The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca
Abstract
1. Introduction
2. Results
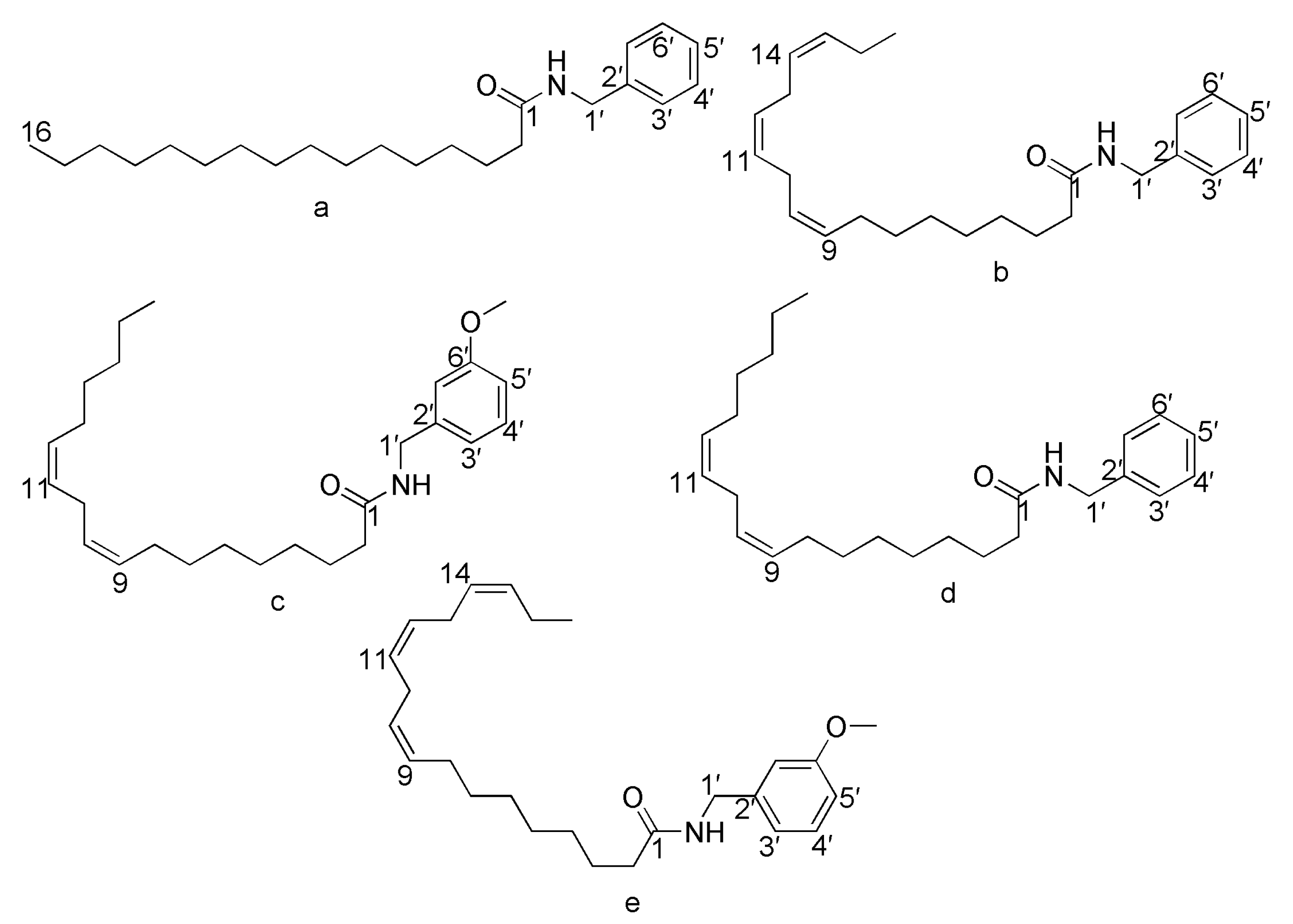
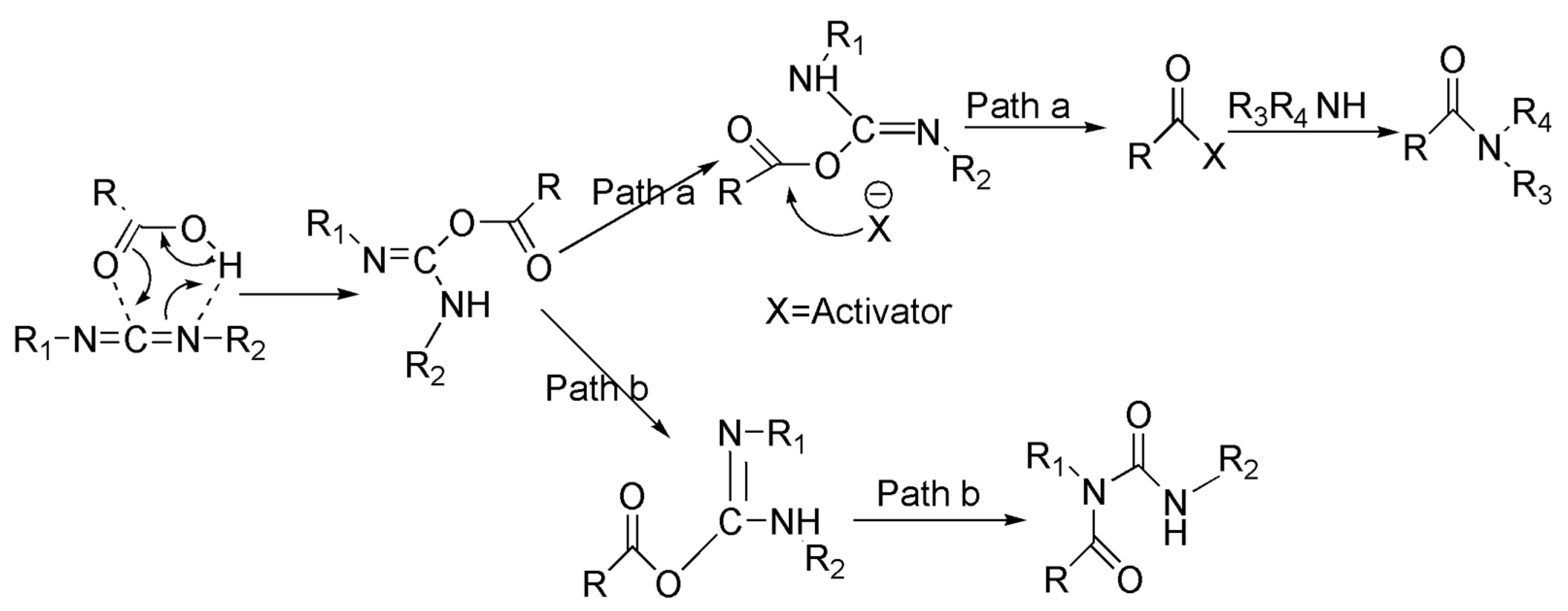
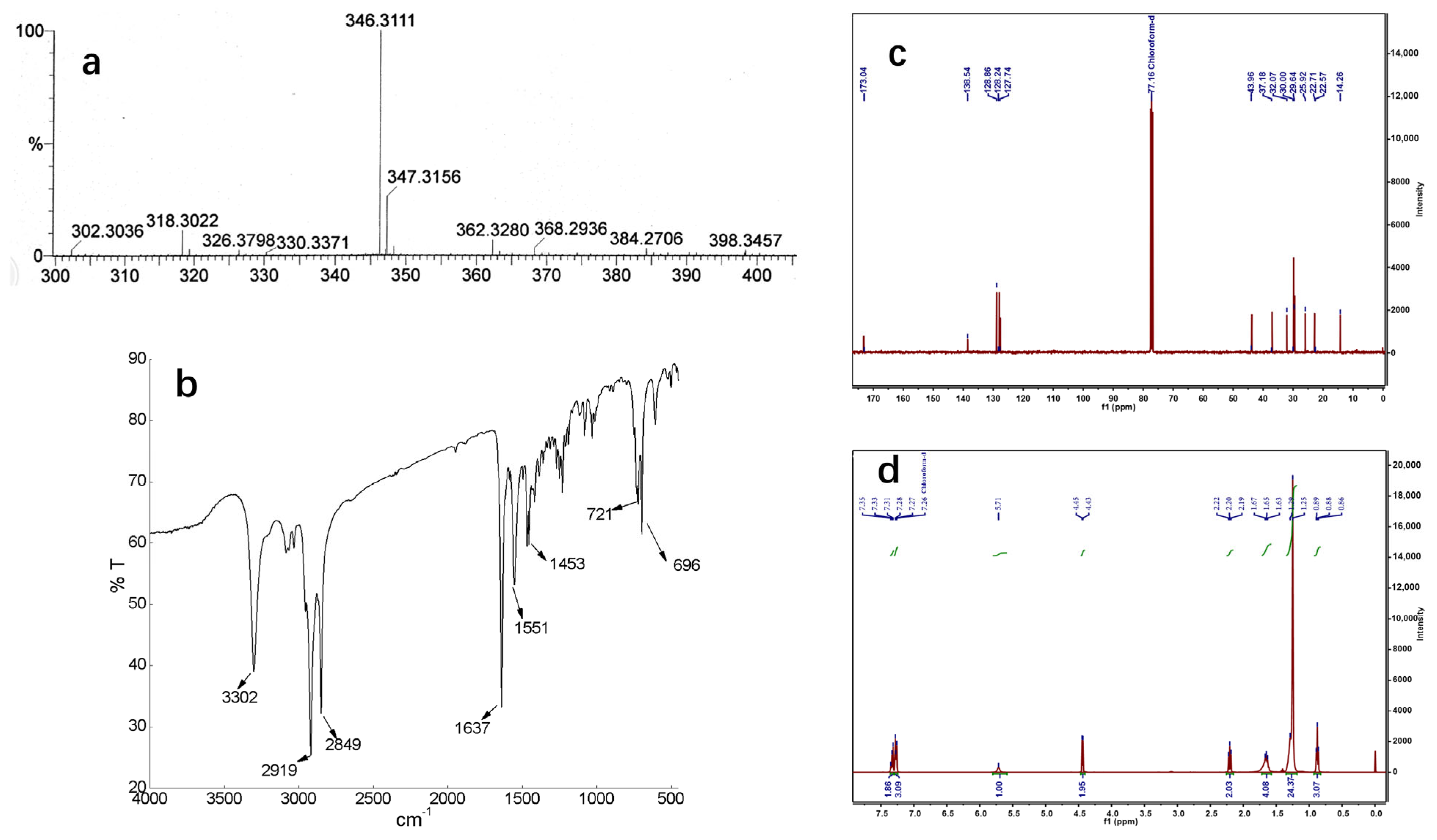
2.1. Synthesis, Purification and Identification of Macamides
2.2. Anti-Fatigue Activity of NBH
2.2.1. Forced Swimming Test
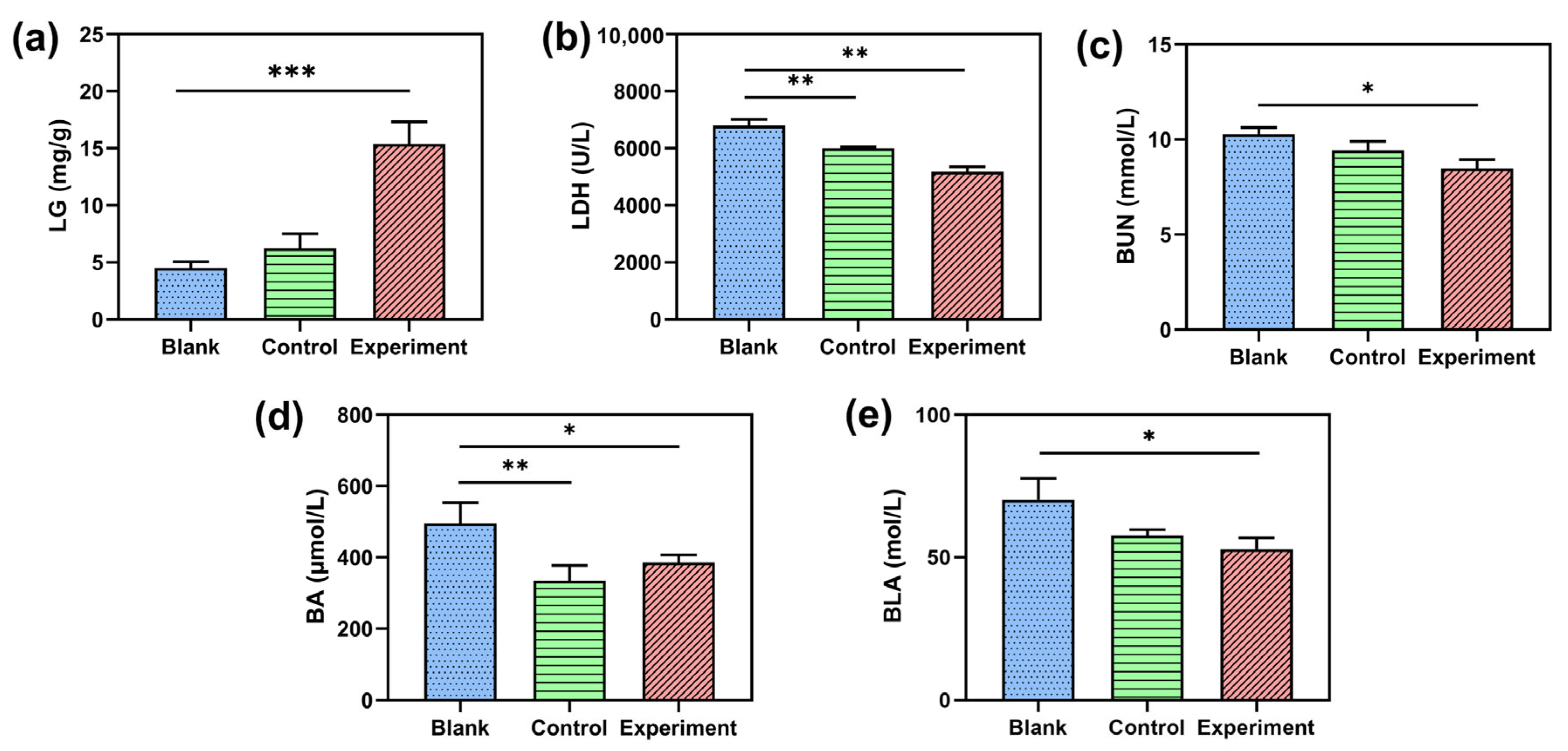
2.2.2. Effect of NBH on Biochemical Paraments Related to Fatigue
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Instrumental and Chromatographic Conditions
4.3. The synthesis of Five Main Macamides
4.3.1. Preparation of NBH (1)
4.3.2. Preparation of N-Benzyl-9Z,12Z,15Z-octadecatrienamide (2)
4.3.3. Preparation of N-(3-Methoxybenzyl)-9Z,12Z-octadecadienamide (3)
4.3.4. Preparation of N-Benzyl-9Z,12Z-octadecadienamide (4)
4.3.5. Preparation of N-(3-Methoxybenzyl)-9Z,12Z,15Z-octadecatrienamide (5)
4.4. The Preparation of Macamides-Rich Extracts
4.5. Anti-Fatigue Activity of NBH
4.5.1. Animals and Groups
4.5.2. Forced Swimming Times and Biochemical Paraments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Esparza, E.; Hadzich, A.; Kofer, W.; Mithöfer, A.; Cosio, E.G. Bioactive maca (Lepidium meyenii) alkamides are a result of traditional Andean postharvest drying practices. Phytochemistry 2015, 116, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Vassetti, A.; Carotenuto, D.; Esposito, L.; Maruccio, L.; Avallone, L.; Ciani, F. Lepidium meyenii (Maca) in male reproduction. Nat. Prod. Res. 2021, 35, 4550–4559. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Cheng, A.F.; Yu, Y.T.; Yu, L.J.; Jin, W. Antidepressant-Like Behavioral, Anatomical, and Biochemical Effects of Petroleum Ether Extract from Maca (Lepidium meyenii) in Mice Exposed to Chronic Unpredictable Mild Stress. J. Med. Food 2014, 17, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, M.; Böhlke, M.; Kelley, C.; Maher, T.; Pino-Figueroa, A. Inhibition of Fatty Acid Amide Hydrolase (FAAH) by Macamides. Mol. Neurobiol. 2019, 56, 1770–1781. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Chang, Y.H. Physicochemical and antioxidant properties of methanol extract from Maca (Lepidium meyenii Walp.) leaves and roots. Food Sci. Technol. 2019, 39, 278–286. [Google Scholar] [CrossRef]
- Li, A.; Liu, J.; Ding, F.; Wu, X.; Pan, C.; Wang, Q.; Gao, M.; Duan, S.; Han, X.; Xia, K.; et al. Maca extracts regulate glucose and lipid metabolism in insulin-resistant HepG2 cells via the PI3K/AKT signalling pathway. Food Sci. Nutr. 2021, 9, 2894–2907. [Google Scholar] [CrossRef]
- Fei, W.; Hou, Y.; Yue, N.; Zhou, X.; Wang, Y.; Wang, L.; Li, A.; Zhang, J. The effects of aqueous extract of Maca on energy metabolism and immunoregulation. Eur. J. Med. Res. 2020, 25, 24. [Google Scholar] [CrossRef]
- Yang, Q.; Jin, W.; Lv, X.; Dai, P.; Ao, Y.; Wu, M.; Deng, W.; Yu, L. Effects of macamides on endurance capacity and anti-fatigue property in prolonged swimming mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Brantner, A.; Wang, H.; Shu, X.; Yang, J.; Si, N.; Han, L.; Zhao, H.; Bian, B. Chemical profiling analysis of Maca using UHPLC-ESI-Orbitrap MS coupled with UHPLC-ESI-QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 7, 44660. [Google Scholar] [CrossRef]
- Jiao, M.; Dong, Q.; Zhang, Y.; Lin, M.; Zhou, W.; Liu, T.; Yuan, B.; Yin, H. Neuroprotection of N-benzyl Eicosapentaenamide in Neonatal Mice Following Hypoxic-Ischemic Brain Injury. Molecules 2021, 26, 3108. [Google Scholar] [CrossRef] [PubMed]
- Zha, R.; Ge, E.; Guo, L.; Gao, Q.; Lin, Q.; Zhou, W.; Jin, X.; Xie, W.; Yin, H.; Liu, T. A newly identified polyunsaturated macamide alleviates dextran sulfate sodium-induced colitis in mice. Fitoterapia 2021, 152, 104916. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gao, Q.; Zhu, J.; Jin, X.; Yin, H.; Liu, T. A Docosahexaenoic Acid Derivative (N-Benzyl Docosahexaenamide) as a Potential Therapeutic Candidate for Treatment of Ovarian Injury in the Mouse Model. Molecules 2022, 27, 2754. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, H.; Li, D.; Chen, X.; Ao, M.; Jin, W.; Yu, L. N-(3-Methozybenzyl)-(9Z,12Z,15Z)-octadecatrienamide from maca (Lepidium meyenii Walp.) ameliorates corticosterone-induced testicular toxicity in rats. Food Funct. 2020, 11, 7762–7774. [Google Scholar] [CrossRef]
- Lamou, B.; Taiwe, G.S.; Hamadou, A.; Abene; Houlray, J.; Atour, M.M.; Tan, P.V. Antioxidant and Antifatigue Properties of the Aqueous Extract of Moringa oleifera in Rats Subjected to Forced Swimming Endurance Test. Oxid. Med. Cell Longev. 2016, 2016, 3517824. [Google Scholar] [CrossRef]
- Wu, H.; Kelley, C.J.; Pino-Figueroa, A.; Vu, H.D.; Maher, T.J. Macamides and their synthetic analogs: Evaluation of in vitro FAAH inhibition. Bioorgan. Med. Chem. 2013, 21, 5188–5197. [Google Scholar] [CrossRef]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and antitumoral activities of isolated macamide and macaene fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef]
- Armario, A. The forced swim test: Historical, conceptual and methodological considerations and its relationship with individual behavioral traits. Neurosci. Biobehav. Rev. 2021, 128, 74–86. [Google Scholar] [CrossRef]
- Zhao, H.P.; Zhang, Y.; Liu, Z.; Chen, J.Y.; Zhang, S.Y.; Yang, X.D.; Zhou, H.L. Acute toxicity and anti-fatigue activity of polysaccharide-rich extract from corn silk. Biomed. Pharmacother. 2017, 90, 686–693. [Google Scholar] [CrossRef]
- Tan, W.; Yu, K.Q.; Liu, Y.Y.; Ouyang, M.Z.; Yan, M.H.; Luo, R.; Zhao, X.S. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int. J. Biol. Macromol. 2012, 50, 59–62. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; Hua, H.; Liu, C.; Cheng, Y.; Guo, Y.; Du, P.; Qian, H. Anti-fatigue activity of Brassica rapa L. extract and correlation among biochemical changes in forced swimming mice. Food Biosci. 2022, 47, 101633. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Meng, Q.; Wang, L.; Xiong, W.; Zhang, L. Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp. (maca). Int. J. Biol. Macromol. 2017, 95, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. The macamide relieves fatigue by acting as inhibitor of inflammatory response in exercising mice: From central to peripheral. Eur. J. Pharmacol. 2022, 917, 174758. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Z.; Huang, B.K.; Liang, J.; Zheng, C.J.; Han, T.; Zhang, Q.Y.; Qin, L.P. Antifatigue activity of the liposoluble fraction from Acanthopanax senticosus. Phytother. Res. 2011, 25, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Williams, A.S.; Wasserman, D.H. Exercise and the Regulation of Hepatic Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 203–225. [Google Scholar] [CrossRef]
- Zhao, X.N.; Liang, J.L.; Chen, H.B.; Liang, Y.E.; Guo, H.Z.; Su, Z.R.; Li, Y.C.; Zeng, H.F.; Zhang, X.J. Anti-Fatigue and Antioxidant Activity of the Polysaccharides Isolated from Millettiae speciosae Champ. Leguminosae. Nutrients 2015, 7, 8657–8669. [Google Scholar] [CrossRef]
- Gbadebo, O.; Fox, K.; Sutton, G.; Murphy, P.V.; Smith, D.; O’Leary, P. Novel versatile synthesis method for amides, carbamates and ureas employing a Grignard base, an amine and an ester. Results Chem. 2022, 4, 100253. [Google Scholar] [CrossRef]
- Leggio, A.; Bagalà, J.; Belsito, E.L.; Comandè, A.; Greco, M.; Liguori, A. Formation of amides: One-pot condensation of carboxylic acids and amines mediated by TiCl4. Chem. Cent. J. 2017, 11, 87. [Google Scholar] [CrossRef]
- Zhao, J.; Muhammad, I.; Dunbar, D.C.; Mustafa, J.; Khan, I.A. New Alkamides from Maca (Lepidium meyenii). J. Agric. Food Chem. 2005, 53, 690. [Google Scholar] [CrossRef]
- McCollom, M.M.; Villinski, J.R.; McPhail, K.L.; Craker, L.E.; Gafner, S. Analysis of macamides in samples of Maca (Lepidium meyenii) by HPLC-UV-MS/MS. Phytochem. Anal. 2005, 16, 463–469. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, P.; Zhang, Z.J.; Wang, B.; Zhang, Z.F. A practical model for efficient anti-fatigue design and selection of metallic materials: II. Parameter analysis and fatigue strength improvement. J. Mater. Sci. Technol. 2021, 70, 250–267. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, Y.H.; Kim, Y.R.; Lee, B.-R.; Shin, S.; Kim, J.Y.; Jung, I.C.; Lee, M.Y. Anti-fatigue potential of Pinus koraiensis leaf extract in an acute exercise-treated mouse model. Biomed. Pharmacother. 2022, 153, 113501. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhu, H.; Xu, L.; Wang, J.; Xu, J.; Li, Z.; Yang, K.; Wu, J.; Sun, P. Structure, anti-fatigue activity and regulation on gut microflora in vivo of ethanol-fractional polysaccharides from Dendrobium officinale. Int. J. Biol. Macromol. 2023, 234, 123572. [Google Scholar] [CrossRef]
- Chen, J.; Lu, X.; Chen, P.; Shen, Y.; Zheng, B.; Guo, Z. Anti-fatigue effect of glycoprotein from hairtail (Trichiurus lepturus) by-products in a behavioral mouse model. Food Chem. X 2023, 18, 100645. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Wu, D.; Lichtenstein, A.H.; Tai, A.K.; Matthan, N.R.; Maddipati, K.R.; Lamon-Fava, S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis 2021, 316, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Shi, H.-H.; Zhang, L.-Y.; Ding, L.; Xue, C.-H.; Yanagita, T.; Zhang, T.-T.; Wang, Y.-M. The rapid effects of eicosapentaenoic acid (EPA) enriched phospholipids on alleviating exercise fatigue in mice. RSC Adv. 2019, 9, 33863–33871. [Google Scholar] [CrossRef]
- Lin, L.; Huang, J.; Sun-Waterhouse, D.; Zhao, M.; Zhao, K.; Que, J. Maca (Lepidium meyenii) as a source of macamides and polysaccharide in combating of oxidative stress and damage in human erythrocytes. Int. J. Food Sci. Technol. 2018, 53, 304–312. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, B.; Hua, H.; Liu, C.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Macamides: A review of structures, isolation, therapeutics and prospects. Food Res. Int. 2020, 138, 109819. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, F. Evaluation of silymarin extract from Silybum marianum in mice: Anti-fatigue activity. Food Sci. Hum. Well 2022, 11, 914–921. [Google Scholar] [CrossRef]
- Li, W.; Luo, C.; Huang, Y.; Zhan, J.; Lei, J.; Li, N.; Huang, X.; Luo, H. Evaluation of antifatigue and antioxidant activities of the marine microalgae Isochrysis galbana in mice. Food Sci. Biotechnol. 2020, 29, 549–557. [Google Scholar] [CrossRef]
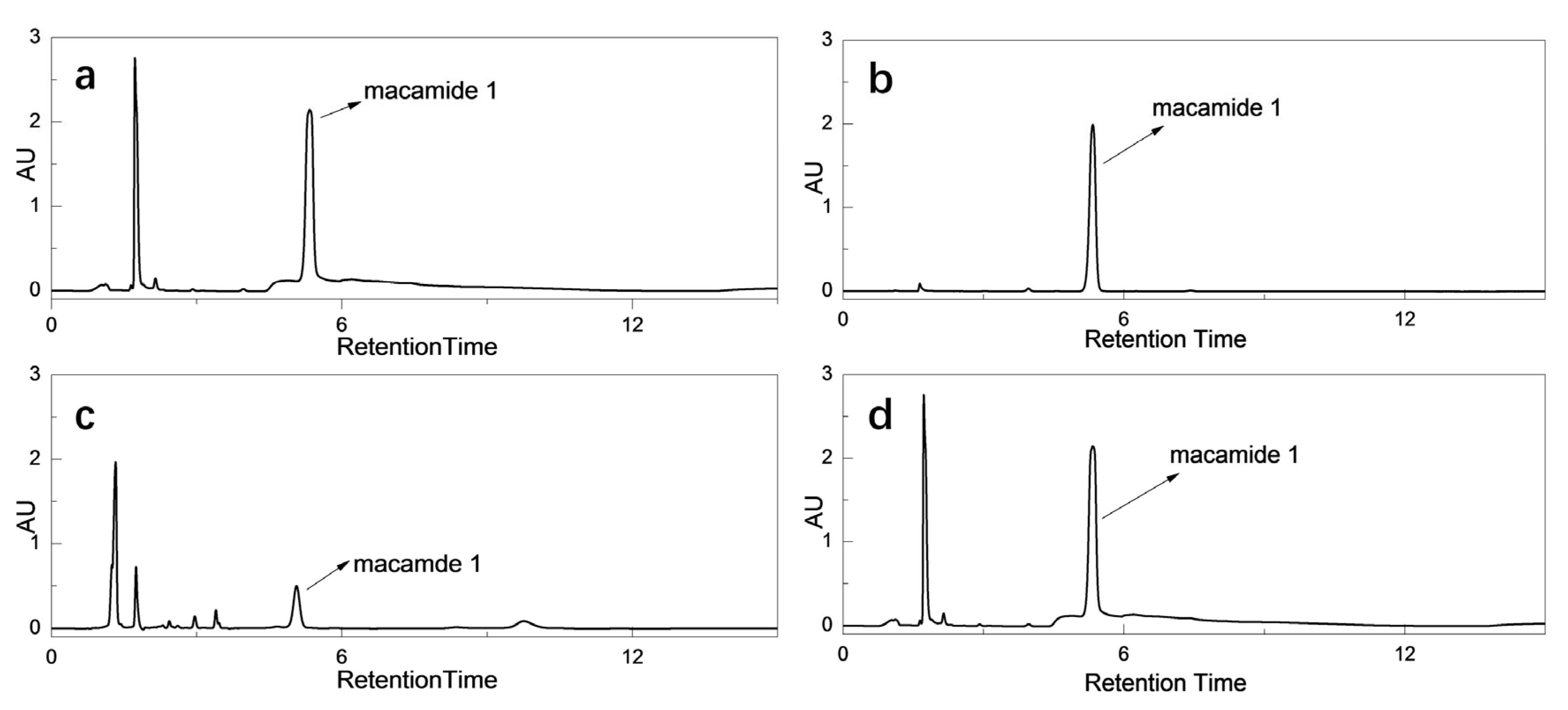


| No. | EDAC | HOBt·H2O | Triethylamine | Acid-Base Reaction | Precipitate Yield (%) | Purity (%) |
|---|---|---|---|---|---|---|
| 1 | Y * | Y | Y | Y | 39.16 | 97.63 |
| 2 | N ** | Y | Y | Y | 0 | 0 |
| 3 | Y | N | Y | Y | 0 | 0 |
| 4 | Y | Y | N | Y | 100.37 | 13.21 |
| 5 | Y | Y | Y | N | 64.25 | 61.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Peng, Z.; Lai, W.; Shao, Y.; Gao, Q.; He, M.; Zhou, W.; Guo, L.; Kang, J.; Jin, X.; et al. The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca. Molecules 2023, 28, 3943. https://doi.org/10.3390/molecules28093943
Liu T, Peng Z, Lai W, Shao Y, Gao Q, He M, Zhou W, Guo L, Kang J, Jin X, et al. The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca. Molecules. 2023; 28(9):3943. https://doi.org/10.3390/molecules28093943
Chicago/Turabian StyleLiu, Tao, Ziyan Peng, Wei Lai, Yan Shao, Qing Gao, Miaoxin He, Wan Zhou, Lirong Guo, Jiyao Kang, Xiaobao Jin, and et al. 2023. "The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca" Molecules 28, no. 9: 3943. https://doi.org/10.3390/molecules28093943
APA StyleLiu, T., Peng, Z., Lai, W., Shao, Y., Gao, Q., He, M., Zhou, W., Guo, L., Kang, J., Jin, X., & Yin, H. (2023). The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca. Molecules, 28(9), 3943. https://doi.org/10.3390/molecules28093943








