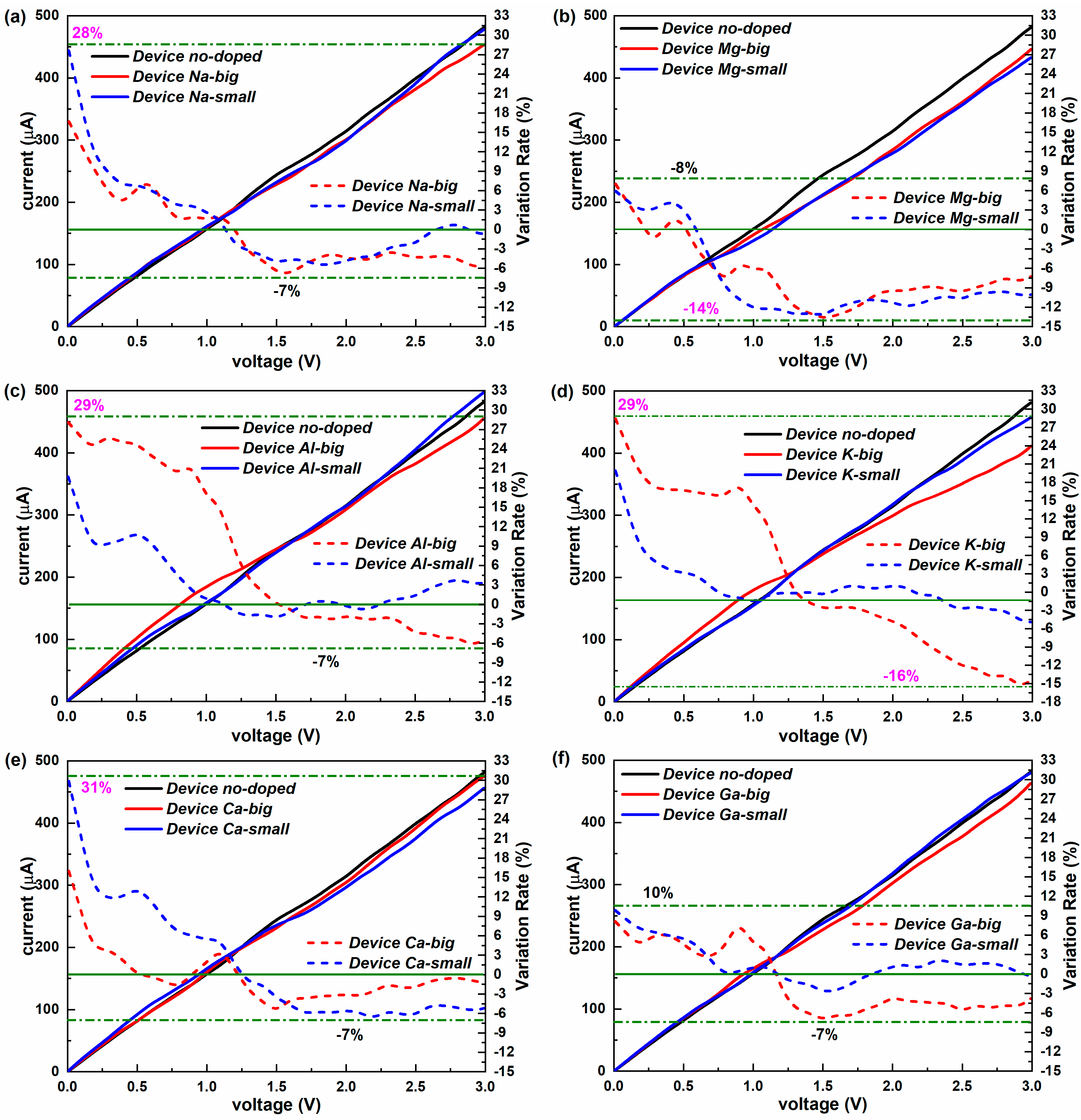
| Dopants | Doping Site | Changes in Con (G0) | Variation Rate (%) | Changes in TS | Variation Rate (%) | Changes in DDOS (eV−1) | Variation Rate (%) |
|---|---|---|---|---|---|---|---|
| Na | big | 0.2874 | 13.36% | 0.4623 | 23.01% | 4.6973 | 24.15% |
| small | 0.4903 | 22.78% | 0.6594 | 32.82% | 4.8661 | 25.01% | |
| K | big | 0.5877 | 27.31% | 0.6279 | 31.25% | 4.8935 | 25.15% |
| small | 0.3463 | 16.09% | 0.5428 | 27.02% | 6.4726 | 33.27% | |
| Mg | big | 0.1417 | 6.58% | 0.1293 | 6.44% | 3.7493 | 19.27% |
| small | 0.0775 | 3.60% | 0.0849 | 4.22% | 2.5120 | 12.91% | |
| Ca | big | 0.2442 | 11.35% | 0.4250 | 21.15% | 10.9708 | 56.39% |
| small | 0.4883 | 22.69% | 0.5842 | 29.08% | 2.8705 | 14.76% | |
| Al | big | 0.6207 | 28.84% | 0.6949 | 34.59% | 6.4266 | 33.04% |
| small | 0.3648 | 16.95% | 0.4781 | 23.80% | 4.9305 | 25.35% | |
| Ga | big | 0.1880 | 8.73% | 0.2142 | 10.66% | 3.9206 | 20.15% |
| small | 0.1637 | 7.61% | 0.1835 | 9.14% | 0.1126 | 0.58% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhou, Y.; Liu, K.; Wang, Y.; Li, H.; Okulov, A. Tunable Electronic Transport of New-Type 2D Iodine Materials Affected by the Doping of Metal Elements. Molecules 2023, 28, 7159. https://doi.org/10.3390/molecules28207159
Li J, Zhou Y, Liu K, Wang Y, Li H, Okulov A. Tunable Electronic Transport of New-Type 2D Iodine Materials Affected by the Doping of Metal Elements. Molecules. 2023; 28(20):7159. https://doi.org/10.3390/molecules28207159
Chicago/Turabian StyleLi, Jie, Yuchen Zhou, Kun Liu, Yifan Wang, Hui Li, and Artem Okulov. 2023. "Tunable Electronic Transport of New-Type 2D Iodine Materials Affected by the Doping of Metal Elements" Molecules 28, no. 20: 7159. https://doi.org/10.3390/molecules28207159
APA StyleLi, J., Zhou, Y., Liu, K., Wang, Y., Li, H., & Okulov, A. (2023). Tunable Electronic Transport of New-Type 2D Iodine Materials Affected by the Doping of Metal Elements. Molecules, 28(20), 7159. https://doi.org/10.3390/molecules28207159







