Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Profiling
2.2. Effect of B. officinalis Extracts on Skin Cell Viability
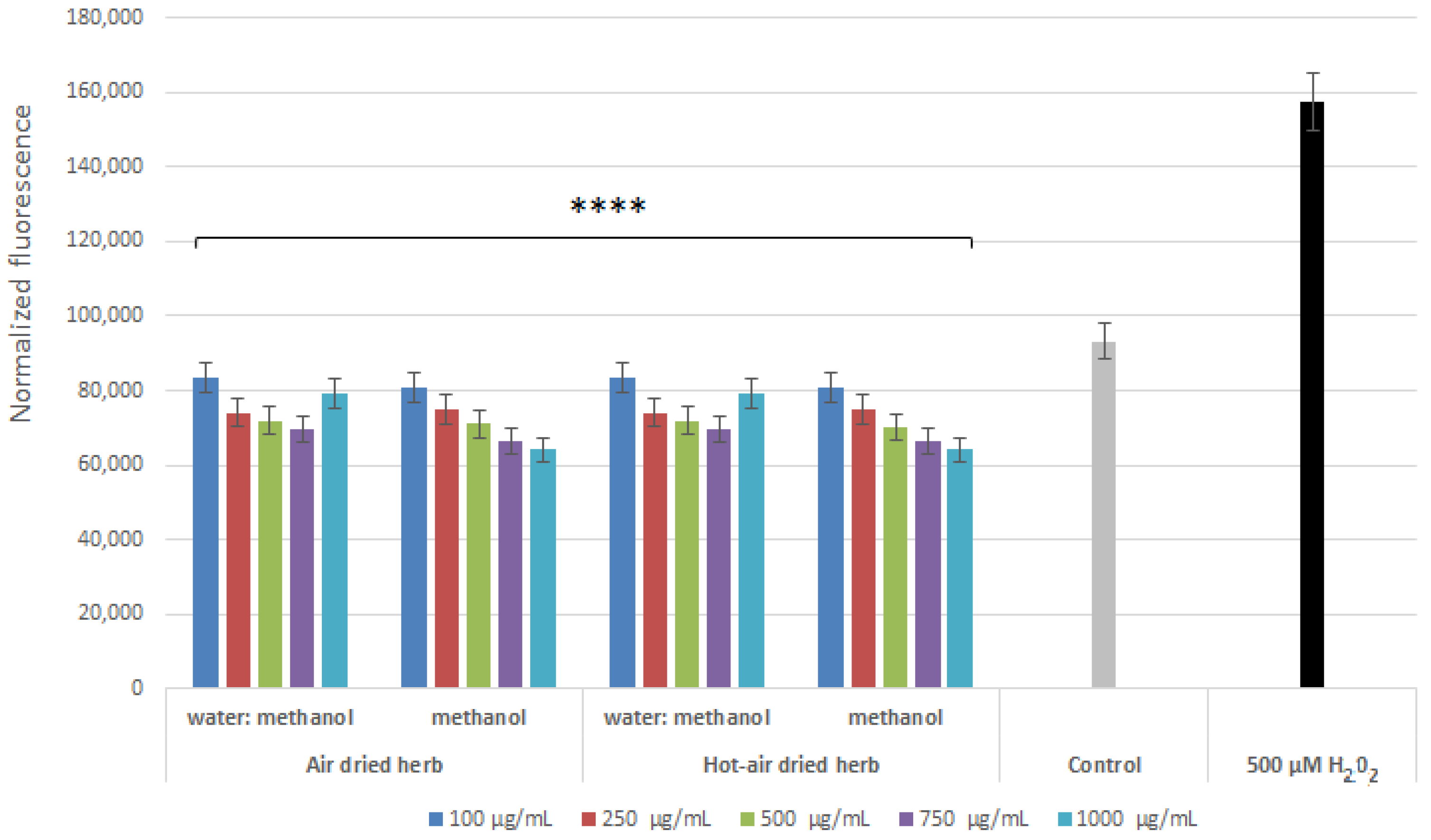
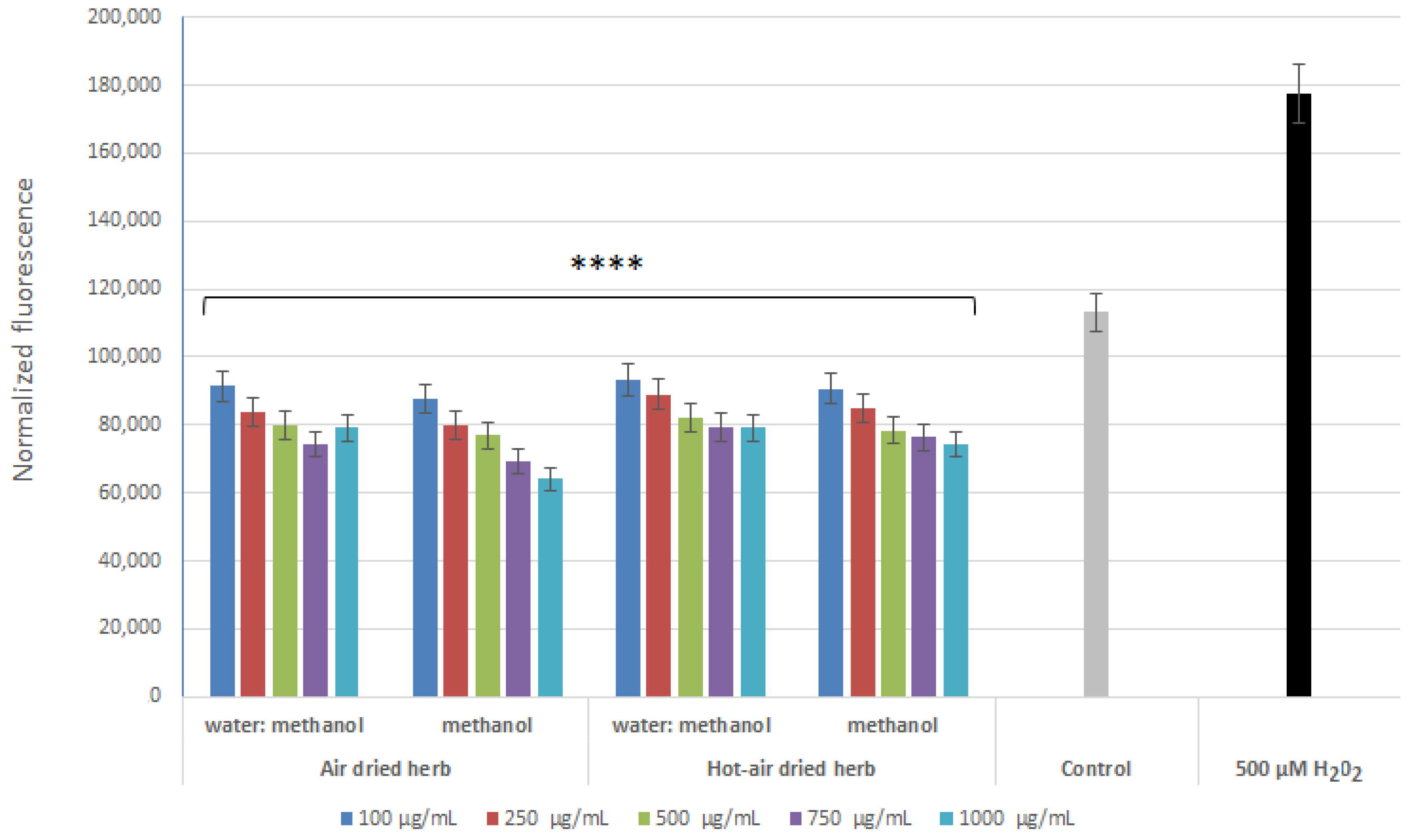
2.3. Intracellular ROS Levels in Skin Cells
2.4. Anti-Inflammatory Properties of B. officinalis
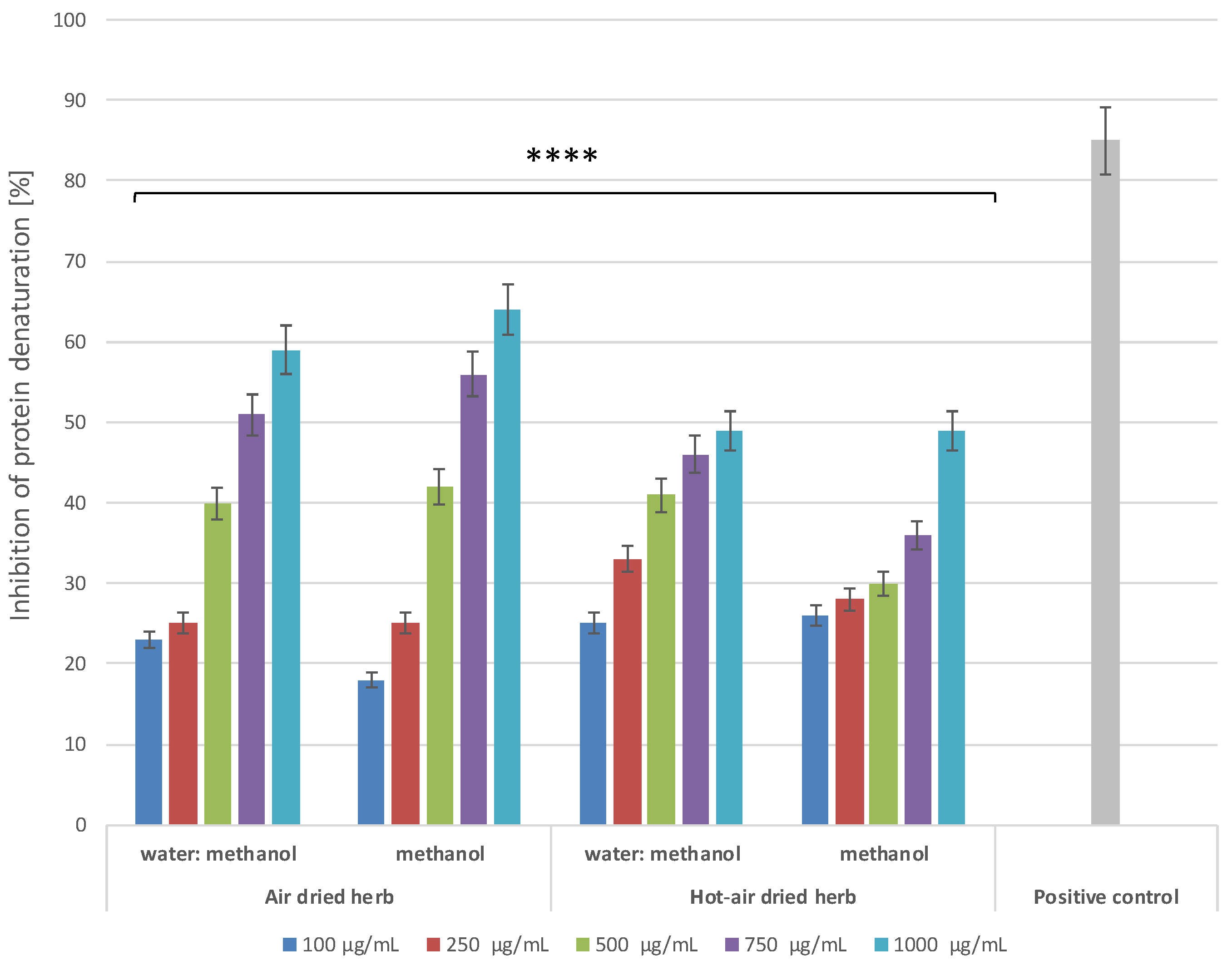
2.4.1. Inhibition of Protein Denaturation
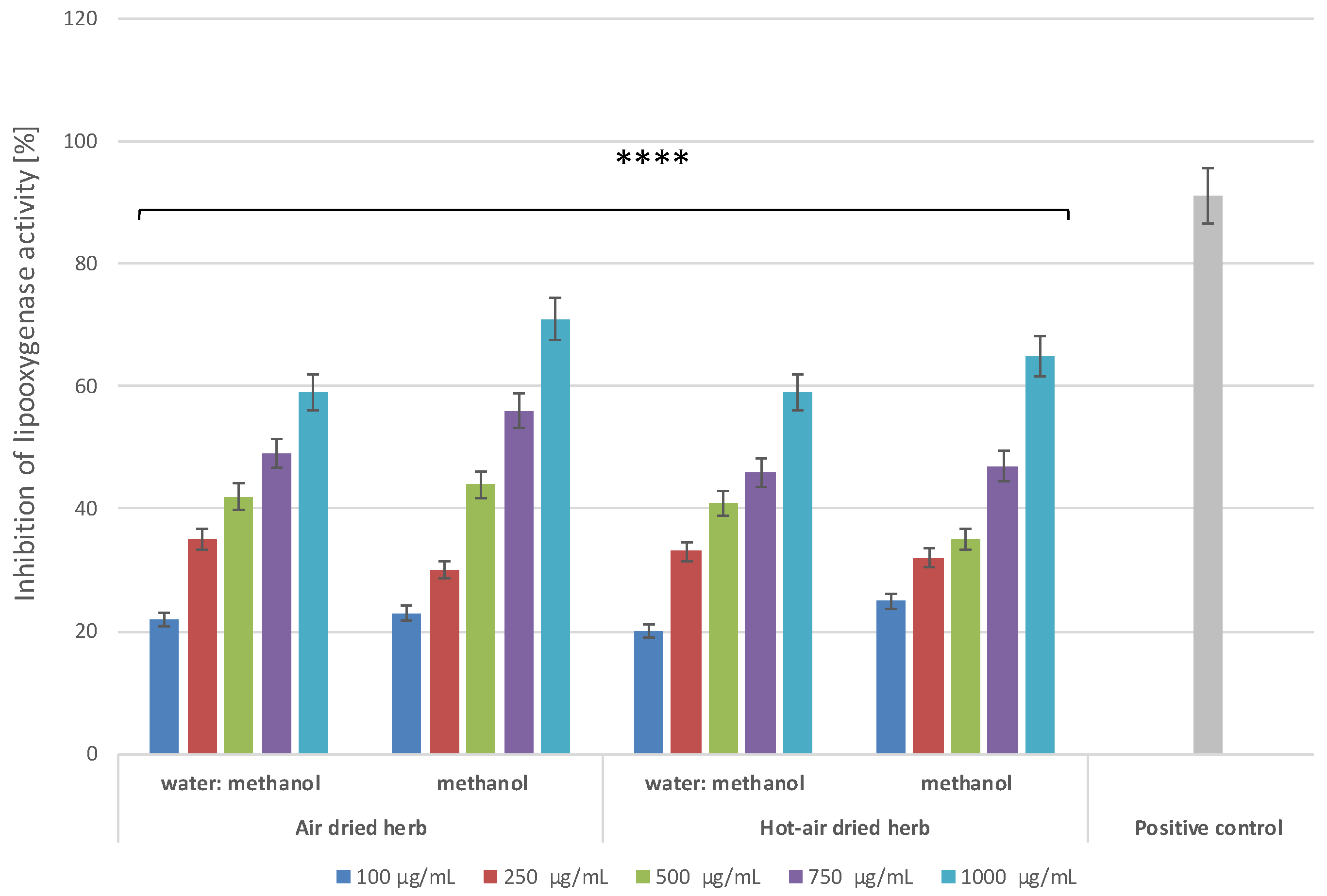
2.4.2. Lipoxygenase Inhibitory Activity
2.4.3. Proteinase Inhibitory Activity
2.5. Anti-Ageing Properties of B. officinalis
3. Materials and Methods
3.1. Plant Material
3.2. Extract Preparation
3.3. Determination of Bioactive Compounds with HPLC-DAD
3.4. Cell Culture
3.5. Assessment of Cytotoxicity—Alamar Blue Assay
3.6. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
3.7. Anti-Inflammatory Activity
3.7.1. Assessment of Inhibition of Protein Denaturation
- As—the absorbance of the test sample,
- Ac—the absorbance of the control sample.
3.7.2. Assessment of Inhibition of Lipoxygenase Activity
- As—the absorbance of the test sample,
- Ac—the absorbance of the control sample.
3.7.3. Assessment of Inhibition of Proteinase Activity
- A1—the absorbance of the control sample,
- A2—the absorbance of the test sample.
3.8. Anti-Ageing Activity
3.8.1. Determination of Anti-Collagenase Activity
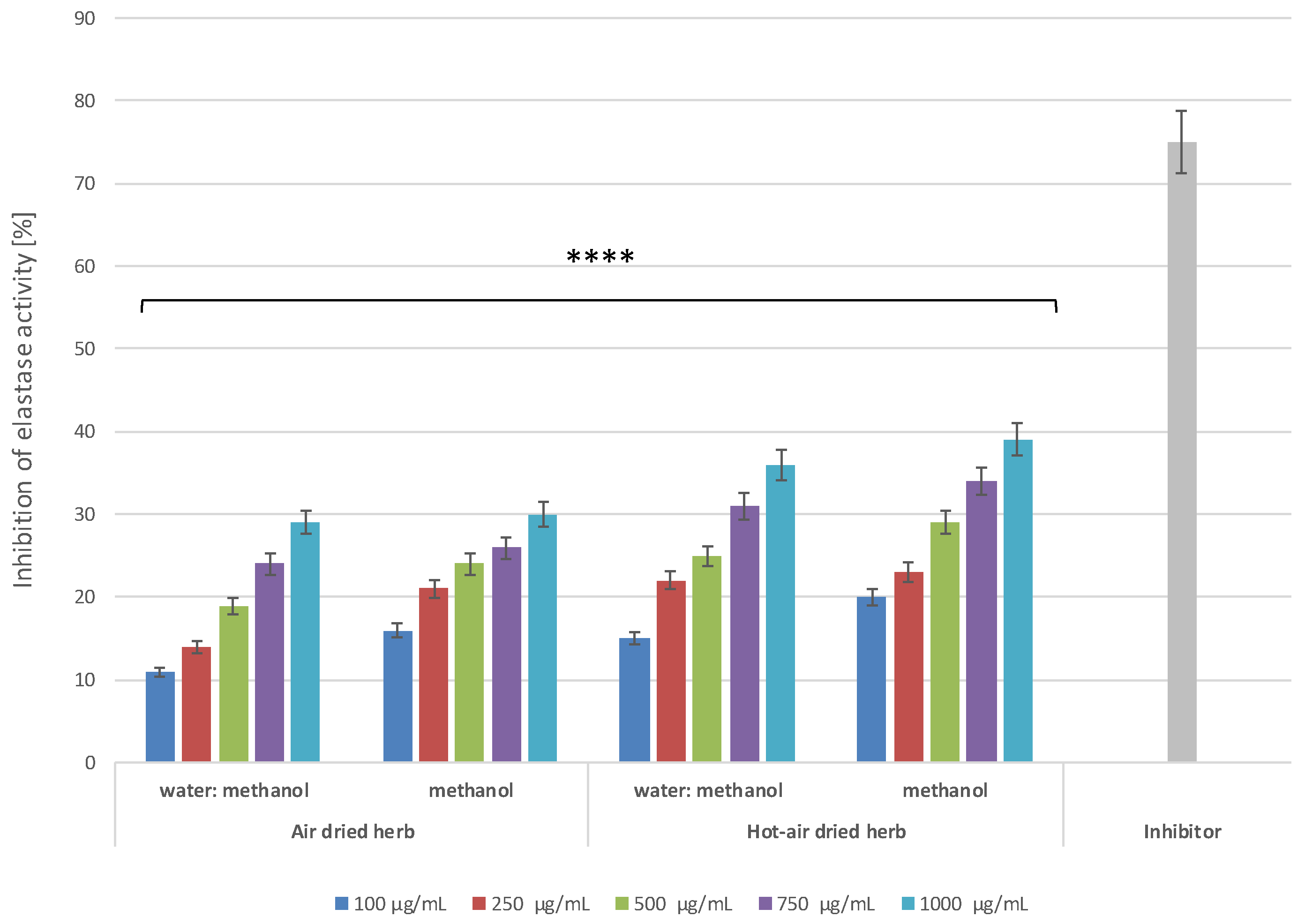
3.8.2. Determination of Anti-Elastase Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Karimi, E.; Oskoueian, E.; Karimi, A.; Noura, R.; Ebrahimi, M. Borago officinalis L. flower: A comprehensive study on bioactive compounds and its health-promoting properties. J. Food. Meas. Charact. 2017, 12, 826–838. [Google Scholar] [CrossRef]
- Pieszak, M.; Mikołajczak, P.Ł.; Manikowska, K. Borage (Borago officinalis L.)—A valuable medicinal plant used in herbal medicine. Herba Pol. 2012, 58, 95–103. [Google Scholar]
- Asadi-Samani, M.; Bahmani, M.; Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: A review. Asian Pac. J. Trop. Med. 2014, 7, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kareem, A.T.; Hamad, M.N. Separation and identification of phenolic acid from Borago officinalis (F:Boraginaceae) cultivated in Iraq. Iraqi J. Pharm. Sci. 2020, 29, 139–151. [Google Scholar] [CrossRef]
- Abu-Qaoud, H.; Shawarb, N.; Hussen, F.; Jaradat, N.; Shtaya, M. Comparison of qualitative, quantitative analysis and antioxidant potential between wild and cultivated Borago officinalis leaves from palestine. Pak. J. Pharm. Sci. 2018, 31, 953–959. [Google Scholar] [PubMed]
- Zemmouri, H.; Ammar, S.; Boumendjel, A.; Messarah, M.; El Feki, A.; Bouaziz, M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria. Arab. J. Chem. 2019, 12, 1954–1963. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Cytoprotective Effect of Ascorbic Acid and Rutin against Oxidative Changes in the Proteome of Skin Fibroblasts Cultured in a Three-Dimensional System. Nutrients 2020, 12, 1074. [Google Scholar] [CrossRef]
- Fernando, P.M.D.J.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.K.M.; Chae, S.W.; Hyun, J.W. Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells. Biomol. Ther. 2016, 24, 75–84. [Google Scholar] [CrossRef]
- You, O.H.; Shin, E.A.; Lee, H.; Kim, J.-H.; Sim, D.Y.; Kim, J.H.; Kim, Y.; Khil, J.-H.; Baek, N.-I.; Kim, S.-H. Apoptotic effect of astragalin in melanoma skin cancers via activation of caspases and inhibition of sry-related HMg-box gene 10. Phytother. Res. 2017, 31, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Qattan, M.Y.; Khan, M.I.; Alharbi, S.H.; Verma, A.K.; Al-Saeed, F.A.; Abduallah, A.M.; Al Areefy, A.A. Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signalling Pathways. Molecules 2022, 27, 8864. [Google Scholar] [CrossRef] [PubMed]
- Gado, F.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Manera, C. Phenolic Compounds in Prevention and Treatment of Skin Cancers: A review. Curr. Med. Chem. 2021, 28, 6730–6752. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Zhang, L.; Cheng, Z.; Deng, X.; Shu, G. Astragalin reduces hexokinase 2 through increasing miR-125b to inhibit the proliferation of hepatocellular carcinoma cells in vitro and in vivo. J. Agric. Food Chem. 2017, 65, 5961–5972. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cai, F.; Zha, D.; Wang, X.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.-C. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-κB pathway. Oncotarget 2017, 8, 26941–26958. [Google Scholar] [CrossRef] [PubMed]
- Moliner, C.; Cásedas, G.; Barros, L.; Finimundy, T.C.; Gómez-Rincón, C.; López, V. Neuroprotective Profile of Edible Flowers of Borage (Borago officinalis L.) in Two Different Models: Caenorhabditis elegans and Neuro-2a Cells. Antioxidants 2022, 11, 1244. [Google Scholar] [CrossRef]
- Seo, S.A.; Park, B.; Hwang, E.; Park, S.-Y.; Yi, T.-H. Borago officinalis L. attenuates UVB-induced skin photodamage via regulation of AP-1 and Nrf2/ARE pathway in normal human dermal fibroblasts and promotion of collagen synthesis in hairless mice. Exp. Gerontol. 2018, 107, 178–186. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago offcinalis L.) seeds. Food Chem. 1999, 67, 399–414. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Lupše, B.; Maedler, K.; Sarma, B.; Radtke, A.; Belge, G.; Dorsch, M.; Wedekind, D.; McCawley, L.J.; Boehm, G.; et al. Matrix Metalloproteinase-3 is Key Effector of TNF-α-Induced Collagen Degradation in Skin. Int. J. Mol. Sci. 2019, 20, 5234. [Google Scholar] [CrossRef]
- Chou, C.T. The antiinflammatory effect of an extract of Tripterygium wilfordii hook F on adjuvant-induced paw oedema in rats and inflammatory mediators release. Phytother. Res. 1997, 11, 152–154. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ma, S. Recent development of lipoxygenase inhibitors as anti-inflammatory agents. Med. Chem. Comm. 2017, 29, 212–225. [Google Scholar] [CrossRef]
- Assiry, A.A.; Bhavikatti, S.K.; Althobaiti, F.A.; Mohamed, R.N.; Karobari, M.I. Evaluation of In Vitro Antiprotease Activity of Selected Traditional Medicinal Herbs in Dentistry and Its In Silico PASS Prediction. Biomed. Res. Int. 2022, 2022, 5870443. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Perużyńska, M.; Cybulska, K.; Kucharska, E.; Ossowicz-Rupniewska, P.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł.; Sulikowski, T.; et al. Assessment of the Anti-Inflammatory, Antibacterial and Anti-Aging Properties and Possible Use on the Skin of Hydrogels Containing Epilobium angustifolium L. Extracts. Front. Pharmacol. 2022, 13, 896706. [Google Scholar] [CrossRef] [PubMed]
- Asaad, G.F.; Redai, A.Q.; Hakami, A.O.; Ghazwani, F.H.; Nomier, Y.; Alshahrani, S. Potential analgesic and anti-inflammatory effect of Cuminum cyminum and Borago officinalis in rats and mice. Asian J. Pharm. Clin. Res. 2020, 13, 216–218. [Google Scholar]
- Barati, E.; Soleimani Asl, S.; Pourbakhsh, S.A.; Jamshidian, M.; Shahidi, S. Investigating the effect of Borago officnale on hipocampal IL-1 beta protein and gene in the amyloid β-peptide (25-35)-induced of inflammation in rat. Biomed. Pharmacol. J. 2015, 8, 937–943. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as Anti-Inflammatory Agents:Implications in Cancer and Cardiovascular Disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Alcaraz, M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions 1991, 32, 283–288. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Lembo, S.; Balato, A.; Di Caprio, R.; Cirillo, T.; Giannini, V.; Gasparri, F.; Monfrecola, G. The modulatory effect of ellagic acid and rosmarinic acid on ultraviolet-B-induced cytokine/chemokine gene expression in skin keratinocyte (HaCaT) cells. Biomed. Res. Int. 2014, 2014, 346793. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Koh, J.; Kim, Y.S.; Park, D. Effect of rosmarinic acid on atopic dermatitis. J. Dermatol. 2008, 35, 768–771. [Google Scholar] [CrossRef]
- Ha, S.J.; Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Song, K.-M.; Chang, P.-S.; Jeong, C.-H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Kim, Y.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin-aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Szopa, A.; Starzec, A.; Ekiert, H. The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J. Photochem. Photobiol. B Biol. 2018, 179, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, H. In vitro cultures of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine)—A potential biotechnological rich source of therapeutically important phenolic acids. Appl. Biochem. Biotechnol. 2012, 166, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Furman-Toczek, D.; Szczepanek, D.; Sowa, I. In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts. Int. J. Mol. Sci. 2022, 23, 13755. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Zagórska-Dziok, M. Antioxidant activity and cytotoxicity of Jerusalem artichoke tubers and leaves extract on HaCaT and BJ fibroblast cells. Lipids Health Dis. 2018, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Sarveswaran, R.; Jayasuriya, W.J.A.B.; Suresh, T.S. In vitro assays to investigate the anti-inflammatory activity of herbal extracts: A review. World J. Pharm. Res. 2017, 6, 131–141. [Google Scholar]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, A.; Sakat, S.; Wankhede, S.; Juvekar, M.; Gambhire, M. Evaluation of Antioxidant and Anti-Inflammatory Activity of Methanol Extract of Oxalis Corniculata. Planta Med. 2009, 75, PJ178. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Bujak, T.; Zagórska-Dziok, M.; Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T. Effect of Fermentation Time on Antioxidant and Anti-Ageing Properties of Green Coffee Kombucha Ferments. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef]


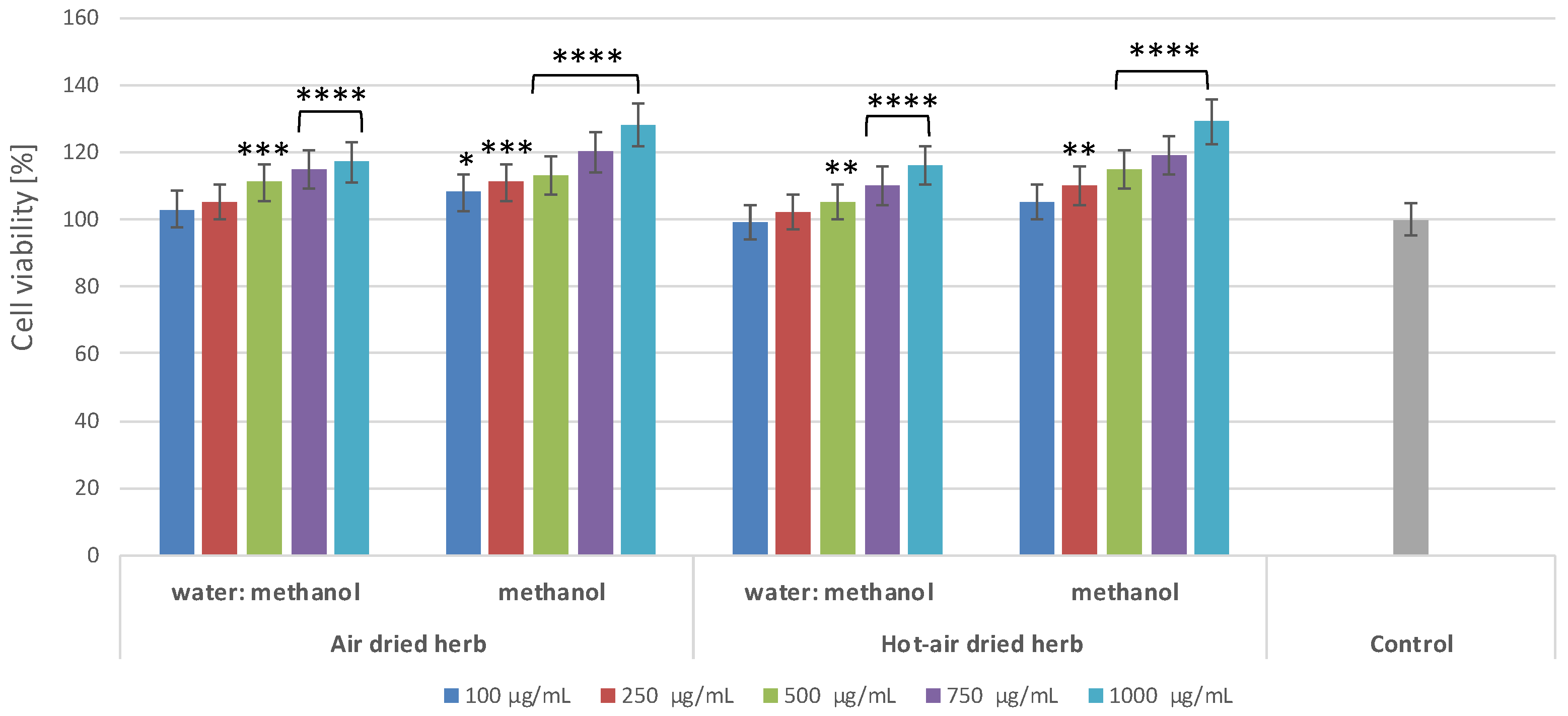

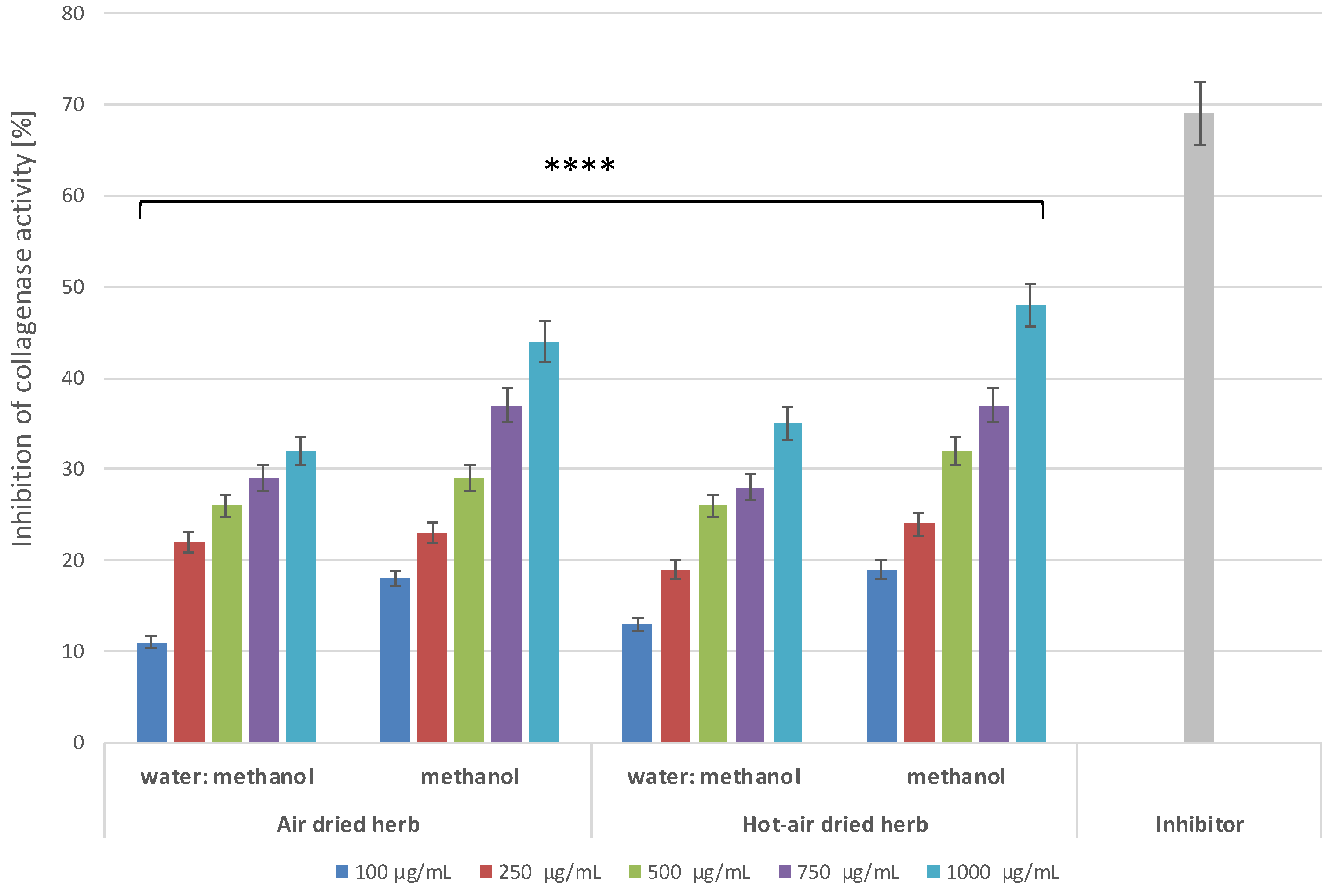
| Flavonoids | Air-Dried Herb | Hot-Air-Dried Herb | ||
|---|---|---|---|---|
| Methanol Extract | Water:Methanol Extract (70:30) | Methanol Extract | Water:Methanol Extract (70:30) | |
| Astragalin | 147.40 ± 13.77 bcd | 48.96 ± 1.95 ac | 248.60 ± 12.47 abd | 61.54 ± 1.21 ac |
| Kaempferol 4-glucoside | 70.73 ± 11.58 bcd | 39.79 ± 1.38 acd | 141.08 ± 1.43 abd | 26.69 ± 1.36 ac |
| Rutoside | 77.09 ± 11.31 cd | 66.06 ± 2.72 cd | 95.71 ± 1.70 abd | 42.22 ± 0.65 abc |
| Vitexin | 40.84 ± 4.28 bd | 3.28 ± 0.30 ac | 42.84 ± 6.07 bd | 1.41 ± 0.08 ac |
| Total content | 336.06 ± 40.94 bcd | 158.09 ± 6.35 ac | 528.23 ± 21.67 abd | 131.86 ± 3.30 ac |
| Phenolic Acids | Air-Dried Herb | Hot-Air-Dried Herb | ||
|---|---|---|---|---|
| Methanol Extract | Water:Methanol Extract (70:30) | Methanol Extract | Water:Methanol Extract (70:30) | |
| Caffeic acid | 18.56 ± 0.94 bd | 28.66 ± 5.17 ac | 20.42 ± 5.29 bd | 25.13 ± 0.66 ac |
| Chlorogenic acid | 13.71 ± 4.78 bd | 33.90 ± 3.63 abc | 11.13 ± 2.76 bd | 23.63 ± 2.26 abc |
| 3,4-Dihydroxyphenylacetic acid | 101.48 ± 5.48 bcd | 260.17 ± 8.68 ad | 243.61 ± 18.25 ad | 189.88 ± 6.79 abc |
| Ferulic acid | 171.50 ± 16.49 bcd | 9.95 ± 0.63 ac | 205.30 ± 6.64 abd | 7.95 ± 0.41 ac |
| p-Hydroxybenzoic acid | 4.68 ± 0.56 bcd | 6.64 ± 0.40 acd | 5.71 ± 0.59 abd | 5.85 ± 0.17 ab |
| Protocatechuic acid | 8.80 ± 1.23 c | 10.30 ± 0.23 cd | 4.16 ± 0.42 abd | 8.06 ± 0.25 bc |
| Rosmarinic acid | 1173.80 ± 14.03 bcd | 309.22 ± 14.34 acd | 1783.55 ± 54.07 abd | 227.58 ± 9.93 abc |
| Syringic acid | 17.81 ± 1.08 bcd | 10.72 ± 1.06 ad | 7.31 ± 1.19 ad | 0.96 ± 0.07 abc |
| Total content | 1510.34 ± 44.59 bcd | 669.56 ± 34.14 acd | 2281.19 ± 89.21 abd | 489.04 ± 20.54 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells. Molecules 2023, 28, 868. https://doi.org/10.3390/molecules28020868
Michalak M, Zagórska-Dziok M, Klimek-Szczykutowicz M, Szopa A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells. Molecules. 2023; 28(2):868. https://doi.org/10.3390/molecules28020868
Chicago/Turabian StyleMichalak, Monika, Martyna Zagórska-Dziok, Marta Klimek-Szczykutowicz, and Agnieszka Szopa. 2023. "Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells" Molecules 28, no. 2: 868. https://doi.org/10.3390/molecules28020868
APA StyleMichalak, M., Zagórska-Dziok, M., Klimek-Szczykutowicz, M., & Szopa, A. (2023). Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells. Molecules, 28(2), 868. https://doi.org/10.3390/molecules28020868









