Impact of Dye Encapsulation in ZIF-8 on CO2, Water, and Wet CO2 Sorption
Abstract
:1. Introduction
2. Results
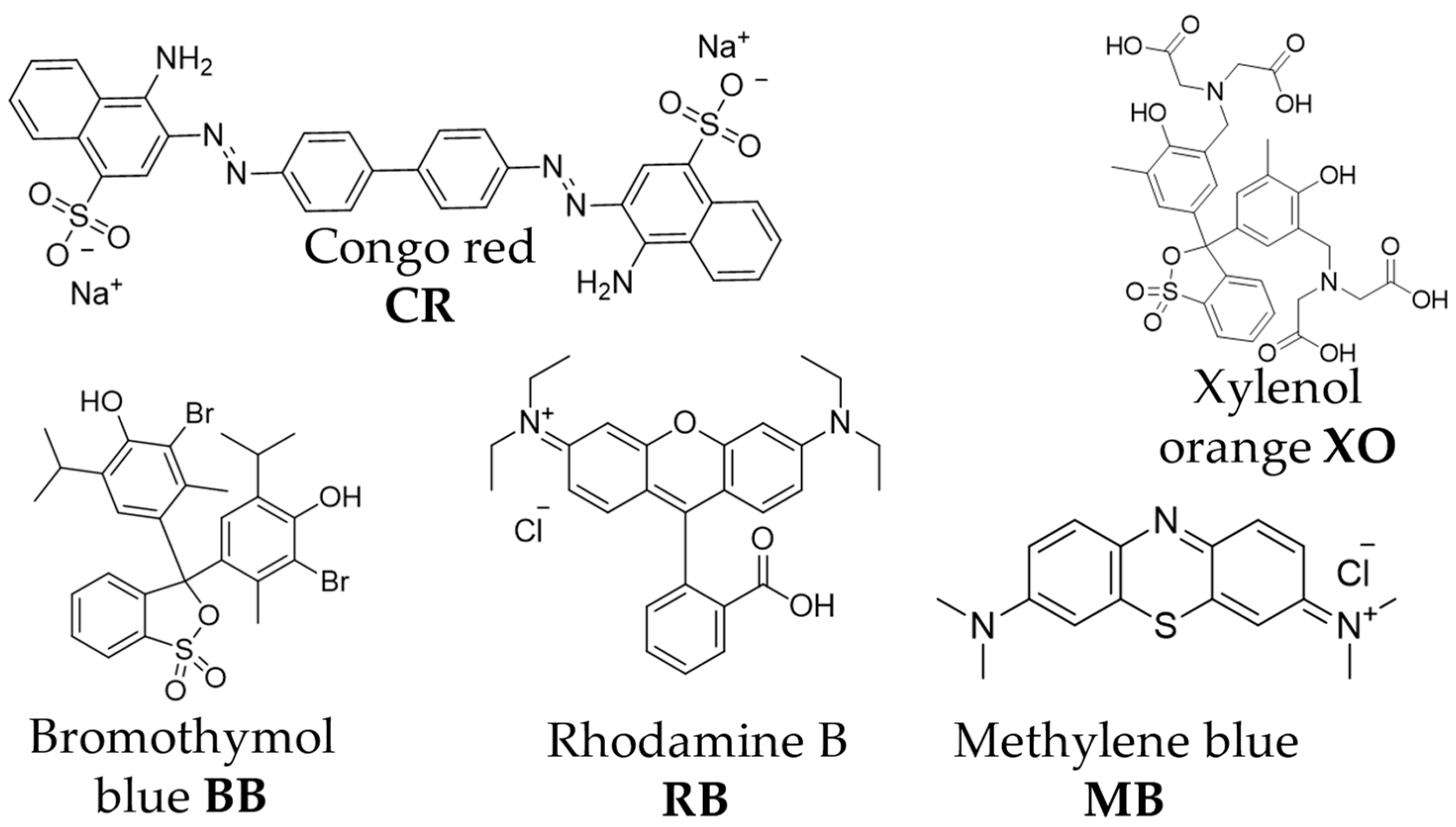
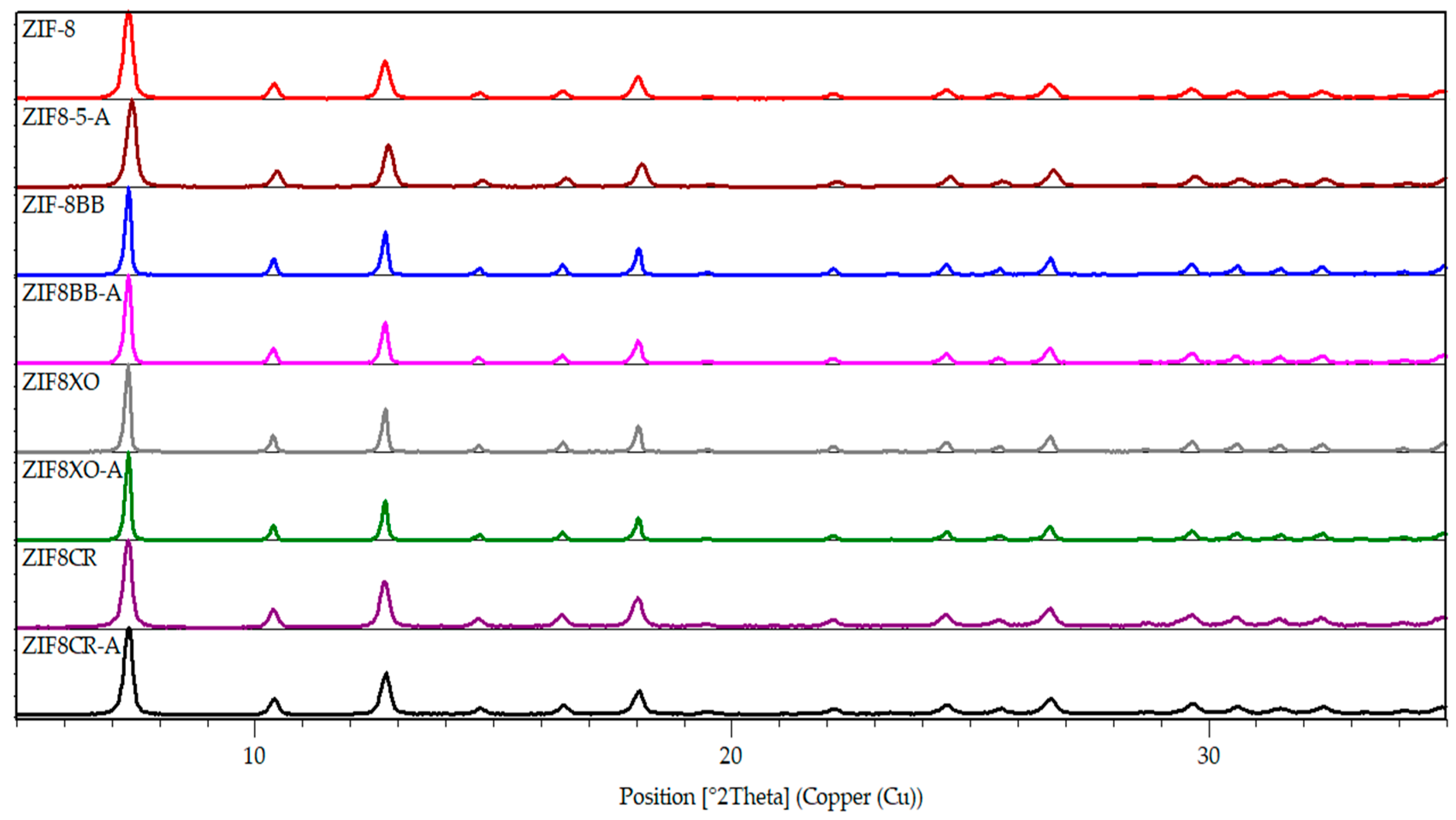
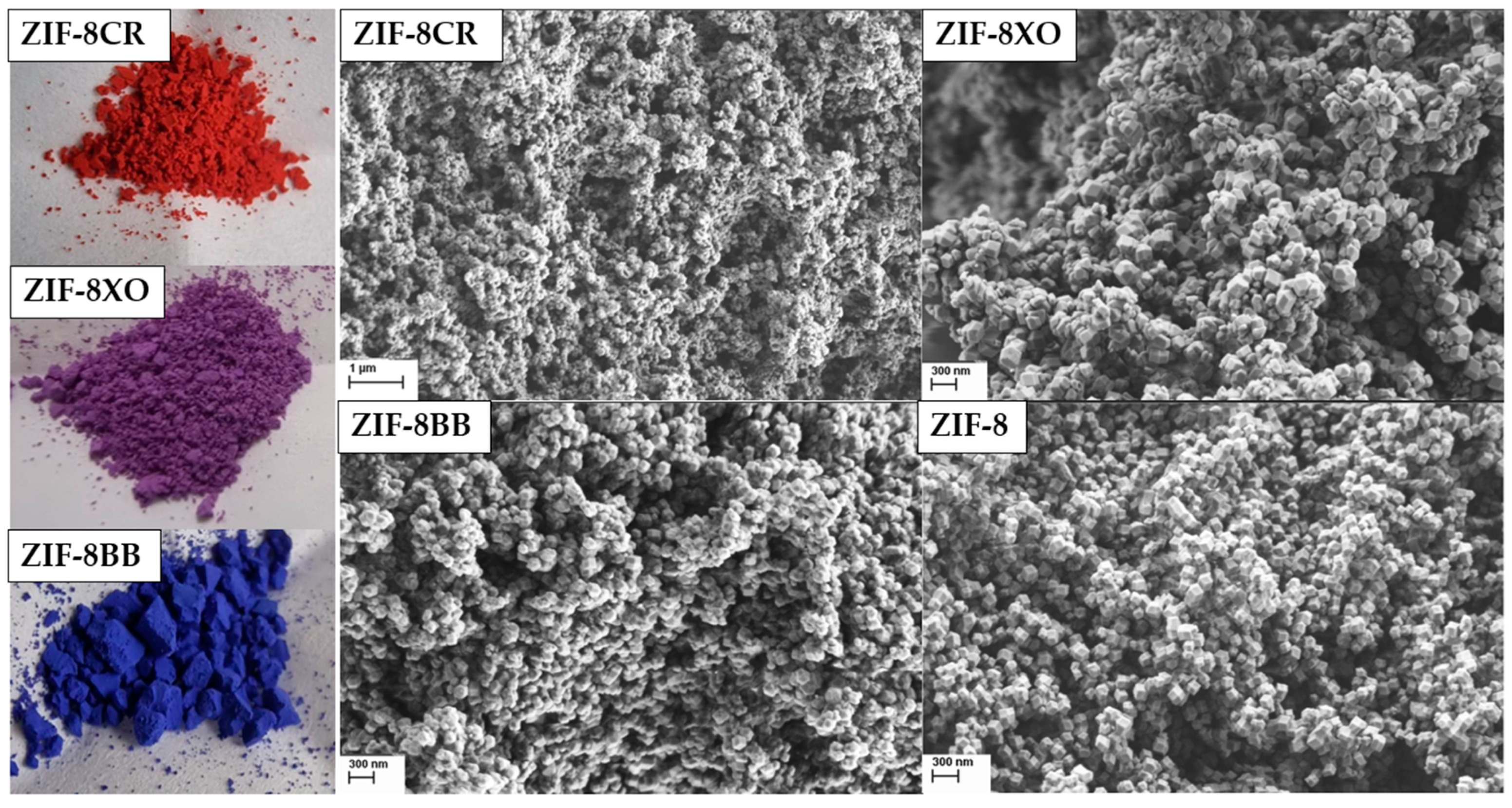
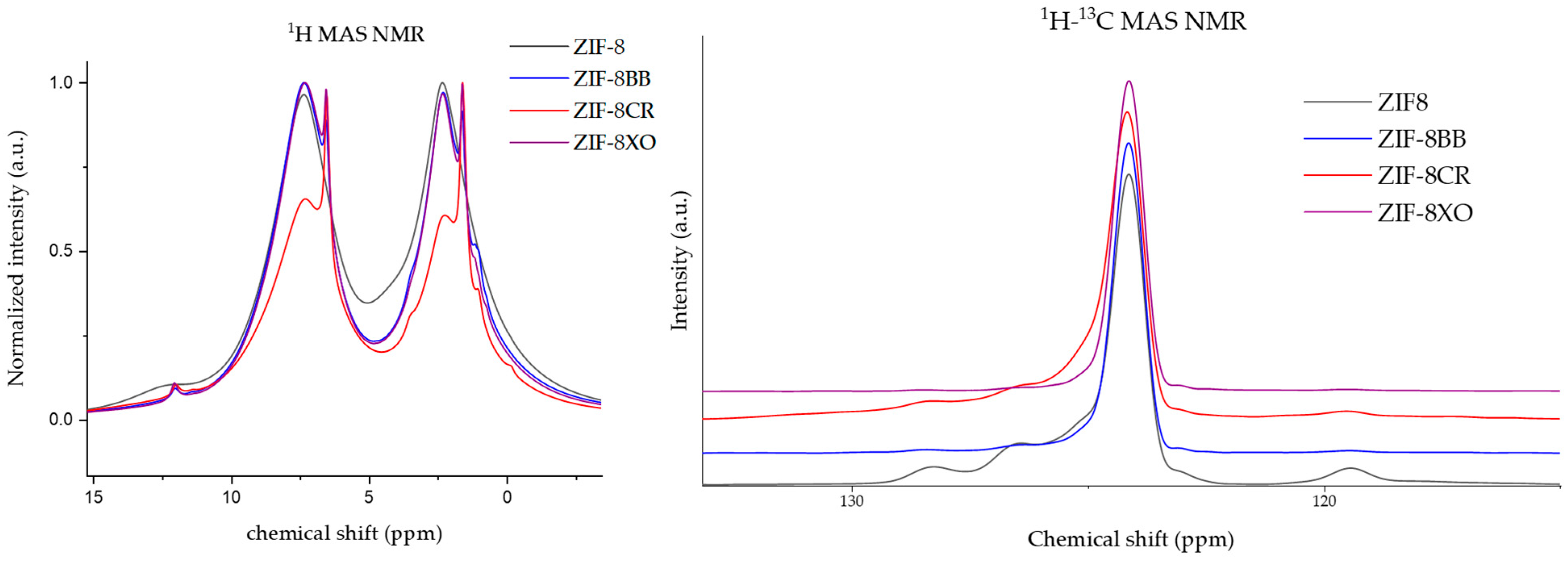
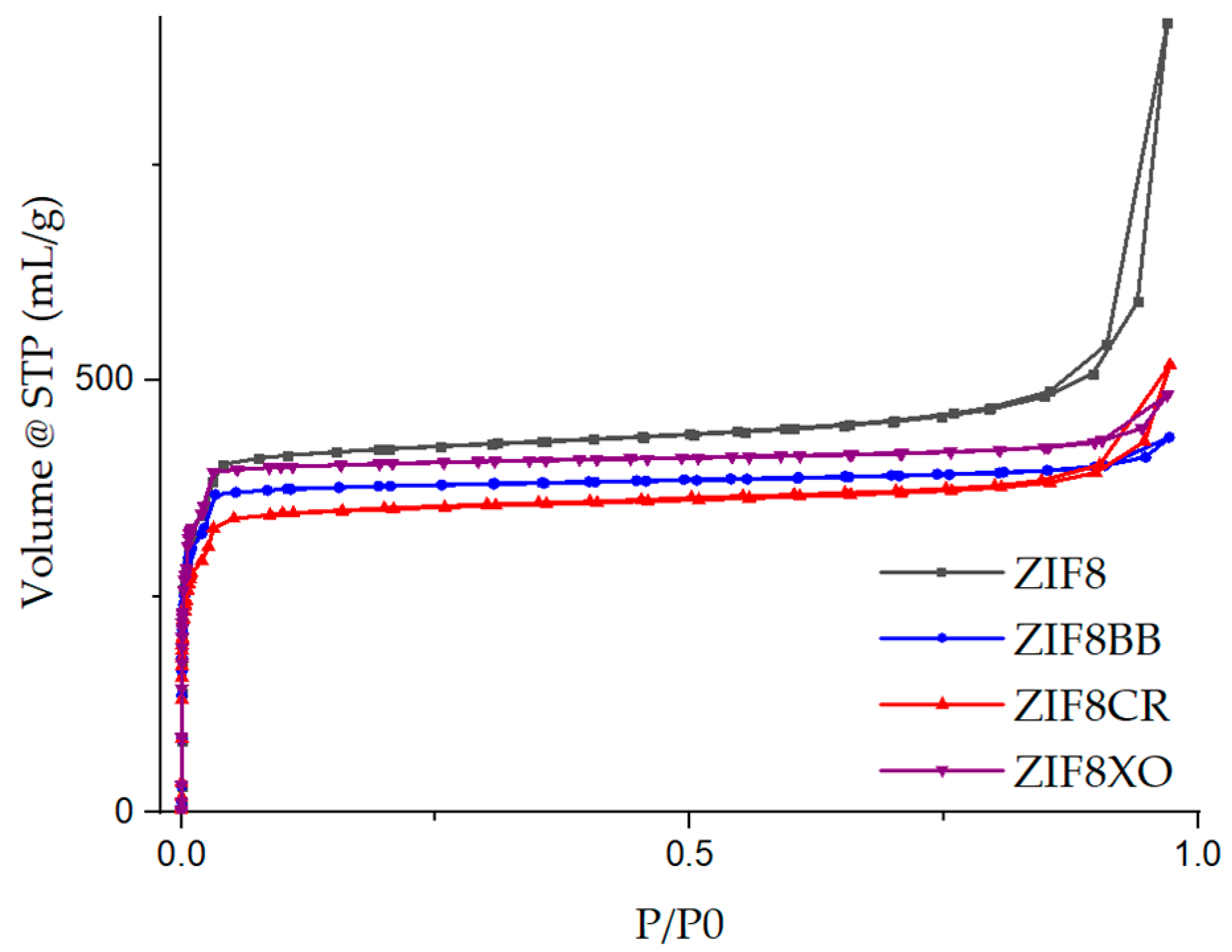
2.1. Synthesis and Characterization
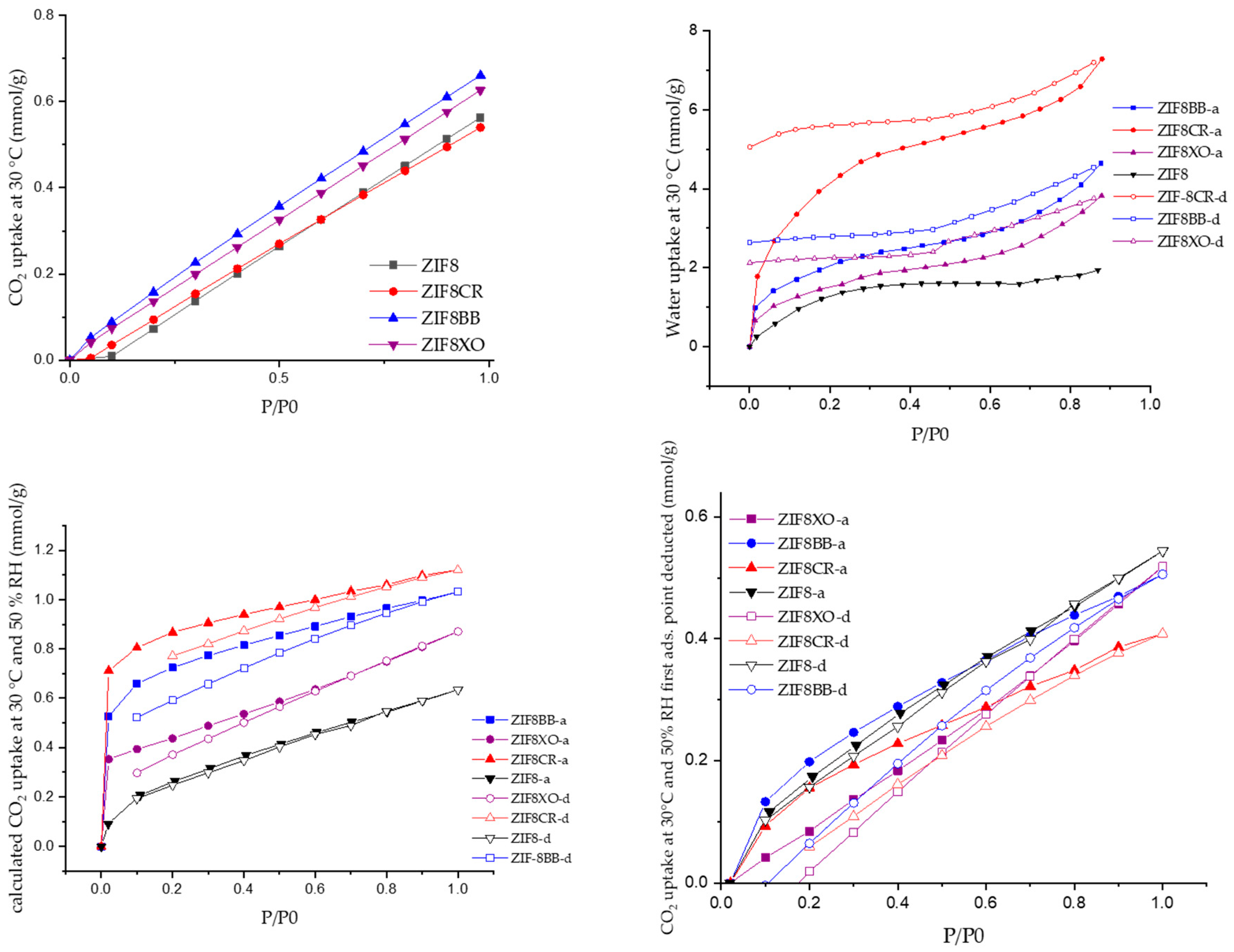
2.2. Sorption Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. ZIF-8 and ZIF-8 OD Synthesis
4.3. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; Okeeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.; Benzaqui, M.; Gkaniatsou, E.; Orsi, A.; Lozinska, M.M.; Sicard, C.; Johnson, T.; Steunou, N.; Wright, P.A.; et al. Influence of Filler Pore Structure and Polymer on the Performance of MOF-Based Mixed-Matrix Membranes for CO2 Capture. Chem. A Eur. J. 2018, 24, 7949–7956. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Yang, R.F.; Zhang, W.Y.; Yan, Y.T.; Gou, X.J.; Huang, W.H.; Wang, Y.Y. Zeolitic Metal Cluster Carboxylic Framework for Selective Carbon Dioxide Chemical Fixation through the Superlarge Cage. ACS Appl. Mater. Interfaces 2020, 59, 3912–3918. [Google Scholar] [CrossRef]
- Schweinefuß, M.E.; Springer, S.; Baburin, I.A.; Hikov, T.; Huber, K.; Leoni, S.; Wiebcke, M. Zeolitic imidazolate framework-71 nanocrystals and a novel SOD-type polymorph: Solution mediated phase transformations, phase selection via coordination modulation and a density functional theory derived energy landscape. Dalt. Trans. 2014, 43, 3528–3536. [Google Scholar] [CrossRef] [PubMed]
- Škrjanc, A.; Byrne, C.; Zabukovec Logar, N. Green Solvents as an Alternative to DMF in ZIF-90 Synthesis. Molecules 2021, 26, 1573. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Leung, B.; Furukawa, H.; Yaghi, O.K.; He, N.; Hayashi, H.; Houndonougbo, Y.; Asta, M.; Laird, B.B.; Yaghi, O.M. A Combined Experimental–Computational Investigation of Carbon Dioxide Capture in a Series of Isoreticular Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2010, 132, 11006–11008. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, J.; Rong, Z.; Shi, Q.; Dong, J. A combined experimental-computational investigation on water adsorption in various ZIFs with the SOD and RHO topologies. RSC Adv. 2018, 8, 39627–39634. [Google Scholar] [CrossRef]
- Gao, W.; Wang, S.; Zheng, W.; Sun, W.; Zhao, L. Computational evaluation of RHO-ZIFs for CO2 capture: From adsorption mechanism to swing adsorption separation. Sep. Purif. Technol. 2023, 313, 123469. [Google Scholar] [CrossRef]
- Švegovec, M.; Škrjanc, A.; Krajnc, A.; Logar, N.Z. Green Synthesis Approaches toward Preparation of ZIF-76 and Its Thermal Behavior. Cryst. Growth Des. 2023, 23, 3754–3760. [Google Scholar] [CrossRef]
- Zhou, C.; Longley, L.; Krajnc, A.; Smales, G.J.; Qiao, A.; Erucar, I.; Doherty, C.M.; Thornton, A.W.; Hill, A.J.; Ashling, C.W.; et al. Metal-organic framework glasses with permanent accessible porosity. Nat. Commun. 2018, 9, 5042. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qiao, A.; Bumstead, A.M.; Bennett, T.D.; Yue, Y.; Tao, H. Impact of 1-Methylimidazole on Crystal Formation, Phase Transitions, and Glass Formation in a Zeolitic Imidazolate Framework. Cryst. Growth Des. 2020, 20, 6528–6534. [Google Scholar] [CrossRef]
- Li, R.; Ren, X.; Ma, H.; Feng, X.; Lin, Z.; Li, X.; Hu, C.; Wang, B. Nickel-substituted zeolitic imidazolate frameworks for time-resolved alcohol sensing and photocatalysis under visible light. J. Mater. Chem. A 2014, 2, 5724–5729. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Ding, F.; Song, C.; Zhang, G.; Guo, X. Hydrothermally stable MOFs for CO2 hydrogenation over iron-based catalyst to light olefins. J. CO2 Util. 2016, 15, 89–95. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Li, M.; Li, J.; Zhuang, W.; Yang, X.; Wu, S.; Zhang, J. Construction of Single Ni Atom-Immobilized ZIF-8 with Ordered Hierarchical Pore Structures for Selective CO2 Photoreduction. ACS Catal. 2023, 4, 6630–6640. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Gugin, N.; Jose Villajos, A.; Feldmann, I.; Emmerling, F. Mix and wait—A relaxed way for synthesizing ZIF-8. RSC Adv. 2022, 12, 8940–8944. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Li, M.; Si, R. Adsorption mechanism on metal organic frameworks of Cu-BTC, Fe-BTC and ZIF-8 for CO2 capture investigated by X-ray absorption fine structure. RSC Adv. 2016, 6, 62705–62716. [Google Scholar] [CrossRef]
- Lee, Y.R.; Jang, M.S.; Cho, H.Y.; Kwon, H.J.; Kim, S.; Ahn, W.S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Hobday, C.L.; Woodall, C.H.; Lennox, M.J.; Frost, M.; Kamenev, K.; Düren, T.; Morrison, C.A.; Moggach, S.A. Understanding the adsorption process in ZIF-8 using high pressure crystallography and computational modelling. Nat. Commun. 2018, 9, 1429. [Google Scholar] [CrossRef]
- Moggach, S.A.; Bennett, T.D.; Cheetham, A.K. The effect of pressure on ZIF-8: Increasing pore size with pressure and the formation of a high-pressure phase at 1.47 GPa. Angew. Chem. Int. Ed. 2009, 48, 7087–7089. [Google Scholar] [CrossRef]
- Bose, R.; Ethiraj, J.; Sridhar, P.; Varghese, J.J.; Kaisare, N.S.; Selvam, P. Adsorption of hydrogen and carbon dioxide in zeolitic imidazolate framework structure with SOD topology: Experimental and modelling studies. Adsorption 2020, 26, 1027–1038. [Google Scholar] [CrossRef]
- Mohajer, F.; Niknam Shahrak, M. Simulation study on CO2 diffusion and adsorption in zeolitic imidazolate framework-8 and -90: Influence of different functional groups. Heat Mass Transf. 2019, 55, 2017–2023. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Y.; Xia, Q.; Li, Z.; Xi, H. Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 2013, 19, 25–37. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Removal of carbon dioxide using zeolitic imidazolate frameworks: Adsorption and conversion via catalysis. Appl. Organomet. Chem. 2022, 36, e6753. [Google Scholar] [CrossRef]
- Tsai, C.W.; Niemantsverdriet, J.W.; Langner, E.H.G. Enhanced CO2 adsorption in nano-ZIF-8 modified by solvent assisted ligand exchange. Microporous Mesoporous Mater. 2018, 262, 98–105. [Google Scholar] [CrossRef]
- Abraha, Y.W.; Tsai, C.-W.; Niemantsverdriet, J.W.H.; Langner, E.H.G. Optimized CO2 Capture of the Zeolitic Imidazolate Framework ZIF-8 Modified by Solvent-Assisted Ligand Exchange. ACS Omega 2021, 6, 21850–21860. [Google Scholar] [CrossRef]
- Jiang, Z.; Xue, W.; Huang, H.; Zhu, H.; Sun, Y.; Zhong, C. Mechanochemistry-assisted linker exchange of metal-organic framework for efficient kinetic separation of propene and propane. Chem. Eng. J. 2023, 454, 140093. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, W.; Yang, K.; Dai, Y.; Ruan, X.; Yan, X.; He, G. Amino-functional ZIF-8 nanocrystals by microemulsion based mixed linker strategy and the enhanced CO2/N2 separation. Sep. Purif. Technol. 2020, 236, 116209. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Dye encapsulated hierarchical porous zeolitic imidazolate frameworks for carbon dioxide adsorption. J. Environ. Chem. Eng. 2020, 8, 104008. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; He, Z.; Su, F.; Zhang, N.; Xin, Y.; Wang, H.; Tian, X.; Zheng, Y.; Yao, D.; et al. Zeolitic imidazolate frameworks-based porous liquids with low viscosity for CO2 and toluene uptakes. Chem. Eng. J. 2021, 417, 129239. [Google Scholar] [CrossRef]
- Philip, F.A.; Henni, A. Enhancement of post-combustion CO2 capture capacity by incorporation of task-specific ionic liquid into ZIF-8. Microporous Mesoporous Mater. 2021, 330, 111580. [Google Scholar] [CrossRef]
- Li, X.; Chen, K.; Guo, R.; Wei, Z. Ionic Liquids Functionalized MOFs for Adsorption. Chem. Rev. 2023, 123, 10432–10467. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Guo, C.; Chen, T.; Feng, C.; Qiao, S.; Wang, J. Construction of defective Zeolitic Imidazolate Frameworks with improved photocatalytic performance via Vanillin as modulator. Chem. Eng. J. 2021, 421, 127839. [Google Scholar] [CrossRef]
- Massahud, E.; Ahmed, H.; Babarao, R.; Ehrnst, Y.; Alijani, H.; Darmanin, C.; Murdoch, B.J.; Rezk, A.R.; Yeo, L.Y. Acoustomicrofluidic Defect Engineering and Ligand Exchange in ZIF-8 Metal–Organic Frameworks. Small Methods 2023, 28, 2201170. [Google Scholar] [CrossRef]
- Lai, W.H.; Zhuang, G.L.; Tseng, H.H.; Wey, M.Y. Creation of tiny defects in ZIF-8 by thermal annealing to improve the CO2/N2 separation of mixed matrix membranes. J. Memb. Sci. 2019, 572, 410–418. [Google Scholar] [CrossRef]
- Kolle, J.M.; Fayaz, M.; Sayari, A. Understanding the Effect of Water on CO2 Adsorption. Chem. Rev. 2021, 121, 7280–7345. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C.; Lucy, C.A. Quantitative Chemical Analysis, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2010; ISBN 1-4292-1815-0. [Google Scholar]
- Saeed, M.; Muneer, M.; Haq, A.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2021, 29, 293–311. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Huang, Z.; El-Zohry, A.M.; Zheng, H.; Zou, X. A Fast and Scalable Approach for Synthesis of Hierarchical Porous Zeolitic Imidazolate Frameworks and One-Pot Encapsulation of Target Molecules. Inorg. Chem. 2017, 56, 9139–9146. [Google Scholar] [CrossRef]
- Anh Tran, V.; Vu, K.B.; Thi Vo, T.T.; Thuan Le, V.; Do, H.H.; Bach, L.G.; Lee, S.W. Experimental and computational investigation on interaction mechanism of Rhodamine B adsorption and photodegradation by zeolite imidazole frameworks-8. Appl. Surf. Sci. 2021, 538, 148065. [Google Scholar] [CrossRef]
- Martínez-Zepeda, D.L.; Meza-González, B.; Álvarez-Hernández, M.L.; Bazany-Rodríguez, I.J.; Vilchis Néstor, A.R.; Cortés-Guzmán, F.; Gómez-Espinosa, R.M.; Valdes-García, J.; Dorazco-González, A. Efficient naked eye sensing of tartrate/malate based on a Zn-Xylenol orange complex in water and membrane-based test strips. Dye. Pigment. 2021, 188, 109239. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, T. Determination of equilibrium structures of bromothymol blue revealed by using quantum chemistry with an aid of multivariate analysis of electronic absorption spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 104–110. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, P.; Wang, Y.; Yang, J.; Li, J.; Li, L. CO2 Capture from High-Humidity Flue Gas Using a Stable Metal–Organic Framework. Molecules 2022, 27, 5608. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, X.; Liang, X.; Wu, L.; Zhang, F.; Shi, Q.; Yang, J.; Dong, J.; Li, J. Superhydrophobic zeolitic imidazolate framework with suitable SOD cage for effective CH4/N2 adsorptive separation in humid environments. AIChE J. 2022, 68, e17589. [Google Scholar] [CrossRef]
- Wee, L.H.; Vandenbrande, S.; Rogge, S.M.J.; Wieme, J.; Asselman, K.; Jardim, E.O.; Silvestre-Albero, J.; Navarro, J.A.R.; Van Speybroeck, V.; Martens, J.A.; et al. Chlorination of a Zeolitic-Imidazolate Framework Tunes Packing and van der Waals Interaction of Carbon Dioxide for Optimized Adsorptive Separation. J. Am. Chem. Soc. 2021, 143, 4962–4968. [Google Scholar] [CrossRef]

| N2 Physisorption | Gas/Vapour Uptake | |||||
|---|---|---|---|---|---|---|
| SBET [m2/g] | Vmicro [mL/g] | Vtotal [mL/g] | CO2 [mmol/g] | H2O [mmol/g(wt%)] | Wet CO2 c [mmol/g] | |
| ZIF-8 | 1566 | 0.59 | 1.41 | 0.56 | 1.9 (3.4) | 0.54 |
| ZIF-8BB | 1457 | 0.56 | 0.67 | 0.66 | 4.6 (8.4) | 0.51 |
| ZIF-8CR | 1314 | 0.50 | 0.80 | 0.54 | 7.3 (13.1) | 0.41 |
| ZIF-8XO | 1560 | 0.59 | 0.75 | 0.62 | 3.8 (6.9) | 0.52 |
| ZIF-8RB a | 1000 | 0.32 | 0.62 | 0.79 b | / | / |
| ZIF-8MB a | 400 | 0.12 | 0.27 | 0.41 b | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrjanc, A.; Opresnik, M.; Gabrijelčič, M.; Šuligoj, A.; Mali, G.; Zabukovec Logar, N. Impact of Dye Encapsulation in ZIF-8 on CO2, Water, and Wet CO2 Sorption. Molecules 2023, 28, 7056. https://doi.org/10.3390/molecules28207056
Škrjanc A, Opresnik M, Gabrijelčič M, Šuligoj A, Mali G, Zabukovec Logar N. Impact of Dye Encapsulation in ZIF-8 on CO2, Water, and Wet CO2 Sorption. Molecules. 2023; 28(20):7056. https://doi.org/10.3390/molecules28207056
Chicago/Turabian StyleŠkrjanc, Aljaž, Mojca Opresnik, Matej Gabrijelčič, Andraž Šuligoj, Gregor Mali, and Nataša Zabukovec Logar. 2023. "Impact of Dye Encapsulation in ZIF-8 on CO2, Water, and Wet CO2 Sorption" Molecules 28, no. 20: 7056. https://doi.org/10.3390/molecules28207056
APA StyleŠkrjanc, A., Opresnik, M., Gabrijelčič, M., Šuligoj, A., Mali, G., & Zabukovec Logar, N. (2023). Impact of Dye Encapsulation in ZIF-8 on CO2, Water, and Wet CO2 Sorption. Molecules, 28(20), 7056. https://doi.org/10.3390/molecules28207056








