Development and Comparative Evaluation of Two Highly Sensitive Immunosensor Platforms for Trace Determination of Copper Ions in Drinking Water Using a Monoclonal Antibody Specific to Copper-EDTA Complex
Abstract
:1. Introduction
2. Results and Discussion
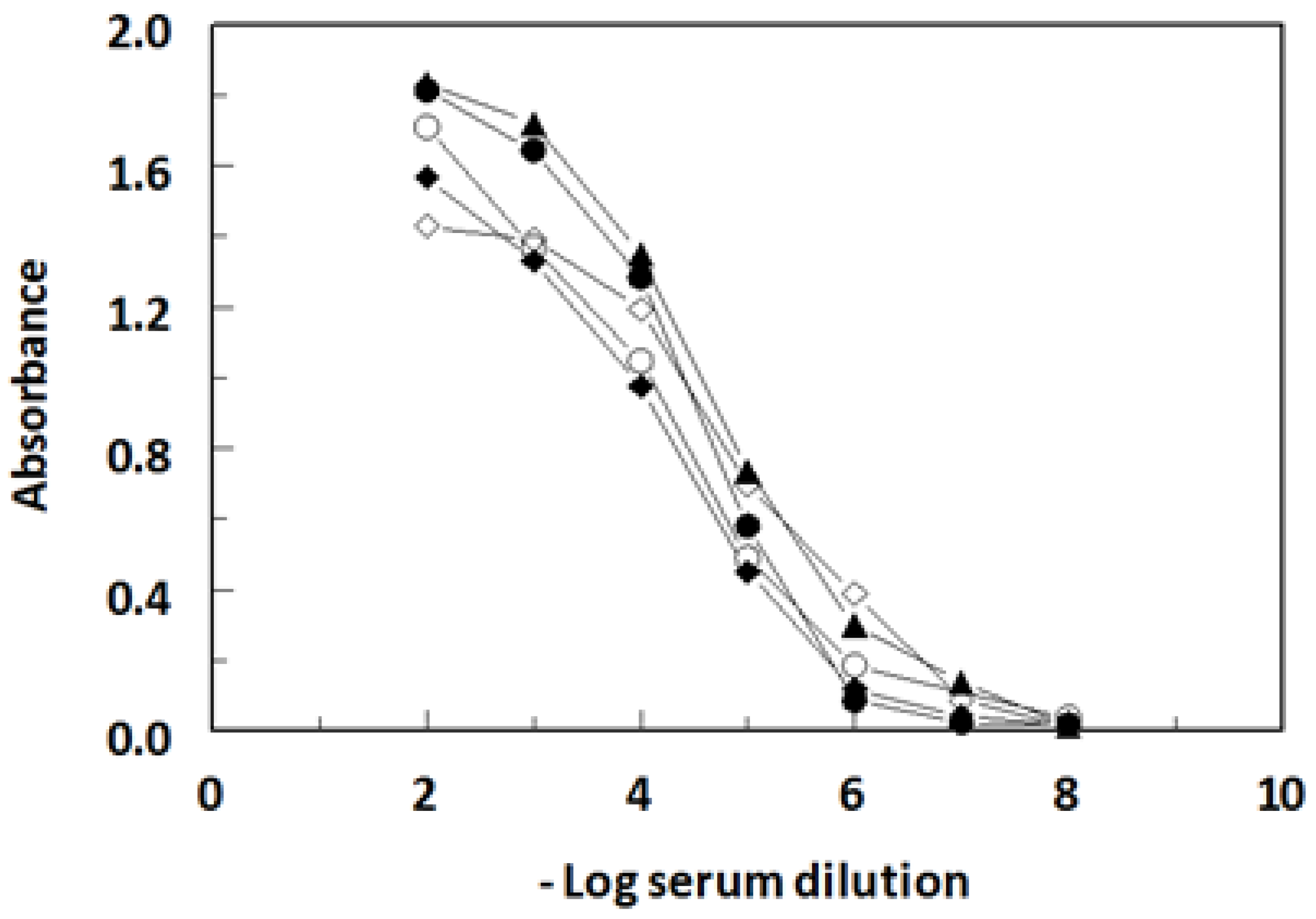
2.1. Immunization and Immune Response
2.2. Hybridoma Screening
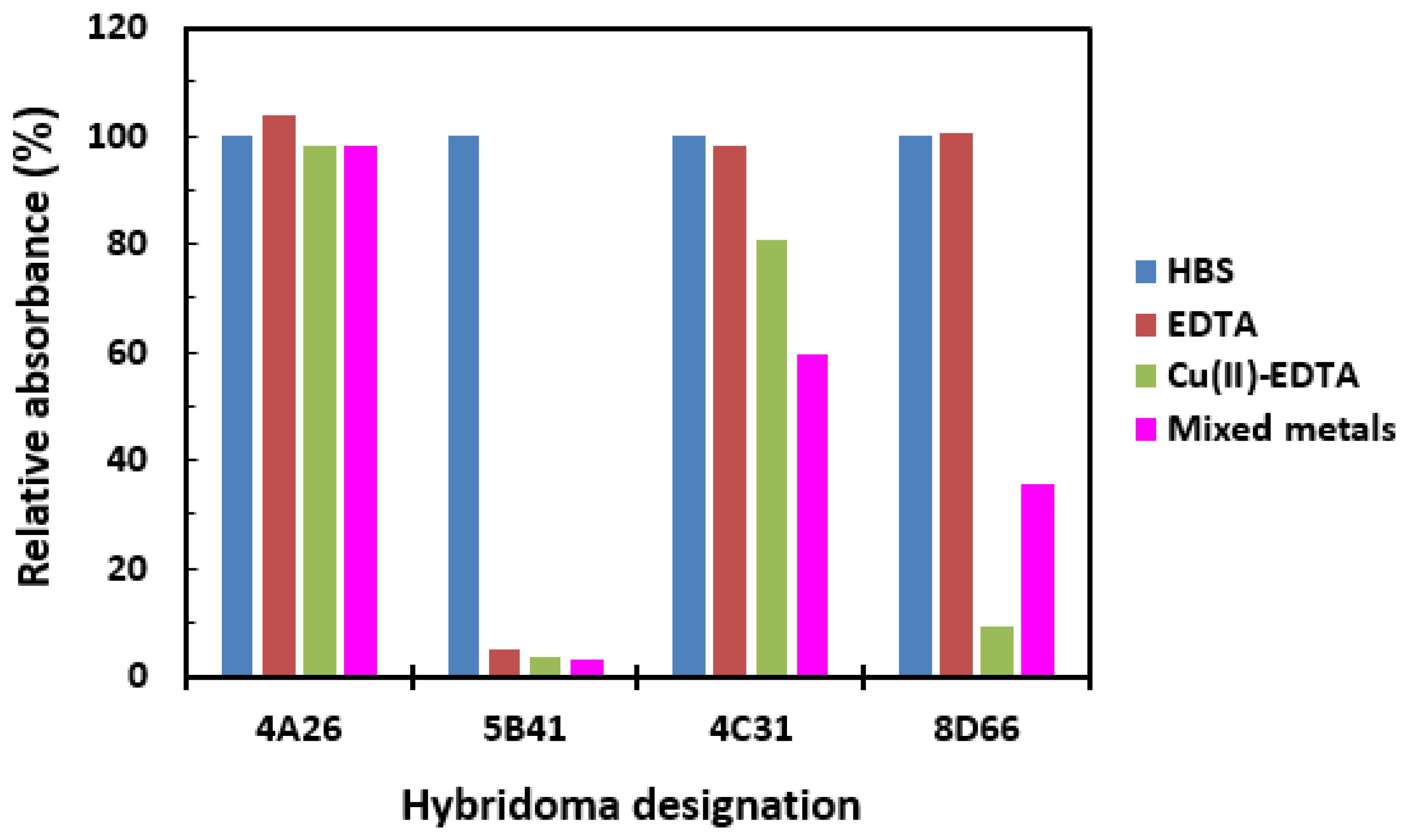
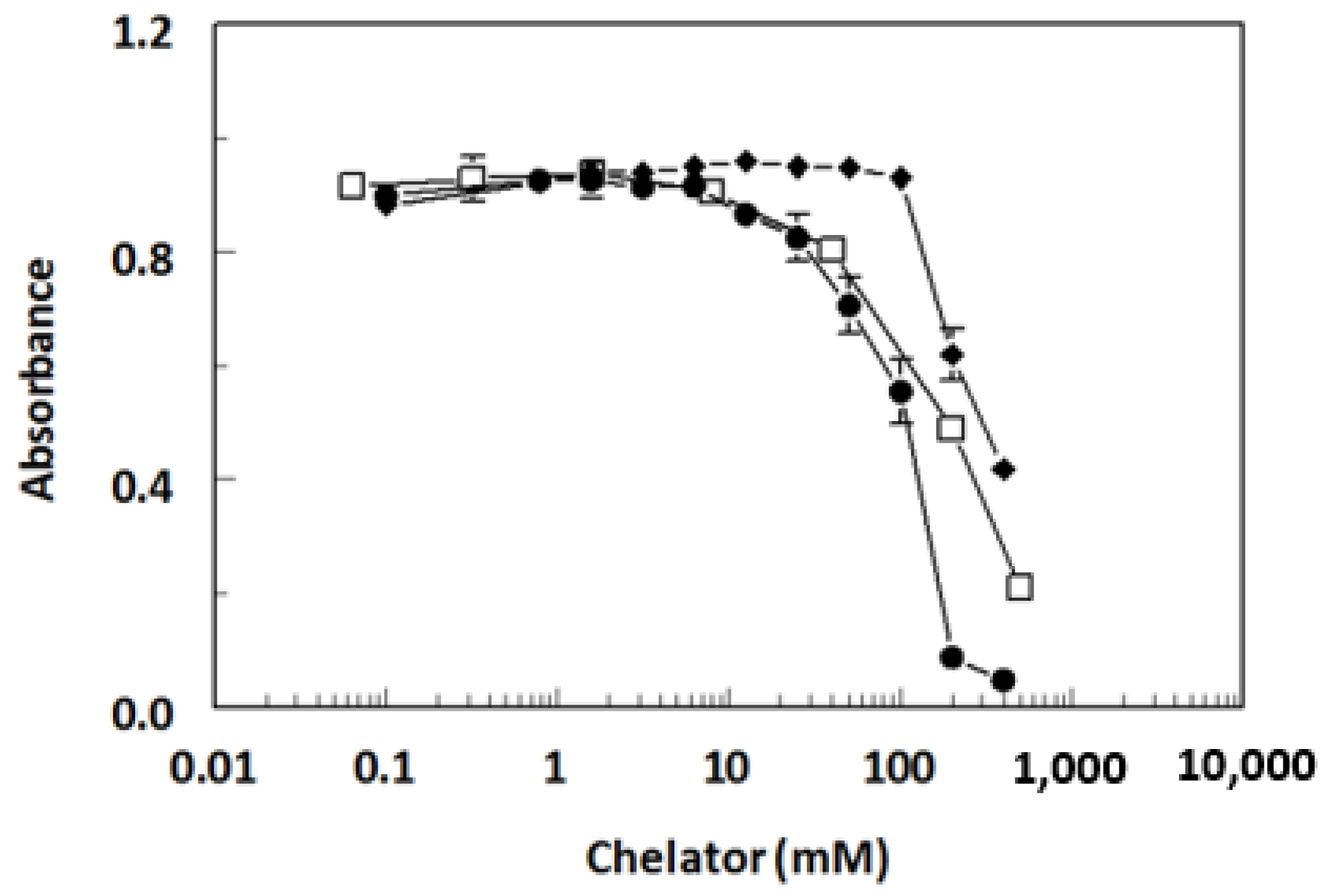
2.3. Antibody Specificity
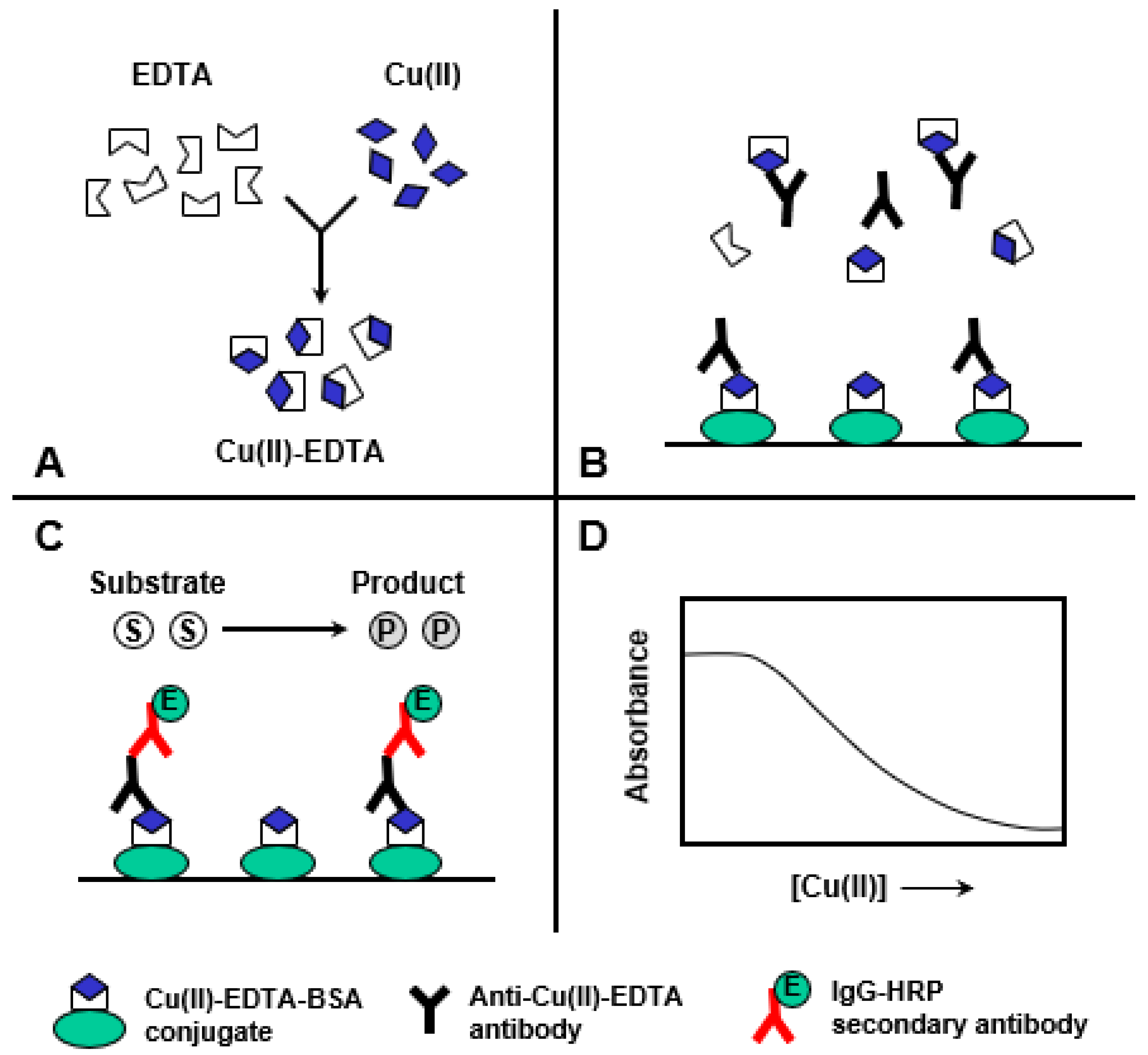
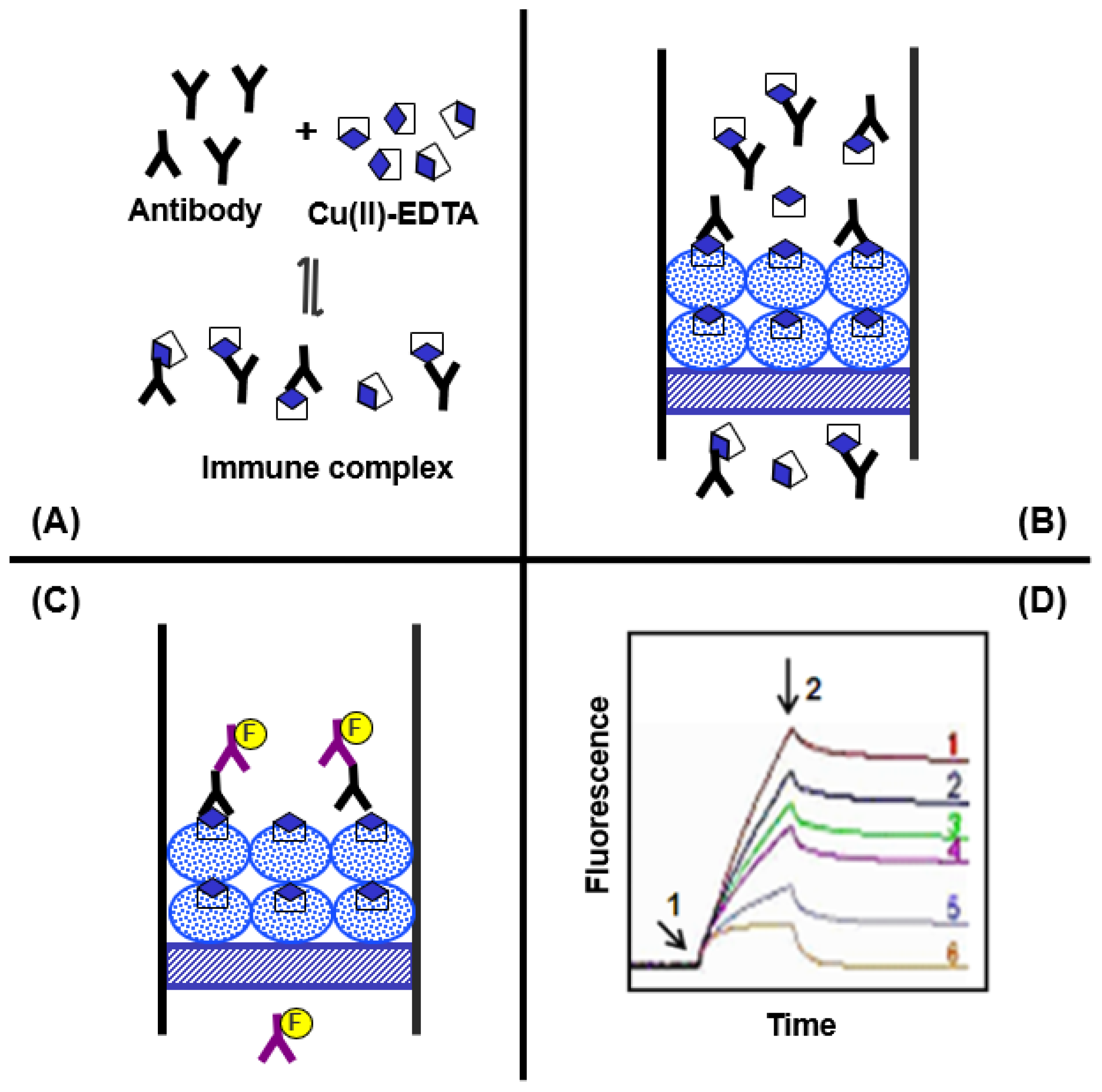
2.4. Description of ELISA and KinExA
2.5. Optimization of Reagent Concentrations for Development of ELISA
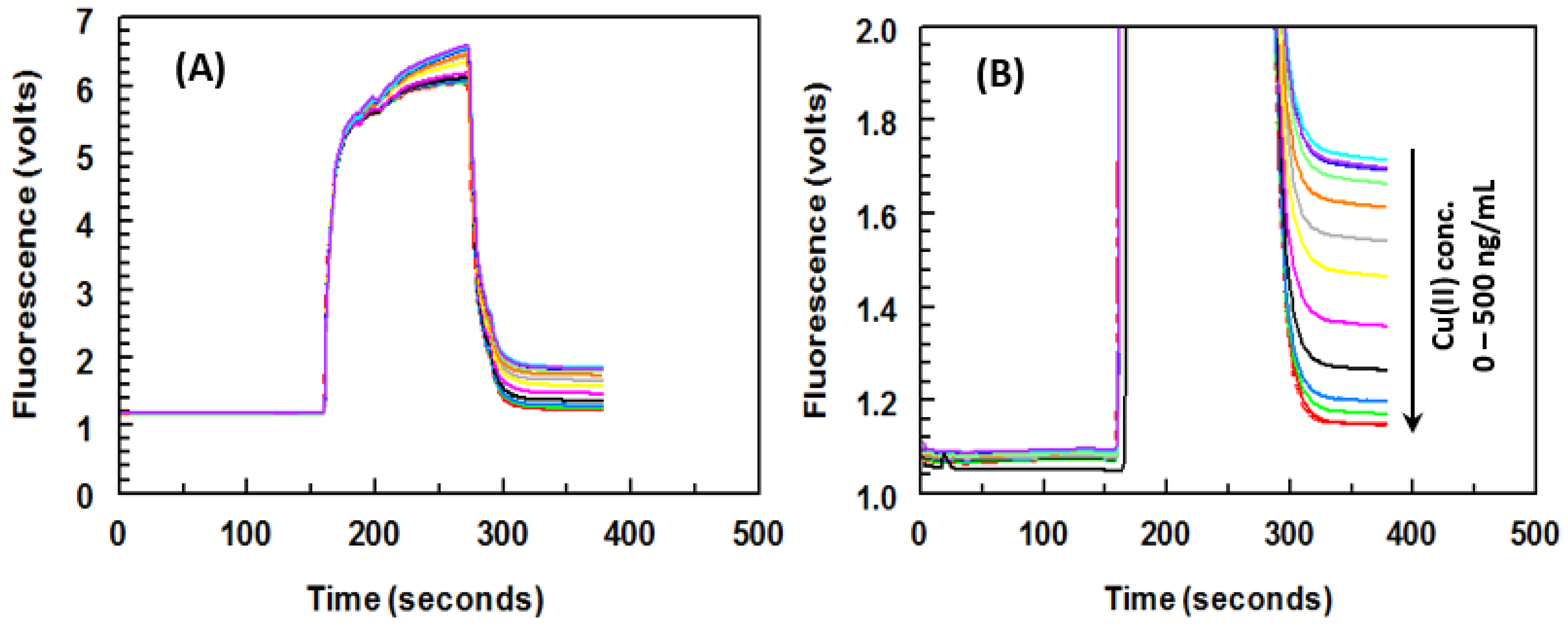
2.6. Optimization of KinExA Conditions and Its Running
2.7. Validation of ELISA and KinExA
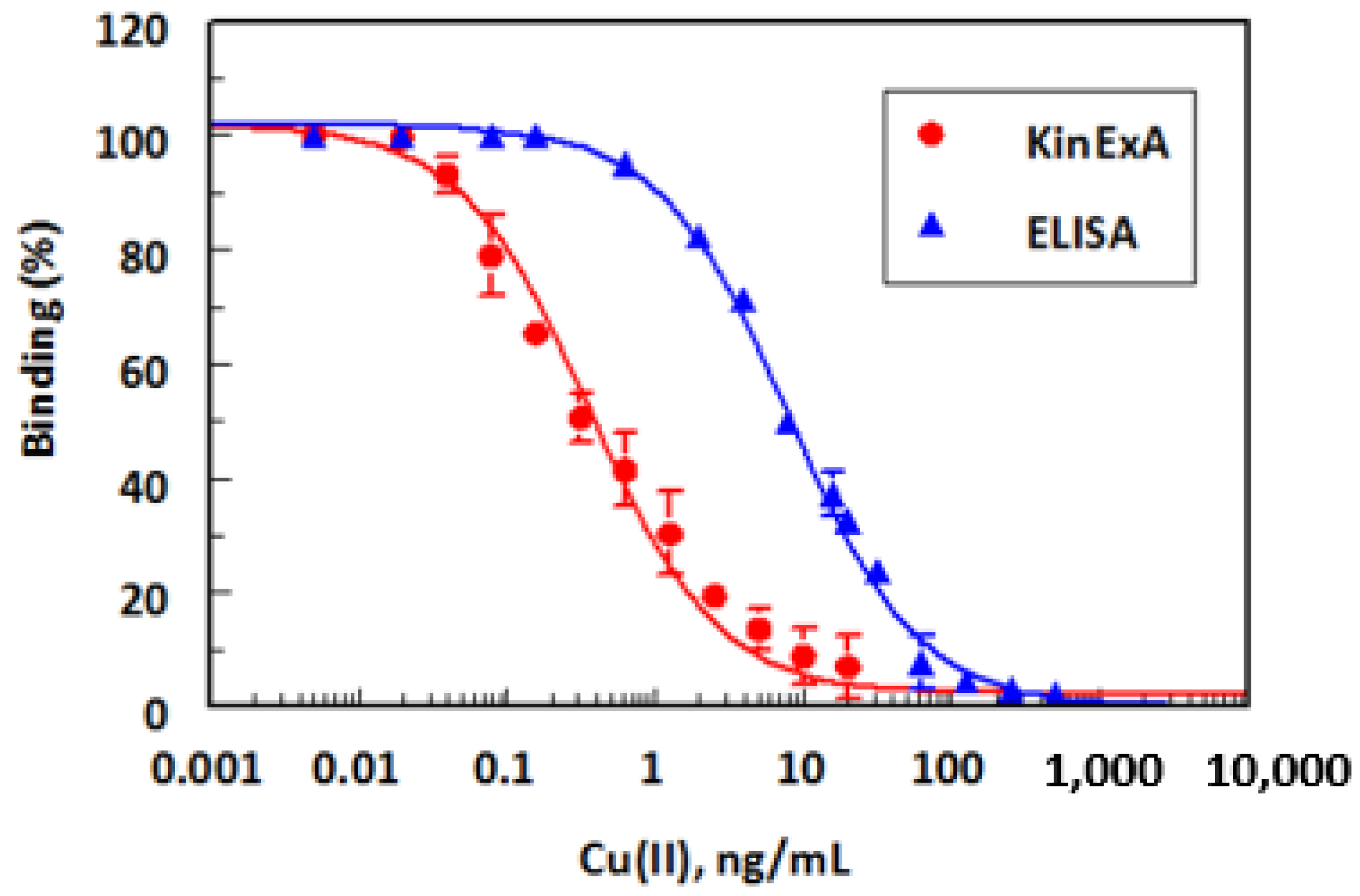
2.7.1. Calibration Curves and Detection Limits
2.7.2. Precision and Recovery
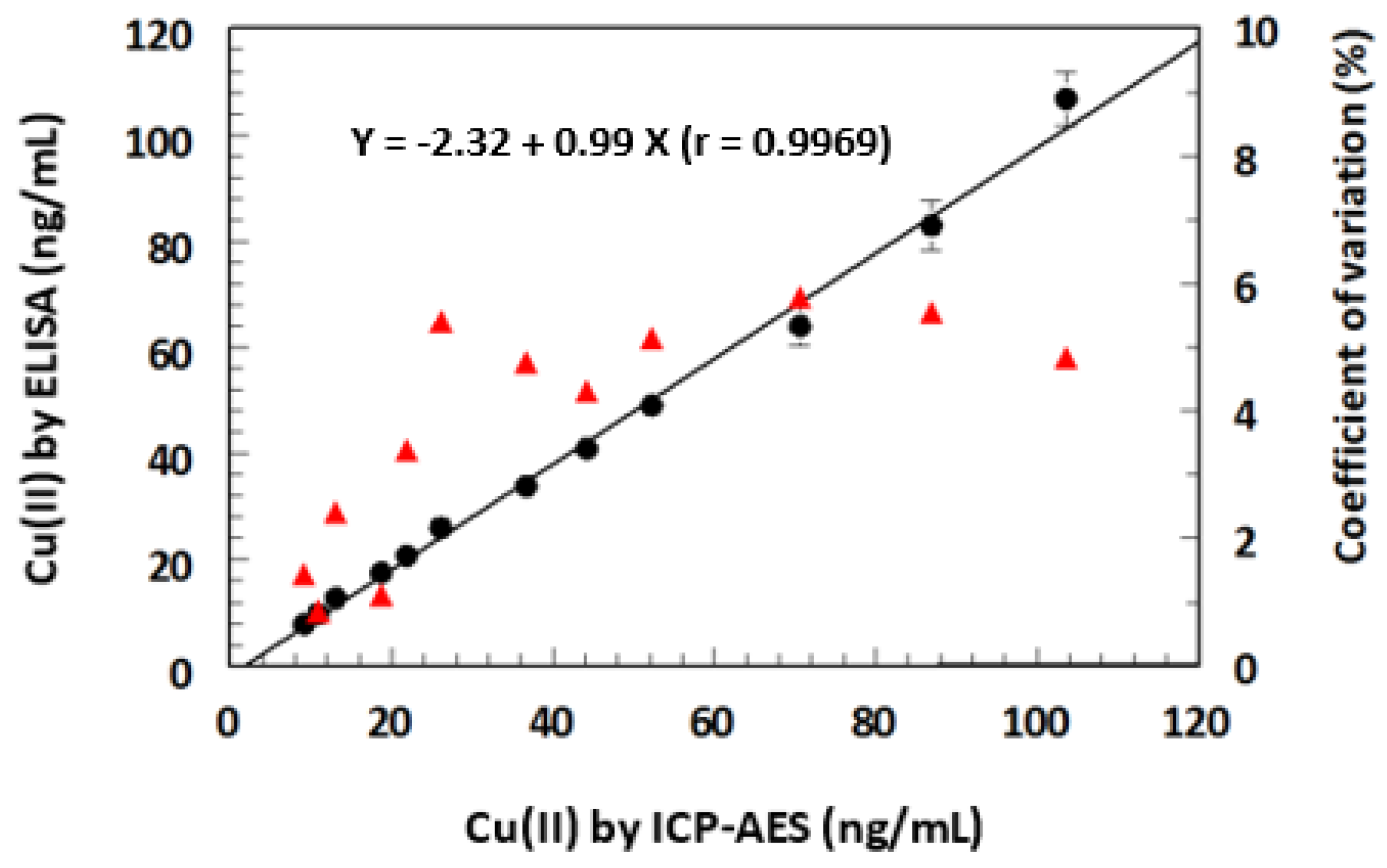
2.8. Comparison of ELISA and KinExA with ICP-AES
2.9. Comparative Evaluation of ELISA and KinExA
3. Experimental
3.1. Instruments
3.2. Materials
3.3. Preparation of Copper-EDTA-Protein Conjugates
3.4. Immunization and Cell Fusion
3.5. Non-Competitive and Competitive ELISA for Screening
3.6. Analysis of Cu(II) by ELISA
3.7. Analysis of Cu(II) by KinExA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Underwood, E.J. Trace Elements in Human and Animal Nutrition, 4th ed.; Academic Press: New York, NY, USA, 1977; pp. 56–108. [Google Scholar]
- Goyer, R.A. Toxic effects of metals. In Casarrett and Doull’s Toxicology: The Basic Science of Poisons, 4th ed.; Amdur, M.O., Doull, J., Klaassen, C.D., Eds.; Pergamon Press: New York, NY, USA, 1991; pp. 623–680. [Google Scholar]
- Federal register. Review of the National Primary Drinking Water Regulation: Lead and Copper Rule Revisions (LCRR). Available online: https://www.federalregister.gov/documents/2021/12/17/2021-27457/review-of-the-national-primary-drinking-water-regulation-lead-and-copper-rule-revisions-lcrr (accessed on 5 October 2023).
- NIH. Copper in Drinking Water. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225402/#:~:text=Many%20pipes%20and%20plumbing%20fixtures,household%20pipes%20to%20ground%20appliances (accessed on 5 October 2023).
- Knobelock, L.; Ziarnik, M.; Howad, J.; Theis, B.; Farmer, D.; Anderson, H.; Proctor, M. Gastrointestinal upsets associated with ingestion of copper-contaminated water. Environ. Health Perspect. 1994, 102, 958–969. [Google Scholar] [CrossRef]
- Pizarro, F.; Olivares, M.; Uauy, R.; Contreras, P.; Rebelo, A.; Gidi, V. Acute gastrointestinal effects of graded levels of copper in drinking water. Environ. Health Perspect. 1999, 107, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Schilsky, M.L. Wilson disease: Genetic basis of copper toxicity and natural history. Semin. Liver Dis. 1996, 16, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, I.H.; Steernlieb, I. Is non-Indian childhood cirrhosis caused by excess dietary copper? Lancet 1994, 344, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Bhave, S. Present interpretation of the role of copper in Indian childhood cirrhosis. Am. J. Clin. Nutr. 1996, 63, 830S–8305S. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Müller, W.; Feichtinger, H. Idiopathic copper toxicosis. Am. J. Clin. Nutr. 1998, 6, 1082S–1086S. [Google Scholar] [CrossRef]
- Zhong, G.; He, Y.; Wan, F.; Wu, S.; Jiang, X.; Tang, Z.; Hu, L. Effects of long-term exposure to copper on the keap1/nrf2 signaling pathway and Msr-related redox status in the kidneys of rats. Biol. Trace Elem. Res. 2021, 199, 4205–4217. [Google Scholar] [CrossRef]
- Husain, N.; Hasan, S.; Khan, A.A.; Mahmood, R. Copper chloride inhibits brush border membrane enzymes, alters antioxidant and metabolic status and damages DNA in rat intestine: A dose-dependent study. Environ. Sci. Pollut. Res. Int. 2021, 28, 43711–43724. [Google Scholar] [CrossRef]
- Niu, B.; Mackness, B.C.; Zitzewitz, J.A.; Matthews, C.R.; Gross, M.L. Trifluoroethanol partially unfolds G93A SOD1 leading to protein aggregation: A study by native mass spectrometry and FPOP protein footprinting. Biochemistry 2020, 59, 3650–3659. [Google Scholar] [CrossRef]
- Khan, A.; Dobson, J.P.; Exley, C. Redox cycling of iron by Aβ. Free. Radic. Biol. Med. 2006, 40, 557–569. [Google Scholar] [CrossRef]
- Qazmooz, H.A.; Smesam, H.N.; Mousa, R.F.; Al-Hakeim, H.K.; Maes, M. Trace element, immune and opioid biomarkers of unstable angina, increased atherogenicity and insulin resistance: Results of machine learning. J. Trace Elem. Med. Biol. 2021, 64, 126703. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Sant’Ana, M.A.; de Carvalho, T.C.; da Silva, I.F. Concentration of heavy metals in UHT dairy milk available in the markets of Sao Luis, Brazil, and potential health risk to children. Food Chem. 2021, 346, 128961. [Google Scholar] [CrossRef]
- Jo, G.; Todorov, T.I. Distribution of nutrient and toxic elements in brown and polished rice. Food Chem. 2019, 289, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhu, T.; Wang, X.; Cheng, X.; Yuan, Y.; Jin, M.; Betancor, M.B.; Tocher, D.R.; Zhou, Q. Toxicological mechanism of excessive copper supplementation: Effects on coloration, copper bioaccumulation and oxidation resistance in mud crab Scylla paramamosain. J. Hazard. Mater. 2020, 395, 122600. [Google Scholar] [CrossRef] [PubMed]
- Campana, O.; Simpson, S.L.; Spadaro, D.A.; Blasco, J. Sub-lethal effects of copper to benthic invertebrates explained by sediment properties and dietary exposure. Environ. Sci. Technol. 2012, 46, 6835–6842. [Google Scholar] [CrossRef] [PubMed]
- Draghici, G.A.; Dehelean, C.; Pinzaru, I.; Bordean, D.M.; Borozan, A.; Tsatsakis, A.M.; Kovatsi, L.; Nica, D. Soil copper uptake by land snails: A semi-field experiment with juvenile Cantareus aspersus snails. Environ. Toxicol. Pharmacol. 2019, 72, 103243. [Google Scholar] [CrossRef]
- Liao, P.; Shu, X.; Tang, M.; Tan, B.; Yin, Y. Effect of dietary copper source (inorganic vs. chelated) on immune response, mineral status, and fecal mineral excretion in nursery piglets. Food Agric. Immunol. 2018, 29, 548–563. [Google Scholar] [CrossRef]
- Perestrelo, A.P.; Miranda, G.; Goncalves, M.I.; Belino, C.; Ballesteros, R. Chronic copper sulfate poisoning. Eur. J. Case Rep. Intern. Med. 2021, 8, 002309. [Google Scholar]
- Ghazaryan, K.; Movsesyan, H.; Ghazaryan, N.; Watts, B.A. Copper phytoremediation potential of wild plant species growing in the mine polluted areas of Armenia. Environ. Pollut. 2019, 249, 491–501. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Copper in Drinking Water. 1, Introduction. In Copper in Drinking Water; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Elkhatat, A.M.; Soliman, M.; Ismail, R.; Ahmed, S.; Mubashir, S.; Fouladi, S.; Khraisheh, M. Recent trends of copper detection in water samples. Bull. Natl. Res. Cent. 2021, 45, 218. [Google Scholar] [CrossRef]
- Arjomandi, M.; Hamid, S. A review: Analytical methods for heavy metals determination in environment and human samples. Anal. Methods Environ. Chem. J. 2019, 2, 97–126. [Google Scholar] [CrossRef]
- EPA. Analytical Methods Recommended for Drinking Water Compliance Monitoring of Secondary Contaminants. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100WD5Z.PDF?Dockey=P100WD5Z.PDF (accessed on 23 September 2023).
- Szurdoki, F.; Jaeger, L.; Harris, A.; Kido, H.; Wengatz, I.; Goodrow, M.H.; Szekacs, A.; Wortberg, M.; Zheng, J.; Stoutamire, D.W.; et al. Rapid assays for environmental and biological monitoring. J. Environ. Sci. Health B 1996, 31, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.A.; Blake, R.C., II; Khosraviani, M.; Pavlov, A.R. Immunoassays for metal ions. Anal. Chim. Acta 1998, 376, 13–19. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liu, F. Antibody developments for metal ions and their applications. Food Agric. Immunol. 2020, 31, 1079–1103. [Google Scholar] [CrossRef]
- Zhao, H.; Nan, T.; Tan, G.; Gao, W.; Cao, Z.; Sun, S.; Li, Z.; Li, Q.X.; Wang, B. Development of two highly sensitive immunoassays for detection of copper ions and a suite of relevant immunochemicals. Anal. Chim. Acta 2011, 702, 102–108. [Google Scholar] [CrossRef]
- Hamidaddin, M.M.; AlRabiah, H.; Darwish, I.A. Development and comparative evaluation of two immunoassay platforms for bioanalysis of crizotinib: A potent drug used for the treatment of non-small cell lung cancer. Talanta 2019, 201, 217–225. [Google Scholar] [CrossRef]
- Blake, R.C.; Pavlov, A.R.; Blake, D.A. Automated kinetic exclusion assays to quantify protein binding interactions in homogeneous solution. Anal. Biochem. 1999, 272, 123–134. [Google Scholar] [CrossRef]
- Nygren, H.; Stenberg, M. Calibration by ellipsometry of the enzyme linked immunosorbent assay. J. Immunol. Methods 1985, 80, 15–24. [Google Scholar] [CrossRef]
- Schots, A.; Gommers, F.J.; Egberts, E. Quantitative ELISA for the detection of potato cyst nematodes in soil samples. Fundam. Appl. Nematol. 1992, 15, 55–61. [Google Scholar]
- Chakrabarti, P.; Hatcher, F.M.; Blake, R.C., II; Ladd, P.A.; Blake, D.A. Enzyme immunoassay to determine heavy metals using antibodies to specific metal-EDTA complexes: Optimization and validation of an immunoassay for soluble indium. Anal. Biochem. 1994, 217, 70–75. [Google Scholar] [CrossRef]
- Habeeb, A.F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]


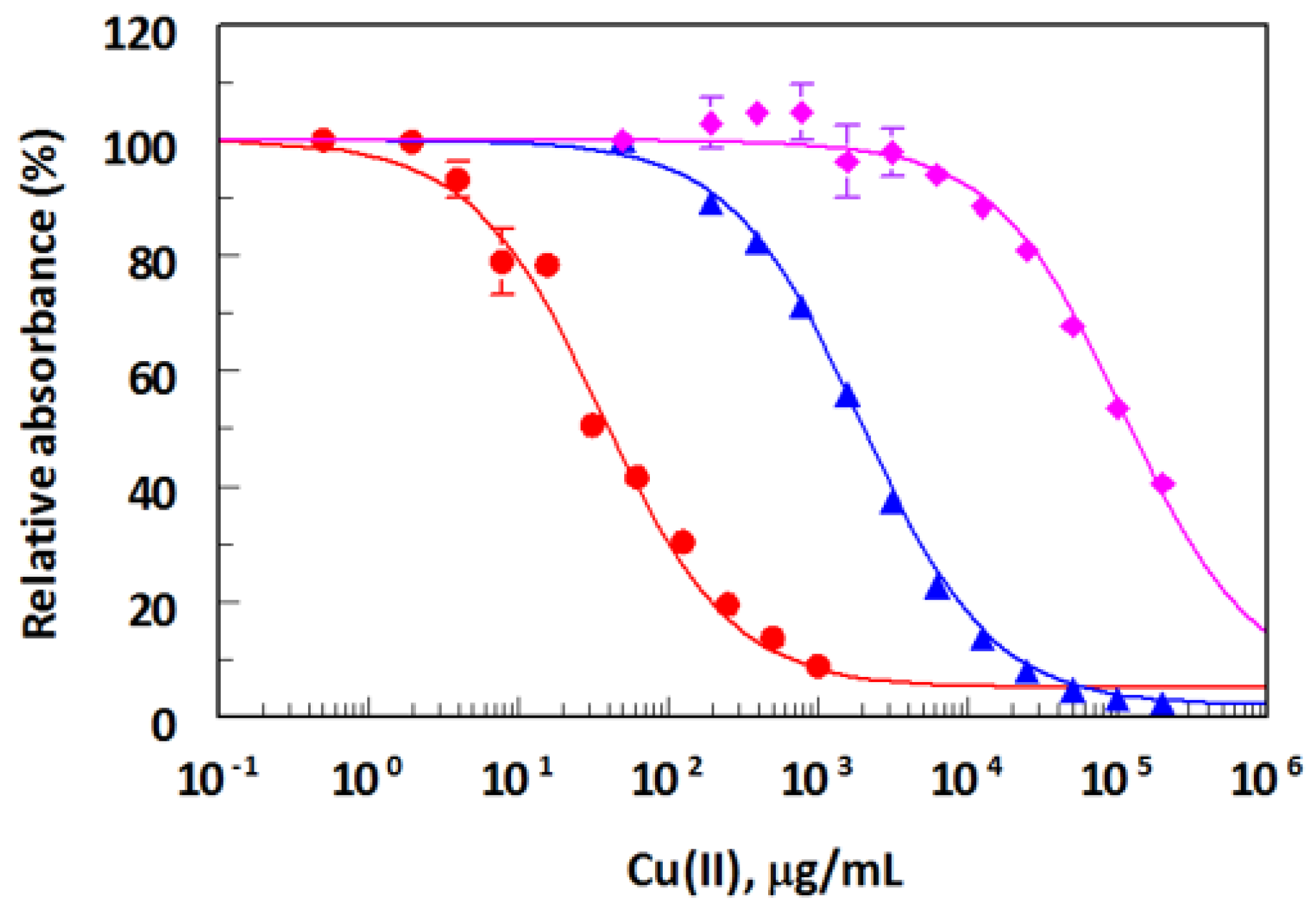
| Metal Ions | IC50 (ng/mL) a | Cross Reactivity (%) b |
|---|---|---|
| Cu | 4.7 c | 100 c |
| Zn | 73.4 | 6.4 |
| Ni | 184.2 | 2.6 |
| Hg | 467.8 | 1.0 |
| Cd | ND d | ND |
| Pb | ND | ND |
| Mn | ND | ND |
| Mg | ND | ND |
| Fe | ND | ND |
| Ca | ND | ND |
| Parameter/Condition | Optimum Value |
|---|---|
| ELISA | |
| Coating Cu(II)-EDTA-BSA conc. (μg/mL) | 0.5 |
| Coating buffer | HBS |
| Coating time (h)/temperature (°C) | 2/37 |
| BSA concentration for blocking (%, w/v) | 3 |
| Blocking with BSA: time (min)/temperature (°C) | 1/37 |
| Concentration of EDTA (mM) | 10 |
| 8D66 antibody concentration (μg/mL) | 0.25 |
| IgG-HRP concentration (μg/mL) | 1 |
| Binding of IgG-HRP: time (h)/temperature (°C) | 1/37 |
| TMB: time (min)/temperature (°C) | 15/25 |
| Reagent for stopping the color reaction (M) | HCl (2) |
| Measuring wavelength (nm) | 450 |
| KinExA | |
| Coated Cu(II)-EDTA-BSA (µg/mL) | 1 |
| Volume of beads suspension (µL) | 583 |
| Flow rate of beads suspension (mL/min) | 1 |
| Time of beads drawing (s) | 35 |
| Concentration of 8D66 antibody (μg/mL) | 0.5 |
| Volume of Cu(II sample (µL) | 500 |
| Time of samples drawing (s) | 120 |
| Concentration of IgG-FITC (µg/mL) | 0.25 |
| Volume of IgG-FITC (µL) | 500 |
| Flow rate (mL/min) | 0.25 |
| ELISA (n = 5) | KinExA (n = 3) | ||||
|---|---|---|---|---|---|
| Concentration (ng/mL) | Intra-Assay | Inter-Assay | Concentration (ng/mL) | Intra-Assay | Inter-Assay |
| 0.5 | 5.4 a | 6.1 | 0.1 | 4.2 | 6.2 |
| 2 | 3.5 | 4.3 | 0.5 | 3.2 | 3.8 |
| 20 | 6.2 | 6.8 | 2 | 4.7 | 5.6 |
| ELISA | KinExA | ||
|---|---|---|---|
| Added (ng/mL) | Recovery a (% ± CV) | Added (ng/mL) | Recovery a (% ± CV) |
| 1.25 | 105.2 ± 5.4 | 0.25 | 103.5 ± 6.2 |
| 2.5 | 96.4 ± 4.2 | 0.5 | 102.6 ± 5.3 |
| 5 | 101.8 ± 4.1 | 1 | 96.7 ± 4.9 |
| 10 | 98.6 ± 5.9 | 2 | 102.5 ± 4.8 |
| 20 | 102.6 ± 6.2 | 4 | 97.8 ± 4.2 |
| Average | 100.9 ± 3.5 | 100.6 ± 3.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, I.A.; Wang, Z.; Darling, R.J. Development and Comparative Evaluation of Two Highly Sensitive Immunosensor Platforms for Trace Determination of Copper Ions in Drinking Water Using a Monoclonal Antibody Specific to Copper-EDTA Complex. Molecules 2023, 28, 7017. https://doi.org/10.3390/molecules28207017
Darwish IA, Wang Z, Darling RJ. Development and Comparative Evaluation of Two Highly Sensitive Immunosensor Platforms for Trace Determination of Copper Ions in Drinking Water Using a Monoclonal Antibody Specific to Copper-EDTA Complex. Molecules. 2023; 28(20):7017. https://doi.org/10.3390/molecules28207017
Chicago/Turabian StyleDarwish, Ibrahim A., Zongzhi Wang, and Ryhan J. Darling. 2023. "Development and Comparative Evaluation of Two Highly Sensitive Immunosensor Platforms for Trace Determination of Copper Ions in Drinking Water Using a Monoclonal Antibody Specific to Copper-EDTA Complex" Molecules 28, no. 20: 7017. https://doi.org/10.3390/molecules28207017
APA StyleDarwish, I. A., Wang, Z., & Darling, R. J. (2023). Development and Comparative Evaluation of Two Highly Sensitive Immunosensor Platforms for Trace Determination of Copper Ions in Drinking Water Using a Monoclonal Antibody Specific to Copper-EDTA Complex. Molecules, 28(20), 7017. https://doi.org/10.3390/molecules28207017







