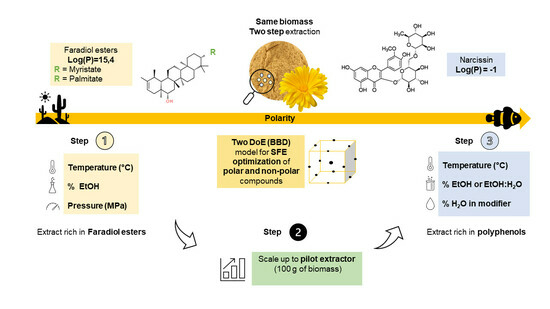
Exploring the Sequential-Selective Supercritical Fluid Extraction (S3FE) of Flavonoids and Esterified Triterpenoids from Calendula officinalis L. Flowers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Calendula Non-Polar Extracts (CNPE)
2.1.1. Statistical Analysis, Model, and Factor Significance
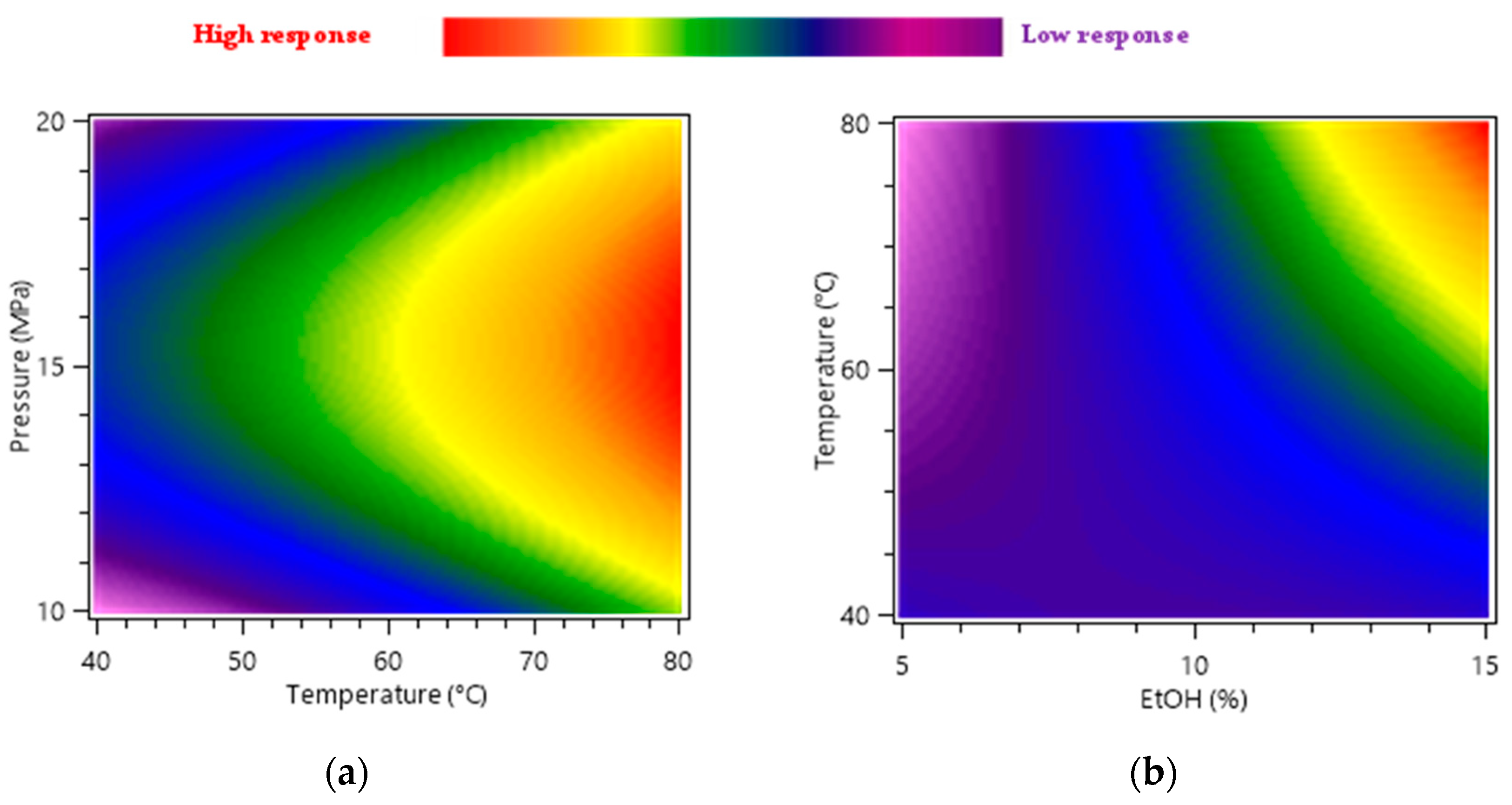
2.1.2. Effects of the Extraction Parameters on CNPE Assessed Using the Box-Behnken Design
2.1.3. Optimal Selective Extraction of Faradiol Esters
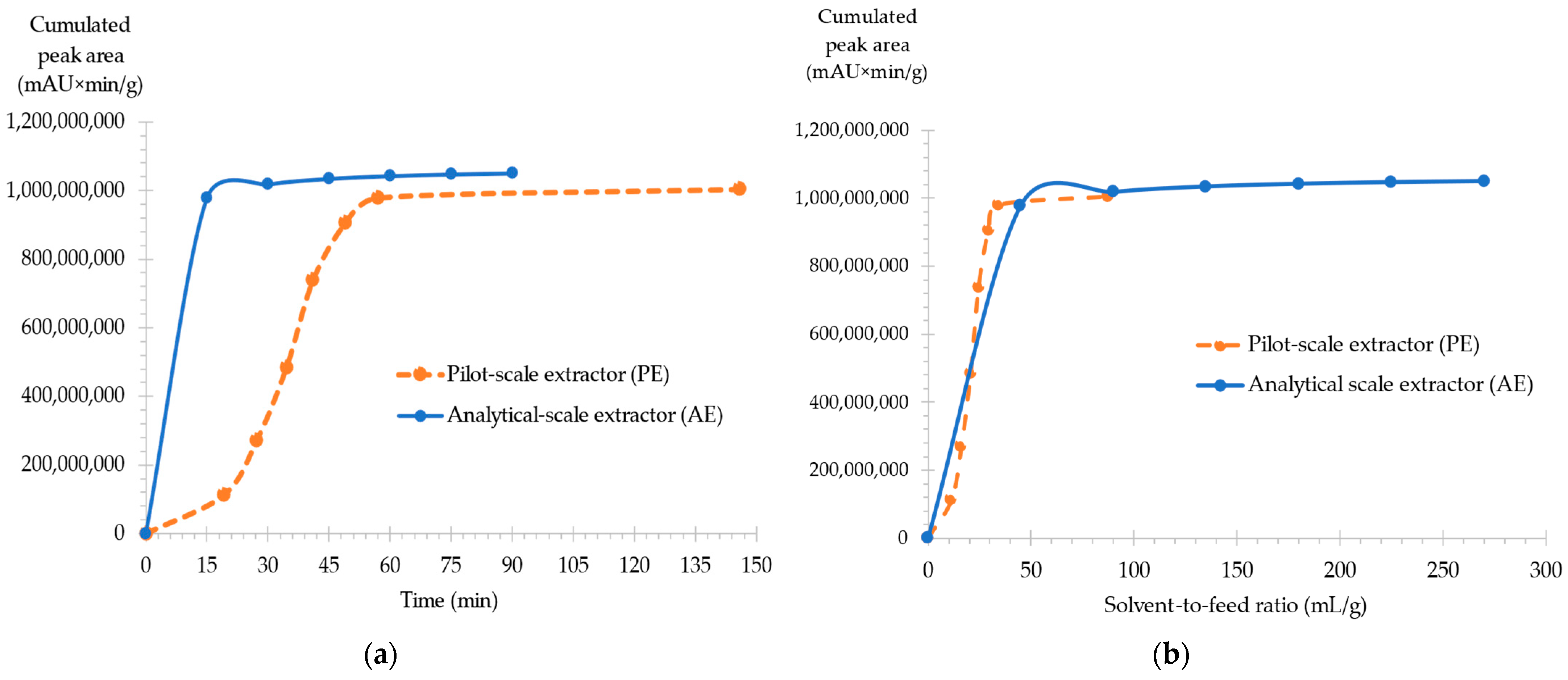
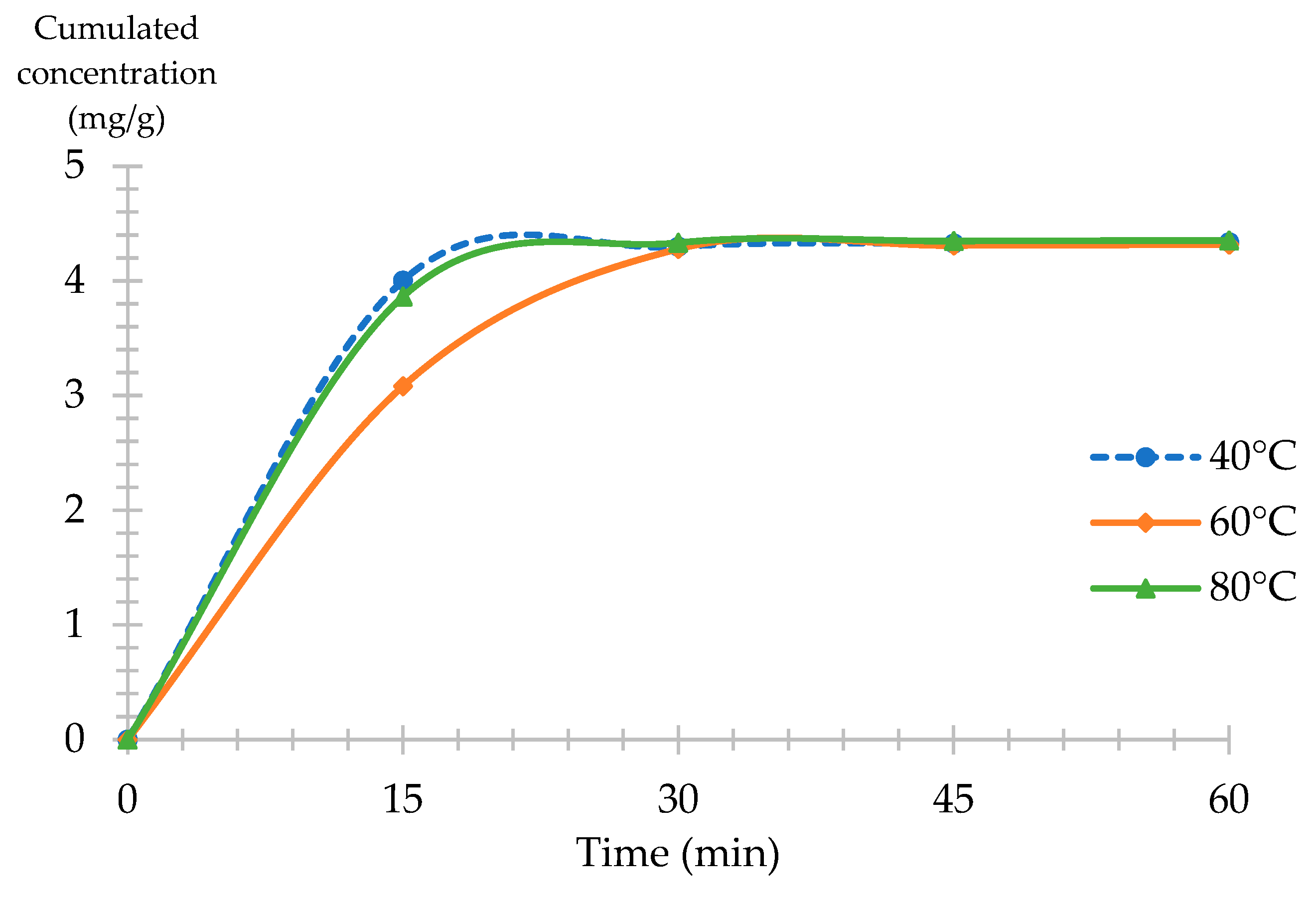
2.1.4. Experimental Design Validation: Extraction Kinetics CNPE
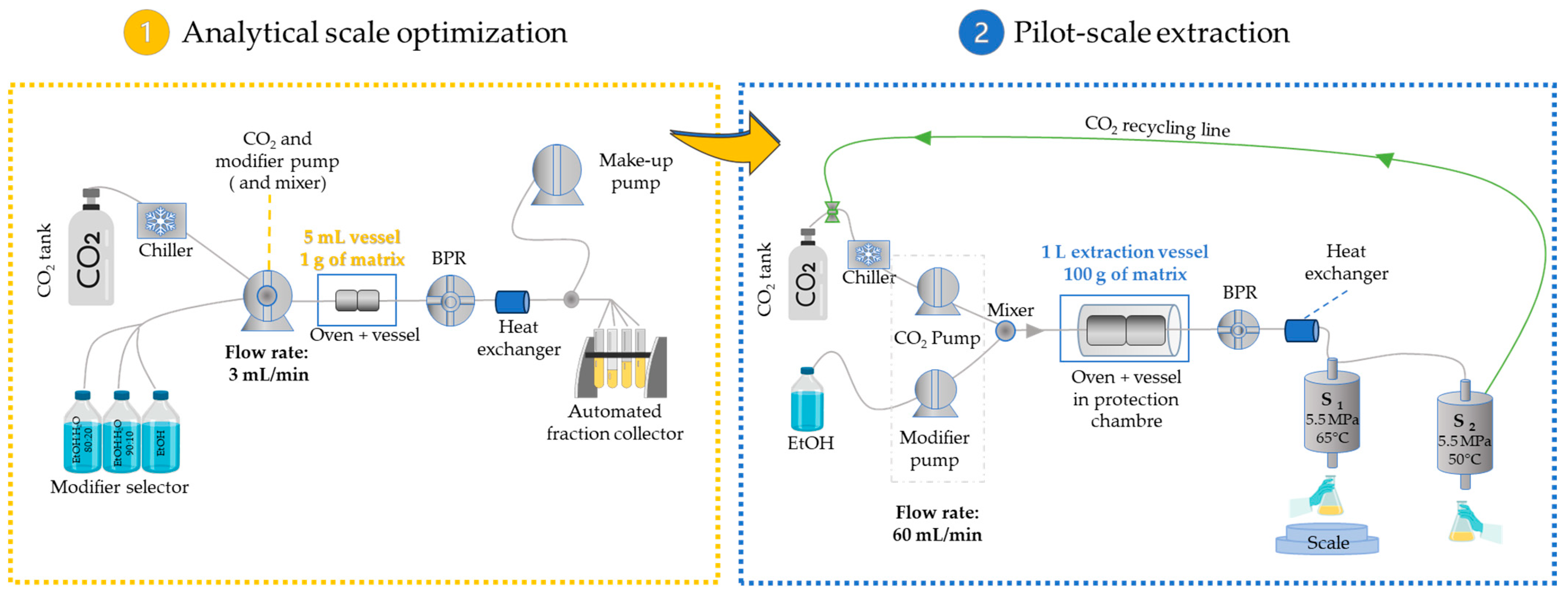
2.1.5. Pilot-Scale Supercritical Extraction of CNPE
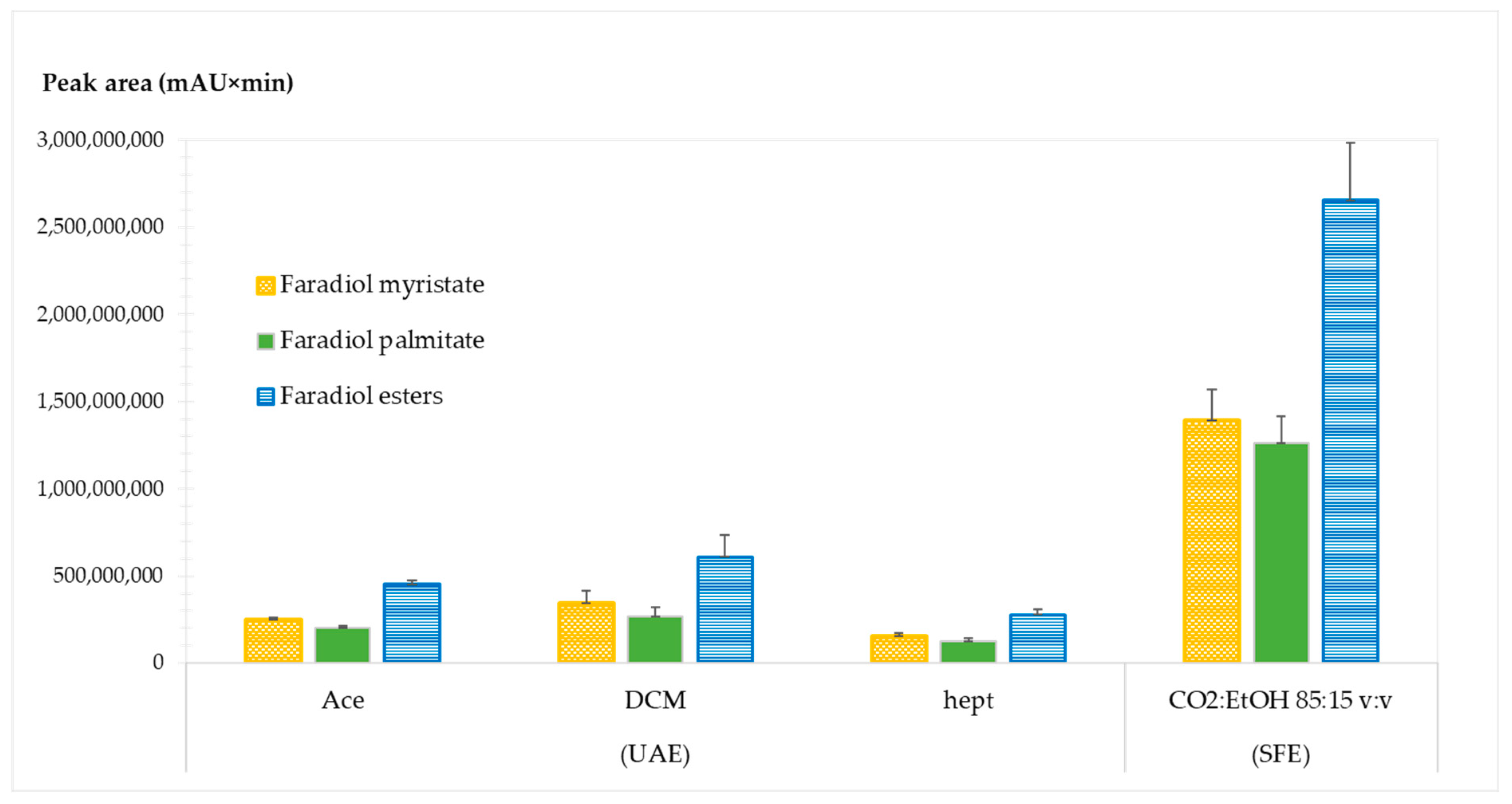
2.1.6. Comparison between Supercritical Fluid Extraction (SFE) and Ultrasound-Assisted Extraction (UAE) for CNPE
2.2. Optimization of Marigold Polar Compounds Extraction (CPE)
2.2.1. Statistical Analysis, Model, and Factor Significance
2.2.2. Extraction Parameters Influence on CPE Assessed with Box-Behnken Design
2.2.3. Optimal Selective Extraction of Narcissin
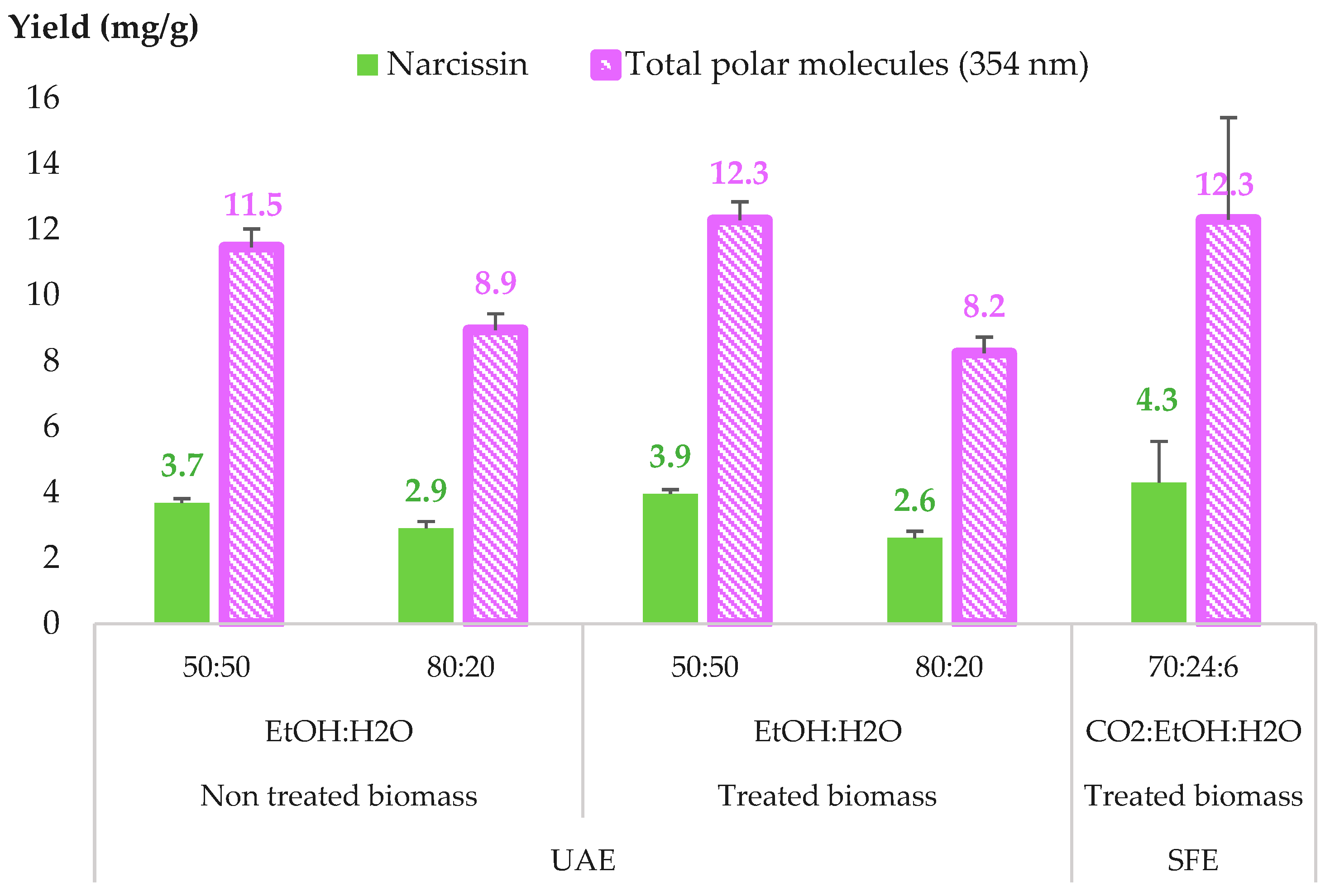
2.2.4. Comparison between SFE and UAE for CPE
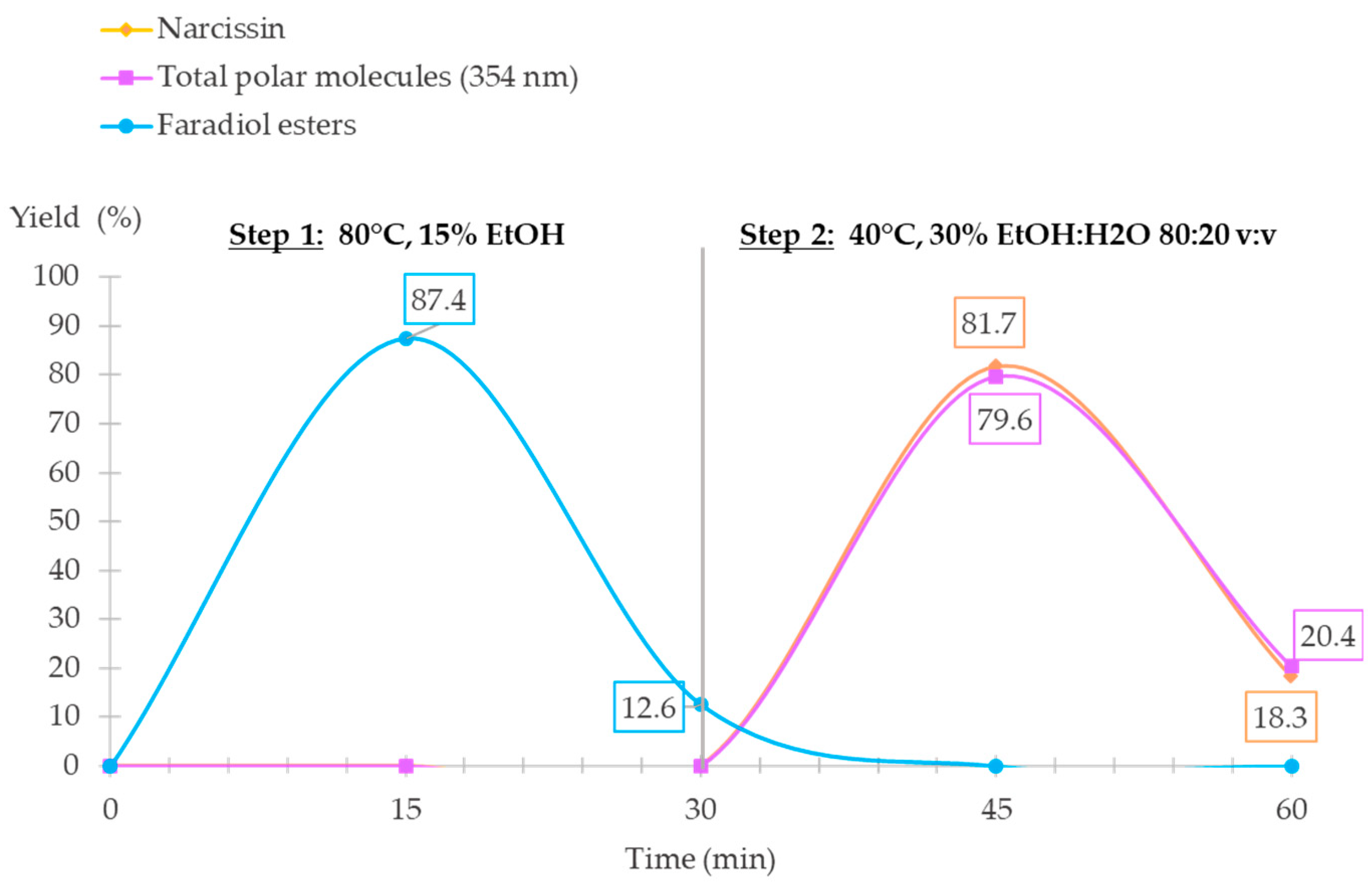
2.3. Validation of S3FE
3. Methodology
3.1. Plant Material
3.2. Chemical and Reagents
3.3. SC-CO2 Extraction
3.4. Ultra-Sound Assisted Extraction (UAE)
3.5. SC-CO2 Extracts Sample Treatment
3.6. Calendula Non-Polar Extracts (CNPE) Chromatographic Analysis
3.6.1. Ultra-High Efficiency Low-Pressure/Supercritical Fluid Chromatography (UHLP/SFC-ELSD): Terpenoid Esters Analysis in CNPE
3.6.2. Unified Chromatography UC-DAD: Analysis of Polar Compounds in CNPE
3.7. Calendula Polar Extracts (CPE): Chromatographic Analysis of Polyphenols
3.8. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Mezzomo, N.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction of peach (Prunus persica) almond oil: Kinetics, mathematical modeling and scale-up. J. Supercrit. Fluids 2009, 51, 10–16. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.D.; Basso, R.C.; Meirelles, A.J.A.; Vladimir Oliveira, J.; Batista, E.A.C.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef]
- Prado, J.M.; Prado, G.H.C.; Meireles, M.A.A. Scale-up study of supercritical fluid extraction process for clove and sugarcane residue. J. Supercrit. Fluids 2011, 56, 231–237. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Vafaei, N.; Rempel, C.B.; Scanlon, M.G.; Jones, P.J.H.; Eskin, M.N.A. Application of Supercritical Fluid Extraction (SFE) of Tocopherols and Carotenoids (Hydrophobic Antioxidants) Compared to Non-SFE Methods. AppliedChem 2022, 2, 68–92. [Google Scholar] [CrossRef]
- Larocca, V.; Martino, M.; Trupo, M.; Magarelli, R.A.; Spagnoletta, A.; Ambrico, A. Evaluation of carbon dioxide supercritical fluid extraction (CO2-SFE) on carotenoids recovery from red yeast cells. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Mellinas, A.C.; Espagnol, J.; Hamzaoui, M.; Jiménez, A.; Garrigós, M.C. Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques. Foods 2022, 11, 2408. [Google Scholar] [CrossRef]
- Rajput, S.; Kaur, S.; Panesar, P.S.; Thakur, A. Supercritical fluid extraction of essential oils from Citrus reticulata peels: Optimization and characterization studies. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Atwi-Ghaddar, S.; Zerwette, L.; Destandau, E.; Lesellier, E. Supercritical Fluid Extraction (SFE) of Polar Compounds from Camellia sinensis Leaves: Use of Ethanol/Water as a Green Polarity Modifier. Molecules 2023, 28, 5485. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Scampicchio, M.; Ferrentino, G.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Capsicum chinense Polyphenols Extraction by Supercritical Fluids Using Response Surface Methodology (RSM). Processes 2023, 11, 2055. [Google Scholar] [CrossRef]
- Dauber, C.; Carreras, T.; Fernández Fernández, A.; Irigaray, B.; Albores, S.; Gámbaro, A.; Ibáñez, E.; Vieitez, I. Response surface methodology for the optimization of biophenols recovery from “alperujo” using supercritical fluid extraction. Comparison between Arbequina and Coratina cultivars. J. Supercrit. Fluids 2022, 180, 105460. [Google Scholar] [CrossRef]
- Atwi-Ghaddar, S.; Destandau, E.; Lesellier, E. Optimization of supercritical fluid extraction of polar flavonoids from Robinia pseudoacacia L. heartwood. J. CO2 Util. 2023, 70, 102440. [Google Scholar] [CrossRef]
- John, R.; Jan, D. Calendula officinalis-An Important Medicinal Plant with Potential Biological Properties. Proc. Indian Natl. Sci. Acad. 2017, 93, 769–787. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef] [PubMed]
- Saffari, E.; Mohammad-Alizadeh-Charandabi, S.; Adibpour, M.; Mirghafourvand, M.; Javadzadeh, Y. Comparing the effects of Calendula officinalis and clotrimazole on vaginal Candidiasis: A randomized controlled trial. Women Health 2017, 57, 1145–1160. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, C.; Di Stadio, A.; Vitale, S.; Saccone, G.; Angelis, M.C.D.; Zizolfi, B.; Di Spiezio Sardo, A. Use of calendula ointment after episiotomy: A randomized clinical trial. J. Matern. Fetal Neonatal Med. 2022, 35, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Buzzi, M.; de Freitas, F.; de Barros Winter, M. Therapeutic effectiveness of a Calendula officinalis extract in venous leg ulcer healing. J. Wound Care 2016, 25, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Giostri, G.S.; Novak, E.M.; Buzzi, M.; Guarita-Souza, L.C. Treatment of acute wounds in hand with Calendula officinalis L.: A randomized trial. Tissue Barriers 2022, 10, 1994822. [Google Scholar] [CrossRef]
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M.; et al. Antioxidant capacity of Calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: A randomized controlled clinical study. DARU J. Pharm. Sci. 2013, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Pommier, P.; Gomez, F.; Sunyach, M.; D’Hombres, A.; Carrie, C.; Montbarbon, X. Phase III Randomized Trial of Calendula officinalis Compared With Trolamine for the Prevention of Acute Dermatitis During Irradiation for Breast Cancer. J. Clin. Oncol. 2004, 22, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Sharif, M.R.; Sharif, A.; Beiraghdar, F.; Zahiri, Z.; Amirchoopani, G.; Marzony, E.T.; Sahebkar, A. A Randomized Comparative Trial on the Therapeutic Efficacy of Topical Aloe vera and Calendula officinalis on Diaper Dermatitis in Children. Sci. World J. 2012, 2012, e810234. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, Z.; Pyrek, J. Triterpenic alcohols of Calendula officinalis L. flowers. Phytochemistry 1968, 7, 1631–1639. [Google Scholar] [CrossRef]
- Neukirch, H.; D’Ambrosio, M.; Via, J.D.; Guerriero, A. Simultaneous quantitative determination of eight triterpenoid monoesters from flowers of 10 varieties of Calendula officinalis L. and characterisation of a new triterpenoid monoester. Phytochem. Anal. 2004, 15, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Reznicek, G.; Zitterl-Eglseer, K. Quantitative determination of the faradiol esters in marigold flowers and extracts. Sci. Pharm. 2003, 71, 121–128. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-Inflammatory, Anti-Tumor-Promoting, and Cytotoxic Activities of Constituents of Marigold (Calendula officinalis) Flowers. J. Nat. Prod. 2006, 69, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Naved, T.; Ansari, S.H.; Mukhtar, H.M.; Ali, M. New Triterpenic Esters of Oleanene-Series from the Flowers of Calendula officinalis Linn. ChemInform 2005, 36, 1088–1091. [Google Scholar] [CrossRef]
- Wiłkomirski, B. Pentacyclic triterpene triols from Calendula officinalis flowers. Phytochemistry 1985, 24, 3066–3067. [Google Scholar] [CrossRef]
- Kishimoto, S.; Maoka, T.; Sumitomo, K.; Ohmiya, A. Analysis of Carotenoid Composition in Petals of Calendula (Calendula officinalis L.). Biosci. Biotechnol. Biochem. 2005, 69, 2122–2128. [Google Scholar] [CrossRef]
- Pintea, A. HPLC analysis of carotenoids in four varieties of Calendula officinalis L. flowers. Acta Biol. Szeged. 2003, 47, 37–40. [Google Scholar]
- Ul’chenko, N.T.; Glushenkova, A.I.; Mukhamedova, K.S. Lipids of Calendula officinalis. Chem. Nat. Compd. 1998, 34, 272–274. [Google Scholar] [CrossRef]
- Król, B.; Paszko, T. Harvest date as a factor affecting crop yield, oil content and fatty acid composition of the seeds of calendula (Calendula officinalis L.) cultivars. Ind. Crops Prod. 2017, 97, 242–251. [Google Scholar] [CrossRef]
- Martin, R.J.; Martin, R.J.; Porter, N.G.; Porter, N.G.; Deo, B.; Deo, B. Initial studies on seed oil composition of calendula and lunaria. Agron. Soc. N. Z. 2005, 35, 129–137. [Google Scholar]
- Garaiova, M.; Hua, Q.; Holic, R. Heterologous Production of Calendic Acid Naturally Found in Calendula officinalis by Recombinant Fission Yeast. J. Agric. Food Chem. 2023, 71, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Shakib, A.K.; Nejad, A.R.; Khalighi, A. Changes in seed and oil yield of Calendula officinalis L. as affected by different levels of nitrogen and plant density. Res. Crops 2010, 11, 728–732. [Google Scholar]
- Wilkomirski, B.; Kasprzyk, Z. Free and ester-bound triterpene alcohols and sterols in cellular subfractions of Calendula officinalis flowers. Phytochemistry 1979, 18, 253–255. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R. Nutritional and chemical characterization of edible petals and corresponding infusions: Valorization as new food ingredients. Food Chem. 2017, 220, 337–343. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.Á.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef]
- Niżyński, B.; Alsoufi, A.S.M.; Pączkowski, C.; Długosz, M.; Szakiel, A. The content of free and esterified triterpenoids of the native marigold (Calendula officinalis) plant and its modifications in in vitro cultures. Phytochem. Lett. 2015, 11, 410–417. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Sosa, S.; Jurenitsch, J.; Schubert-Zsilavecz, M.; Della Loggia, R.; Tubaro, A.; Bertoldi, M.; Franz, C. Anti-oedematous activities of the main triterpendiol esters of marigold (Calendula officinalis L.). J. Ethnopharmacol. 1997, 57, 139–144. [Google Scholar] [CrossRef] [PubMed]
- García-Risco, M.R.; Mouhid, L.; Salas-Pérez, L.; López-Padilla, A.; Santoyo, S.; Jaime, L.; Ramírez de Molina, A.; Reglero, G.; Fornari, T. Biological Activities of Asteraceae (Achillea millefolium and Calendula officinalis) and Lamiaceae (Melissa officinalis and Origanum majorana) Plant Extracts. Plant Foods Hum. Nutr. 2017, 72, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.M.A.S.; Michielin, E.M.Z.; Danielski, L.; Ferreira, S.R.S. Experimental data and modeling the supercritical fluid extraction of marigold (Calendula officinalis) oleoresin. J. Supercrit. Fluids 2005, 34, 163–170. [Google Scholar] [CrossRef]
- Hamburger, M.; Adler, S.; Baumann, D.; Förg, A.; Weinreich, B. Preparative purification of the major anti-inflammatory triterpenoid esters from Marigold (Calendula officinalis). Fitoterapia 2003, 74, 328–338. [Google Scholar] [CrossRef]
- Danielski, L.; Campos, L.M.A.S.; Bresciani, L.F.V.; Hense, H.; Yunes, R.A.; Ferreira, S.R.S. Marigold (Calendula officinalis L.) oleoresin: Solubility in SC-CO2 and composition profile. Chem. Eng. Process. Process Intensif. 2007, 46, 99–106. [Google Scholar] [CrossRef]
- López-Padilla, A.; Ruiz-Rodriguez, A.; Reglero, G.; Fornari, T. Supercritical carbon dioxide extraction of Calendula officinalis: Kinetic modeling and scaling up study. J. Supercrit. Fluids 2017, 130, 292–300. [Google Scholar] [CrossRef]
- Villanueva-Bermejo, D.; Vázquez, E.; Villalva, M.; Santoyo, S.; Fornari, T.; Reglero, G.; Rodriguez García-Risco, M. Simultaneous Supercritical Fluid Extraction of Heather (Calluna vulgaris L.) and Marigold (Calendula officinalis L.) and Anti-Inflammatory Activity of the Extracts. Appl. Sci. 2019, 9, 2245. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Kaškonas, P.; Jalinskaitė, M.; Maruška, A. Chemical Composition and Chemometric Analysis of Variation in Essential Oils of Calendula officinalis L. during Vegetation Stages. Chromatographia 2011, 73, 163–169. [Google Scholar] [CrossRef]
- Crabps, N.; Marongiu, B.; Piras, A.; Pivetta, T.; Porcedda, S. Extraction, Separation and Isolation of Volatiles and Dyes from Calendula officinalis L. and Aloysia triphylla (L’Her.) Britton by Supercritical CO2. J. Essent. Oil Res. 2003, 15, 350–355. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Sharova, O.V. Flavonoids from Calendula officinalis flowers. Chem. Nat. Compd. 2007, 43, 216–217. [Google Scholar] [CrossRef]
- Silva, D.; Ferreira, M.S.; Sousa-Lobo, J.M.; Cruz, M.T.; Almeida, I.F. Anti-Inflammatory Activity of Calendula officinalis L. Flower Extract. Cosmetics 2021, 8, 31. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Cakır, O.; Bensari, S.; Yılmaz, M.A.; Gallo, M.; Montesano, D. A Comparative Bio-Evaluation and Chemical Profiles of Calendula officinalis L. Extracts Prepared via Different Extraction Techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; Bassarello, C.; Piacente, S.; Pizza, C.; Çalış, İ. Triterpene Saponins from Calendula arvensis. Z. Für Naturforschung B 2006, 61, 1170–1173. [Google Scholar] [CrossRef]
- Derkach, A.I.; Komissarenko, N.F.; Chernobai, V.T. Coumarins of the inflorescences of Calendula officinalis and Helichrysum arenarium. Chem. Nat. Compd. 1986, 22, 722–723. [Google Scholar] [CrossRef]
- BEKDEŞER, B. Modeling and optimizing microwave-assisted extraction of antioxidant compounds from marigold Calendula officinalis L. using response surface methodology. Turk. J. Chem. 2019, 43, 1457–1471. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Antioxidant properties of marigold extracts. Food Res. Int. 2004, 37, 643–650. [Google Scholar] [CrossRef]
- Ercetin, T.; Senol, F.S.; Erdogan Orhan, I.; Toker, G. Comparative assessment of antioxidant and cholinesterase inhibitory properties of the marigold extracts from Calendula arvensis L. and Calendula officinalis L. Ind. Crops Prod. 2012, 36, 203–208. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Sequential extraction of carnosic acid, rosmarinic acid and pigments (carotenoids and chlorophylls) from Rosemary by online supercritical fluid extraction-supercritical fluid chromatography. J. Chromatogr. A 2021, 1639, 461709. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Baumann, D.; Adler, S.; Grüner, S.; Otto, F.; Weinreich, B.; Hamburger, M. Supercritical carbon dioxide extraction of marigold at high pressures: Comparison of analytical and pilot-scale extraction. Phytochem. Anal. 2004, 15, 226–230. [Google Scholar] [CrossRef]
- Sovová, H. Steps of supercritical fluid extraction of natural products and their characteristic times. J. Supercrit. Fluids 2012, 66, 73–79. [Google Scholar] [CrossRef]
- Moraes, M.L.L.; da Silva, H.D.T.; Blanes, L.; Doble, P.; Tavares, M.F.M. Optimization of chemometric approaches for the extraction of isorhamnetin-3-O-rutinoside from Calendula officinalis L. J. Pharm. Biomed. Anal. 2016, 125, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Gros, Q.; Wolniaczyk, M.; Duval, J.; West, C.; Horie, S.; Toyota, Y.; Funada, Y.; Lesellier, E. Comparison of the triglyceride composition of vegetable samples with ultra-high efficiency / low-pressure supercritical fluid chromatography—Mass spectrometry. J. Food Compos. Anal. 2023, 115, 104960. [Google Scholar] [CrossRef]
- Lesellier, E.; Latos, A.; West, C. Ultra high efficiency/low pressure supercritical fluid chromatography (UHE/LP-SFC) for triglyceride analysis: Identification, quantification, and classification of vegetable oils. Anal. Sci. Adv. 2021, 2, 33–42. [Google Scholar] [CrossRef]
- Lesellier, E.; Latos, A.; de Oliveira, A.L. Ultra high efficiency/low pressure supercritical fluid chromatography with superficially porous particles for triglyceride separation. J. Chromatogr. A 2014, 1327, 141–148. [Google Scholar] [CrossRef]
- Molineau, J.; Meunier, M.; Noireau, A.; Fougère, L.; Petit, A.-M.; West, C. Analysis of flavonoids with unified chromatography-electrospray ionization mass spectrometry—Method development and application to compounds of pharmaceutical and cosmetic interest. Anal. Bioanal. Chem. 2020, 412, 6595–6609. [Google Scholar] [CrossRef] [PubMed]


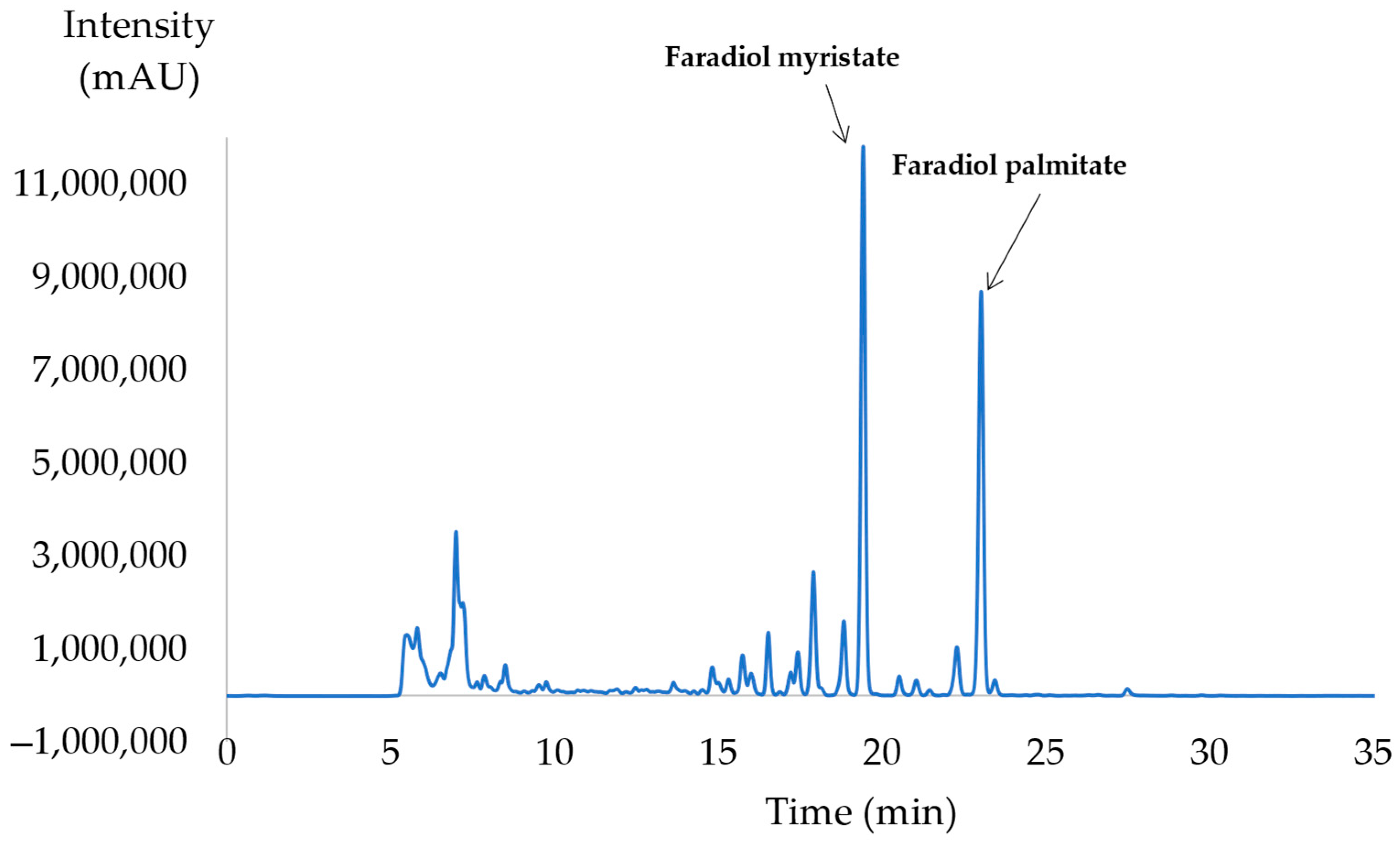
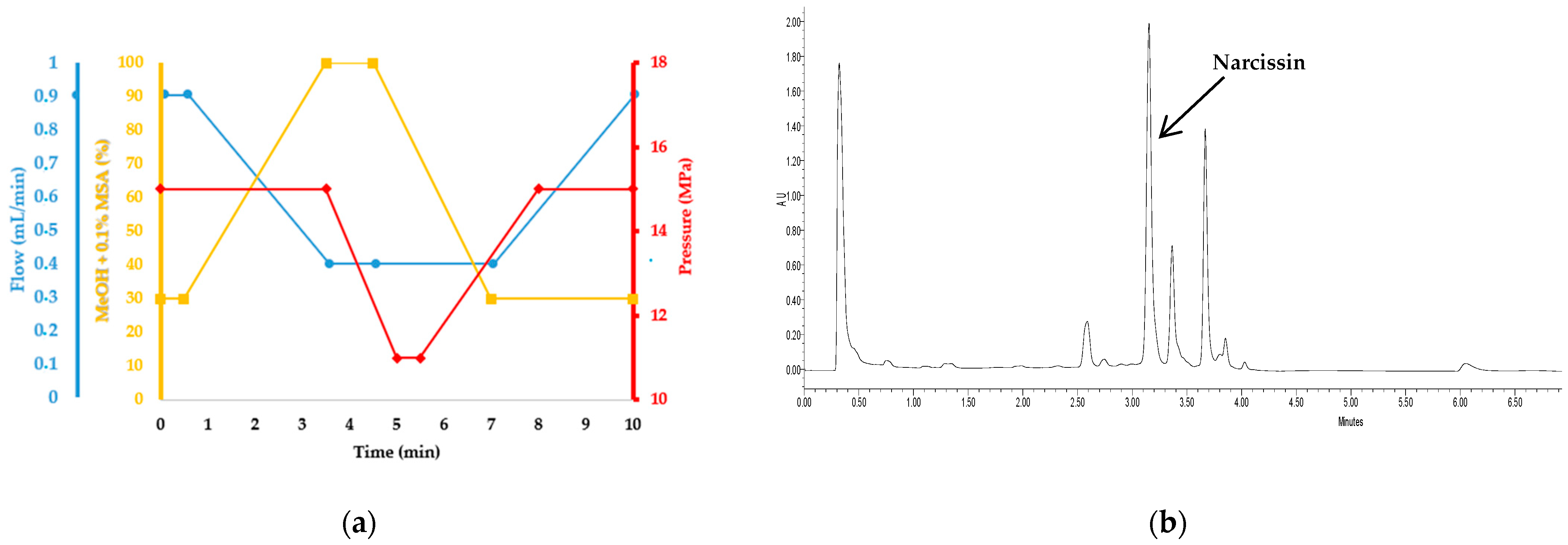
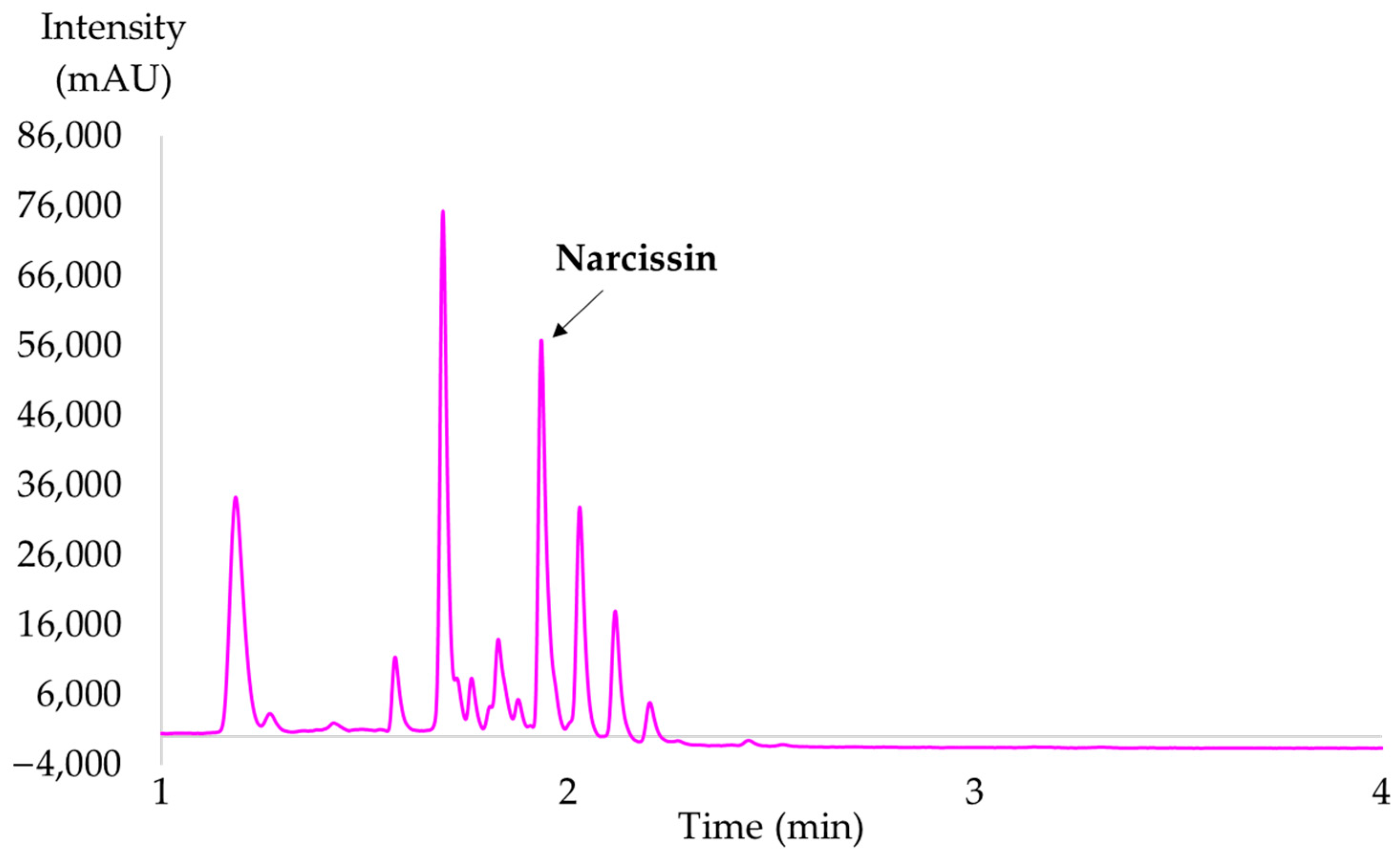
| Experiment N° | Coded Levels | Results (after 30 min of Extraction) | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Faradiol Myristate (mAU × min) | Faradiol Palmitate (mAU × min) | Both Faradiol Esters (Y) (mAU × min) | Narcissin (µv × s) | Dried Extract Yield (mg/g) | |
| Temperature (°C) | Pressure (MPa) | EtOH (%) | ||||||
| 1 | 40 (−1) | 10 (−1) | 10 (0) | 3.11 × 108 | 2.54 × 108 | 5.64 × 108 | 0.00 | 58.9 |
| 2 | 80 (+1) | 10 (−1) | 10 (0) | 5.46 × 108 | 4.65 × 108 | 1.01 × 109 | 4.08 × 105 | 88.5 |
| 3 | 40 (−1) | 20 (+1) | 10 (0) | 2.78 × 108 | 2.31 × 108 | 5.08 × 108 | 0.00 | 62.5 |
| 4 | 80 (+1) | 20 (+1) | 10 (0) | 5.50 × 108 | 4.77 × 108 | 1.03 × 109 | 0.00 | 76.1 |
| 5 | 40 (−1) | 15 (0) | 5 (−1) | 3.94 × 108 | 3.27 × 108 | 7.20 × 108 | 0.00 | 56.5 |
| 6 | 80 (+1) | 15 (0) | 5 (−1) | 1.99 × 108 | 9.61 × 107 | 2.95 × 108 | 0.00 | 41.5 |
| 7 | 40 (−1) | 15 (0) | 15 (+1) | 5.39 × 108 | 4.66 × 108 | 1.00 × 109 | 3.23 × 105 | 69.7 |
| 8 | 80 (+1) | 15 (0) | 15 (+1) | 1.39 × 109 | 1.26 × 109 | 2.66 × 109 | 0.00 | 92.3 |
| 9 | 60 (0) | 10 (−1) | 5 (−1) | 1.30 × 108 | 5.79 × 107 | 1.88 × 108 | 0.00 | 35.2 |
| 10 | 60 (0) | 20 (+1) | 5 (−1) | 3.65 × 108 | 3.13 × 108 | 6.79 × 108 | 0.00 | 55.5 |
| 11 | 60 (0) | 10 (−1) | 15 (+1) | 6.81 × 108 | 5.73 × 108 | 1.25 × 109 | 3.04 × 105 | 85.4 |
| 12 | 60 (0) | 20 (+1) | 15 (+1) | 6.50 × 108 | 5.53 × 108 | 1.20 × 109 | 3.15 × 105 | 102.6 |
| 13 | 60 (0) | 15 (0) | 10 (0) | 5.77 × 108 | 4.93 × 108 | 1.07 × 109 | 0.00 | 73.4 |
| 14 | 60 (0) | 15 (0) | 10 (0) | 5.68 × 108 | 4.68 × 108 | 1.04 × 109 | 0.00 | 70.7 |
| 15 | 60 (0) | 15 (0) | 10 (0) | 5.82 × 108 | 4.82 × 108 | 1.06 × 109 | 0.00 | 68.1 |
| 16 | 60 (0) | 15 (0) | 10 (0) | 6.59 × 108 | 5.49 × 108 | 1.21 × 109 | 0.00 | 71.5 |
| 17 | 60 (0) | 15 (0) | 10 (0) | 4.59 × 108 | 3.86 × 108 | 8.45 × 108 | 0.00 | 65.5 |
| Source | DF | SS | MS | F Value | p-Value | Contribution (%) | Conclusion |
|---|---|---|---|---|---|---|---|
| Regression model | 9 | 4.3485 × 10 18 | 6.2121 × 1017 | 17.5737 | 0.0001 | Significant | |
| X1—Temperature (°C) | 1 | 5.9963 × 1017 | 9.8515 × 1016 | 2.7869 | 0.1294 | 3.75 | Not significant |
| X2—Pressure (MPa) | 1 | 1.9942 × 1016 | 4.0094 × 1017 | 11.3423 | 0.0083 | 15.3 | Significant |
| X3—EtOH (%) | 1 | 2.2417 × 1018 | 3.0974 × 1017 | 8.7624 | 0.0160 | 11.8 | Significant |
| X12 | 1 | 2.3401 × 1015 | 5.2683 × 1015 | 0.1490 | 0.7084 | 0.2 | Not significant |
| X22 | 1 | 3.7452 × 1017 | 3.8531 × 1017 | 10.9002 | 0.0092 | 14.7 | Significant |
| X32 | 1 | 3.3276 × 1016 | 3.3276 × 1016 | 0.9414 | 0.3573 | 1.27 | Not significant |
| X1× X3 | 1 | 1.0771 × 1018 | 1.0771 × 1018 | 30.4700 | 0.0004 | 41 | Significant |
| Residuals | 7 | 3.1814 × 1017 | 3.5349 × 1016 | 12.1 | |||
| Total | 16 | 4.6666 × 1018 | |||||
| Degree of freedom | Sum of squares | Mean square |
| Experiment N° | Coded Levels | Results (Concentration mg/g of Biomass after 15 min of Extraction at 354 nm) | |||
|---|---|---|---|---|---|
| X′1 | X′2 | X′3 | Narcissin (Y′) mg/g of Biomass) | Total Polar Compounds (mg/g of Biomass) | |
| Temperature (°C) | Modifier (%) | H2O in Modifier (%) | |||
| 1 | 40 (−1) | 10 (−1) | 10 (0) | 0.00 | 0.01 |
| 2 | 80 (+1) | 10 (−1) | 10 (0) | 0.00 | 0.00 |
| 3 | 40 (−1) | 30 (+1) | 10 (0) | 1.10 | 2.52 |
| 4 | 80 (+1) | 30 (+1) | 10 (0) | 1.71 | 3.85 |
| 5 | 40 (−1) | 20 (0) | 0 (−1) | 0.01 | 0.03 |
| 6 | 80 (+1) | 20 (0) | 0 (−1) | 0.04 | 0.12 |
| 7 | 40 (−1) | 20 (0) | 20 (+1) | 3.31 | 9.18 |
| 8 | 80 (+1) | 20 (0) | 20 (+1) | 2.82 | 8.14 |
| 9 | 60 (0) | 10 (−1) | 0 (−1) | 0.00 | 0.00 |
| 10 | 60 (0) | 30 (+1) | 0 (−1) | 0.05 | 0.12 |
| 11 | 60 (0) | 10 (−1) | 20 (+1) | 0.00 | 0.00 |
| 12 | 60 (0) | 30 (+1) | 20 (+1) | 5.30 | 14.64 |
| 13 | 60 (0) | 20 (0) | 10 (0) | 0.11 | 0.33 |
| 14 | 60 (0) | 20 (0) | 10 (0) | 0.13 | 0.37 |
| 15 | 60 (0) | 20 (0) | 10 (0) | 0.14 | 0.42 |
| 16 | 60 (0) | 20 (0) | 10 (0) | 0.21 | 0.57 |
| Source | DF | SS | MS | F Value | p-Value | Contribution (%) | Conclusion |
|---|---|---|---|---|---|---|---|
| Regression model | 7 | 36.096 | 5.1566 | 34.3206 | 0.0000 | Significant | |
| X′1—Temperature (°C) | 1 | 0.0028125 | 0.56408 | 3.7544 | 0.0887 | 3.02 | Not significant |
| X′2—Modifier (%) | 1 | 8.3232 | 0.22289 | 1.4835 | 0.2579 | 1.19 | Not significant |
| X′3—H2O in modifier (%) | 1 | 16.046 | 4.9439 | 32.9049 | 0.0004 | 26.5 | Significant |
| X′12 | 1 | 0.58141 | 0.58141 | 3.8697 | 0.0847 | 3.12 | Not significant |
| X′22 | 1 | 0.12076 | 0.12076 | 0.8037 | 0.3962 | 0.647 | Not significant |
| X′32 | 1 | 4.1311 | 4.1311 | 27.4951 | 0.0008 | 22.1 | Significant |
| X′2 × X′3 | 1 | 6.8906 | 6.8906 | 45.8620 | 0.0001 | 36.9 | Significant |
| Residuals | 8 | 1.202 | 0.15025 | 6.44 | |||
| Total | 15 | 37.298 | |||||
| Degree of freedom | Sum of squares | Mean square |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atwi-Ghaddar, S.; Zerwette, L.; Destandau, E.; Lesellier, E. Exploring the Sequential-Selective Supercritical Fluid Extraction (S3FE) of Flavonoids and Esterified Triterpenoids from Calendula officinalis L. Flowers. Molecules 2023, 28, 7060. https://doi.org/10.3390/molecules28207060
Atwi-Ghaddar S, Zerwette L, Destandau E, Lesellier E. Exploring the Sequential-Selective Supercritical Fluid Extraction (S3FE) of Flavonoids and Esterified Triterpenoids from Calendula officinalis L. Flowers. Molecules. 2023; 28(20):7060. https://doi.org/10.3390/molecules28207060
Chicago/Turabian StyleAtwi-Ghaddar, Sirine, Lydie Zerwette, Emilie Destandau, and Eric Lesellier. 2023. "Exploring the Sequential-Selective Supercritical Fluid Extraction (S3FE) of Flavonoids and Esterified Triterpenoids from Calendula officinalis L. Flowers" Molecules 28, no. 20: 7060. https://doi.org/10.3390/molecules28207060
APA StyleAtwi-Ghaddar, S., Zerwette, L., Destandau, E., & Lesellier, E. (2023). Exploring the Sequential-Selective Supercritical Fluid Extraction (S3FE) of Flavonoids and Esterified Triterpenoids from Calendula officinalis L. Flowers. Molecules, 28(20), 7060. https://doi.org/10.3390/molecules28207060