Novel Strigolactone Mimics That Modulate Photosynthesis and Biomass Accumulation in Chlorella sorokiniana
Abstract
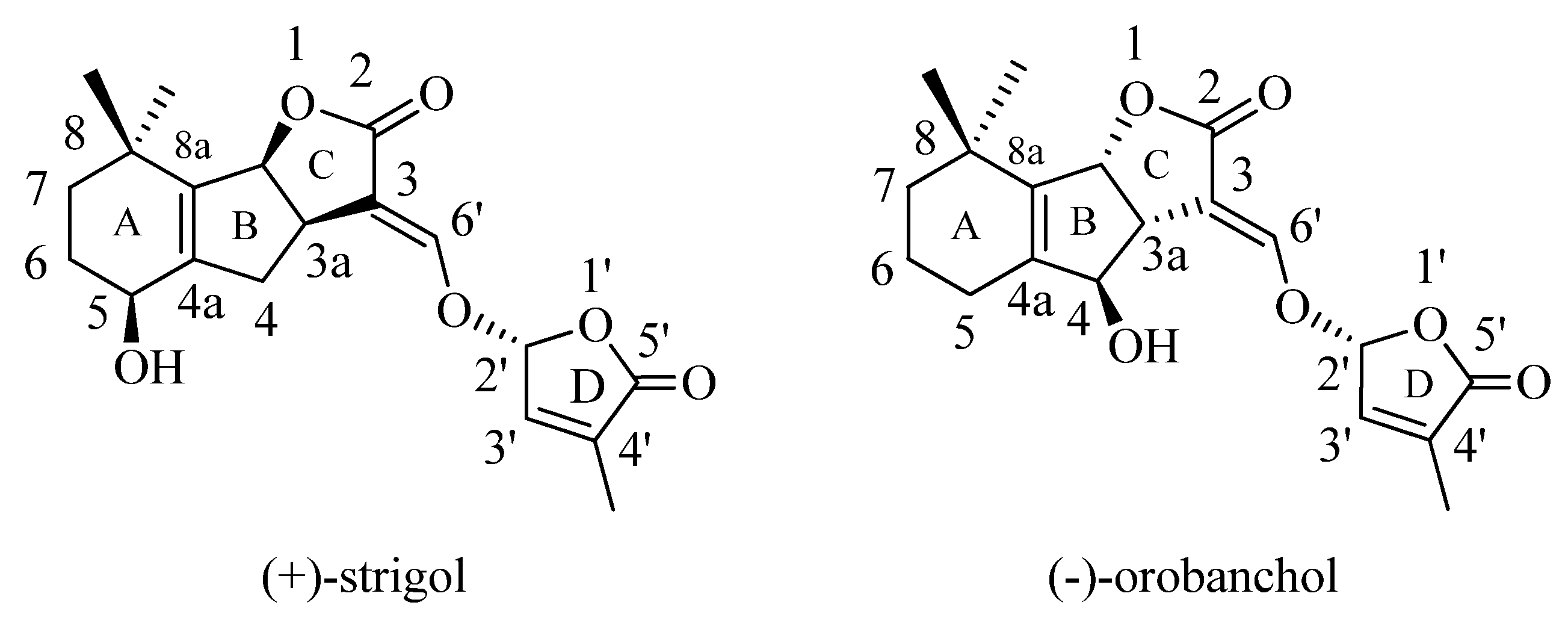
:1. Introduction
2. Results
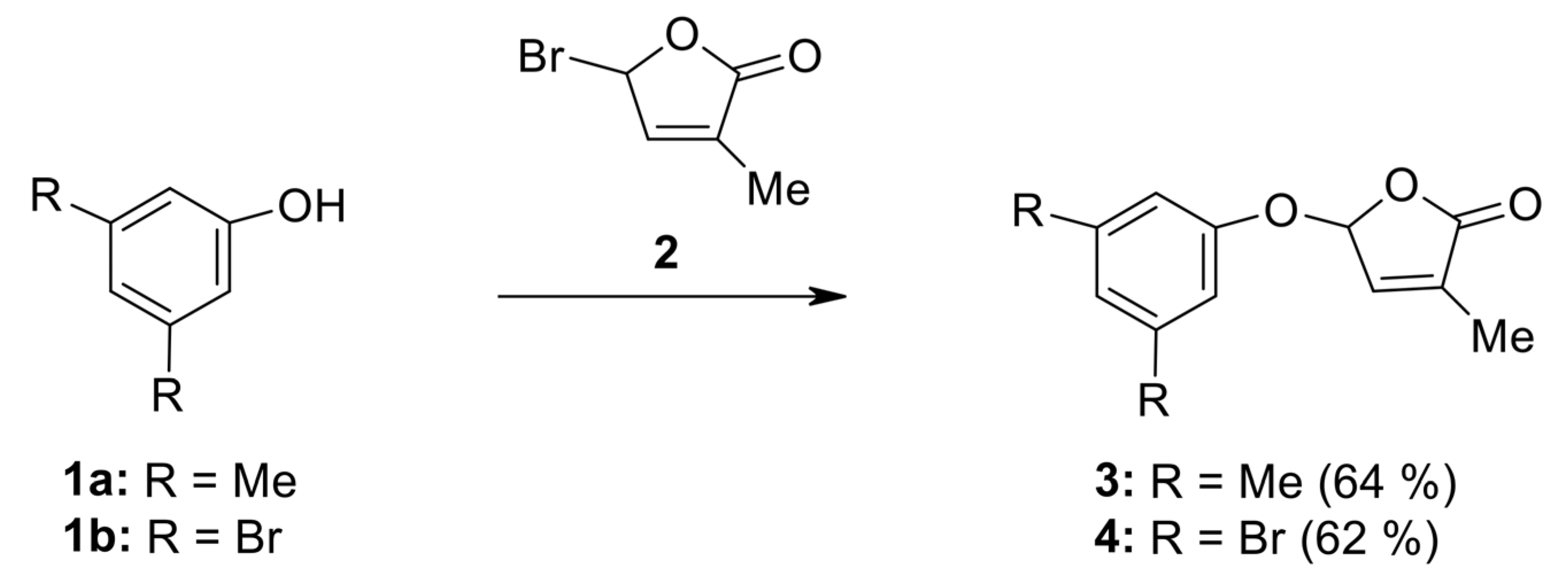
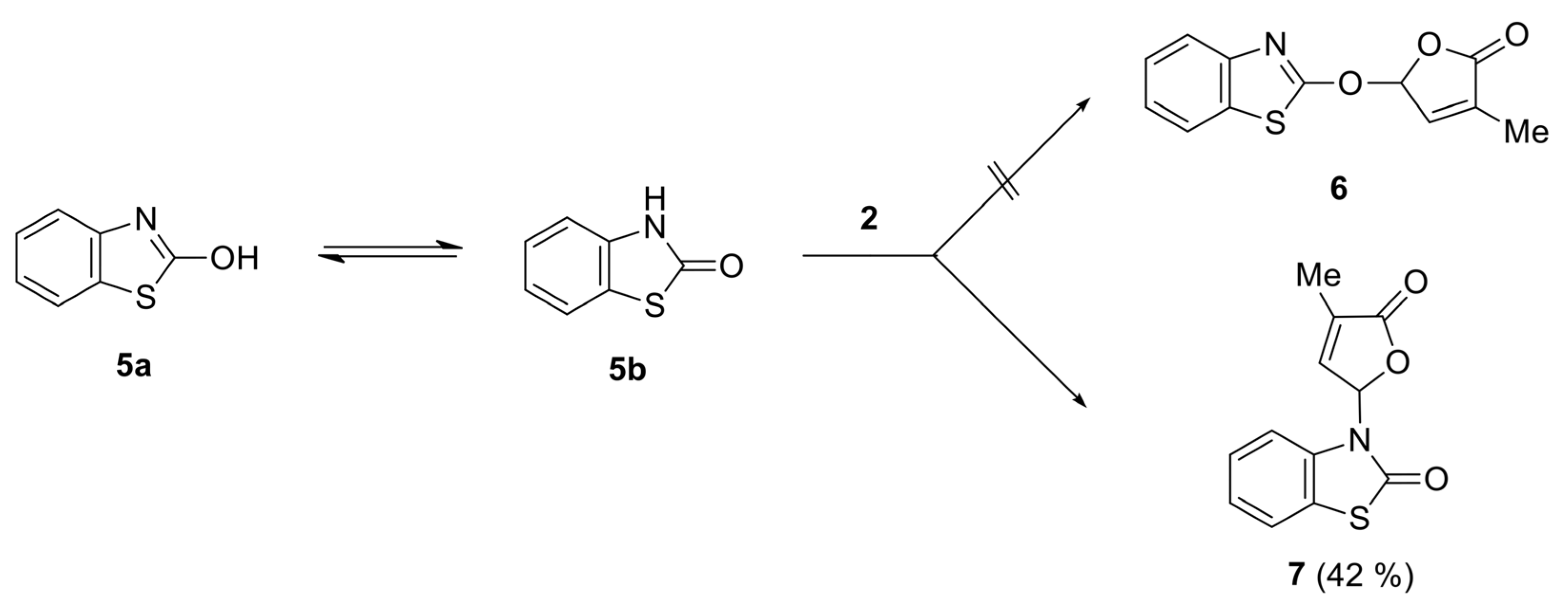
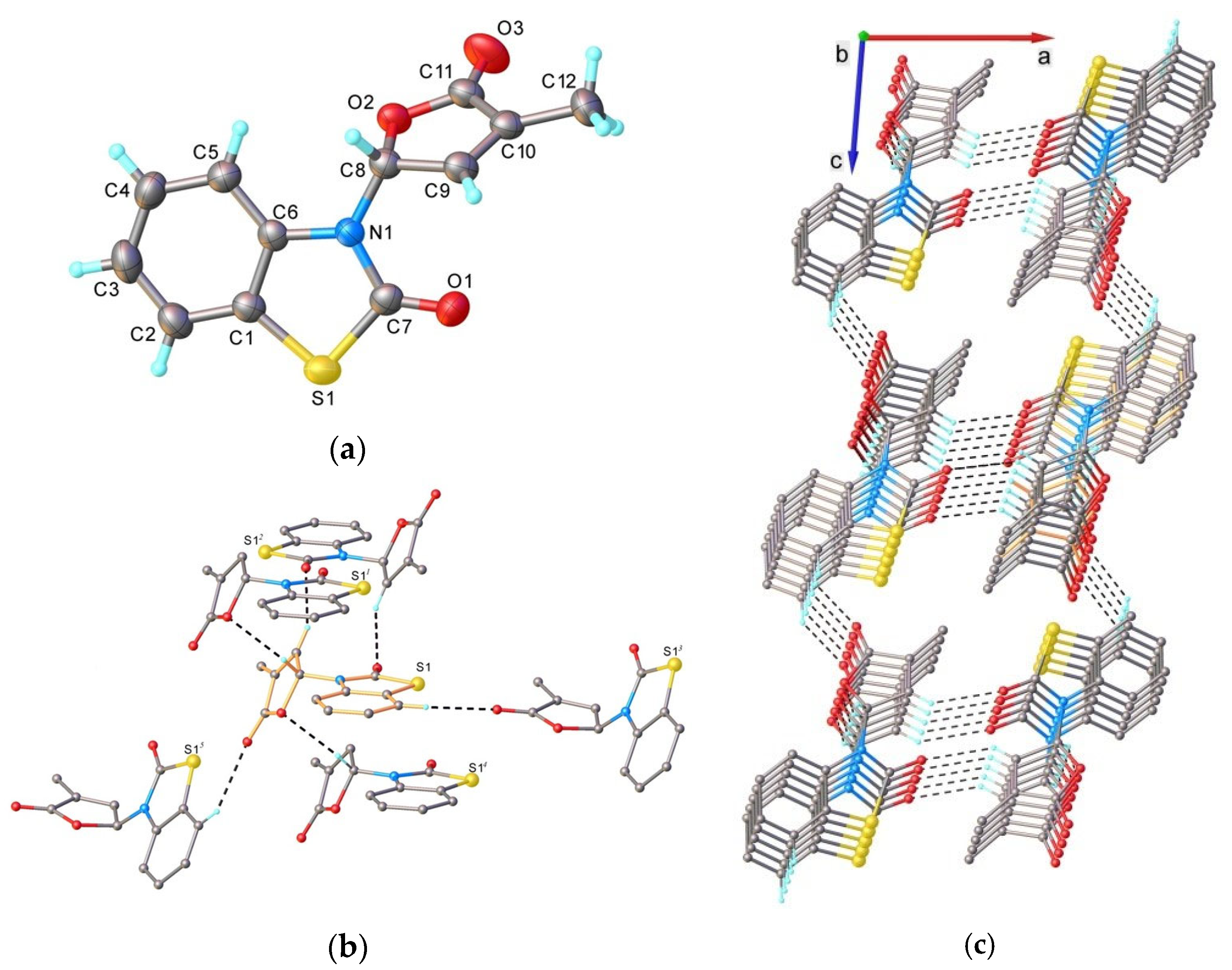
2.1. Synthesis of New Strigolactone Mimics
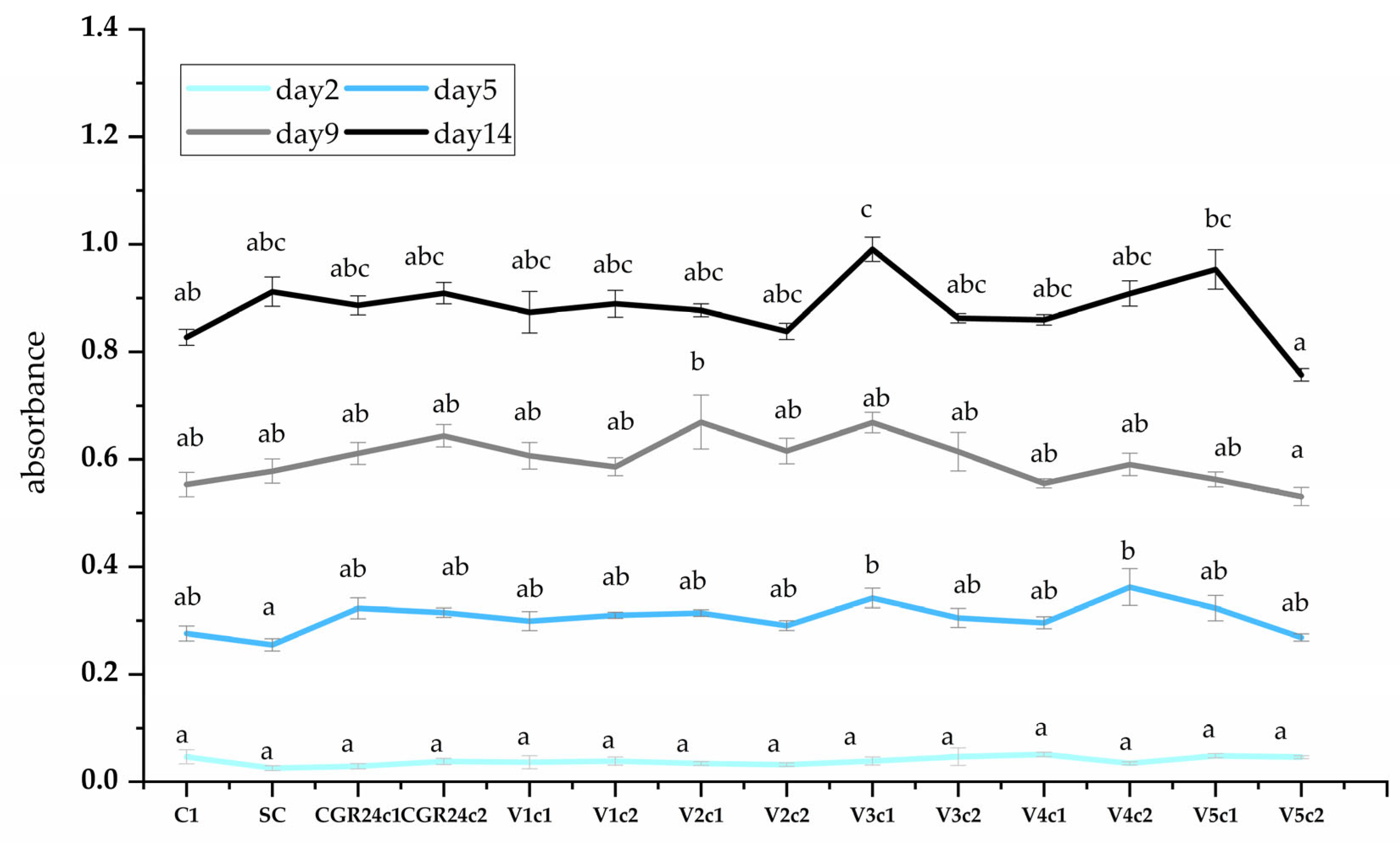
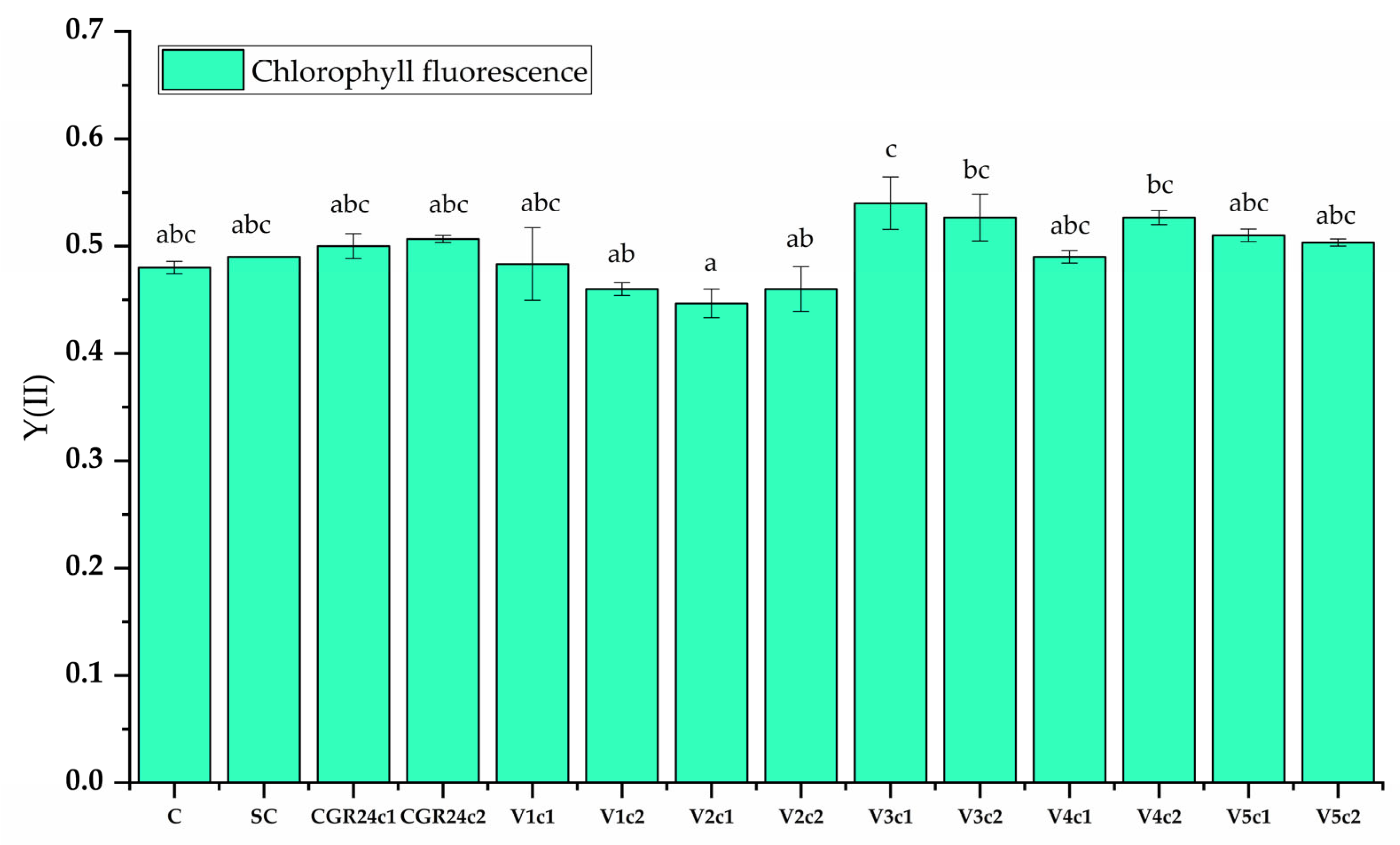
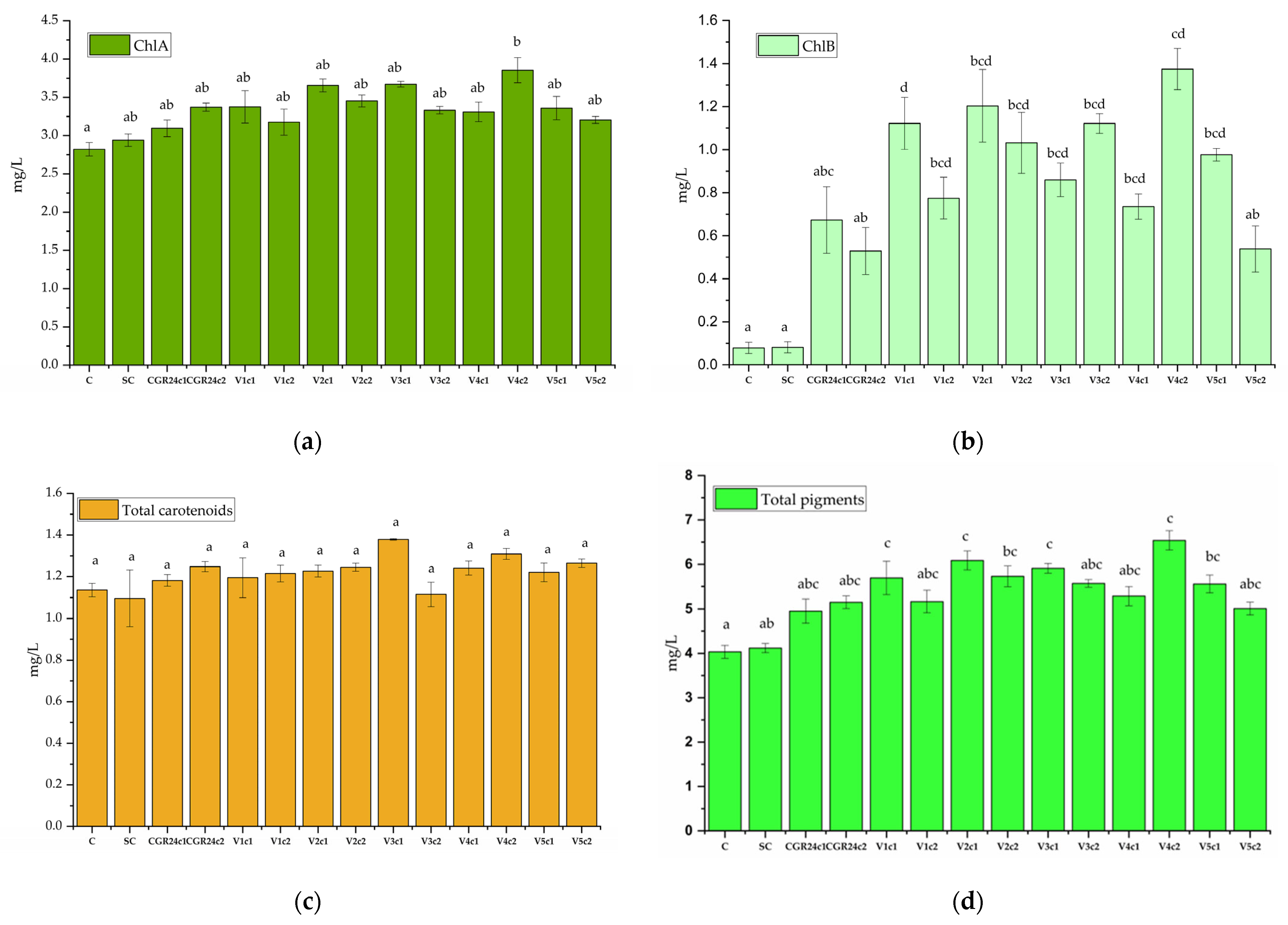
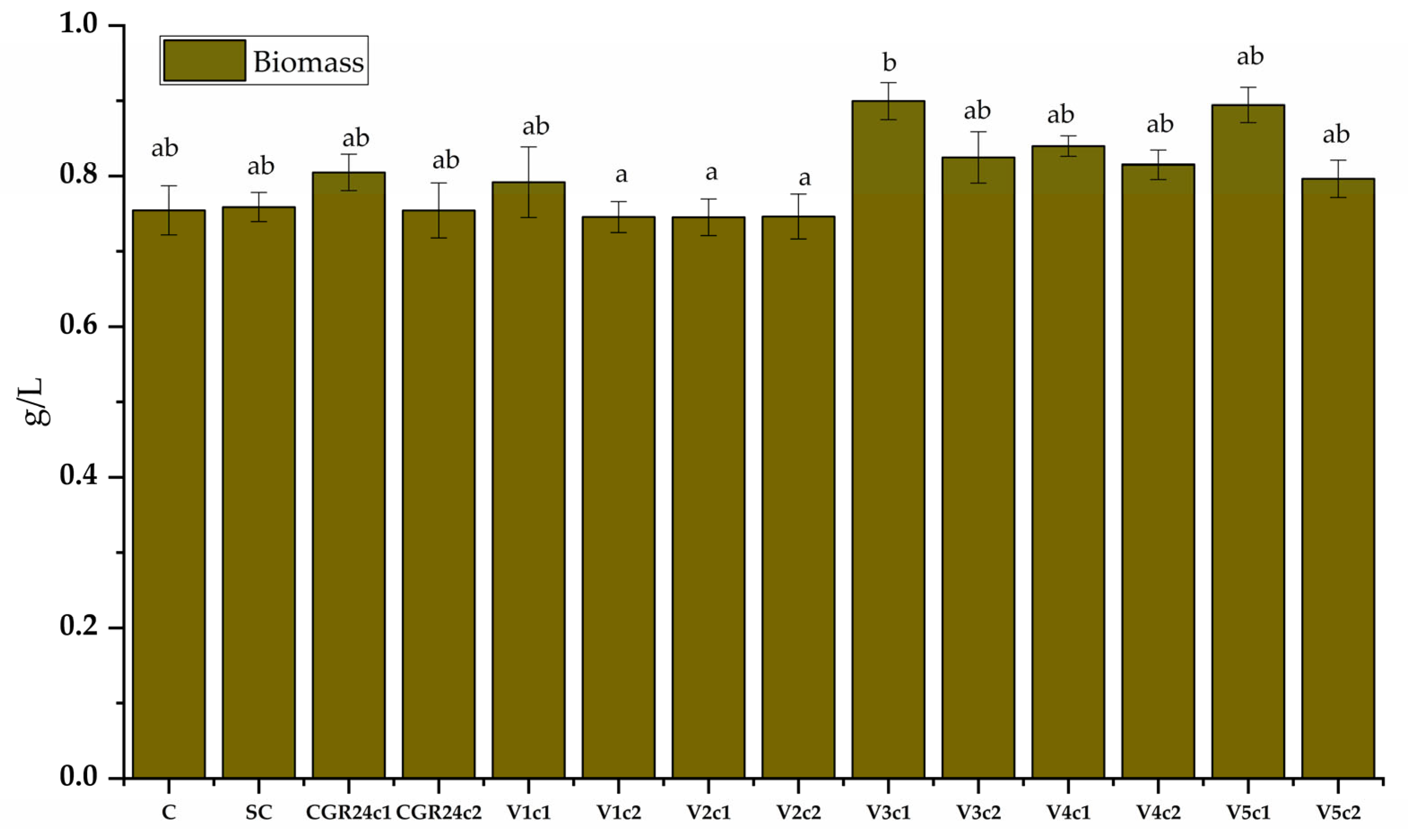
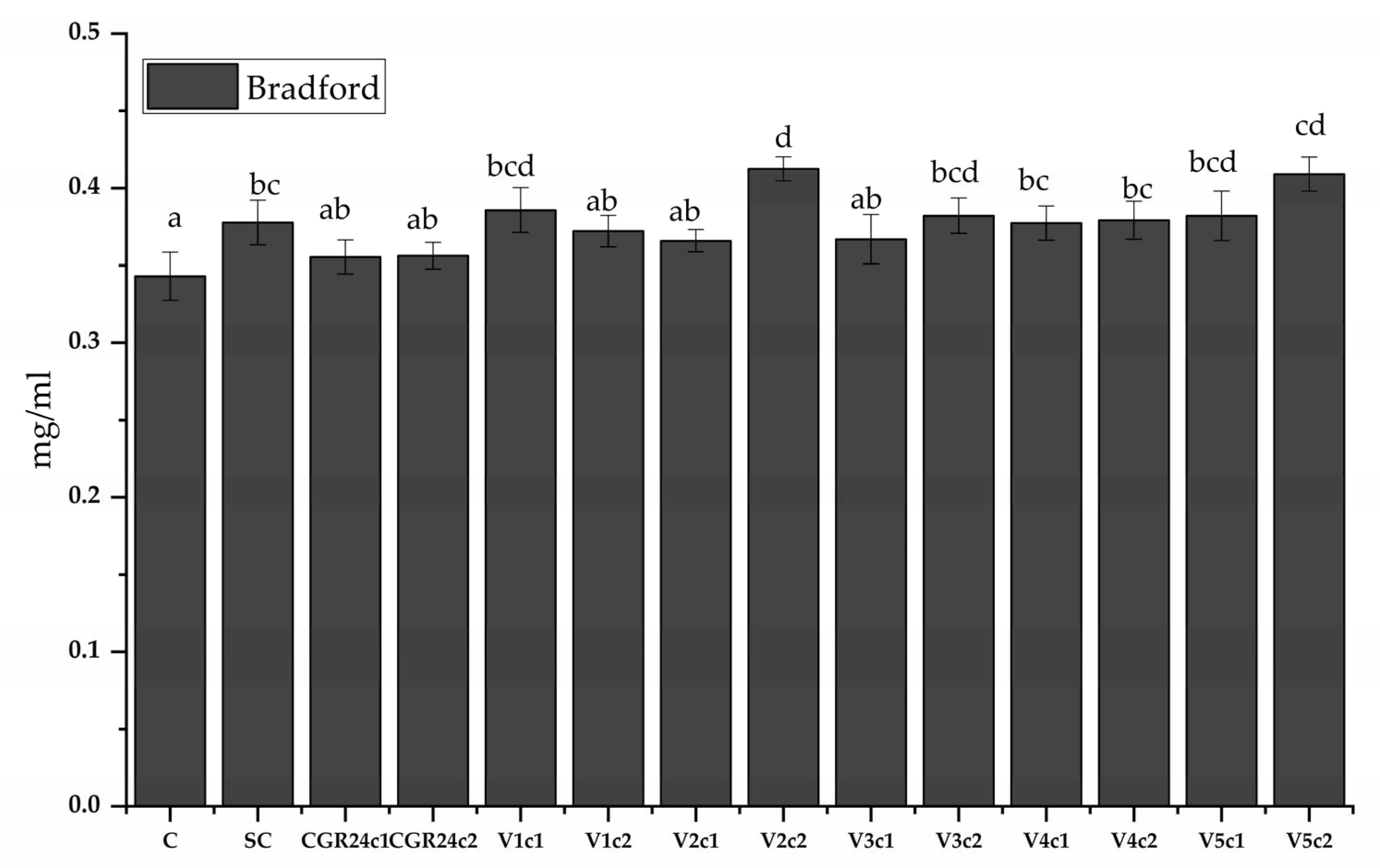
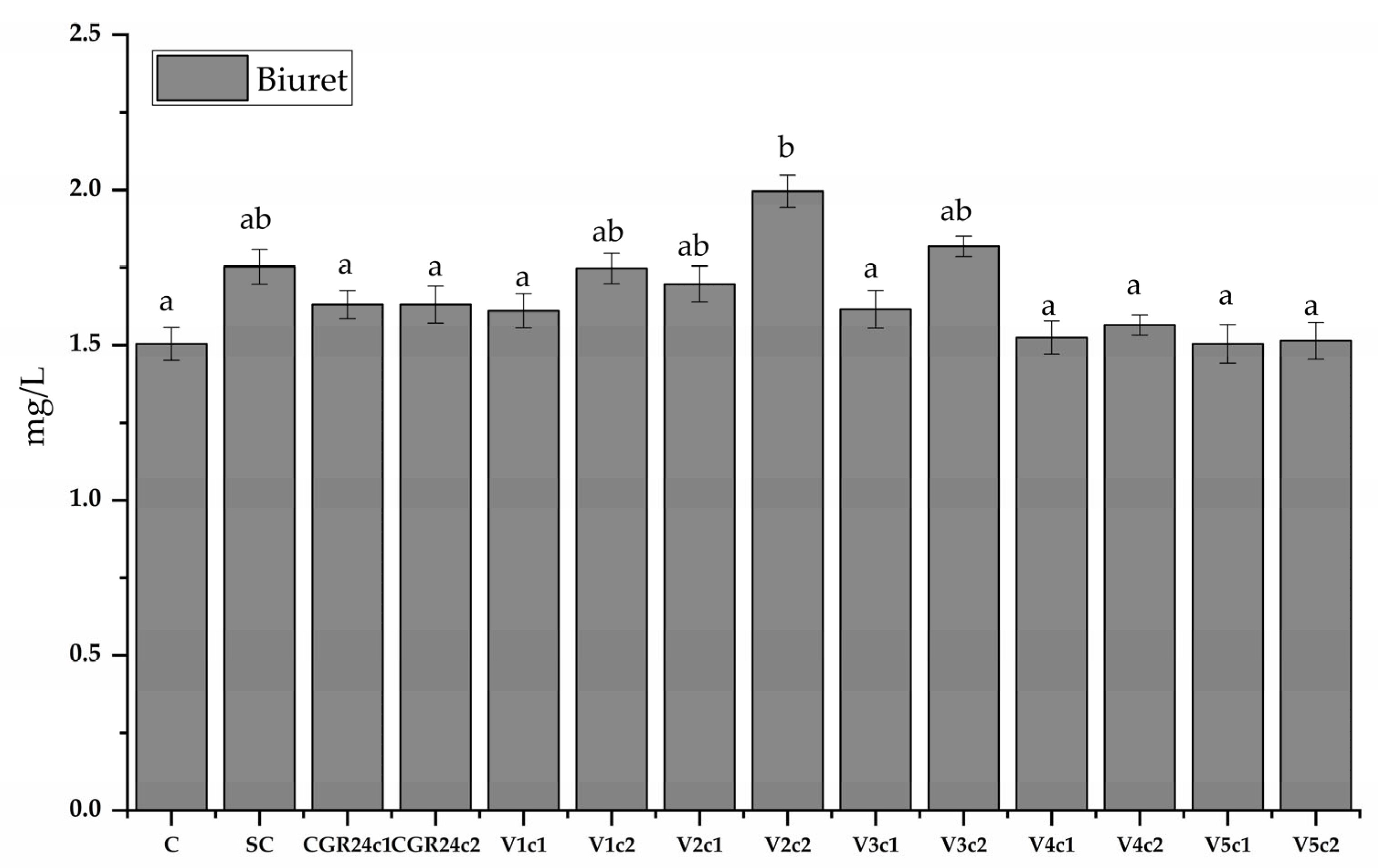
2.2. The Biological Activity of Synthesized Compounds
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Information
4.3. General Procedure for Preparation of SL Mimics Derived from 3,5-Disubstituted Phenols
- 5-(3,5-Dimethylphenoxy)-3-methyl-5H-furan-2-one (3) [SL-F1]. Viscous, pale yellow liquid after purification using column chromatography. Yield 64%. Anal. Calcd. for C13H14O3 (218.25): C 71.54, H 6.47%; Found: C 71.62, H 6.56%. ATR-FTIR (solid): 1771 cm−1 (νC=O). 1H NMR (300 MHz, CDCl3) δ (ppm): 2.12 (s, 3H, Me), 2.44 (s, 6H, 2Me, phenyl), 6.38 (s, 1H, H-5), 6.87 (3H, H-4, 2H phenyl), 7.10 (s, 1H, phenyl). 13C NMR (75 MHz, CDCl3) δ (ppm): 10.5 (Me), 21.3 (2Me, phenyl), 99.1 (C-5), 114.4 (C-2, C-6, phenyl), 125.1(C-4, phenyl), 133.9 (C-3), 139.4 (C-3, C-5, phenyl), 142.6 (C-4), 156.4 (C-1, phenyl), 171.4 (C=O).
- 5-(3,5-Dibromophenoxy)-3-methyl-5H-furan-2-one (4) [SL-F2]. The compound was crystallized from cyclohexane as white crystals with mp 88–91 °C; yield 62%. Anal. Calcd. for C11H8Br2O3 (347.99): C 37.97, H 2.32%; Found: C 38.91, H 2.40%. ATR-FTIR (solid): 1776 cm−1 (νC=O). 1H NMR (300 MHz, CDCl3) δ (ppm): 2.02 (t, 3H, Me, J = 1.4 Hz), 6.23 (quintet, J = 1.4 Hz, 1H, H-5), 6.97 (quintet, J = 1.5 Hz, 1H, H-4), 7.24 (d, 2H, J = 1.4 Hz, H-2, H-6, phenyl), 7.24 (t, 1H, J = 1.4 Hz, H-4 phenyl). 13C NMR (75 MHz, CDCl3) δ (ppm): 10.8 (Me), 98.5 (C-5), 113.2 (C-2, C-6, phenyl), 123.3 (C-3, C-5, phenyl), 129.4 (C-4, phenyl), 134.9 (C-3), 141.7 (C-4), 157.3 (C-1, phenyl), 170.9 (C=O).
4.4. General Procedure for Preparation of SL Mimics Derived from Benzothiazoles
- 3-(4-Methyl-5-oxo-2,5-dihydrofuran-2-yl)-3H-benzothiazol-2-one (7) [SL-F3]. The compound was crystallized from 2-propanol as white crystals with mp 153–155 °C; yield 42%. Anal. Calcd. for C12H9NO3S (M 247.27): C 58.29, H 3.67, N 5.66%; Found: C 58.21, H 3.42, N 5.71%. ATR-FTIR (solid): 1764 cm−1 (νC=O, lactone), 1673 cm−1 (νC=O, lactam). 1H NMR (300 MHz, CDCl3) δ (ppm): 2.28 (s, 3H, Me), 7.07 (d, 1H, J = 7.7 Hz, benzothiazole), 7.16 (bs, 1H, H-5), 7.35–7.42 (m, 3H, H-4 and 2H-benzothiazole), 7.58–7.60 (m, 1H, benzothiazole). 13C NMR (75 MHz, CDCl3) δ (ppm): 10.5 (Me), 81.5 (C-5), 111.9 (C-4, benzothiazole), 122.0 (C-7a, benzothiazole), 123.0, 124.2, 126.8 (C-5, C-6, C-7, benzothiazole), 134.4 (C-3), 135.0 (C-3a, benzothiazole), 143.1 (C-4), 170.0, 171.2 (2 C=O).
- 6-Bromo-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl)-3H-benzothiazol-2-one (9) [SL-F5]. The compound was crystallized from 2-propanol as white crystals with mp 176–177 °C, yielding 44%. Anal. Calcd. for C12H8BrNO3S (M 326.17): C 44.19, H 2.47, N 4.29%; Found: C 44.14, H 2.53, N 4.34. ATR-FTIR (solid): 1755 cm−1 (νC=O, lactone), 1690 cm−1 (νC=O, lactam). 1H NMR (300 MHz, CDCl3) δ (ppm): 2.27 (t, 3H, Me, J = 1.9 Hz), 6.94 (d, 1H, H-4, J = 8.8 Hz, benzothiazole), 7.10 (quintet, J = 1.9 Hz, 1H, H-5), 7.34 (quintet, J = 1.6 Hz, 1H, H-4), 7.53 (dd, 1H, H-5, J = 8.8, 1.9 Hz, benzothiazole), 7.72 (d, 1H, H-7, J = 1.9 Hz, benzothiazole). 13C NMR (75 MHz, CDCl3) δ (ppm): 11.1 (Me), 81.5 (C-5), 113.1 (C-4, benzothiazole), 117.0 (C-6, benzothiazole), 124.0 (C-7a, benzothiazole), 124.5, 128.8 (C-5, C-7, benzothiazole), 134.0 (C-3), 134.6 (C-3a, benzothiazole), 142.7 (C-4), 169.2, 170.9 (2 C=O).
- 6-Nitro-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl)-3H-benzothiazol-2-one (11) [SL-F6]. The compound was crystallized from acetonitrile as white crystals with mp 183–185 °C, yielding 39%. Anal. Calcd. for C12H8N2O5S (M 292.27): C 49.31, H 2.76, N 9.58%; Found: C 49.52, H 2.87, N 9.34%. ATR-FTIR (solid): 1769 cm−1 (νC=O, lactone), 1730 cm−1 (νC=O, lactam). 1H NMR (500 MHz, CDCl3) δ (ppm): 2.13 (t, 3H, Me, J = 1.7 Hz), 6.98 (t, J = 1.8 Hz, 1H, H-5), 7.05 (d, 1H, H-4, J = 9.0 Hz, benzothiazole), 7.23 (t, J = 1.7 Hz, 1H, H-4), 8.19 (dd, 1H, H-5, J = 9.0, 1.3 Hz, benzothiazole), 8.36 (d, 1H, H-7, J = 1.3 Hz, benzothiazole). 13C NMR (100 MHz, CDCl3) δ (ppm): 10.9 (Me), 81.4 (C-5), 111.6 (C-4, benzothiazole), 118.0 (C-5, benzothiazole), 122.8 (C-7, benzothiazole), 123.3 (C-7a, benzothiazole), 134.9 (C-3), 139.6 (C-3a, benzothiazole), 142.1 (C-4), 144.1(C-5, benzothiazole), 169.1, 170.5 (2 C=O).
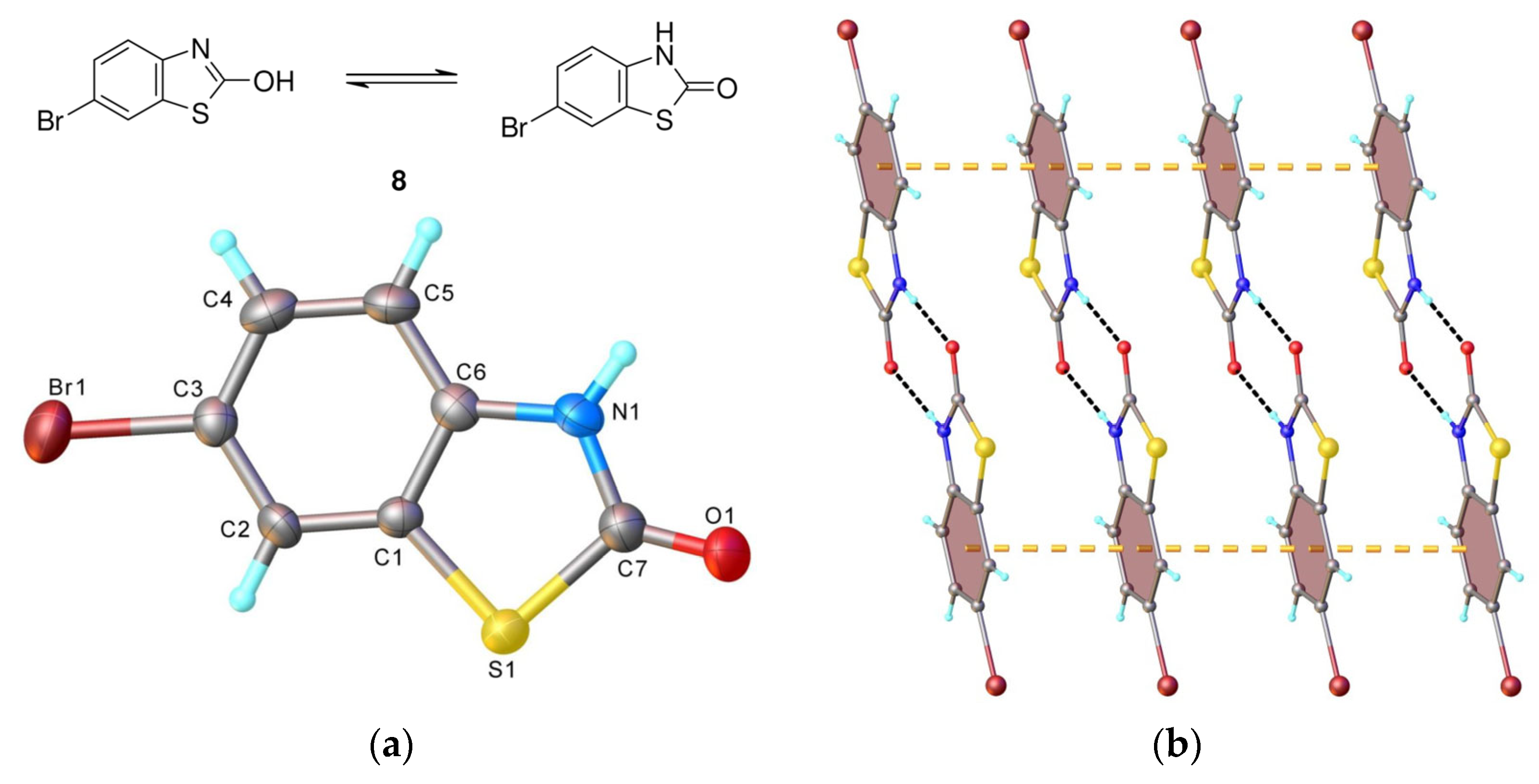
4.5. Single Crystal X-ray Diffraction
4.6. Biological Activities Tests
4.6.1. Bioassay of Strigolactones Mimics
4.6.2. Growth Media
4.6.3. Treatment Solutions
4.6.4. Culturing Conditions
4.6.5. Growth Parameters
4.6.6. Biomass
4.6.7. Pigment Concentrations after Extraction
4.6.8. Protein Content
4.6.9. Chlorophyll Fluorescence
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Compound Number (Synthesis Series) | Compound Code as Strigolactone Mimic/Analog | Chemical Name |
|---|---|---|
| 3 | SL-F1 | 5-(3,5-dimethylphenoxy)-3-methylfuran-2(5H)-one |
| 4 | SL-F2 | 5-(3,5-dibromophenoxy)-3-methylfuran-2(5H)-one |
| 7 | SL-F3 | 3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl)-3H-benzothiazol-2-one |
| 9 | SL-F5 | 6-bromo-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl)-3H-benzothiazol-2-one |
| 11 | SL-F6 | 6-nitro-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl)-3H-benzothiazol-2-one |
| - | GR24 (strigolactone analog)—reference compound | (rac)-GR24; [(±)-(3aR,8bS,E)-3-(((4-methyl-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno [1,2-b]furan-2-one] |
References
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Wall, M.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A.T. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pages, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Becard, G.; Sejalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Boyer, F.D.; de Saint Germain, A.; Pillot, J.P.; Pouvreau, J.B.; Chen, V.X.; Ramos, S.; Stevenin, A.; Simier, P.; Delavault, P.; Beau, J.M.; et al. Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: Molecule design for shoot branching. Plant Physiol. 2012, 159, 1524–1544. [Google Scholar] [CrossRef] [PubMed]
- Kohlen, W.; Ruyter-Spira, C.; Bouwmeester, H.J. Strigolactones: A new musician in the orchestra of plant hormones. Botany 2011, 89, 827–840. [Google Scholar] [CrossRef]
- Seto, Y.; Kameoka, H.; Yamaguchi, S.; Kyozuka, J. Recent advances in strigolactone research: Chemical and biological aspects. Plant Cell Physiol. 2012, 53, 1843–1853. [Google Scholar] [CrossRef]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospisil, T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol. Plant 2013, 6, 38–62. [Google Scholar] [CrossRef]
- Waldie, T.; McCulloch, H.; Leyser, O. Strigolactones and the control of plant development: Lessons from shoot branching. Plant J. 2014, 79, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H. Strigolactones are regulators of root development. New Phytol. 2011, 190, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: Plant Hormones with Promising Features. Angew. Chem. Int. Ed. Engl. 2019, 58, 12778–12786. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Koltai, H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol. 2014, 166, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Kleman, J.; Matusova, R. Strigolactones: Current research progress in the response of plants to abiotic stress. Biologia 2023, 78, 307–318. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, N.; Sugimoto, Y.; Kato, M.; Inanaga, S.; Yoneyama, K. (+)-Strigol, a witchweed seed germination stimulant, from Menispermum dauricum root culture. Phytochemistry 2003, 62, 1115–1119. [Google Scholar] [CrossRef]
- Xie, X.; Kusumoto, D.; Takeuchi, Y.; Yoneyama, K.; Yamada, Y.; Yoneyama, K. 2′-Epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J. Agric. Food Chem. 2007, 55, 8067–8072. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Zwanenburg, B. Tentative molecular mechanism for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogs. J. Agric. Food Chem. 2002, 40, 1066–1070. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones: Structures and biological activities. Pest. Manag. Sci. 2009, 65, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest. Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Hayashi, H. Strigolactones: Chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006, 97, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Becard, G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010, 51, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073–1081. [Google Scholar] [CrossRef]
- McAdam, E.L.; Hugill, C.; Fort, S.; Samain, E.; Cottaz, S.; Davies, N.W.; Reid, J.B.; Foo, E. Determining the Site of Action of Strigolactones during Nodulation. Plant Physiol. 2017, 175, 529–542. [Google Scholar] [CrossRef]
- Soto, M.J.; Fernández-Aparicio, M.; Castellanos-Morales, V.; García-Garrido, J.M.; Ocampo, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil. Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Liu, F.; Rice, J.H.; Lopes, V.; Grewal, P.; Lebeis, S.L.; Hewezi, T.; Staton, M.E. Overexpression of strigolactone-associated genes exerts fine-tuning selection on soybean rhizosphere bacterial and fungal microbiome. Phytobiomes J. 2020, 4, 239–251. [Google Scholar] [CrossRef]
- Kim, B.; Westerhuis, J.A.; Smilde, A.K.; Flokova, K.; Suleiman, A.K.A.; Kuramae, E.E.; Bouwmeester, H.J.; Zancarini, A. Effect of strigolactones on recruitment of the rice root-associated microbiome. FEMS Microbiol. Ecol. 2022, 98, fiac010. [Google Scholar] [CrossRef]
- Wheeldon, C.D.; Hamon-Josse, M.; Lund, H.; Yoneyama, K.; Bennett, T. Environmental strigolactone drives early growth responses to neighboring plants and soil volume in pea. Curr. Biol. 2022, 32, 3593–3600.e3593. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Nomura, T.; Yoneyama, K.; Bennett, T. Supra-organismal regulation of strigolactone exudation and plant development in response to rhizospheric cues in rice. Curr. Biol. 2022, 32, 3601–3608.e3603. [Google Scholar] [CrossRef] [PubMed]
- Waters, M. Plant development: Sizing up the competition with strigolactones. Curr. Biol. 2022, 32, R884–R886. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Li, C.; Bouwmeester, H.J.; Al-Babili, S. Strigolactone Biosynthesis and Signal Transduction; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.K.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 2012, 139, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Xue, Y.L.; Miyakawa, T.; Hou, F.; Qin, H.M.; Fukui, K.; Shi, X.; Ito, E.; Ito, S.; Park, S.H.; et al. Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 2013, 4, 2613. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Uchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Flematti, G.R.; Scaffidi, A.; Waters, M.T.; Smith, S.M. Stereospecificity in strigolactone biosynthesis and perception. Planta 2016, 243, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Tadokoro, E.; Matsuura, M.; Iwasaki, K.; Sugimoto, Y.; Miyake, H.; Takikawa, H.; Sasaki, M. Synthesis and seed germination stimulating activity of some imino analogs of strigolactones. Biosci. Biotechnol. Biochem. 2007, 71, 2781–2786. [Google Scholar] [CrossRef]
- Lachia, M.; Wolf, H.C.; Jung, P.J.; Screpanti, C.; De Mesmaeker, A. Strigolactam: New potent strigolactone analogues for the germination of Orobanche cumana. Bioorg. Med. Chem. Lett. 2015, 25, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Kisugi, T.; Nomura, T.; Nakatani, Y.; Akiyama, K.; McErlean, C.S.P. Which are the major players, canonical or non-canonical strigolactones? J. Exp. Bot. 2018, 69, 2231–2239. [Google Scholar] [CrossRef]
- Johnson, A.W.; Gowada, G.; Hassanali, A.; Knox, J.; Monaco, S.; Razavi, Z.; Rosebery, G. The preparation of synthetic analogues of strigol. J. Chem. Soc. Perkin Trans. 1 1981, 1734–1743. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Zwanenburg, B. Synthesis, structural characterization, and biological evaluation of all four enantiomers of strigol analog GR7. J. Agric. Food Chem. 2002, 40, 697–700. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Van Vliet, L.A.; Vandenput, D.A.L.; Zwanenburg, B. Structural modifications of strigol analogs. Influence of the B and C rings on the bioactivity of the germination stimulant GR24. J. Agric. Food Chem. 2002, 40, 1222–1229. [Google Scholar] [CrossRef]
- Boyer, F.D.; de Saint Germain, A.; Pouvreau, J.B.; Clavé, G.; Pillot, J.P.; Roux, A.; Rasmussen, A.; Depuydt, S.; Lauressergues, D.; Frey, N.F.D.; et al. New Strigolactone Analogs as Plant Hormones with Low Activities in the Rhizosphere. Mol. Plant 2014, 7, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Nefkens, G.H.L.; Thuring, J.W.J.F.; Beenakkers, M.F.M.; Zwanenburg, B. Synthesis of a Phthaloylglycine-Derived Strigol Analogue and Its Germination Stimulatory Activity toward Seeds of the Parasitic Weeds Striga hermonthica and Orobanche crenata. J. Agric. Food Chem. 1997, 45, 2273–2277. [Google Scholar] [CrossRef]
- Mwakaboko, A.S.; Zwanenburg, B. Strigolactone analogs derived from ketones using a working model for germination stimulants as a blueprint. Plant Cell Physiol. 2011, 52, 699–715. [Google Scholar] [CrossRef]
- Mwakaboko, A.S.; Zwanenburg, B. Single step synthesis of strigolactone analogues from cyclic keto enols, germination stimulants for seeds of parasitic weeds. Bioorg. Med. Chem. 2011, 19, 5006–5011. [Google Scholar] [CrossRef]
- Lachia, M.; Jung, P.M.J.; De Mesmaeker, A. A novel approach toward the synthesis of strigolactones through intramolecular [2+2] cycloaddition of ketenes and ketene-iminiums to olefins. Application to the asymmetric synthesis of GR-24. Tetrahedron Lett. 2012, 53, 4514–4517. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S. Strigolactone analogues and mimics derived from phthalimide, saccharine, p-tolylmalondialdehyde, benzoic and salicylic acid as scaffolds. Bioorg. Med. Chem. 2011, 19, 7394–7400. [Google Scholar] [CrossRef]
- Fukui, K.; Ito, S.; Ueno, K.; Yamaguchi, S.; Kyozuka, J.; Asami, T. New branching inhibitors and their potential as strigolactone mimics in rice. Bioorg. Med. Chem. Lett. 2011, 21, 4905–4908. [Google Scholar] [CrossRef]
- Fukui, K.; Ito, S.; Asami, T. Selective mimics of strigolactone actions and their potential use for controlling damage caused by root parasitic weeds. Mol. Plant 2013, 6, 88–99. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Nayak, S.K.; Charnikhova, T.V.; Bouwmeester, H.J. New strigolactone mimics: Structure-activity relationship and mode of action as germinating stimulants for parasitic weeds. Bioorg. Med. Chem. Lett. 2013, 23, 5182–5186. [Google Scholar] [CrossRef]
- Dvorakova, M.; Soudek, P.; Vanek, T. Triazolide Strigolactone Mimics Influence Root Development in Arabidopsis. J. Nat. Prod. 2017, 80, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Cala, A.; Ghooray, K.; Fernandez-Aparicio, M.; Molinillo, J.M.; Galindo, J.C.; Rubiales, D.; Macias, F.A. Phthalimide-derived strigolactone mimics as germinating agents for seeds of parasitic weeds. Pest. Manag. Sci. 2016, 72, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Oancea, F.; Georgescu, E.; Matusova, R.; Georgescu, F.; Nicolescu, A.; Raut, I.; Jecu, M.-L.; Vladulescu, M.-C.; Vladulescu, L.; Deleanu, C. New Strigolactone Mimics as Exogenous Signals for Rhizosphere Organisms. Molecules 2017, 22, 961. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.B.; Screpanti, C.; De Mesmaeker, A.; Munnik, T.; Bouwmeester, H.J. Science and application of strigolactones. New Phytol. 2020, 227, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Screpanti, C.; Fonne-Pfister, R.; Lumbroso, A.; Rendine, S.; Lachia, M.; De Mesmaeker, A. Strigolactone derivatives for potential crop enhancement applications. Bioorg. Med. Chem. Lett. 2016, 26, 2392–2400. [Google Scholar] [CrossRef]
- Walker, C.H.; Siu-Ting, K.; Taylor, A.; O’Connell, M.J.; Bennett, T. Strigolactone synthesis is ancestral in land plants, but canonical strigolactone signalling is a flowering plant innovation. BMC Biol. 2019, 17, 70. [Google Scholar] [CrossRef]
- Bonhomme, S.; Guillory, A. Synthesis and signalling of strigolactone and KAI2-ligand signals in bryophytes. J. Exp. Bot. 2022, 73, 4487–4495. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Wang, Z.; Li, Y.; Zheng, Z. Supplementation of 5-deoxystrigol for higher pollutant removal and biogas quality improvement by different microalgae-based technologies. Water Environ. Res. 2023, 95, e10895. [Google Scholar] [CrossRef]
- Shen, X.; Xue, Z.; Sun, L.; Zhao, C.; Sun, S.; Liu, J.; Zhao, Y.; Liu, J. Effect of GR24 concentrations on biogas upgrade and nutrient removal by microalgae-based technology. Bioresour. Technol. 2020, 312, 123563. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, B.; Jiang, N.; Liu, J.; Zhao, Y.; Xu, J.; Wang, H. The effects of influent chemical oxygen demand and strigolactone analog concentration on integral biogas upgrading and pollutants removal from piggery wastewater by different microalgae-based technologies. Bioresour. Technol. 2023, 370, 128483. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Z.; Zhao, C.; Sun, S.; Xu, J.; Zhao, Y. Effect of GR24 concentrations on tetracycline and nutrient removal from biogas slurry by different microalgae-based technologies. Bioresour. Technol. 2023, 369, 128400. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones Improve Plant Growth, Photosynthesis, and Alleviate Oxidative Stress under Salinity in Rapeseed (Brassica napus L.) by Regulating Gene Expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Su, Q.; Jiang, H.; Cui, J.; He, X.; Wu, Z.; Zhang, Z.; Liu, J.; Zhao, Y. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci. Rep. 2020, 10, 6183. [Google Scholar] [CrossRef]
- Oancea, A.; Georgescu, E.; Georgescu, F.; Nicolescu, A.; Oprita, E.I.; Tudora, C.; Vladulescu, L.; Vladulescu, M.C.; Oancea, F.; Deleanu, C. Isoxazole derivatives as new nitric oxide elicitors in plants. Beilstein J. Org. Chem. 2017, 13, 659–664. [Google Scholar] [CrossRef]
- Georgescu, E.; Oancea, A.; Georgescu, F.; Nicolescu, A.; Oprita, E.I.; Vladulescu, L.; Vladulescu, M.C.; Oancea, F.; Shova, S.; Deleanu, C. Schiff bases containing a furoxan moiety as potential nitric oxide donors in plant tissues. PLoS ONE 2018, 13, e0198121. [Google Scholar] [CrossRef]
- Ali, R.; Siddiqui, N. Biological Aspects of Emerging Benzothiazoles: A Short Review. J. Chem. 2013, 2013, 345198. [Google Scholar] [CrossRef]
- Zhilitskaya, L.V.; Shainyan, B.A.; Yarosh, N.O. Modern Approaches to the Synthesis and Transformations of Practically Valuable Benzothiazole Derivatives. Molecules 2021, 26, 2190. [Google Scholar] [CrossRef] [PubMed]
- MacAlpine, G.A.; Raphael, R.A.; Shaw, A.; Taylor, A.W.; Wild, H.-J. Synthesis of the germination stimulant (±)-strigol. J. Chem. Soc. Perkin Trans. 1 1976, 410–416. [Google Scholar] [CrossRef]
- D’Amico, J.J.; Bellinger, F.G.; Freeman, J.J. Synthesis of 2-oxo and 2-thioxo-3(2H)-benzothiazoleethanimic acid anhydride with acetic acid and related products. J. Heterocycl. Chem. 1988, 25, 1503–1509. [Google Scholar] [CrossRef]
- Loos, D.; Sidoova, E.; Sutoris, V. Benzothiazole Derivatives. 48. Synthesis of 3-Alkoxycarbonylmethyl-6-bromo-2-benzothiazolones and 3-Alkoxycarbonylmethyl-6-nitro-2-benzothiazolones as Potential Plant Growth Regulators. Molecules 1999, 4, 81–93. [Google Scholar] [CrossRef]
- Ma, S.; Zeng, W.; Huang, Y.; Zhu, X.; Xia, A.; Zhu, X.; Liao, Q. Revealing the synergistic effects of cells, pigments, and light spectra on light transfer during microalgae growth: A comprehensive light attenuation model. Bioresour. Technol. 2022, 348, 126777. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.L.; Soares, J.; Coimbra, J.; Leite, M.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, A.; Kilikian, B. Comparative study of Lowry and Bradford methods: Interfering substances. Biotechnol. Tech. 1999, 13, 149–154. [Google Scholar] [CrossRef]
- Martínez, I.; Herrera, A.; Tames-Espinosa, M.; Bondyale-Juez, D.R.; Romero-Kutzner, V.; Packard, T.T.; Gómez, M. Protein in marine plankton: A comparison of spectrophotometric methods. J. Exp. Mar. Biol. Ecol. 2020, 526, 151357. [Google Scholar] [CrossRef]
- Robinson, T. The determination of proteins in plant extracts that contain polyphenols. Plant Sci. Lett. 1979, 15, 211–216. [Google Scholar] [CrossRef]
- Mitsuda, H.; Mitsunaga, T. Evaluation and elimination of the interference by starch in the biuret determination of wheat protein. Agric. Biol. Chem. 1974, 38, 1649–1655. [Google Scholar]
- Dupré, C.; Burrows, H.D.; Campos, M.G.; Delattre, C.; Encarnação, T.; Fauchon, M.; Gaignard, C.; Hellio, C.; Ito, J.; Laroche, C. Microalgal biomass of industrial interest: Methods of characterization. In Handbook on Characterization of Biomass, Biowaste and Related By-Products; Springer: Berlin/Heidelberg, Germany, 2020; pp. 537–639. [Google Scholar]
- Barbier, F.; Fichtner, F.; Beveridge, C. The strigolactone pathway plays a crucial role in integrating metabolic and nutritional signals in plants. Nat. Plants 2023, 9, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Guo, J.; Li, K.; Wang, Y.; Han, X.; Fu, W.; Miao, Y.; Jia, K.P. Carotenoid-derived bioactive metabolites shape plant root architecture to adapt to the rhizospheric environments. Front. Plant Sci. 2022, 13, 986414. [Google Scholar] [CrossRef] [PubMed]
- Halouzka, R.; Zeljkovic, S.C.; Klejdus, B.; Tarkowski, P. Analytical methods in strigolactone research. Plant Methods 2020, 16, 76. [Google Scholar] [CrossRef]
- Charnikhova, T.V.; Gaus, K.; Lumbroso, A.; Sanders, M.; Vincken, J.P.; De Mesmaeker, A.; Ruyter-Spira, C.P.; Screpanti, C.; Bouwmeester, H.J. Zealactones. Novel natural strigolactones from maize. Phytochemistry 2017, 137, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Cavar Zeljkovic, S.; Pospisil, T. Synthesis of strigolactones, a strategic account. Pest. Manag. Sci. 2016, 72, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Strategies for the synthesis of canonical, non-canonical and analogues of strigolactones, and evaluation of their parasitic weed germination activity. Phytochem. Rev. 2022, 21, 1627–1659. [Google Scholar] [CrossRef]
- Seto, Y.; Sado, A.; Asami, K.; Hanada, A.; Umehara, M.; Akiyama, K.; Yamaguchi, S. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. USA 2014, 111, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Pospíšil, T.; Ćavar Zeljković, S. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Dommerholt, F.J.; De Jong, R.L.; Zwanenburg, B. Improved synthesis of strigol analog GR24 and evaluation of the biological activity of its diastereomers. J. Agric. Food Chem. 1992, 40, 1230–1235. [Google Scholar] [CrossRef]
- Moges, M.E.; Heistad, A.; Heidorn, T. Nutrient recovery from anaerobically treated blackwater and improving its effluent quality through microalgae biomass production. Water 2020, 12, 592. [Google Scholar] [CrossRef]
- Mihaila, E.-G.; Popa, D.G.; Dima, M.D.; Stoian, I.M.; Dinca, C.F.; Constantinescu-Aruxandei, D.; Oancea, F. Experimental Model for High-Throughput Screening of Microalgae Strains Useful for CO2 Fixation. Chem. Proc. 2022, 7, 25. [Google Scholar]
- Zoccali, M.; Giuffrida, D.; Salafia, F.; Socaciu, C.; Skjanes, K.; Dugo, P.; Mondello, L. First Apocarotenoids Profiling of Four Microalgae Strains. Antioxidants 2019, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Y.; Li, T.; Han, B.; Zhao, P.; Xu, J.W.; Yu, X. Enhancement of lipid accumulation in Monoraphidium sp. QLY-1 by induction of strigolactone. Bioresour. Technol. 2019, 288, 121607. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, W.; Taidi, B.; Lacombe, R.; Perré, P.; Pozzobon, V. Impact of seconds to minutes photoperiods on Chlorella vulgaris growth rate and chlorophyll a and b content. Algal Res. 2018, 36, 10–16. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Popa, D.G.; Lupu, C.; Constantinescu-Aruxandei, D.; Oancea, F. Humic Substances as Microalgal Biostimulants-Implications for Microalgal Biotechnology. Mar. Drugs 2022, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- Faraon, V.A.; Popa, D.G.; Tudor-Popa, I.; Mihăilă, E.G.; Oancea, F. Effect of humic acids from biomass biostimulant on microalgae growth. Chem. Proc. 2022, 7, 51. [Google Scholar]
- Constantinescu-Aruxandei, D.; Vlaicu, A.; Marinaș, I.C.; Vintilă, A.C.N.; Dimitriu, L.; Oancea, F. Effect of betaine and selenium on the growth and photosynthetic pigment production in Dunaliella salina as biostimulants. FEMS Microbiol. Lett. 2019, 366, fnz257. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Lim, J.W.; Rawindran, H.; Chai, Y.H.; Tan, I.S.; Fui Chin, B.L.; Kiew, P.L. The potential of waste chicken feather protein hydrolysate as microalgae biostimulant using organic fertilizer as nutrients source. IOP Conf. Ser. Earth Environ. Sci. 2022, 1074, 012028. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Rawindran, H.; Lim, J.W.; Pa’ee, K.F.; Yong, K.T.L.; Tan, I.S.; Chin, B.L.F.; Show, P.L.; Lee, K.T. Optimizing extraction of antioxidative biostimulant from waste onion peels for microalgae cultivation via response surface model. Energy Convers. Manag. 2023, 286, 117023. [Google Scholar] [CrossRef]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.Q.; Wang, L.R.; Zhang, Z.X.; Sun, X.M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, B.-F.; Kong, F.; Ren, N.-Q.; Ren, H.-Y. Overview on stress-induced strategies for enhanced microalgae lipid production: Application, mechanisms and challenges. Resour. Conserv. Recycl. 2022, 183, 106355. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.P.; Han, B.; Yu, X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Bioresour. Technol. 2019, 274, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qi, M.; Guo, J.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The active phytohormone in microalgae: The characteristics, efficient detection, and their adversity resistance applications. Molecules 2021, 27, 46. [Google Scholar] [CrossRef] [PubMed]
- Proust, H.; Hoffmann, B.; Xie, X.; Yoneyama, K.; Schaefer, D.G.; Yoneyama, K.; Nogue, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011, 138, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Rich, M.K.; Yoda, A.; Shimazaki, S.; Xie, X.; Akiyama, K.; Mizuno, Y.; Komatsu, A.; Luo, Y.; Suzuki, H.; et al. An ancestral function of strigolactones as symbiotic rhizosphere signals. Nat. Commun. 2022, 13, 3974. [Google Scholar] [CrossRef] [PubMed]
- Kee, Y.J.; Ogawa, S.; Ichihashi, Y.; Shirasu, K.; Yoshida, S. Strigolactones in rhizosphere communication: Multiple molecules with diverse functions. Plant Cell Physiol. 2023, 64, 955–966. [Google Scholar] [CrossRef]
- Ito, S.; Braguy, J.; Wang, J.Y.; Yoda, A.; Fiorilli, V.; Takahashi, I.; Jamil, M.; Felemban, A.; Miyazaki, S.; Mazzarella, T.; et al. Canonical strigolactones are not the major determinant of tillering but important rhizospheric signals in rice. Sci. Adv. 2022, 8, eadd1278. [Google Scholar] [CrossRef]
- Sattar, A.; Ul-Allah, S.; Ijaz, M.; Sher, A.; Butt, M.; Abbas, T.; Irfan, M.; Fatima, T.; Alfarraj, S.; Alharbi, S.A. Exogenous application of strigolactone alleviates drought stress in maize seedlings by regulating the physiological and antioxidants defense mechanisms. Cereal Res. Commun. 2021, 50, 263–272. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, Z.; Zhou, Y.; Guo, S.; Sang, T.; Wang, Y.; Shu, S. Physiological mechanism of strigolactone enhancing tolerance to low light stress in cucumber seedlings. BMC Plant Biol. 2022, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.I.; Zehra, A.; Choudhary, S.; Naeem, M.; Khan, M.M.A.; Khan, R.; Aftab, T. Exogenous Strigolactone (GR24) Positively Regulates Growth, Photosynthesis, and Improves Glandular Trichome Attributes for Enhanced Artemisinin Production in Artemisia annua. J. Plant Growth Regul. 2022, 42, 4606–4615. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Jerez, C.G.; Korbee, N. Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat. Am. J. Aquat. Res. 2013, 41, 801–819. [Google Scholar] [CrossRef]
- Solovchenko, A.; Lukyanov, A.; Vasilieva, S.; Lobakova, E. Chlorophyll fluorescence as a valuable multitool for microalgal biotechnology. Biophys. Rev. 2022, 14, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence--a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.; Shahbaz, M.; Wahid, M.A. Potential Role of Foliage Applied Strigolactone (GR24) on Photosynthetic Pigments, Gas Exchange Attributes, Mineral Nutrients and Yield Components of Zea mays (L.) Under Saline Regimes. Gesunde Pflanz. 2022, 75, 577–591. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, L.; Wang, Z.; Xu, J.; Wei, J.; Zhao, Y. Performance of cocultivation of Chlorella vulgaris and four different fungi in biogas slurry purification and biogas upgrading by induction of strigolactone (GR24) and endophytic bacteria. Water Environ. Res. 2023, 95, e10896. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, W.K.; Lee, B.; Seon, G.; Suh, W.I.; Moon, M.; Chang, Y.K. Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Sci. Rep. 2019, 9, 19383. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Chai, S.; Shi, J.; Huang, T.; Guo, Y.; Wei, J.; Guo, M.; Li, L.; Dou, S.; Liu, L.; Liu, G. Characterization of Chlorella sorokiniana growth properties in monosaccharide-supplemented batch culture. PLoS ONE 2018, 13, e0199873. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, D.G.; Georgescu, F.; Dumitrascu, F.; Shova, S.; Constantinescu-Aruxandei, D.; Draghici, C.; Vladulescu, L.; Oancea, F. Novel Strigolactone Mimics That Modulate Photosynthesis and Biomass Accumulation in Chlorella sorokiniana. Molecules 2023, 28, 7059. https://doi.org/10.3390/molecules28207059
Popa DG, Georgescu F, Dumitrascu F, Shova S, Constantinescu-Aruxandei D, Draghici C, Vladulescu L, Oancea F. Novel Strigolactone Mimics That Modulate Photosynthesis and Biomass Accumulation in Chlorella sorokiniana. Molecules. 2023; 28(20):7059. https://doi.org/10.3390/molecules28207059
Chicago/Turabian StylePopa, Daria Gabriela, Florentina Georgescu, Florea Dumitrascu, Sergiu Shova, Diana Constantinescu-Aruxandei, Constantin Draghici, Lucian Vladulescu, and Florin Oancea. 2023. "Novel Strigolactone Mimics That Modulate Photosynthesis and Biomass Accumulation in Chlorella sorokiniana" Molecules 28, no. 20: 7059. https://doi.org/10.3390/molecules28207059
APA StylePopa, D. G., Georgescu, F., Dumitrascu, F., Shova, S., Constantinescu-Aruxandei, D., Draghici, C., Vladulescu, L., & Oancea, F. (2023). Novel Strigolactone Mimics That Modulate Photosynthesis and Biomass Accumulation in Chlorella sorokiniana. Molecules, 28(20), 7059. https://doi.org/10.3390/molecules28207059







