The Content of Bioactive Compounds and Technological Properties of Matcha Green Tea and Its Application in the Design of Functional Beverages
Abstract
1. Introduction
2. Results
2.1. Physicochemical and Bioactive Properties of Matcha Green Tea
2.1.1. Physicochemical Properties of Matcha Green Tea
Organoleptic Evaluation of Matcha Green Tea Powders
Instrumental Measurement of the Color Parameters of Matcha Green Tea Powders
Water Activity, Water Solubility Index (WSI) and Water Holding Capacity (WHC) of Matcha Green Tea Powders
pH, Soluble Solids Content and Osmolality in 2.5% Aqueous Solutions of Matcha Green Tea
2.1.2. Bioactive Properties of Matcha Green Tea
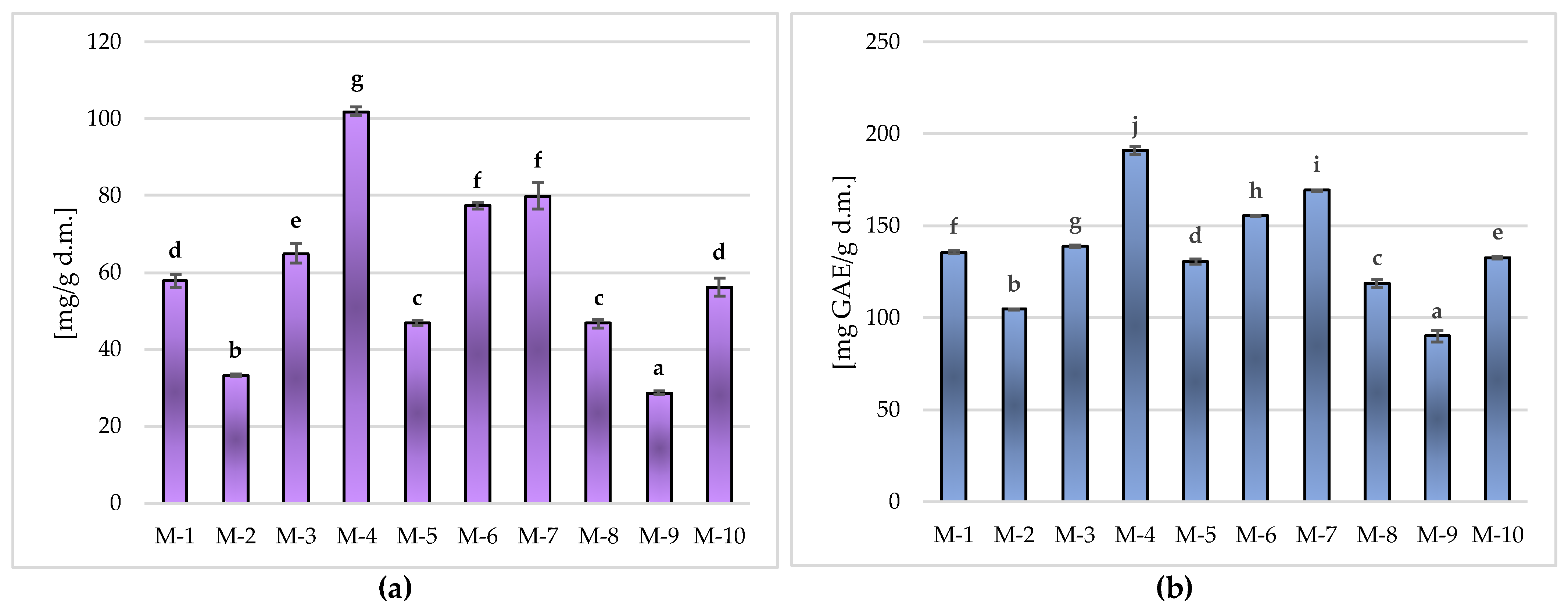
Phenolic Ingredients in Matcha Green Tea
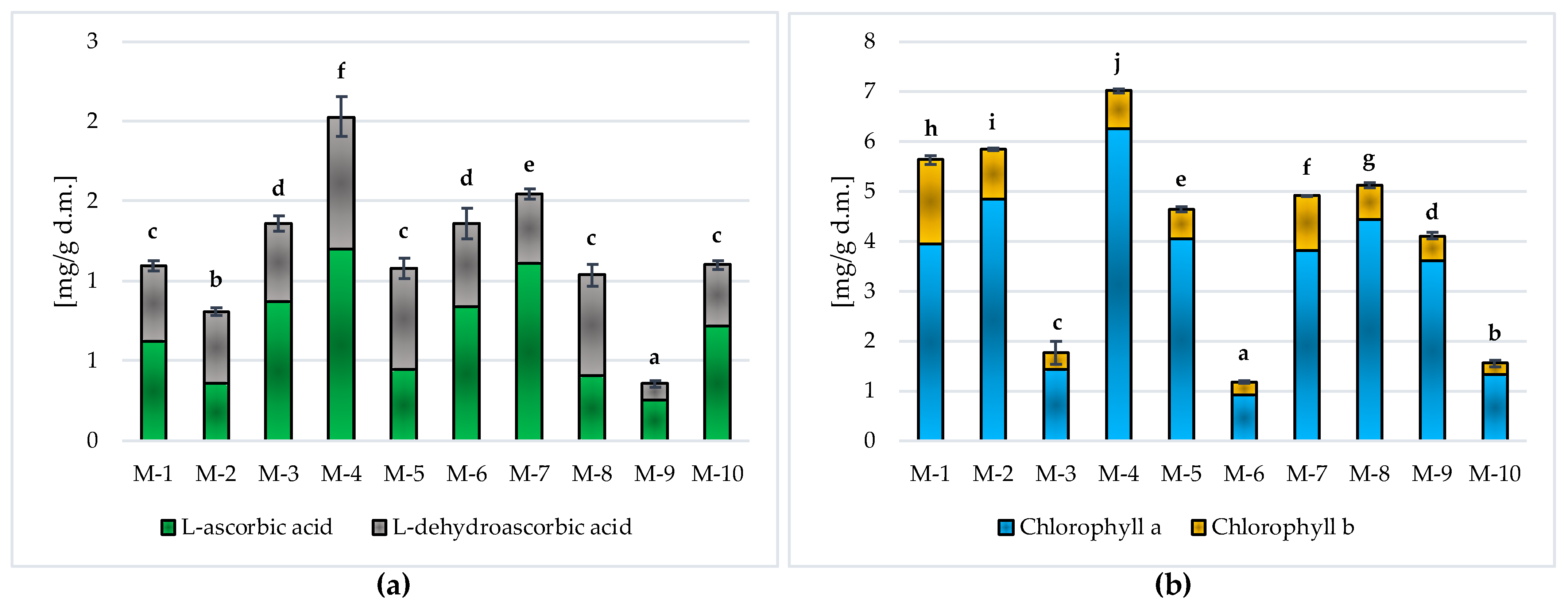
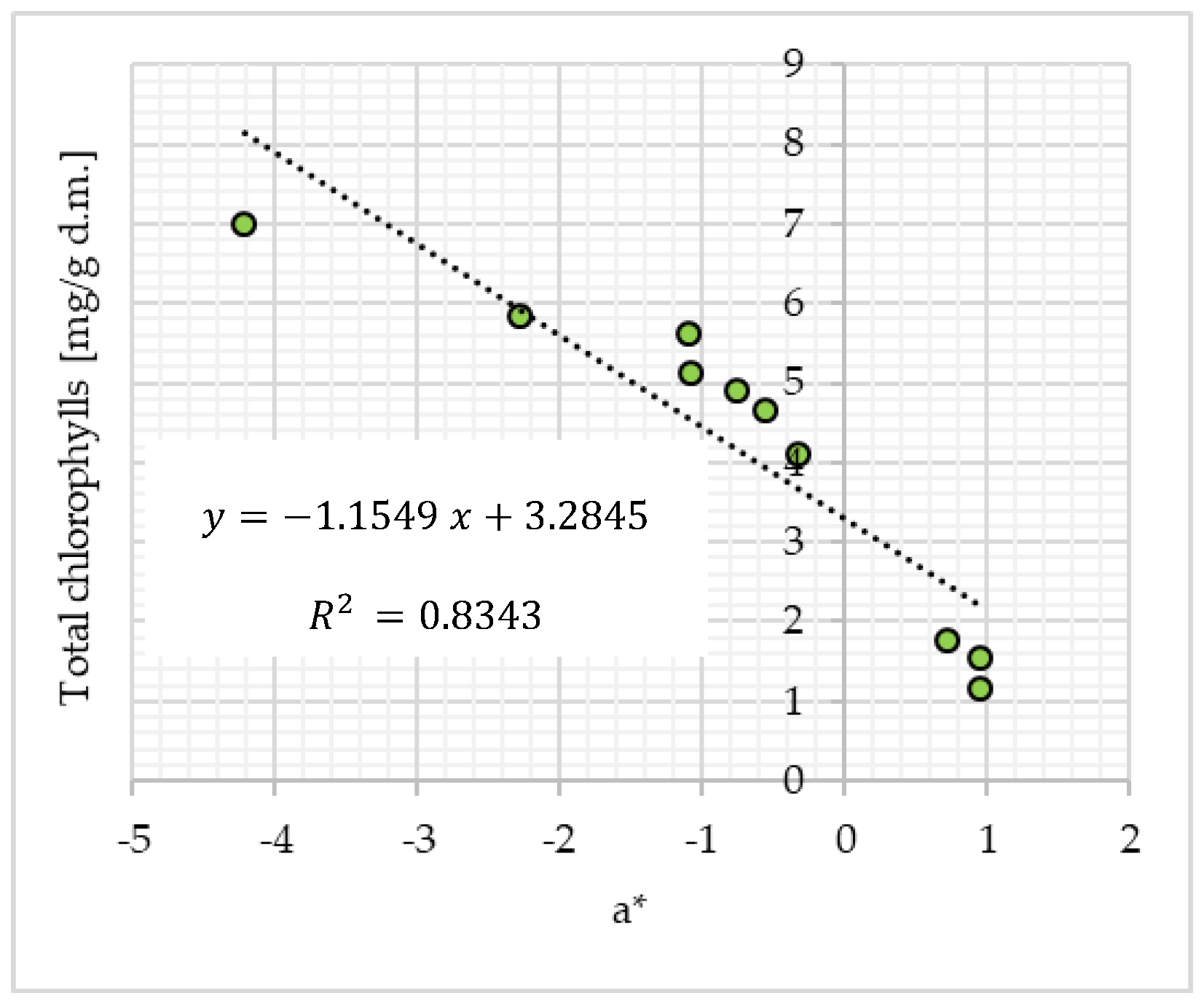
Vitamin C and Chlorophylls in Matcha Green Tea
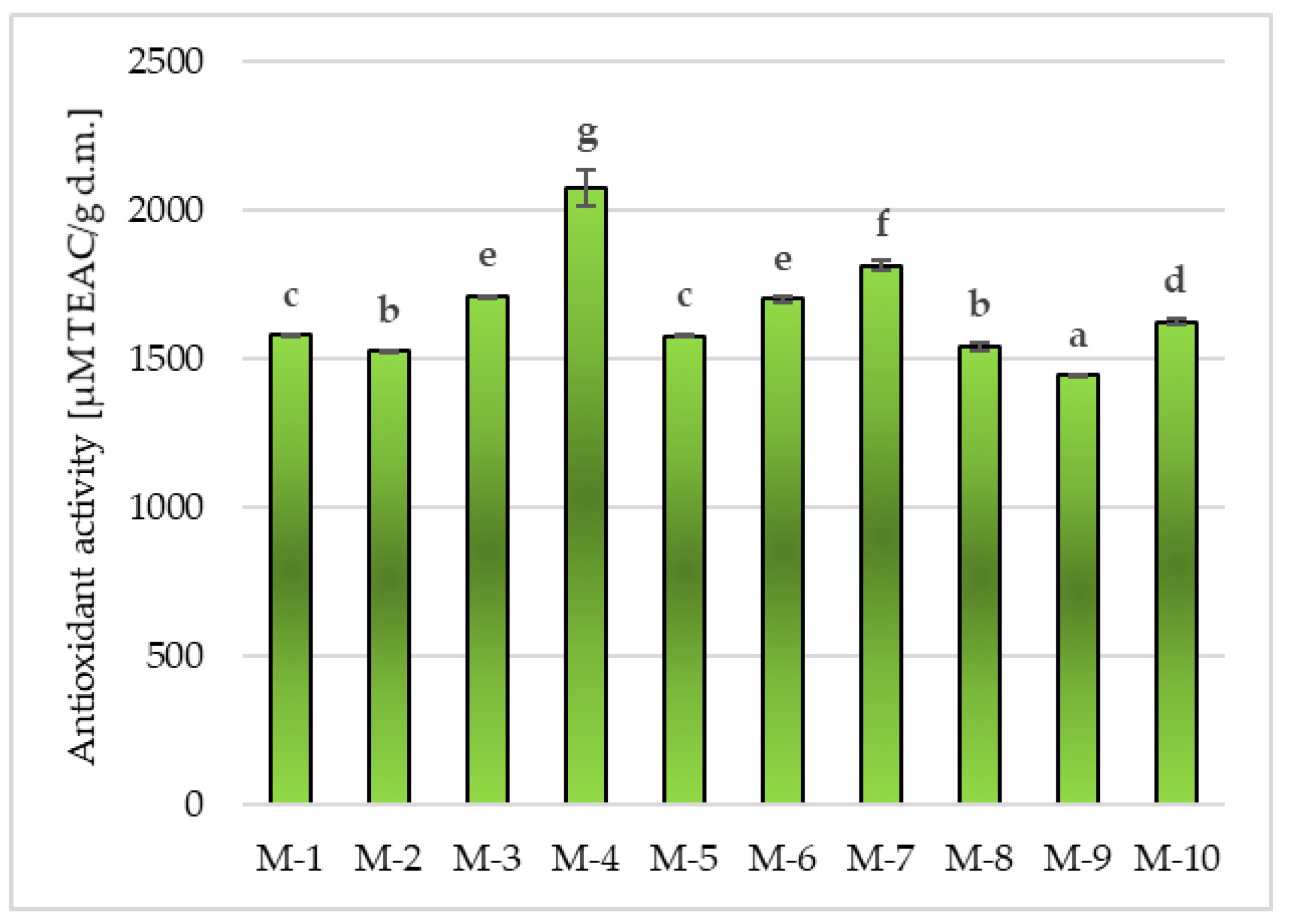
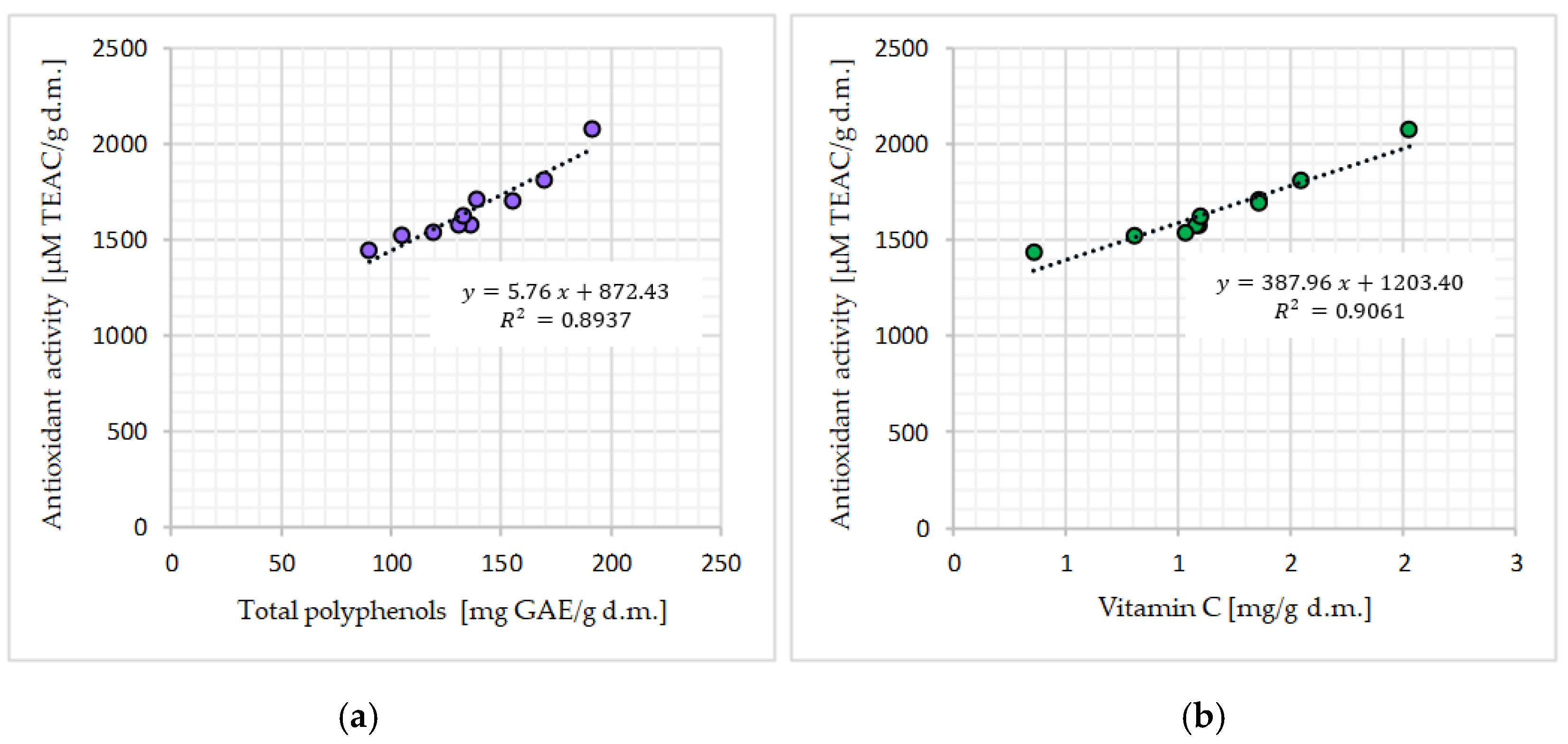
Antioxidant Activity in Matcha Green Tea
2.2. The Possibility of Using Matcha Green Tea in the Design of Functional Protein Drinks
2.2.1. Physicochemical Evaluation of Designed Functional Protein Drinks
2.2.2. Sensory Evaluation of Designed Functional Protein Drinks
2.2.3. Nutritional Value of Designed Functional Protein Drinks
3. Discussion
3.1. Physicochemical and Bioactive Properties of Matcha Green Tea
3.2. The Possibility of Using Matcha Green Tea in the Design of Functional Protein Drinks
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Instrumental Color Measurement (CIE L*a*b*) of Powders
4.2.2. Water Activity (aw) of Powders
4.2.3. Water Solubility Index (WSI) of Powders
4.2.4. Water Holding Capacity (WHC) of Powders
4.2.5. pH
4.2.6. Soluble Solids Content (°Brix)
4.2.7. Osmolality
4.2.8. Selected Phenolic Acids and Flavonoids Identified by the HPLC Method
4.2.9. Vitamin C Content
4.2.10. Chlorophylls Content
4.2.11. Preparation of Water Extracts for Total Polyphenol Content and Antioxidant Activity Assay
4.2.12. Total Polyphenol Content
4.2.13. Antioxidant Activity
4.2.14. Sensory Evaluation of Functional Matcha Drinks
4.2.15. Nutritional Value of Functional Matcha Drinks
4.2.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of Matcha green tea: A review. Molecules 2021, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Schröder, L.; Marahrens, P.; Koch, J.G.; Heidegger, H.; Vilsmeier, T.; Phan-Brehm, T.; Hofmann, S.; Mahner, S.; Jeschke, U.; Richter, D.U. Effects of green tea, matcha tea and their components epigallocatechin gallate and quercetin on MCF-7 and MDA-MB-231 breast carcinoma cells. Oncol. Rep. 2019, 41, 387–396. [Google Scholar] [CrossRef]
- Horie, H.; Kaori, E.; Sumikawa, O. Chemical components of Matcha and powdered green tea. J. Cook. Sci. Jpn. 2017, 50, 182–188. [Google Scholar] [CrossRef]
- Yüksel, K.A.; Yüksel, M.İ.; Şat, G.İ. Determination of certain physicochemical characteristics and sensory properties of green tea powder (Matcha) added ice creams and detection of their organic acid and mineral contents. GIDA—J. Food 2017, 42, 116–126. [Google Scholar] [CrossRef]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Chun, S.; Oh, J.W. Retrospecting the antioxidant activity of Japanese Matcha green tea Lack of enthusiasm? Appl. Sci. 2021, 11, 5087. [Google Scholar] [CrossRef]
- Ahmed, S.; Stepp, J. Chapter 2—Green Tea: The Plants, Processing, Manufacturing and Production. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 19–31. [Google Scholar] [CrossRef]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Horie, H.; Nakamura, Y. Stress-reducing function of Matcha green tea in animal experiments and clinical trials. Nutrients 2018, 10, 1468. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of shading intensity on morphological and color traits and on chemical components of new tea (Camellia sinensis L.) shoots under direct covering cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef]
- Queiroz, C.; Lopes, M.L.M.; Fialho, E.L.; Valente-Mesquita, V.L. Polyphenol oxidase: Characteristics and mechanisms of browning control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Chen, C.; Yang, C.Y.; Tzen, J.T.C. Molecular characterization of polyphenol oxidase between small and large leaf tea cultivars. Sci. Rep. 2022, 12, 12870. [Google Scholar] [CrossRef]
- Jyotismita, K.; Chandan, R.; Arindam, R. Determination of tannin content by titrimetric method from different types of tea. J. Chem. Pharm. Res. 2015, 7, 238–241. [Google Scholar]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.-S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałduńska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant properties and nutritional composition of Matcha green tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef]
- Tang, M.-G.; Zhang, S.; Xiong, L.-G.; Zhou, J.-H.; Huang, J.-A.; Zhao, A.-Q.; Liu, Z.-H.; Liu, A.-L. A comprehensive review of polyphenol oxidase in tea (Camellia sinensis): Physiological characteristics, oxidation manufacturing, and biosynthesis of functional constituents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2267–2291. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Juneja, L.R.; Chu, D.; Kim, M. Chemistry and Applications of Green Tea; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Farooq, S.; Sehgal, A. Antioxidant activity of different forms of green tea: Loose leaf, bagged and Matcha. Curr. Res. Nutr. Food Sci. 2018, 6, 35–40. [Google Scholar] [CrossRef]
- Koláčková, T.; Kolofiková, K.; Sytařová, I.; Snopek, L.; Sumczynski, D.; Orsavová, J. Matcha tea: Analysis of nutritional composition, phenolics and antioxidant activity. Plant Foods Hum. Nutr. 2020, 75, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J.A. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A review of the antiviral role of green tea catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Xu, P.; Ying, L.; Hong, G.; Wang, Y. The effects of the aqueous extract and residue of Matcha on the antioxidant status and lipid and glucose levels in mice fed a high-fat diet. Food Funct. 2016, 7, 294–300. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shioi, Y. Identification of chlorophylls and carotenoids in major teas by high-performance liquid chromatography with photodiode array detection. J. Agric. Food Chem. 2003, 51, 5307–5314. [Google Scholar] [CrossRef] [PubMed]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Krawczyk, M.; Zgoła-Grześkowiak, A. Determination of antioxidant activity, rutin, quercetin, phenolic acids and trace elements in tea infusions: Influence of citric acid addition on extraction of metals. J. Food Compos. Anal. 2015, 40, 70–77. [Google Scholar] [CrossRef]
- Fujioka, K.; Iwamoto, T.; Shima, H.; Tomaru, K.; Saito, H.; Ohtsuka, M.; Yodhidome, A.; Kawamura, Y.; Monome, Y. The powdering process with a set of ceramic mills for green tea promoted catechin extraction and the ROS inhibition effect. Molecules 2016, 21, 474. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ai, Z.; Qu, F.; Chen, Y.; Ni, D. Effect of steeping temperature on antioxidant and inhibitory activities of green tea extracts against α-amylase, α-glucosidase and intestinal glucose uptake. Food Chem. 2017, 234, 168–173. [Google Scholar] [CrossRef]
- Cardoso, G.A.; Salgado, J.M.; Cesar, M.C.; Pestana-Donado, C.M. The effects of green tea consumption and resistance training on body composition and resting metabolic rate in overweight or obese women. J. Med. Food 2013, 16, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Y.; Wang, Y. Matcha green tea alleviates non-alcoholic fatty liver disease in high-fat diet-induced obese mice by regulating lipid metabolism and inflammatory responses. Nutrients 2021, 13, 1950. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green tea catechins: Their use in treating and preventing infectious diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Mahmood, M.S.; Mártinez, J.L.; Aslam, A.; Rafique, A.; Vinet, R.; Laurido, C.; Hussain, I.; Abbas, R.Z.; Khan, A.; Ali, S. Antiviral effects of green tea (Camellia sinensis) against pathogenic viruses in human and animals (a Mini-Review). Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 176. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Dietz, C.; Dekker, M.; Piqueras-Fiszman, B. An intervention study on the effect of matcha tea, in drink and snack bar formats, on mood and cognitive performance. Food Res. Int. 2017, 99, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Čížková, H.; Voldřich, M.; Mlejnecká, J.; Kvasnička, F. Authenticity evaluation of tea-based products. Czech. J. Food Sci. 2008, 26, 259–267. [Google Scholar] [CrossRef]
- Topuz, A.; Dinçer, G.; Torun, M.; Tontul, İ.; Şahin-Nadeem, H.; Haznedar, A.; Ozdemir, F. Physicochemical properties of Turkish green tea powder: Effects of shooting period, shading, and clone. Turk. J. Agric. For. 2014, 38, 233–241. [Google Scholar] [CrossRef]
- Ujihara, T.; Hayashi, N.; Ikezaki, H. Objective evaluation of astringent and umami taste intensities of matcha using a taste sensor system. Food Sci. Technol. Res. 2013, 19, 1099–1105. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. The European Parliament and Council of the European Union, 30 December 2006, L 404/9-25. Off. J. Eur. Union. 2006. Available online: http://data.europa.eu/eli/reg/2006/1924/oj (accessed on 30 December 2006).
- Szwedziak, K.; Polańczyk, E.; Dąbrowska-Molenda, M.; Płuciennik, K. The impact of brewing time and the fineness of the color of black tea. Postępy Tech. Przetwórstwa Spożywczego 2016, 2, 76–79. (In Polish) [Google Scholar]
- Maskan, M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Maskan, M. Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: Colour degradation and kinetics. J. Food Eng. 2006, 72, 218–224. [Google Scholar] [CrossRef]
- Ceni, G.C.; Baldissera, E.M.; Primo, M.S.; Antunes, O.A.C.; Dariva, C.; Oliveira, J.V.; Oliveira, D. Influence of application of microwave energy on quality parameters of mate tea leaves (Ilex paraguariensis St. Hill.). Food Technol. Biotechnol. 2009, 47, 221–226. [Google Scholar]
- Erkmen, O.; Bozoglu, T.F. (Eds.) Factors affecting microbial growth in foods. In Food Microbiology: Principles into Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; Volume 1, Section 2; pp. 91–106. [Google Scholar] [CrossRef]
- Kowalska, J.; Majewska, E.; Lenart, A. Water activity of powdered cocoa beverage with a modified composition of raw materials. Zywnosc Nauka Technol. Jakosc. 2011, 4, 57–65. (In Polish) [Google Scholar] [CrossRef]
- Gisolfi, C.V.; Summers, R.W.; Lambert, G.P.; Xia, T. Effect of beverage osmolality on intestinal fluid absorption during exercise. J. Appl. Physiol. 1998, 85, 1595–2000. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, D.L.; Hopkins, W.G. Effects of hypotonic and isotonic sports drinks on endurance performance and physiology. Sportscience 2010, 14, 63–70. [Google Scholar]
- Shishikura, Y.; Khokhar, S. Factors affecting the levels of catechins and caffeine in tea beverage: Estimated daily intakes and antioxidant activity. J. Sci. Food Agric. 2005, 85, 2125–2133. [Google Scholar] [CrossRef]
- Park, J.H.; Back, C.N.; Kim, J.K. Recommendation of packing method to delay the quality decline of green tea powder stored at room temperature. Korean J. Hortic. Sci. 2005, 23, 499–506. [Google Scholar] [CrossRef]
- Koch, W.; Kukula-Koch, W.; Głowniak, K. Catechin composition and antioxidant activity of black teas in relation to brewing time. J. AOAC Int. 2017, 100, 1694–1699. [Google Scholar] [CrossRef]
- Yamabe, N.; Kang, K.S.; Hur, J.M.; Yokozawa, T. Matcha, a powdered green tea, ameliorates the progression of renal and hepatic damage in type 2 diabetic OLETF rats. J. Med. Food 2009, 12, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Liu, J.; Lv, Y.J.; Jiang, Y.L.; Pan, J.X.; Zhu, Y.J.; Huang, M.G.; Zhang, S.K. Changes in intestinal microbiota of type 2 diabetes in mice in response to dietary supplementation with instant tea or Matcha. Can. J. Diabetes 2020, 44, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Chiba, T.; Tomita, I.; Koizumi, H.; Miura, S.; Umegaki, K.; Hara, Y.; Ikeda, M.; Tomita, T. Tea catechins prevent the development of atherosclerosis in apoprotein E—Deficient mice. J. Nutr. 2001, 131, 27–32. [Google Scholar] [CrossRef]
- Orrù, S.; Imperlini, E.; Nigro, E.; Alfieri, A.; Cevenini, A.; Polito, R.; Daniele, A.; Buono, P.; Mancini, A. Role of functional beverages on sport performance and recovery. Nutrients 2018, 10, 1470. [Google Scholar] [CrossRef]
- Spaccarotella, K.J.; Andzel, W.D. Building a beverage for recovery from endurance activity: A review. J. Strength Cond. Res. 2011, 25, 3198–3204. [Google Scholar] [CrossRef]
- Leidy, H.J. Consumption of protein beverages as a strategy to promote increased energy intake in older adults. Am. J. Clin. Nutr. 2017, 106, 715–716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kulczyński, B.; Gramza-Michałowska, A. Health benefits of inulin-type fructans. Med. Rodz. 2016, 19, 86–90. (In Polish) [Google Scholar]
- Rashidinejad, A.; Birch, J.; Sun-Waterhouse, D.; Everett, D.W. Addition of milk to tea infusions: Helpful or harmful? Evidence from in vitro and in vivo studies on antioxidant properties. Crit. Food Sci. Nutr. 2017, 57, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Birch, E.J.; Everett, D.W. Interactions between milk fat globules and green tea catechins. Food Chem. 2016, 199, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Tireki, S. A review on packed non-alcoholic beverages: Ingredients, production, trends and future opportunities for functional product development. Trends Food Sci. 2021, 112, 442–454. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Schramm, L. Going green: The role of the green tea component EGCG in chemoprevention. J. Carcinog. Mutagen. 2013, 4, 1000142. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Yousf, N.; Nazir, F.; Salim, R.; Ahsan, H.; Adnan Sirwal, A. Water solubility index and water absorption index of extruded product from rice and carrot blend. J. Pharmacogn. Phytochem. 2017, 6, 2165–2168. [Google Scholar]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- PN-EN 12143:2000; Determination of the Content of Soluble Substances—Refractometric Method. Department of Standardization Publishing, Polish Committee for Standardization: Warsaw, Poland, 2000.
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The nutritive value of organic and conventional white cabbage (Brassica oleracea L. var. Capitata) and anti-apoptotic activity in gastric adenocarcinoma cells of sauerkraut juice produced therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef] [PubMed]
- Ponder, A.; Hallmann, E. The nutritional value and vitamin C content of different raspberry cultivars from organic and conventional production. J. Food Compos. Anal. 2020, 87, 103429. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]

| Sample | L* | a* | b* |
|---|---|---|---|
| M-1 | 63.15 ± 0.58 d | −1.10 ± 0.02 c | 30.17 ± 0.74 d |
| M-2 | 59.82 ± 0.31 c | −2.29 ± 0.11 b | 34.04 ± 0.36 g |
| M-3 | 55.25 ± 1.45 b | 0.71 ± 0.09 g | 26.69 ± 0.58 b |
| M-4 | 56.28 ± 2.10 b | −4.21 ± 0.15 a | 31.51 ± 0.69 ef |
| M-5 | 56.44 ± 0.31 b | −0.55 ± 0.04 e | 30.40 ± 1.11 de |
| M-6 | 46.35 ± 1.45 a | 0.96 ± 0.06 h | 19.78 ± 0.73 a |
| M-7 | 64.10 ± 0.72 d | −0.74 ± 0.04 d | 34.35 ± 0.62 g |
| M-8 | 68.76 ± 2.51 e | −1.07 ± 0.05 c | 31.99 ± 0.39 f |
| M-9 | 57.02 ± 0.35 b | −0.33 ± 0.07 f | 28.80 ± 0.60 c |
| M-10 | 45.90 ± 0.58 a | 0.94 ± 0.04 h | 19.28 ± 0.45 a |
| Sample | Water Activity (aw) | WSI (%) | WHC (g/g) |
|---|---|---|---|
| M-1 | 0.2655 ± 0.001 c | 25.45 ± 0.56 f | 2.04 ± 0.01 b |
| M-2 | 0.3000 ± 0.000 d | 22.14 ± 0.48 d | 2.04 ± 0.16 b |
| M-3 | 0.3024 ± 0.000 d | 23.30 ± 0.45 e | 3.06 ± 0.18 e |
| M-4 | 0.4082 ± 0.003 h | 23.33 ± 0.20 e | 2.19 ± 0.21 bc |
| M-5 | 0.2238 ± 0.001 b | 21.05 ± 0.37 c | 2.44 ± 0.16 cd |
| M-6 | 0.3926 ± 0.003 g | 17.43 ± 0.76 a | 3.71 ± 0.02 f |
| M-7 | 0.3743 ± 0.001 f | 25.80 ± 0.22 f | 1.48 ± 0.09 a |
| M-8 | 0.3375 ± 0.001 e | 22.72 ± 0.18 de | 2.01 ± 0.14 b |
| M-9 | 0.1546 ± 0.002 a | 20.89 ± 0.23 e | 2.47 ± 0.14 d |
| M-10 | 0.3956 ± 0.004 g | 18.80 ± 0.21 b | 3.56 ± 0.26 f |
| Sample | pH | °Brix (%) | Osmolality (mOsm/kg·H2O) |
|---|---|---|---|
| M-1 | 5.84 ± 0.01 c | 1.23 ± 0.06 abc | 1.00 ± 0.00 a |
| M-2 | 5.83 ± 0.02 c | 1.30 ± 0.10 cde | 2.33 ± 0.58 bc |
| M-3 | 5.59 ± 0.02 a | 1.13 ± 0.06 a | 1.00 ± 0.00 a |
| M-4 | 5.94 ± 0.03 d | 1.40 ± 0.10 e | 5.67 ± 0.58 d |
| M-5 | 5.61± 0.02 a | 1.27 ± 0.06 bcd | 3.33 ± 0.58 c |
| M-6 | 5.63 ± 0.09 a | 1.17 ± 0.06 ab | 1.67 ± 0.58 ab |
| M-7 | 5.72 ± 0.05 b | 1.37 ± 0.06 de | 5.33 ± 0.58 d |
| M-8 | 5.75 ± 0.02 b | 1.27 ± 0.06 bcd | 3.00 ± 0.00 c |
| M-9 | 5.61 ± 0.04 a | 1.13 ± 0.06 a | 0.67 ± 0.58 a |
| M-10 | 5.58 ± 0.04 a | 1.17 ± 0.06 ab | 1.67 ± 0.58 ab |
| Sample | Gallic Acid | P-Coumaric Acid | Catechin | Epigallocatechin | Gallate Epigallocatechin | Quercetin | Rutoside-3-O-quercetin |
|---|---|---|---|---|---|---|---|
| M-1 | 3.75 ± 0.19 b | 20.64 ± 0.53 f | 3.41 ± 0.10 e | 3.43 ± 0.33 d | 22.67 ± 1.03 b | 0.45 ± 0.01 d | 3.46 ± 0.18 a |
| M-2 | 2.96 ± 0.03 a | 5.78 ± 0.17 ab | 2.49 ± 0.15 d | 5.51 ± 0.11 f | 10.41 ± 0.15 a | 0.75 ± 0.02 e | 5.22 ± 0.37 b |
| M-3 | 2.86 ± 0.06 a | 15.94 ± 0.77 e | 1.68 ± 0.05 c | 4.43 ± 0.21 e | 26.13 ± 1.35 c | 0.95 ± 0.01 g | 13.00 ± 3.08 c |
| M-4 | 25.10 ± 0.47 h | 41.16 ± 0.33 g | 0.80 ± 0.01 a | 6.61 ± 0.08 g | 25.66 ± 0.70 c | 0.29 ± 0.00 bc | 2.31 ± 0.34 a |
| M-5 | 6.58 ± 0.08 c | 7.32 ± 1.31 cd | 1.14 ± 0.05 b | 1.39 ± 0.24 b | 27.39 ± 0.42 c | 0.31 ± 0.01 c | 2.66 ± 0.13 a |
| M-6 | 22.65 ± 0.05 f | 6.27 ± 0.37 bc | 0.69 ± 0.01 a | 6.68 ± 0.03 g | 38.45 ± 0.26 d | 0.28 ± 0.01 b | 2.30 ± 0.12 a |
| M-7 | 24.16 ± 0.50 g | 7.54 ± 0.61 d | 4.70 ± 0.05 f | 4.25 ± 0.40 e | 36.79 ± 3.63 d | 0.23 ± 0.01 a | 2.23 ± 0.34 a |
| M-8 | 7.33 ± 0.39 d | 6.48 ± 0.67 bcd | 3.52 ± 0.22 e | 1.00 ± 0.02 a | 25.49 ± 0.11 c | 0.44 ± 0.03 d | 2.51 ± 0.09 a |
| M-9 | 3.63 ± 0.19 b | 7.15 ± 0.43 cd | 1.03 ± 0.06 b | 2.38 ± 0.09 c | 11.62 ± 0.10 a | 0.29 ± 0.00 bc | 2.52 ± 0.28 a |
| M-10 | 18.79 ± 0.46 e | 4.95 ± 0.08 a | 2.51 ± 0.06 d | 4.19 ± 0.12 e | 22.46 ± 2.08 b | 0.84 ± 0.03 f | 2.29 ± 0.07 a |
| Component | Matcha Drink Based on Milk | Matcha Drink Based on Water | ||
|---|---|---|---|---|
| General appearance of Matcha drinks |  |  | ||
| [g/100 g drink] | [g/100 g powder] | [g/100 g of drink] | [g/100 g powder] | |
| Water | 43 | - | 90 | - |
| Milk 1.5% fat | 43 | - | - | - |
| Inulin | 3 | 21 | 2.5 | 25 |
| Collagen hydrolyzate | 3 | 21 | 3 | 30 |
| Whey isolate | 3.5 | 25 | - | - |
| Maltodextrin | 2.5 | 18 | 2.5 | 25 |
| Sugar | 1.5 | 11 | 1.5 | 15 |
| Matcha M-4 | 0.5 | 4 | 0.5 | 5 |
| Parameter | Matcha Drink Based on Milk | Matcha Drink Based on Water |
|---|---|---|
| Water activity (aw) | 0.19 ± 0.00 a | 0.27 ± 0.01 b |
| pH | 6.46 ± 0.05 a | 6.58 ± 0.02 b |
| °Brix | 16.33 ± 1.03 b | 8.83 ± 1.24 a |
| Osmolality (mOsm/kg·H2O) | 313.00 ± 5.65 b | 147.00 ± 1.63 a |
| Sensory Features | Matcha Drink Based on Milk | Matcha Drink Based on Water |
|---|---|---|
| Appearance | light green color with a delicate sediment and foam on the surface | dark green color, cloudy drink |
| Points (0–5) | 4.8 | 4.0 |
| Smell | milk, tea, vegetable, herbal, sweet | herbal, tea, spicy, sweet, vegetable, grassy |
| Points (0–5) | 4.5 | 4.2 |
| Taste | milk, tea, herbal, slightly sweet, slightly bitter, vegetable | vegetable, tea, herbal, almond, slightly sweet, bitter, burning, astringent, raw, grassy |
| Points (0–5) | 4.8 | 4.0 |
| Consistency | liquid, causing astringency on the tongue, perceptible fine particles suspended in the drink | fluid, slightly viscous, causing tightening on the tongue |
| Points (0–5) | 4.7 | 4.0 |
| Overall score (0–5) | 4.8 | 4.0 |
| Nutritional Value/ Nutrition Claim | Matcha Drink Based on Milk | Matcha Drink Based on Water |
|---|---|---|
| Energy value (kJ/kcal) | 265.19/63.34 | 139.29/33.27 |
| Fat (g) | 0.7 | <0.1 |
| Saturated fatty acids (g) | 0.47 | <0.01 |
| Carbohydrates (g) | 8.8 | 6.25 |
| Sugars (g) | 4.28 | 2.18 |
| Proteins (g) | 6.7 | 2.7 |
| Fiber (g) | 2.7 | 2.25 |
| Salt (g) | 0.05 | <0.01 |
| Nutrition claims according to Regulation (EC) 1924/2006 | High protein content Low fat Low salt content High content of dietary fiber | High protein content Does not contain fat It does not contain salt High content of dietary fiber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najman, K.; Sadowska, A.; Wolińska, M.; Starczewska, K.; Buczak, K. The Content of Bioactive Compounds and Technological Properties of Matcha Green Tea and Its Application in the Design of Functional Beverages. Molecules 2023, 28, 7018. https://doi.org/10.3390/molecules28207018
Najman K, Sadowska A, Wolińska M, Starczewska K, Buczak K. The Content of Bioactive Compounds and Technological Properties of Matcha Green Tea and Its Application in the Design of Functional Beverages. Molecules. 2023; 28(20):7018. https://doi.org/10.3390/molecules28207018
Chicago/Turabian StyleNajman, Katarzyna, Anna Sadowska, Monika Wolińska, Katarzyna Starczewska, and Krzysztof Buczak. 2023. "The Content of Bioactive Compounds and Technological Properties of Matcha Green Tea and Its Application in the Design of Functional Beverages" Molecules 28, no. 20: 7018. https://doi.org/10.3390/molecules28207018
APA StyleNajman, K., Sadowska, A., Wolińska, M., Starczewska, K., & Buczak, K. (2023). The Content of Bioactive Compounds and Technological Properties of Matcha Green Tea and Its Application in the Design of Functional Beverages. Molecules, 28(20), 7018. https://doi.org/10.3390/molecules28207018







