Abstract
The in situ-generated N-aryl nitrile imines derived from trifluoroacetonitrile smoothly undergo (3+2)-cycloadditions onto the enone fragment of the levoglucosenone molecule, yielding the corresponding, five-membered cycloadducts. In contrast to the ‘classic’ C(Ph),N(Ph) nitrile imine, reactions with fluorinated C(CF3),N(Ar) analogues lead to stable pyrazolines in a chemo- and stereoselective manner. Based on the result of X-ray single crystal diffraction analysis, their structures were established as exo-cycloadducts with the location of the N-Ar terminus of the 1,3-dipole at the α-position of the enone moiety. The DFT computation demonstrated that the observed reaction pathway results from the strong dominance of kinetic control over thermodynamic control.
1. Introduction
The (3+2)-cycloaddition reactions developed by R. Huisgen in the 1960s are recognized as one of the most general methods widely applied for the preparation of five-membered heterocyclic rings, which in many cases are of great importance for the manufacturing of drugs, agrochemicals, and a plethora of other practically useful organic materials [1,2,3,4]. In addition to the great practical importance of (3+2)-cycloaddition reactions, lively discussion on their mechanisms significantly contributed to the development of a general theory of the mechanisms of organic reactions in recent decades [2,3,4].
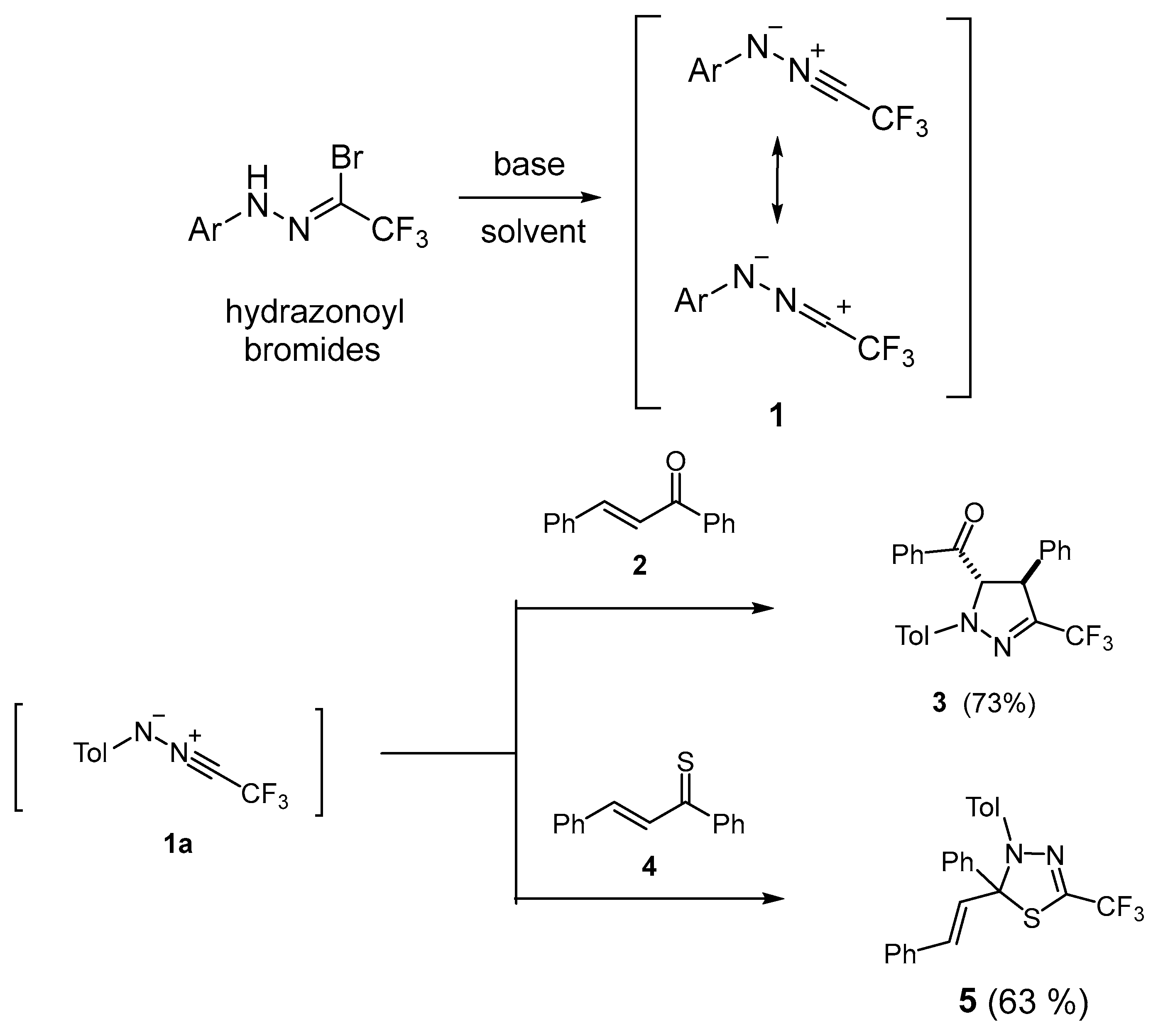
One of the most important and widely applied groups of so-called ‘N-centered 1,3-dipoles’ used in these reactions is nitrile imines, which are classified as ‘propargylic 1,3-dipoles’ [5,6,7,8]. In general, they cannot be isolated as stable compounds, but when generated in situ, they can be trapped with suitable dipolarophiles, yielding the corresponding (3+2)-cycloadducts. In recent years, fluorinated nitrile imines 1, derived from trifluoroacetonitrile, have been readily available from hydrazonoyl halides by treatment with a base. They were widely useful tools for the preparation of bioactive, trifluoromethyl-substituted pyrazolines of type 3 by means of the (3+2)-cycloaddition with a suitable olefinic dipolarophile 2 (Scheme 1). Recently published results demonstrated that fluorinated nitrile imines 1 smoothly undergo (3+2)-cycloadditions not only with electron-rich but also with diverse electron-deficient olefinic dipolarophiles [9,10,11,12,13]. For example, nitrile imine 1a reacts with chalcone 2, yielding stereoselectively 3-trifluoromethyl-substituted pyrazoline 3 as an exclusive product in a good yield [12] (Scheme 1).
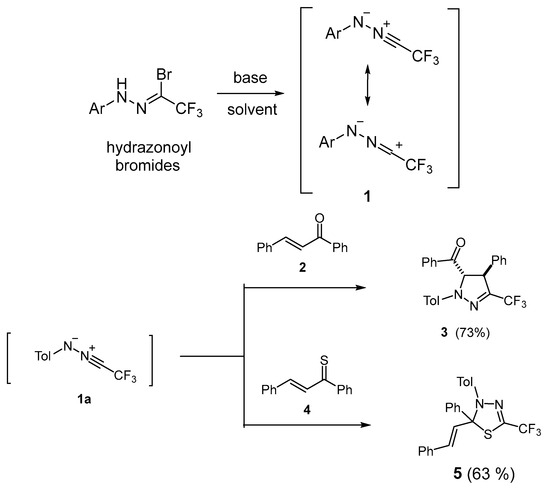
Scheme 1.
Generation of fluorinated nitrile imines 1 and two literature examples of their (3+2)-cycloadditions with chalcone 2 and thiochalcone 4, leading to pyrazoline 3 and 1,3,4-thiadiazoline derivative 5, respectively [7,14].
Moreover, some spirobispyrazolines were obtained through a double (3+2)-cycloaddition of nitrile imines 1 onto cumulated C=C bonds in alkoxyallenes [13].
Interestingly, the (3+2)-cycloaddition with sulfur analogues of chalcones, i.e., with thiochalcones of type 4, occurred with inversed chemoselectivity along the C=S bond, and corresponding 1,3,4-thiadiazole derivatives of type 5 were obtained as exclusive products [14] (Scheme 1). In addition, diverse thioketones (aromatic, ferrocenyl, and cycloaliphatic) react as typical ‘superdipolarophiles’ [2] with nitryl imines 1, yielding highly substituted analogues of heterocycles 5 in a regioselective manner [15,16] Electron deficient fluorinated thioamides and fluorinated nitroalkenes also react regioselectively, with 1 yielding highly fluorinated 2-trifluoromethyl-1,3-dihydro-1,3,4-thiadiazole and 3-trifluoromethyl-5-fluoropyrazole derivatives, respectively [17,18]. In a very recent publication, the synthesis of fused 3-trifluoromethyl-1,2,4-triazoles by means of a multi-step reaction of nitrile imines 1 with enolizable 2,3-dihydro-benzoimidazole-2-thiones was reported [19].
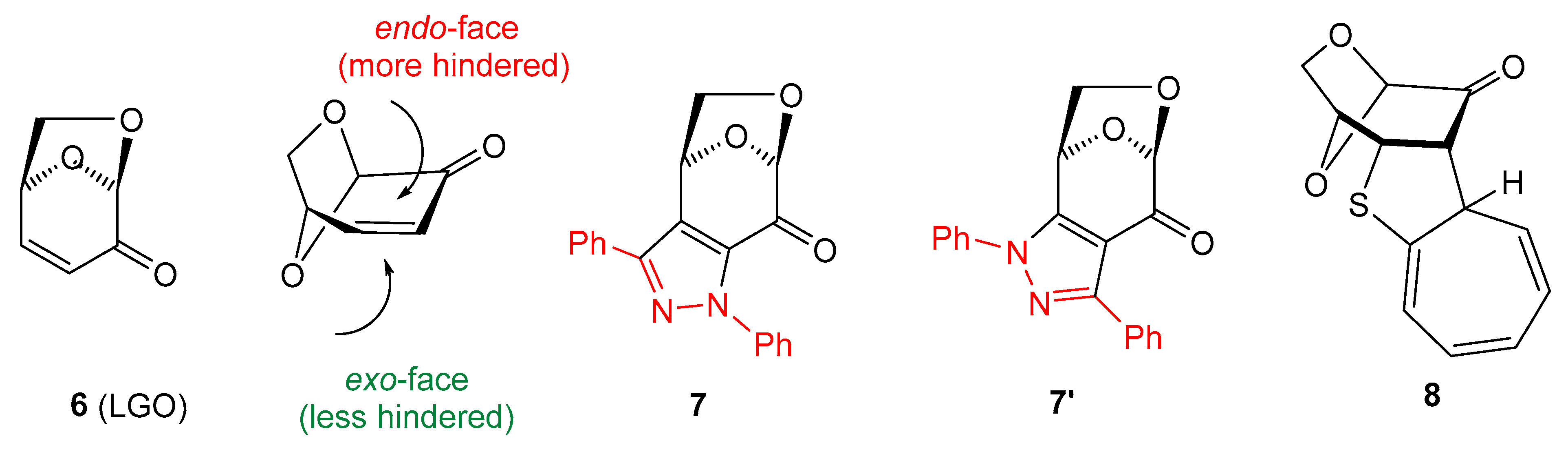
Levoglucosenone (LGO, 6) (Figure 1), with an α,β-unsaturated ketone unit incorporated in its structure, is an exceptional carbohydrate derivative that offers unique opportunities for exploration in asymmetric synthesis as a chiral dipolarophile and/or dienophile. It was identified in 1970 as one of the products of acid-catalyzed cellulose pyrolysis [20]. Modified methods for manufacturing LGO, based either on low-temperature pyrolysis of microcrystalline cellulose or on fast pyrolysis of acid-impregnated cellulose, were described in recent publications [21,22]. The rapid development of LGO chemistry during the last five decades and its applications in medicinal and polymer chemistry are reported in review articles and original publications, which have regularly appeared in recent years [23,24,25,26,27,28,29]. Diverse transformations of LGO were achieved based, among others, on 1,3-dipolar cycloadditions [30,31,32], Diels-Alder reactions [33,34,35], and Michael additions [36,37,38]. For example, in a pioneering work, reactions of 6 with a ‘classic’, non-fluorinated benzonitrile imine 9 (C(Ph),N(Ph)) nitrile imine were reported to yield a mixture of two re-gioisomeric, fused pyrazoles 7 and 7′ (in a ca. 1:8 ratio), which were postulated to be formed as secondary products of a spontaneous oxidation of the initially formed (3+2)-cycloadducts, i.e., the corresponding pyrazolines; very likely, both compounds were formed as products of the preferred exo attack under the harsh reaction conditions (boiling toluene, 1.5 h heating) [30]. Moreover, in a very recent study, LGO 6 was shown to smoothly enter a ‘higher order (8+2)-cycloaddition’ with tropothione, yielding the hitherto unknown polycyclic thiophene derivative 8 [39].
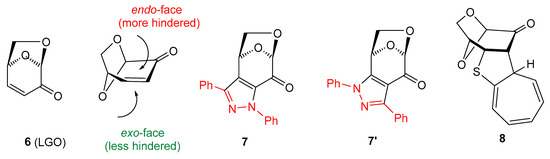
Figure 1.
Levoglucosenone (6), its stereochemical structure, and selected products of cycloaddition reactions with a C(Ph),N(Ph) nitrile imine (9) and tropothione lead to polycylic, isomeric pyrazoles 7/7′ [30] and polycylic thiophene derivative 8 [39], respectively. In structures 7/7′, the skeleton of C(Ph),N(PH) nitrile imine 9 is presented in red.
Prompted by these results, we were motivated to check whether fluorinated nitrile imines 1 bearing differently substituted N-aryl moieties can undergo the anticipated (3+2)-cycloadditions onto the enone fragment of 6, forming the pyrazoline ring attached to the levoglucosenone skeleton. From a mechanistic point of view, the regio- and diastereoselectivity of these cycloaddition reactions and the comparison with the results reported for nitrile imine 9 were also of interest.
2. Results and Discussion
2.1. Experimental Study
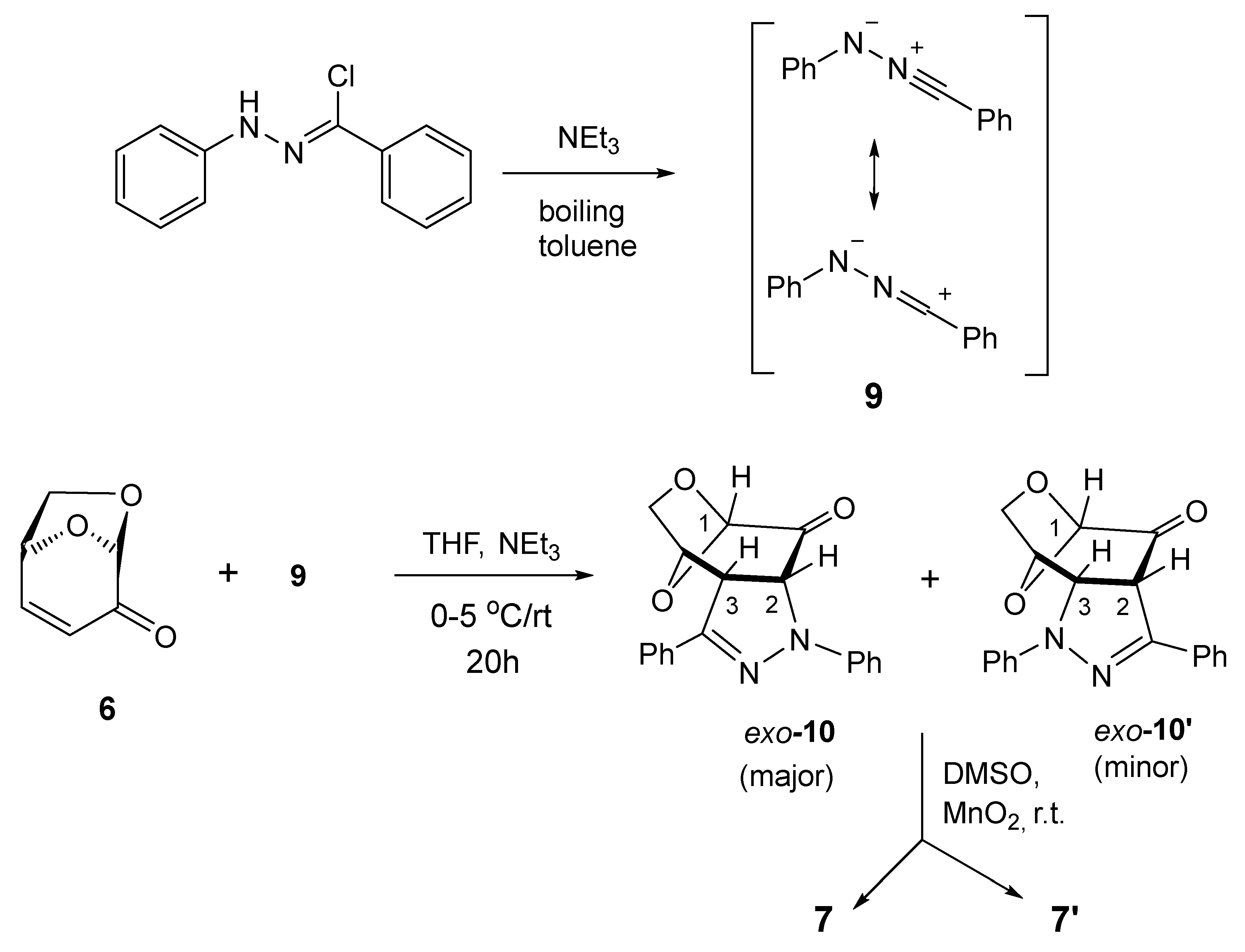
This study started with repetition of the already reported (3+2)-cycloaddition of 6 with benzonitrile N-phenylimine (C(Ph),N(Ph) nitrile imine) (9), but in contrast to the published protocol comprising the generation of the latter in boiling toluene [30], the reaction was performed in THF solution at room temperature using triethylamine as a base (Scheme 2).
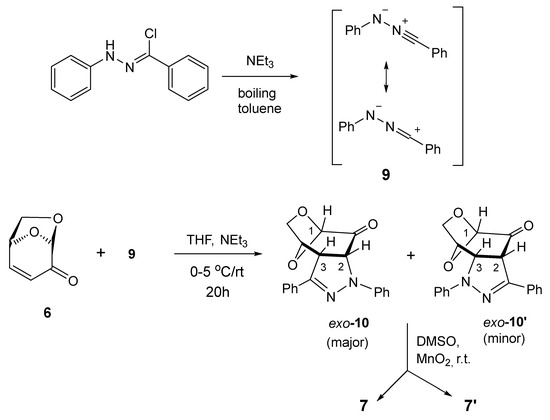
Scheme 2.
Selectivity was observed in the (3+2)-cycloaddition of C(Ph),N(Ph) nitrile imine (9) [7,30] with levoglucosenone (6) in THF solution at room temperature, providing pyrazolines exo-10 and exo-10′ as primary products, followed by oxidation to isolated pyrazole derivatives 7 and 7′.
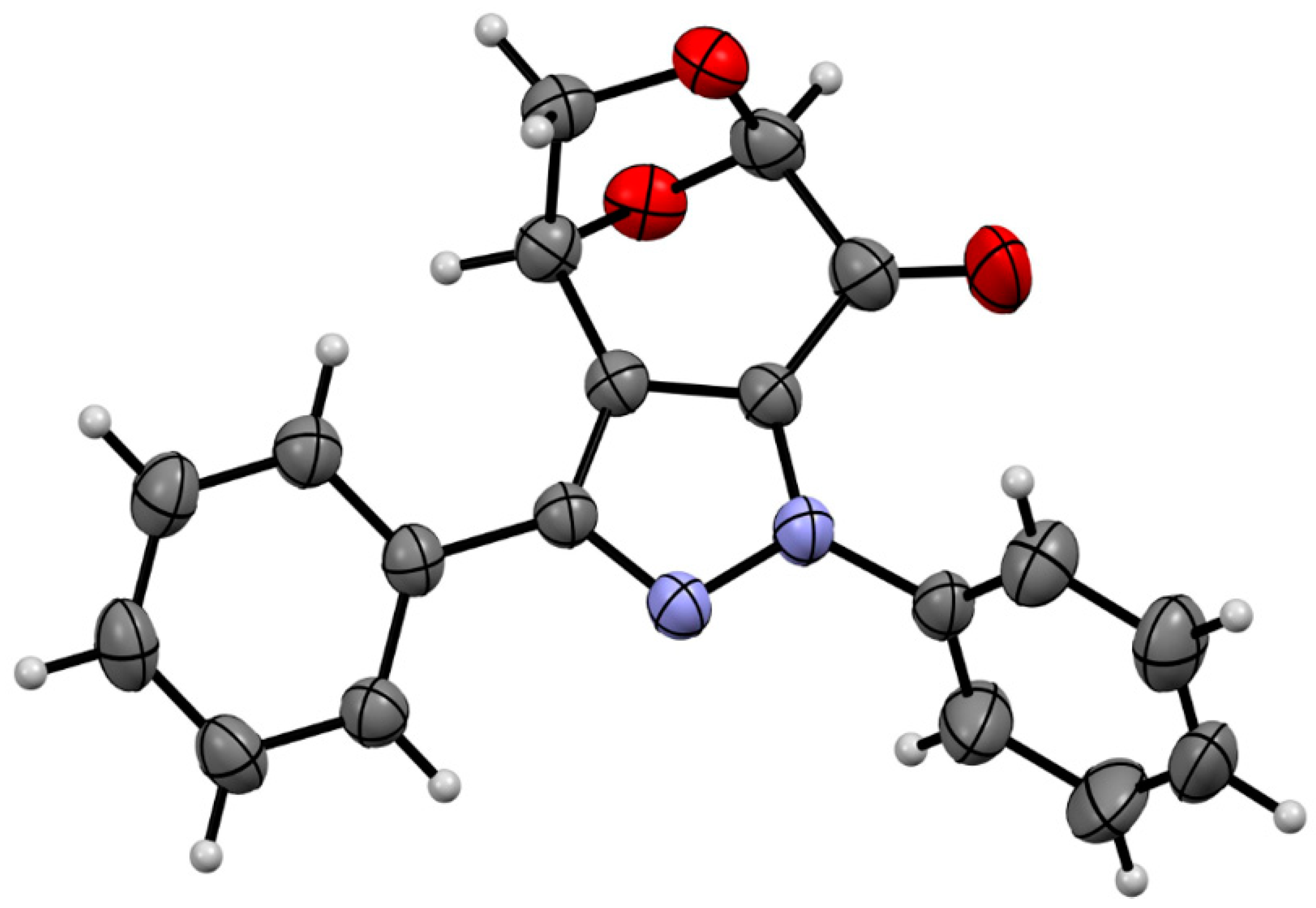
After 24 h, the dipolarophile 6 was completely consumed, and the 1H NMR registered for the crude product revealed the presence of two regioisomeric (3+2)-cycloadducts, which showed characteristic signals assigned to the HC(3) and HC(2) atoms, located at the fused rings. They were found as doublets at 4.22/5.02 ppm (3JH,H = 11.9 Hz) and 4.48/4.69 ppm (3JH,H = 11.3 Hz), respectively, and comparison of the integration lines allowed to estimate the ratio of cycloadducts 10 and 10′ as ca. 75:25. All attempts to separate these isomeric compounds either by crystallization or column chromatography were unsuccessful. For this reason, they were oxidized by treatment with MnO2 in DMSO solution in an overnight experiment. After flash chromatography, a mixture of two products expected to be isomeric fused pyrazoles 7 and 7′ with characteristic absorptions of HC(1) atoms located at 5.57 ppm (s, major) and 5.59 ppm (s, minor), respectively, were found in the NMR spectrum. This time, the ratio of both isomers was calculated to be ca. 65:35. The obtained isomers were separated by preparative layer chromatography (PLC) on silica gel, and the major product was isolated as the less polar fraction. Crystallization from the petroleum ether/dichloromethane mixture gave single crystals suitable for the X-ray analysis, which unambiguously confirmed the structure of isomer 7 (Figure 2).
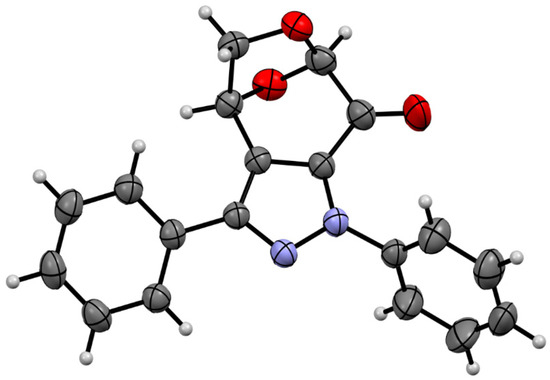
Figure 2.
The molecular structure of polycylic pyrazole 7 was estimated by means of a single crystal X-ray experiment. Atoms are represented by thermal elipsoids (50%) for clarity.
However, further attempts to grow suitable monocrystals of the purified minor isomer 7′ were unsuccessful. The described experiment allows us to conclude that the initial (3+2)-cycloaddition of 6 and 9 led to the formation of two regioisomeric cycloadducts 10 and 10′ with moderate regioselectivity, and this result confirms the previously reported observation [30]. However, our results unambiguously demonstrate that the structure of the major isomer 7 is the opposite of that suggested as the major isomer by the authors of the earlier study [30]. Based on the generally known tendency of LGO 6 to yield sterically favorable exo-cycloadducts, one can anticipate that this orientation of heterocyclic fragments has to be attributed to the initially formed fused pyrazolines exo-10 (major) and exo-10′ (minor).
The test experiment with a representative of the fluorinated nitrile imines of type 1 was performed with the in situ-generated 1,3-dipole 1a, starting with the corresponding hydrazonoyl bromide (Ar = 4-MeC6H4) and 6. In this case, dichloromethane (and not THF or boiling toluene) was applied as a solvent at room temperature, and calcined K2CO3 served as a base. The progress of the reaction was monitored by TLC, and the experiment was stopped when no precursor 6 was detected in the reaction solution (in this case, after 18 h). After filtration of the inorganic salts and evaporation of the solvent, the crude mixture was examined by 1H NMR spectroscopy, which revealed the presence of only one cycloadduct with two characteristic signals of the levoglucosenone skeleton located at 5.28 (H(C-1), s) and 5.24 (H(C-2), d) ppm, and the singlet attributed to a methyl group at 2.34 ppm. The pure product was isolated by column chromatography in 54% yield as pale yellow crystals with m.p. Approximately 132–133 °C and tentatively identified as the expected (3+2)-cycloadduct exo-11a with the three singlet signals registered along with multiplets attributed to the levoglucosenone skeleton and an AB-system of the p-tolyl ring located at 7.09 and 7.13 ppm (3JH,H = 8.5 Hz). In the 13C NMR spectrum, the characteristic absorption of the CF3 group was found as a quartet (1JC,F = 278 Hz) at 120.9 ppm. The anticipated molecular formula, C15H13F3N2O3, was confirmed by the correct elemental analysis.
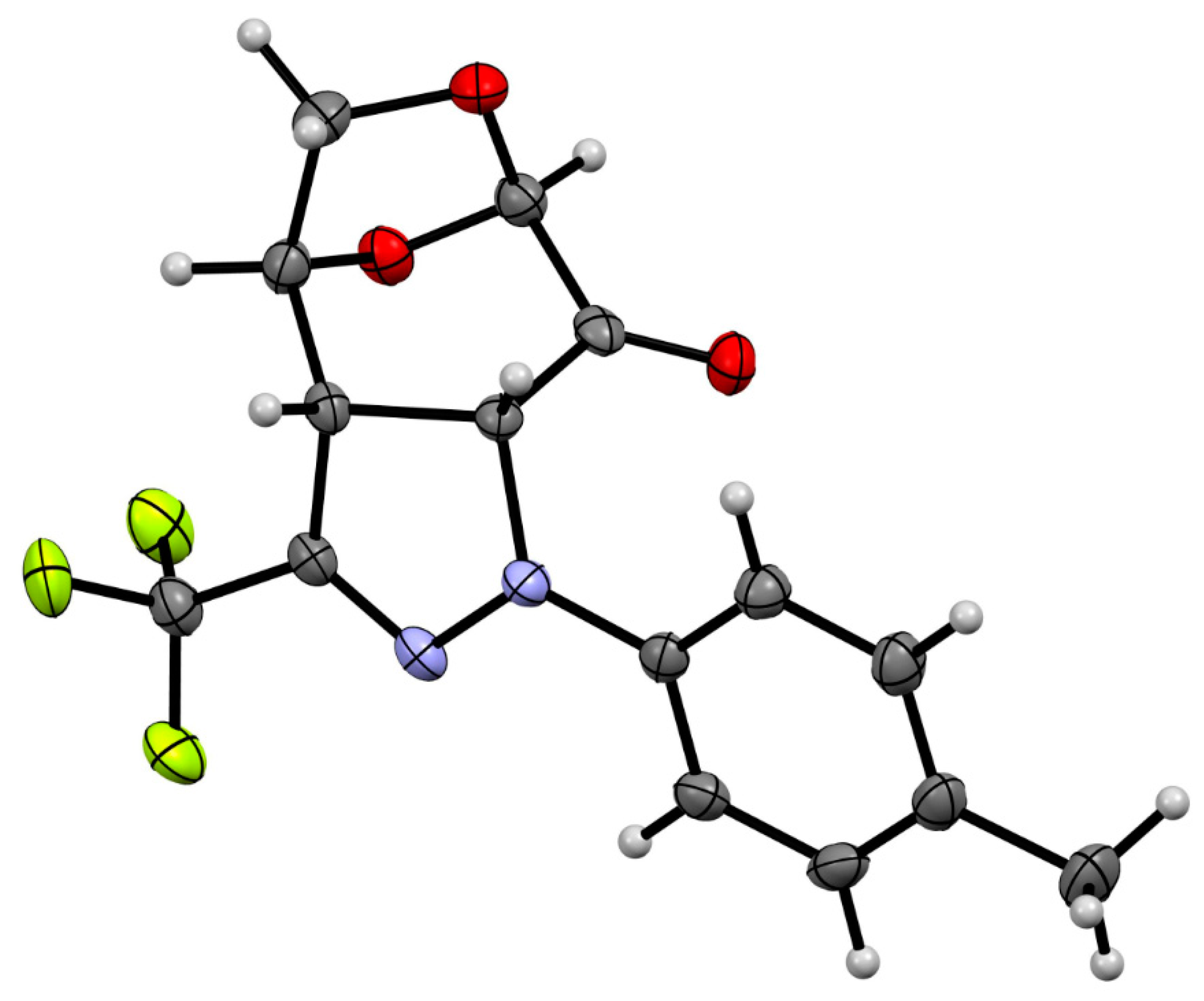
In order to determine the constitution and configuration of this product, single crystals were grown from MeOH, and the X-ray analysis revealed the structure presented in Figure 3.
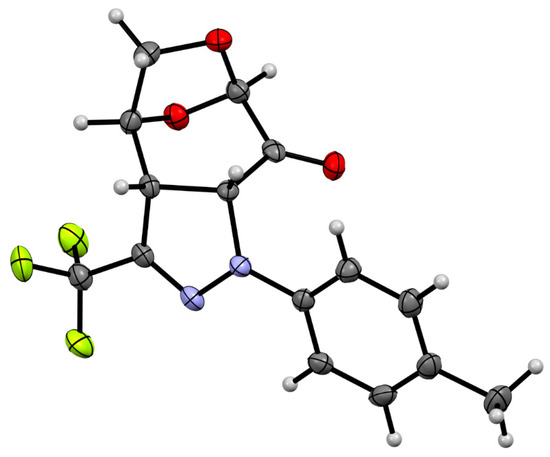
Figure 3.
Molecular structure of polycylic pyrazoline exo-11a, estimated by means of a single crystal X-ray experiment. Atoms are represented by thermal elipsoids (50%) for clarity.
It confirmed the formation of cycloadduct exo-11a in a regioselective manner with the new C–N bond connecting the α-carbon atom of the enone-part of 6 with the terminal N-atom of the 1,3-dipole framework. Thus, the regioselectivity of the (3+2)-cycloaddition corresponds to those observed in the reaction of 6 with C(Ph),N(Ph) nitrile imine 9, leading to the major isomer exo-10. However, whereas 9 and 6 provide a mixture of regioisomeric cycloadducts, the 1,3-dipole 1a undergoes the (3+2)-cycloaddition with complete regioselectivity. In addition, the X-ray analysis unambiguously indicates that the favored (as anticipated) exo-isomer 11a was the only cycloadduct formed in the course of the (3+2)-cycloaddition of 6 with fluorinated nitrile imine 1a (Scheme 3). This stereochemical aspect observed in the (3+2)-cycloadditions of 6 with nitrile imines 1 will be discussed in the part ‘Computational study′, which is based on the results obtained by using DFT calculations.
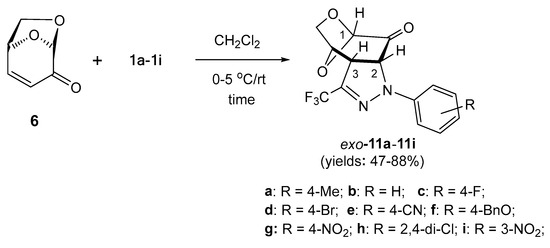
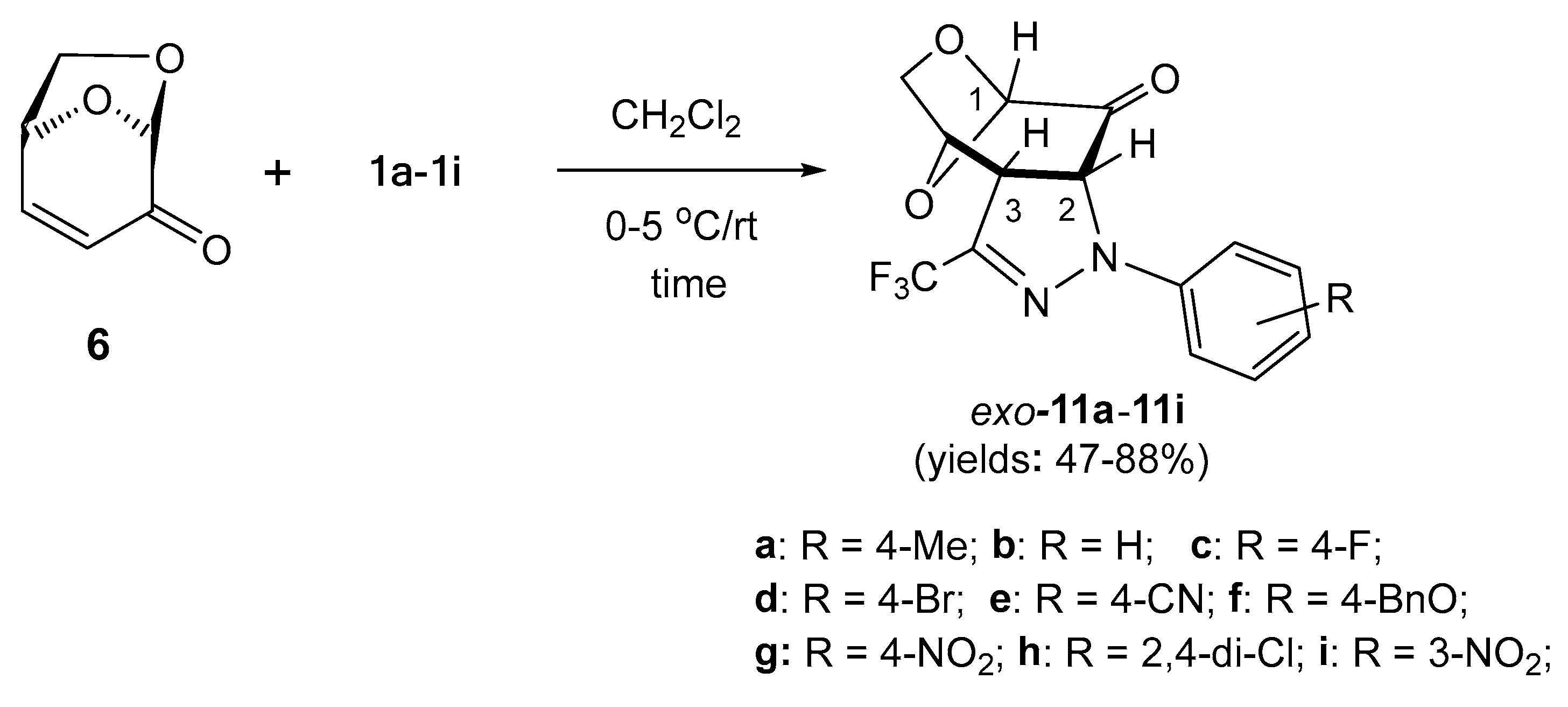
Scheme 3.
Highly selective (3+2)-cycloadditions of fluorinated nitrile imines 1a–1i, derived from trifluoroacetonitrile, with levoglucosenone (6) leading to trifluoromethyl substituted, fused pyrazolines 11a–11i.
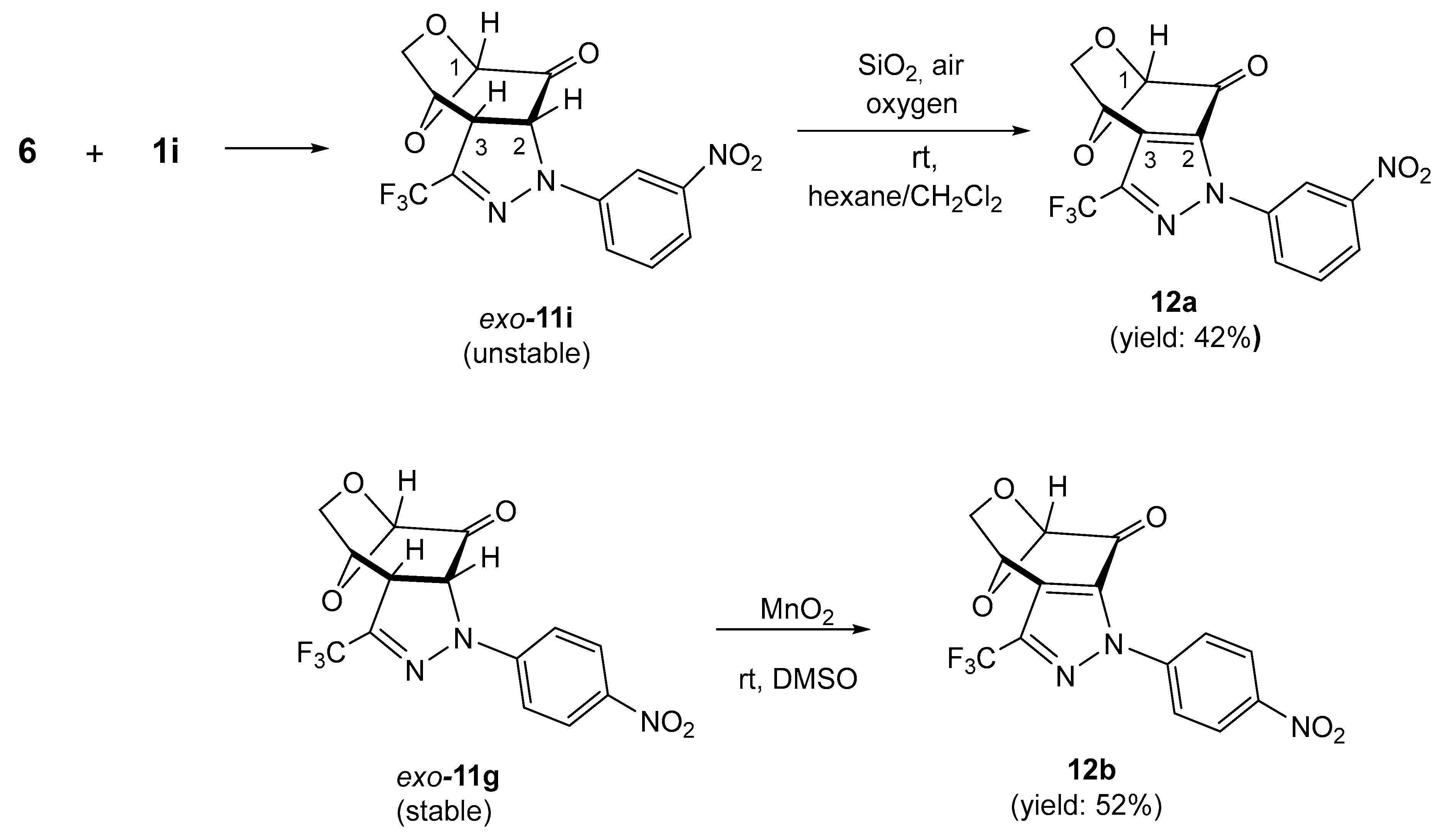
Prompted by the high selectivity observed in the experiment with 1,3-dipole 1a, we decided to test a series of similar (3+2)-cycloadditions starting with 6 as a reactive dipolarophile and differently substituted nitrile imines 1b–1i. Under analogous reaction conditions, in all reactions, the expected tricyclic pyrazolines exo-11b–11i were obtained as sole products and subsequently isolated chromatographically in fair to good yields (47–88%) as stable, solid materials with the tendency to crystallize from the corresponding organic solvents (Scheme 3). In analogy to exo-11a, for all obtained products 11b–11i, the structure of the anticipated exo-cycloadducts was also attributed based on the similarity of their spectroscopic properties (1H NMR and 13C NMR). Notably, the spontaneous oxidation of pyrazolines such as 10/10′ to the corresponding pyrazoles was not observed in most cases of trifluoromethyl-substituted cycloadducts 11, and an exceptional behavior was observed only in the case of pyrazoline 11i, when nitrile imine 1i bearing Ar = m-NO2C6H4 was employed as the reactive 1,3-dipole. In the 1H NMR spectrum of the crude mixture, a characteristic doublet attributed to HC(2) as well as a singlet signal characteristic for HC(1) in the anticipated cycloadduct exo-11i were registered at 5.28 (3JH,H = 11.7 Hz) and 5.21 ppm, respectively. In addition, a tiny singlet was also observed at 5.61 ppm, indicating the presence of an unknown, minor product. To our surprise, after column chromatography, the initially observed doublet disappeared and the later singlet with integration corresponding to one H-atom in the structure of the oxidized pyrazole 12a at 5.61 ppm was present. Finally, this component was isolated by column chromatography as the major product in 42% yield (Scheme 4, above).
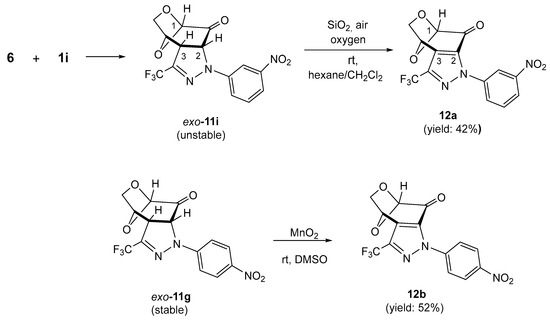
Scheme 4.
Equation above: spontaneous oxidation of pyrazoline exo-11i with air oxygen during chromatographic purification, leading to the fused pyrazole 12a. Equation below: dehydrogenation of pyrazoline exo-11g using MnO2 as an oxidizing reagent, leading to the fused pyrazole 12b.
The collected spectroscopic data allowed us to determine the expected structure 12a. For example, in the 13C NMR, weak absorptions of C(2) and C(3) were localized at 138.7 and 129.4 ppm, respectively. The signal of C=O was found at 178.8 ppm, and the characteristic quartet of CF3 with 1JC,F = 260.7 Hz was found at 120.5 ppm.
In extension of this study, a sample of the stable cycloadduct exo-11g, bearing an Ar = 4-NO2C6H4 substituent, was oxidized by treatment with MnO2 in DMSO solution based on the procedure applied for the mixture of isomeric pyrazolines 10/10′. After 24 h, the expected pyrazole 12b was isolated as a pure material with a 52% yield (Scheme 4, equation below). The reason for the particular sensitivity of pyrazoline exo-11i bearing the 3-NO2C6H4 substituent at the terminal N-atom of the 1,3-dipole to air oxygen is not known at the moment.
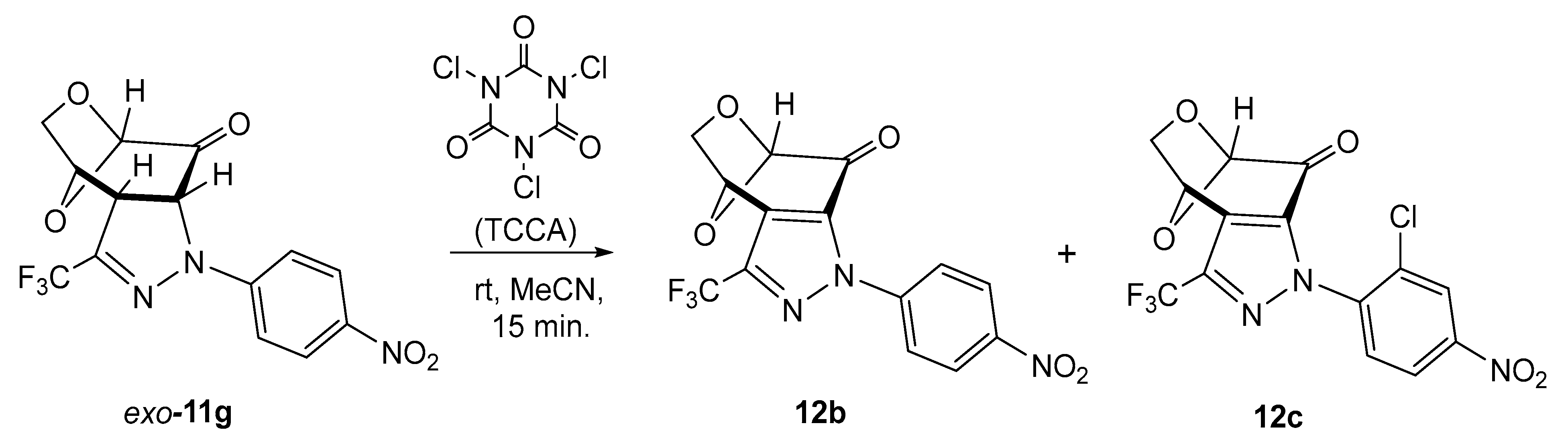
Prompted by the results described in a recent study [40], we decided to check the oxidation of the stable pyrazoline exo-11g with trichloroisocyanuric acid (TCCA) (1.5 mol-equiv.) in acetonitrile solution. A series of experiments performed with different ratios of both substrates demonstrated that, irrespective of the reaction conditions, in all cases, mixtures of the above-described fused pyrazole 12b and its chlorinated derivative 12c were obtained in a ratio of ca. 85:15. The attempted separation of the mixture, either by crystallization or by standard column chromatography, was unsuccessful, and for this reason, both products, i.e., 12b and 12c, were identified spectroscopically in the mixture. This result allowed us to conclude that in the case of fused pyrazolines 11, TCCA acts not only as an oxidant but also as a powerful chlorinating reagent, and the reaction leads to an undesired side product (Scheme 5). This observation fits well with the results described in the above-cited publication [40].
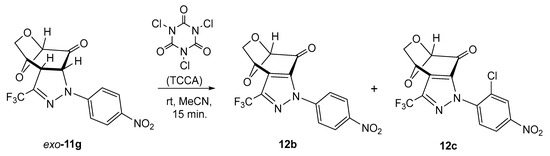
Scheme 5.
Attempted oxidation of pyrazoline exo-11g with TCCA, leading to a mixture of fused pyrazole 12b and its chlorinated derivative 12c.
The structure of the chlorinated derivative can tentatively be postulated as 12c, which is suggested by all the analyzed signals found in the 1H NMR spectrum. Thus, three signals attributed to protons of the aromatic ring were found at 5.80 (dd), 7.97 (dd), and 8.41 (d) ppm, respectively.
In a supplementary experiment aimed at comparing the ability of pyrazolines exo-10/exo-10′ and exo-11g to undergo air oxidation, a sample of the latter compound was heated in boiling toluene under reflux for 1.5h. After this time, the obtained material was analyzed by running the 1H NMR spectrum, which demonstrated the presence of both compounds, starting with pyrazoline exo-11g and target pyrazole 12b in a ratio of ca. 80:20. The oxidation of trifluoromethyl-substituted pyrazolines of type 11 occurs definitively slower than in the case of the known Ph-substituted analogues 10 [30].
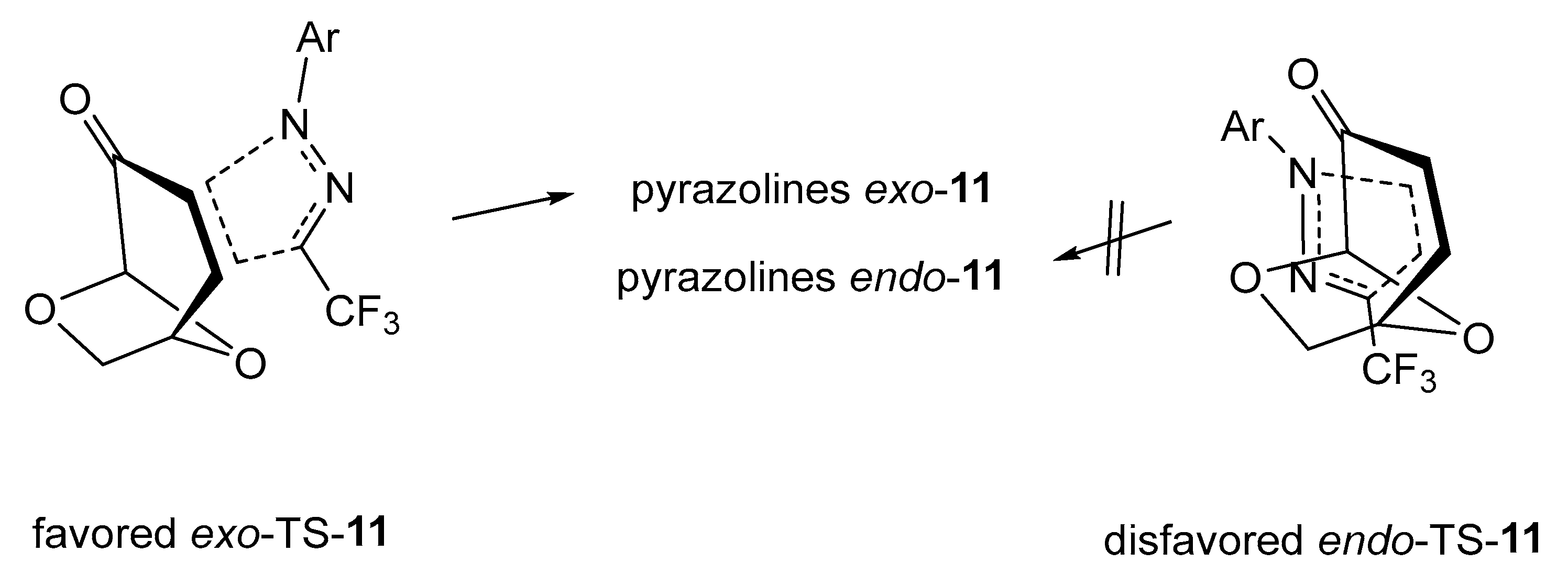
A general mechanistic presentation of the studied (3+2)-cycloadditions of trifluoromethyl-substituted 1,3-dipoles 1 with levoglucosenone 6 is outlined in Figure 4. The sterically favored exo-transition state leads to the formation of tricyclic pyrazolines exo-11 as exclusive products of these reactions. The approach of the 1,3-dipole from the endo-face of 6 is hindered due to the sterically more demanding -O-CH2- moiety compared to the -O- bridge. (See the computational part below).
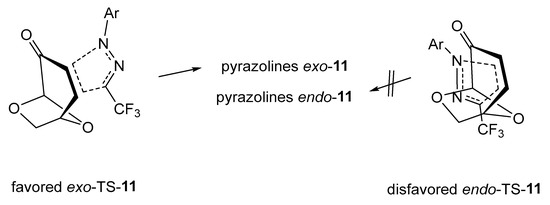
Figure 4.
Competitive exo- and endo-approaches of nitrile imines 1 onto the enone fragment of levoglucosenone 6 in the transition state of the (3+2)-cycloaddition reaction.
The experiments presented above demonstrate that the (3+2)-cycloadditions of fluorinated nitrile imines 1 derived from trifluoroacetonitrile and the non-fluorinated C(Ph),N(Ph) nitrile imine 9 lead to pyrazolines exo-11 and exo-10/10′, respectively, with high diastereoselectivity but with different regioselectivity. These observations prompted us to shed more light on the pathways of these concerted processes depicted in Figure 4. A detailed DFT calculation related to these problems will be discussed in the following part.
2.2. Computational Study
Mechanistic Investigations by DFT Calculations
To understand the observed stereochemistry of the cycloaddition reactions, the experimental results will be discussed on the basis of the calculated Gibbs free energy surface (ΔG298 [kcal/mol]) as investigated by a comprehensive quantum chemical DFT study. The determination of kinetic and thermodynamic properties will be the main focus of this study.
Starting with B3LYP/6-31G(d) [41,42] + GD3BJ [43,44], geometry optimizations for the gas phase and PBE1PBE/def2tzvp [45,46,47,48] + GD3BJ optimizations for the PCM solvent sphere of dichloromethane [49] were performed. For all calculations, the GAUSSIAN 16 package of programs was used (see in Supplementary Materials) [50]. In accordance with the reaction conditions (room temperature, no irradiation), only closed shell calculations were performed, although diradical intermediates principally cannot be excluded (see Haberhauer, Gleiter et al. [51], Firestone [52], and also the comments given in ref. [2,3]). Haberhauer, Gleiter et al. [51] have convincingly shown that 1,3-dipolar cycloadditions of the nitrile oxides, which are quite similar to nitrile imines, involve alkenes in preferentially closed shell transition states, whereas alkynes may also involve diradical intermediates. In the following, we discuss differences in Gibbs free energies (ΔG298) [kcal/mol] (see also Supplementary Materials for details); all relative energies refer to the sum of the starting materials.
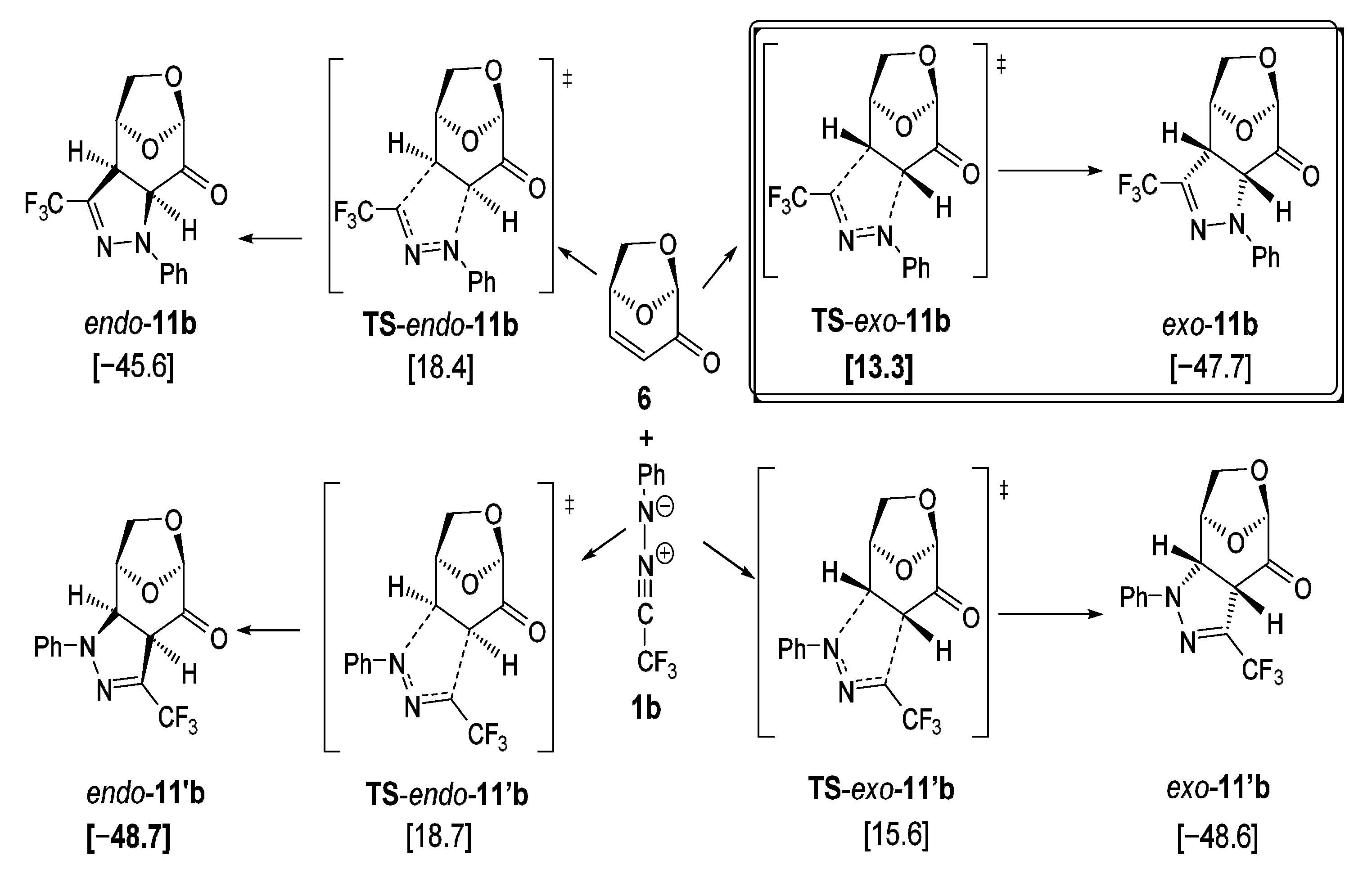
For the calculations of the reaction leading to pyrazoline exo-11b and its hypothetical isomers (Figure 5), the configurations and conformations were derived from the X-ray structure of exo-11a (Figure 3). The transition states were localized by reaction path calculations elongating the respective C–C and C–N bonds stepwise, followed by complete geometry optimization.
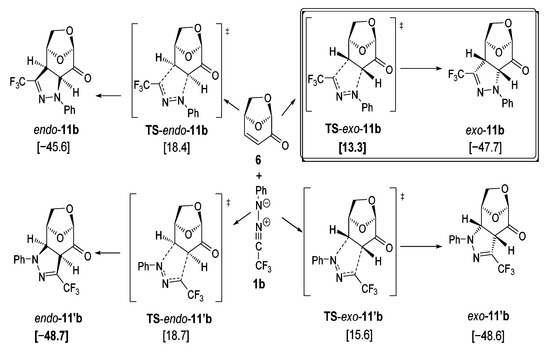
Figure 5.
DFT calculations of the (3+2)-cycloadditions of trifluoromethyl-substituted nitrile imine 1b with levoglucosenone 6 (PBE1PBE/def2tzvp + GD3BJ +PCM-dichloromethane) [kcal/mol] led to the regioisomeric pyrazolines exo-11b/endo-11b and exo-11′b/endo-11′b. Symbol: transition state.
As shown in Figure 5, the trifluoromethyl-phenyl substituted nitrile imine 1b was employed as the standard model for the 1,3-dipolar cycloadditions with LGO 6 used in the experiments. As the calculations show, all four cycloadditions proceed very exothermically (−45.6 to −48.7 kcal/mol). However, the calculated kinetic barriers (Gibbs free activation energies) are quite different, with TS-exo-11b [13.3 kcal/mol] being the significantly lowest transition state calculated. This transition state leads to the experimentally observed product exo-11b, which is not the thermodynamically best isomer. Thus, this reaction is a good example of a kinetically controlled process leading exclusively to the experimentally observed stereoisomer exo-11b. As shown in Figure 4, the 1,3-dipole approaches 6 from the sterically less hindered face (lower side, according to the drawings presented in Figure 5 and Figure 6), leading to the exo-product. The alternative face of 6 is sterically more hindered due to the presence of the bulkier CH2O- moiety. The exo-transition states are found to be lower by ~5 kcal/mol (Figure 5 and Figure 6) compared to the endo-transition states. Significantly shorter distances between the O-CH2-group and the 1,3-dipole lead to much higher repulsion for the endo- compared to the exo-transition states. Thus, the expected diastereoselectivity is nicely reflected by the DFT calculations.
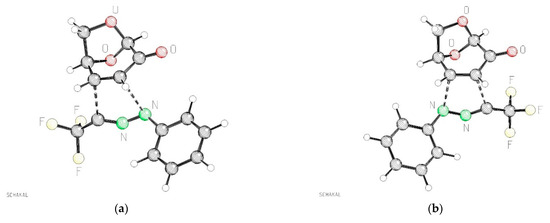
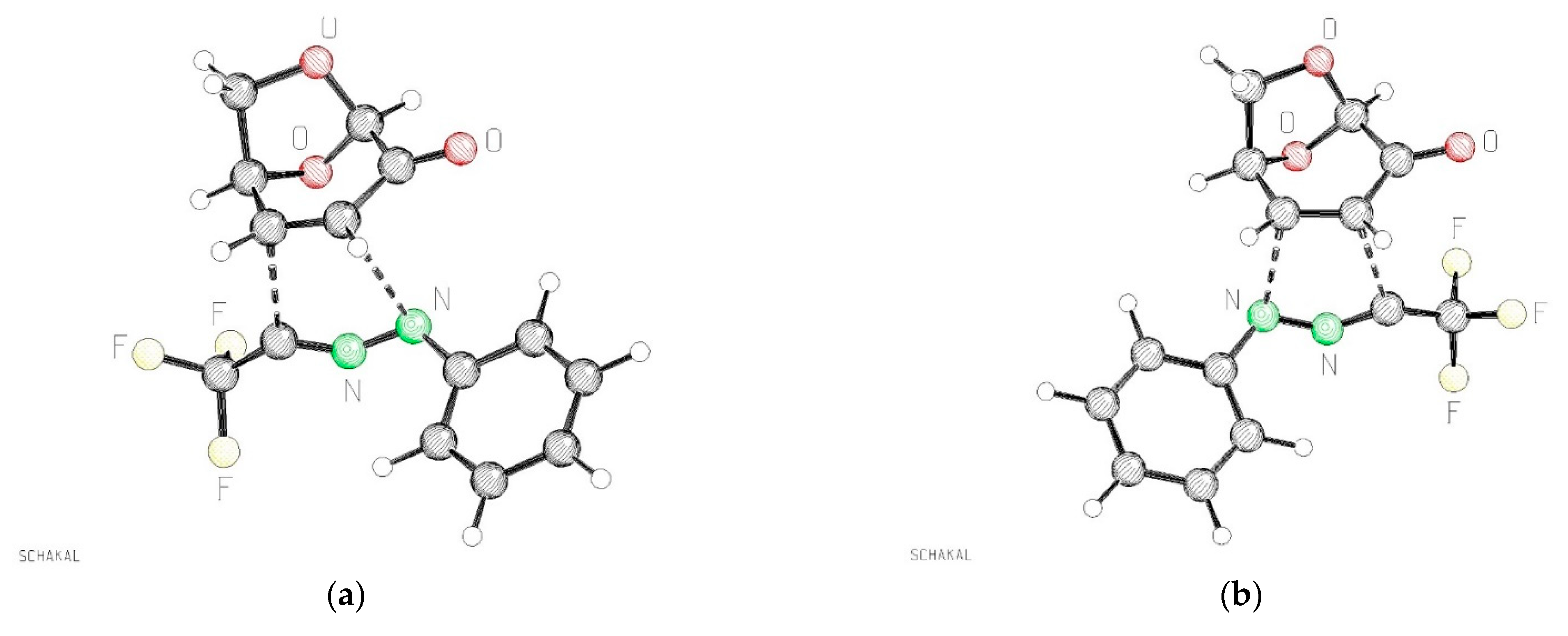
Figure 6.
Calculated atomic distances of the forming bonds of the transition states TS-exo-11b and TS-exo-11′b (PBE1PBE/def2tzvp + GD3BJ +PCM-dichloromethane). (a): TS-exo-11b atomic distances C–N 2.569 Å, C–C 2.253 Å; (b): TS-exo-11′b atomic distances C–N 2.323 Å C–C 2.353 Å.
Concerning the structural properties of the two lowest transition states, TS-exo-11b and TS-exo-11′b, significant geometrical differences were found, as illustrated in Figure 6. While for the lower transition state TS-exo-11b quite different atomic distances between the reacting carbon and nitrogen atoms were observed (C–N 2.569 Å, C–C 2.253 Å), illustrating the fairly unsymmetrical formation of the new bonds, the second-best transition state TS- exo-11′b shows quite similar distances between the reacting atoms: C–N 2.323 Å C–C 2.353 Å. The two other possible approaches of the nitrile imine 1b to the alternative face of 6 show significantly higher barriers (18.4 to 18.7 kcal/mol). The hypothetical product endo-11′b, resulting from the transition state TS-endo-11′b, would be one of the thermodynamically best isomers of all possible products, but it seems to be kinetically not easily accessible.
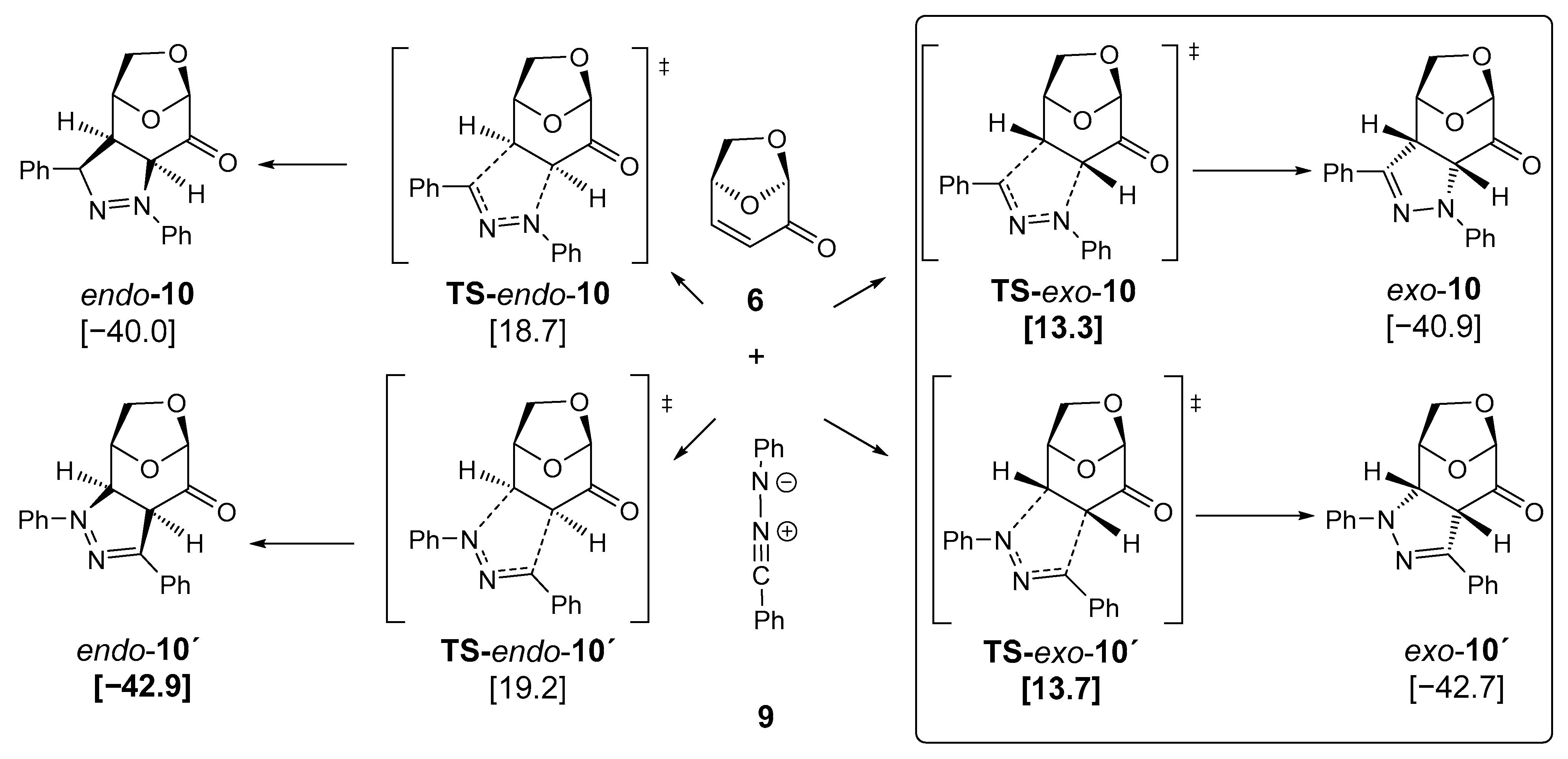
For comparison, quantum chemical calculations were also performed for the corresponding 1,3-dipolar cycloaddition reaction of diphenylnitrilimine 9 [53,54,55] with LGO 6. As shown in Figure 7, the results are mostly similar to the data of the CF3-substituted nitrilimine 1b, with a remarkable difference between the transition state energies of TS-exo-10 and TS-exo-10’. Both activation energies are low but quite similar, thus explaining the experimental observation of both diastereomeric products, exo-10 and exo-10’ (Scheme 2). Again, the endo-isomers are not accessible due to higher barriers. Kinetic control determines the constitution of the products observed, whereas thermodynamics is less important for the reaction products.
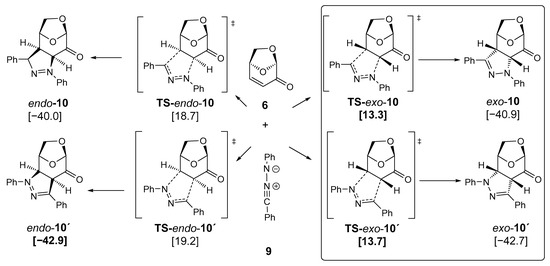
Figure 7.
DFT calculations of the cycloadditions of diphenyl-substituted nitrile imine 9 with levoglucosenone 6 (PBE1PBE/def2tzvp + GD3BJ +PCM-dichloromethane, room temperature) [kcal/mol] led to diastereomeric and regioisomeric pyrazolines exo/endo-10 and exo/endo-10′. Symbol: transition state.
In summary, the computational investigations presented here interpret the experimental findings as the result of the strong dominance of kinetic control over thermodynamic control.
3. Materials and Methods
CCDC Deposition: see Table S1 (see in Supplementary Materials).
General information: Chemicals, hexane, and petroleum ether were purchased and used as received without further purification. Other solvents (CH2Cl2, THF) were purified by distillation prior to their usage. Crude products (reaction mixtures) were purified by standard preparative layer chromatography (PLC) on plates coated with silica gel (60 Å medium pore diameter, PF254, Merck) or alternatively by standard column chromatography (silica gel, 40 mesh, J. T. Baker)). Unless stated otherwise, yields refer to amounts of isolated products.
Melting points were determined in capillaries with a Stuart SMP30 apparatus with automatic temperature monitoring and are uncorrected. The NMR spectra were recorded with a Bruker Avance III 600 MHz instrument (1H NMR: 600 MHz; 13C NMR: 151 MHz). Chemical shifts are reported relative to solvent residual peaks (1H NMR: δ = 7.26 ppm [CHCl3]; 13C NMR: δ = 77.0 ppm [CDCl3]). IR spectra were run on the Agilent Cary 630 FTIR spectrometer. ESI-MS measurements were performed with a Varian 500-MS LC Ion Trap instrument. High-resolution mass spectrometry (HRMS) measurements were performed using a Synapt G2-Si mass spectrometer (Waters) equipped with an ESI source and quadrupole-time-of-flight mass analyzer. Optical rotations were determined with an Anton Paar MCP 500 polarimeter at the temperatures indicated. Elemental analyses were obtained with a Vario EL III (ElementarAnalysensysteme GmbH) instrument.
Starting materials: Levoglucosenone (6) was prepared from cellulose by sulfuric acid-assisted pyrolysis, following a known procedure [56]. Hydrazonoyl bromides used as precursors of the in situ-generated nitrile imines 1 were synthesized by bromination of corresponding trifluoroacetaldehyde arylhydrazones, prepared in the presence of molecular sieves, according to a known procedure [57], using NBS as a brominating reagent [14].
Reactions of nitrile imines 1a–1b with levoglucosenone (6). General procedure: Approximately 1 mmol of 6 and 1.1 mmol of the corresponding hydrazonoyl bromide were dissolved in 2–3 mL of dry dichloromethane, and an excess of freshly dried K2CO3 was added. The mixture was magnetically stirred until 6 was completely consumed (24–72 h). Upon completion of the reaction, potassium carbonate was filtered off, and, after evaporation of the solvent in a vacuum evaporator, the crude mixtures were purified by column chromatography using petroleum ether with increasing amounts of methylene chloride as an eluent. Analytically pure samples were obtained after crystallization of the product in petroleum ether with a small amount of dichloromethane.
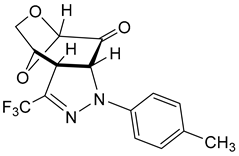
- 5-(p-Tolyl)-3-(trifluoromethyl)-9,11-dioxa-4,5-diazatricyclo[6.2.1.02,6]undec-3-en-7-one (exo-11a). Yield: 175 mg (54%), pale-yellow crystals, mp = 132–133 °C (petroleum ether/CH2Cl2). [α]D20 = –629 (c 0.3, CHCl3).
- 1H NMR (CDCl3): δ 2.34 (s, 3H, CH3); 4.01 (d, JH,H = 12.2 Hz, 1H); 4.07–4.09 (m, 1H); 4.12–4.14 (m, 1H); 5.15 (d, JH,H = 4.7 Hz, 1H); 5.24 (d, JH,H = 12.1 Hz, 1H); 5.28 (s, 1H); 7.09, 7.13 (AB-system, JH,H = 8.5 Hz, 4CHarom);
- 13C NMR (CDCl3): δ 21.1, 51.8, 63.6, 70.9, 100.2, 68.6, 114.5, 129.9, 120.9 (q, 1JC,F = 277.7 Hz, CF3); 131.6, 140.7, 134.0 (q, 2JC,F = 36.9 Hz, C–CF3), 194.5 (C=O);
- 19F NMR: δ –63.9;
- IR: ν 1703s (C=O), 1510s, 1378m, 1299m, 1258s, 1193m, 1123vs, 1083s, 943m, 801s, cm−1;
- EA for C15H13F3N2O3 (326.28): calcd. C 55.22, H 4.02, N 8.59; found C 55.03, H 4.04, N 8.85.
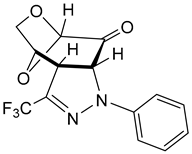
- 5-Phenyl-3-(trifluoromethyl)-9,11-dioxa-4,5-diazatricyclo[6.2.1.02,6]undec-3-en-7-one (exo-11b). Yield: 190 mg (61%), pale yellow crystals, mp = 108–109 °C (petroleum ether/CH2Cl2). [α]D20 = –515 (c 0.3, CHCl3).
- 1H NMR (CDCl3): δ 3.96 (dd, JH,H = 12.0 Hz, JH,H = 1.0 Hz, 1H); 4.04–4.07 (m, 1H); 4.09–4.10 (m, 1H); 5.13 (d, JH,H = 2.3 Hz, 1H); 5.24 (d, JH,H = 11.9 Hz, 1H); 5.26 (s, 1H); 7.00–7.03 (m, 1CHarom); 7.17–7.20 (m, 2CHarom); 7.30–7.34 (m, 2CHarom);
- 13C NMR: δ 51.6, 63.2, 71.1, 100.4, 68.5, 114.4, 121.9, 129.2, 120.8 (q, 1JC,F = 268.1 Hz, CF3); 134.8 (q, 2JC,F = 37.3 Hz, C–CF3); 142.9, 194.6 (C=O).
- 19F NMR (CDCl3): δ –64.0;
- IR: ν 1744s (C=O), 1600s, 1494s, 1312s, 1297m, 1259s, 1185s, 1092vs, 976s, 913s, 799s, 752s, cm−1;
- EA for C14H11F3N2O3 (326.28): calcd. C 53.85, H 3.55, N 8.97; found C 53.78, H 3.54, N 9.22.
4. Conclusions
In recent years, fluorinated and fluoroalkylated pyrazole derivatives have attracted great attention as biologically active compounds, and some representatives have already found practical applications as bioactive components of some drugs and agrochemicals offered on the market, e.g., Celecoxib (Pfizer), Penflufen (Bayer), Isoflucypram (Bayer), etc. [58,59,60,61,62]. On the other hand, levoglucosenone (6) is also extensively studied as a potential biorenewable platform for the development of a plethora of well-promising, bioactive organic compounds [23,24,25,26,27,28,29]. However, it has never been explored as a chiral platform for the preparation of fluorinated, polycylic pyrazole derivatives.
The presented study showed that, in contrast to the widely known ‘classic’ N(Ph), C(Ph)-nitrile imine 9, the (3+2)-cycloadditions of recently developed fluorinated nitrile imines 1, derived from trifluoroacetonitrile, react with levoglucosenone 6 in a highly stereoselective manner, and the expected polycyclic pyrazolines 11 are formed in kinetically controlled reactions with perfect regioselectivity as exo-cycloadducts, exclusively. The DFT calculations fully support the experimental findings.
It was also established that the initially obtained pyrazolines can be smoothly oxidized to the corresponding pyrazoles by treatment with MnO2 in DMSO as a solvent. For all these reasons, the method described therein offers a new, convenient access to optically active, polycylic, trifluoromethyl substituted pyrazoles incorporating the chiral levoglucosenone skeleton, which can be of importance for their further medicinally and/or agrochemically-oriented studies.
This study emphasizes the universality and importance of 1,3-dipolar cycloaddition reactions for the current development of organic synthesis related to the preparation of five membrane nitrogen heterocycles [63,64,65].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28217348/s1. The authors have cited additional references within the Supporting Information [66,67,68,69,70].
Author Contributions
Conceptualization, G.M. and Z.J.W.; methodology, G.M., K.U., M.P. and E.-U.W.; software, G.M. and E.-U.W.; validation, G.M., K.U. and Z.J.W.; formal analysis, G.M.; investigation, K.U., M.P. and E.-U.W.; resources, G.M.; data curation, G.M., M.P., E.-U.W. and K.U.; writing—original draft preparation, G.M. and E.-U.W.; writing—review and editing, G.M. and E.-U.W.; visualization, G.M. and E.-U.W.; supervision, G.M. and E.-U.W.; project administration, G.M.; funding acquisition, G.M. All authors have read and agreed to the published version of this manuscript.
Funding
The presented research was financially supported by the University of Łódź within the grant IDUB (2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this manuscript are available on request from the corresponding authors.
Acknowledgments
G.M. and K.U. acknowledge generous support by the University of Lodz (Poland) within Grant IDUB-2023. All authors thank Hans-Ulrich Reissig (Freie Universität Berlin) for stimulating discussions on the chemistry of nitrile imines and related 1,3-dipolar species as well as for his help in the course of preparation of the final version of this manuscript. We are very thankful to Christian Mück-Lichtenfeld (Universität Münster) for helpful discussions concerning the theoretical part. G.M. thanks Professor Heinz Heimgartner (University of Zurich) and Jakub Wręczycki (Lodz University of Technology) for their help in the course of preparation of the final version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huisgen, R. 1,3-Dipolar Cycloadditions: Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Fišera, L.; Huisgen, R.; Kalwinsch, I.; Langhals, E.; Li, X.; Mloston, G.; Polborn, K.; Rapp, J.; Sicking, W.; Sustmann, R. New thione chemistry. Pure Appl. Chem. 1996, 68, 789–798. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, R.; Domingo, L.R. Unravelling the Mysteries of the [3+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.-U. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Jamieson, C.; Livingstone, K. (Eds.) The Nitrile Imine 1,3-Dipoles: Properties, Reactivity and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Sharp, J.T. Nitrile Ylides and Nitrile Imines. In Synthetic Applications of 1,3-dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products; Padwa, A., Pearson, W.H., Eds.; Wiley: New York, NY, USA, 2020; Chapter 7; pp. 473–538. [Google Scholar]
- Deepthi, A.; Acharjee, N.; Sruthi, S.L.; Meenakshy, C.B. An overview of nitrile imine based [3+2] cycloadditions over half a decade. Tetrahedron 2022, 116, 132812. [Google Scholar] [CrossRef]
- Matiadis, D. Strategies and Methods for the Synthesis of 2-Pyrazolines: Recent Developments (2012–2022). Adv. Synth. Catal. 2023, 365, 1934–1969. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, R.; Yang, F.; Wang, K.-H.; Wang, J.; Huang, D.; Lv, X.; Hu, Y. Synthesis of Difluoromethyl Pyrazolines and Pyrazoles by [3+2] Cycloaddition Reaction of Difluoroacetohydrazonoyl Bromides with Electron-deficient Olefins. Asian J. Org. Chem. 2022, 11, e202200438. [Google Scholar] [CrossRef]
- Li, M.-M.; Huang, H.; Tian, W.; Pu, Y.; Zhang, C.; Yang, J.; Ren, Q.; Tao, F.; Deng, Y.; Lu, J.; et al. Construction of multi-substituted pyrazoles via potassium carbonate-mediated [3+2] cycloaddition of in situ generated nitrile imines with cinnamic aldehydes. RSC Adv. 2022, 12, 13087–13092. [Google Scholar] [CrossRef]
- Rouatbi, F.; Mhiri, C.; Askri, M.; Knorr, M.; Rousselin, Y.; Kubicki, M.M. Regioselective Synthesis of Mono- and Dispiropyrazoline Derivatives via 1,3-dipolar Cycloaddition with Nitrilimines. J. Heterocycl. Chem. 2017, 54, 1152–1160. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Utecht-Jarzyńska, G.; Mlostoń, G.; Jasiński, M. Trifluoromethylated pyrazoles via sequential (3+2)-cycloaddition of fluorinated nitrile imines with chalcones and solvent-dependent deacylative oxidation reaction. Org. Lett. 2022, 24, 2499–2503. [Google Scholar] [CrossRef]
- Utecht, G.; Mlostoń, G.; Jasiński, M. A straightforward access to trifluoromethylated spirobipyrazolines through a double (3+2)-cycloaddition of fluorinated nitrile imines with alkoxyallenes. Synlett 2018, 29, 1753–1758. [Google Scholar] [CrossRef]
- Mlostoń, G.; Grzelak, G.; Utecht, G.; Jasiński, M. First [3+2]-cycloadditions of thiochalcones as C=S dipolarophiles in reactions with fluorinated nitrile imines. Synthesis 2017, 49, 2129–2137. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Utecht, G.; Lentz, D.; Jasiński, M. Trifluoromethylated 2,3-dihydro-1,3,4-thiadiazoles via the regioselective (3+2)-cycloadditions of fluorinated nitrile imines with aryl, hetaryl, and ferrocenyl thioketones. J. Fluorine Chem. 2016, 192, 147–154. [Google Scholar] [CrossRef]
- Utecht, G.; Sioma, J.; Jasiński, M.; Mlostoń, G. Expected and unexpected results in reactions of fluorinated nitrile imines with (cyclo)aliphatic thioketones. J. Fluor. Chem. 2017, 201, 68–75. [Google Scholar] [CrossRef]
- Utecht-Jarzyńska, G.; Mykhaylychenko, S.S.; Rusanov, E.B.; Shermolovich, Y.G.; Jasiński, M.; Mlostoń, G. Highly fluorinated 2,3-dihydro-1,3,4-thiadiazole derivatives via (3+2)-cycloadditions of fluorinated, tertiary thioamides with nitrile imines derived from trifluoroacetonitrile. J. Fluor. Chem. 2021, 242, e109702. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, H.; Cheung, C.W.; Zhang, F.-G.; Jasiński, M.; Ma, J.-A.; Nie, J. Regioselective [3 + 2] cycloaddition of di/trifluoromethylated hydrazonoyl chlorides with fluorinated nitroalkenes: A facile access to 3-di/trifluoroalkyl-5-fluoropyrazoles. Org. Biomol. Chem. 2023, 21, 5040–5045. [Google Scholar] [CrossRef] [PubMed]
- Cen, K.; Wei, J.; Feng, Y.; Liu, Y.; Wang, X.; Liu, Y.; Yin, Y.; Yu, J.; Wang, D.; Cai, J. Synthesis of fused 3-trifluoromethyl-1,2,4-triazoles via base-promoted [3 + 2] cycloaddition of nitrile imines and 1H-benzo[d]imidazole-2-thiols. Org. Biomol. Chem. 2023, 21, 7095–7099. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sumi, K. Thermal Decomposition Products of Cellulose. J. Appl. Polym. Sci. 1970, 14, 2003–2013. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, X.; Liu, C.; Wang, Y.; Xiao, R. Selective low-temperature pyrolysis of microcrystalline cellulose to produce levoglucosan and levoglucosenone in a fixed bed reactor. Fuel Process. Technol. 2017, 167, 484–490. [Google Scholar] [CrossRef]
- Xu, F.; Luo, J.; Jiang, L.; Zhao, Z. Improved production of levoglucosan and levoglucosenonefrom acid-impregnated cellulose via fast pyrolysis. Cellulose 2022, 29, 1463–1472. [Google Scholar] [CrossRef]
- Witczak, Z.J. (Ed.) Levoglucosenone and Levoglucosans: Chemistry and Applications; ATL Press, Inc. Science Publishers: Mount Prospect, IL, USA, 1994. [Google Scholar]
- Sarotti, A.M.; Zanardi, M.M.; Spanevello, R.A. Recent Applications of Levoglucosenone as Chiral Synthon. Curr. Org. Synth. 2012, 9, 439–459. [Google Scholar] [CrossRef]
- Comba, M.B.; Tsai, Y.; Sarotti, A.M.; Mangione, M.I.; Suárez, A.G.; Spanevello, R.A. Levoglucosenone and Its New Applications: Valorization of Cellulose Residues. Eur. J. Org. Chem. 2018, 5, 590–604. [Google Scholar] [CrossRef]
- Liu, X.; Carr, P.; Gardiner, M.G.; Banwell, M.G.; Elbanna, A.H.; Khalil, Z.G.; Capon, R.J. Levoglucosenone and Its Pseudoenantiomer iso-Levoglucosenone as Scaffolds for Drug Discovery and Development. ACS Omega 2020, 5, 13926–13939. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.E.; Greatrex, B.W. Levoglucosenone: Levoglucosenone: Bio-Based Platform for Drug Discovery. Front. Chem. 2022, 10, e902239. [Google Scholar] [CrossRef] [PubMed]
- Warne, C.M.; Fadlallah, S.; Whitwood, A.C.; Sherwood, J.; Mouterde, L.M.M.; Allais, F.; Guebitz, G.M.; McElroy, C.R.; Pellis, A. Levoglucosenone-derived synthesis of bio-based solvents and polyesters. Green Chem. Lett. Rev. 2023, 16, 2154573. [Google Scholar] [CrossRef]
- Banwell, M.G.; Liu, X.; Connal, L.A.; Gardiner, M.G. Synthesis of Functionally and Stereochemically Diverse Polymers via Ring-Opening Metathesis Polymerization of Derivatives of the Biomass-Derived Platform Molecule Levoglucosenone Produced at Industrial Scale. Macromolecules 2020, 53, 5308–5314. [Google Scholar] [CrossRef]
- Blake, A.J.; Cook, T.A.; Forsyth, A.C.; Gould, R.O.; Paton, R.M. 1,3-dipolar cycloaddition reactions of levoglucosenone. Tetrahedron 1992, 48, 8053–8064. [Google Scholar] [CrossRef]
- Novikov, R.A.; Rafikov, R.R.; Shulishov, E.V.; Konyushkin, L.D.; Semenov, V.V.; Tomilov, Y.V. Reactions of levoglucosenone and its derivatives with diazo compounds. Russ. Chem. Bull. Int. Ed. 2009, 58, 327–334. [Google Scholar] [CrossRef]
- Müller, C.; Gómez-Zurita Frau, M.A.; Ballinari, D.; Colombo, S.; Bitto, A.; Martegani, E.; Airoldi, C.; van Neuren, A.S.; Stein, M.; Weiser, J.; et al. Design, Synthesis, and Biological Evaluation of Levoglucosenone-Derived Ras Activation Inhibitors. Chem. Med. Chem. 2009, 4, 524–528. [Google Scholar] [CrossRef]
- Ward, D.D.; Shafizadeh, F. Cycloaddition “4 + 2” reactions of levoglucosenone. Carbohydr. Res. 1981, 95, 155–176. [Google Scholar] [CrossRef]
- Sarotti, A.M.; Joullie, M.M.; Spanevello, R.A.; Suárez, A.G. Microwave-Assisted Regioselective Cycloaddition Reactions between 9-Substituted Anthracenes and Levoglucosenone. Org. Lett. 2006, 8, 5561–5564. [Google Scholar] [CrossRef]
- Zurita, M.A.; Avila, A.; Spanevello, R.A.; Suárez, A.G.; Sarotti, A.M. Montmorillonite K-10 promoted synthesis of chiral dioxa-caged compounds derived from levoglucosenone. Carbohydr. Res. 2015, 402, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Witczak, Z.J.; Mauger, A.; Bielski, R.; Mencer, D.E. Thio-glycomimetics with enhanced lipophilicity and their biological activity. Arkivoc 2021, 4, 268–279. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Sun, J.; Mielguj, R. Synthesis of L-fucopyranosyl-4-thiodisaccharides from levoglucosenone and their inhibitory activity on α-L-fucosidase. Bioorg. Med. Chem. Lett. 1995, 5, 2169–2175. [Google Scholar] [CrossRef]
- Tsai, Y.; Etichetti, C.M.B.; Cicetti, S.; Girardini, J.E.; Spanevello, R.A.; Suarez, A.G.; Sarotti, A.M. Design, synthesis and evaluation of novel levoglucosenone derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2020, 30, e127247. [Google Scholar] [CrossRef] [PubMed]
- Mlostoń, G.; Celeda, M.; Palusiak, M. Higher-order (8+2)-cycloadditions of tropothione with levoglucosenone (LGO) and structurally similar exo-cyclic enones derived from cyrene. Carbohydr. Res. 2023, 529, e108844. [Google Scholar] [CrossRef]
- Feng, Y.; Chang, B.; Ren, Y.; Zhao, F.; Wang, K.-H.; Wang, J.; Huang, D.; Lv, X.; Hu, Y. Synthesis of trifluoromethylpyrrolopyrazole derivatives via [3+2] cycloaddition of trifluoromethyl N-acylhydrazones or trifluoroacetohydrazonoyl bromides with maleimides. Tetrahedron 2023, 136, 133353. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Dispersion-Corrected Mean-Field Electronic Structure Methods. Chem. Rev. 2016, 116, 5105–5154. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Weigend, R.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Haberhauer, G.; Gleiter, R.; Woitschetzki, S. Anti-Diradical Formation in 1,3-Dipolar Cycloadditions of Nitrile Oxides to Acetylenes. J. Org. Chem. 2015, 80, 12321–12332. [Google Scholar] [CrossRef]
- Firestone, R.A. The Low Energy of Concert in Many Symmetry-Allowed Cycloadditions Supports a Stepwise-Diradical Mechanism. Int. J. Chem. Kinet. 2013, 45, 415–428. [Google Scholar] [CrossRef]
- Bégué, D.; Dargelos, A.; Berstermann, H.M.; Netsch, K.P.; Bednarek, P.; Wentrup, C. Nitrile Imines and Nitrile Ylides: Rearrangements of Benzonitrile N-Methylimine and Benzonitrile Dimethylmethylide to Azabutadienes, Carbodiimides, and Ketenimines. Chemical Activation in Thermolysis of Azirenes, Tetrazoles, Oxazolones, Isoxazolones, and Oxadiazolones. J. Org. Chem. 2014, 79, 1247–1253. [Google Scholar] [CrossRef]
- Bégué, D.; Wentrup, C. Carbenic Nitrile Imines: Properties and Reactivity. J. Org. Chem. 2014, 79, 1418–1426. [Google Scholar] [CrossRef]
- Vermeeren, P.; Tiezza, M.D.; Wolf, M.E.; Lahm, M.E.; Allen, W.D.; Schaefer, H.F., III; Hamlin, T.A.; Bickelhaupt, F.M. Pericyclic reaction benchmarks: Hierarchical computations targeting CCSDT(Q)/CBS and analysis of DFT performance. Phys. Chem. Chem. Phys. 2022, 24, 18028–18042. [Google Scholar] [CrossRef] [PubMed]
- Klepp, J.; Dillon, W.; Lin, Y.; Feng, P.; Greatrex, B.W. Preparation of (-)-Levoglucosenone from Cellulose Using Sulfuric Acid in Polyethylene Glycol. Org. Synth. 2020, 97, 38–53. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Jacaszek, N.; Linden, A.; Heimgartner, H. Exploration of fluoral hydrazones derived from carbohydrazides for the synthesis of trifluoromethylated heterocycles. Heterocycles 2014, 88, 387–401. [Google Scholar] [CrossRef]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.V.; Vegi, P.F.; Carvalho Miguita, A.G.; Silva dos Santos, M.; Boechat, N.; Rolim Bernardino, A.M. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simno-Fuentes, A. From 2000 to mid-2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.-P.; Leroux, F.R. Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. J. Fluor. Chem. 2013, 152, 2–11. [Google Scholar] [CrossRef]
- Beutick, S.E.; Vermeeren, P.; Hamlin, T.A. The 1,3 dipolar cycloaddition: From conception to quantum chemical design. Chem. Asian J. 2022, 17, e202200553. [Google Scholar] [CrossRef]
- Martina, K.; Tagliapietra, S.; Veselov, V.V.; Cravotto, G. Green protocols in heterocycle syntheses via 1,3-dipolar cycloadditions. Front. Chem. 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, L.; Algieri, V.; Olivito, F.; De Nino, A. Recent developments on 1,3-dipolar cycloaddition reactions by catalysis in green solvents. Catalysts 2020, 10, 65. [Google Scholar] [CrossRef]
- CrysAlisPRO Software System; Version 1.171; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2015.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. Appl. Cryst. 2009, 42, 39–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Effect of the damping function in dispersion corrected density functional theory. Chem. Rev. 2016, 116, 5105–5154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).